In Vitro Antibacterial Activities and Calf Thymus DNA–Bovine Serum Albumin Interactions of Tridentate NNO Hydrazone Schiff Base–Metal Complexes
Abstract
1. Introduction
2. Results and Discussion
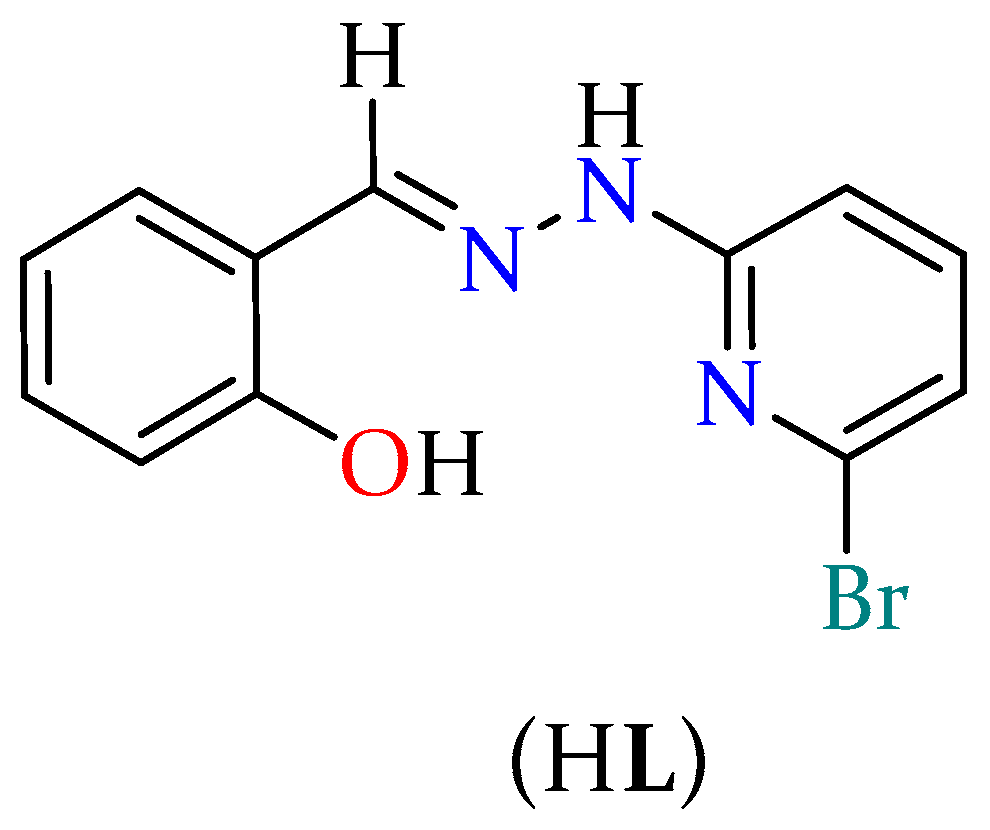
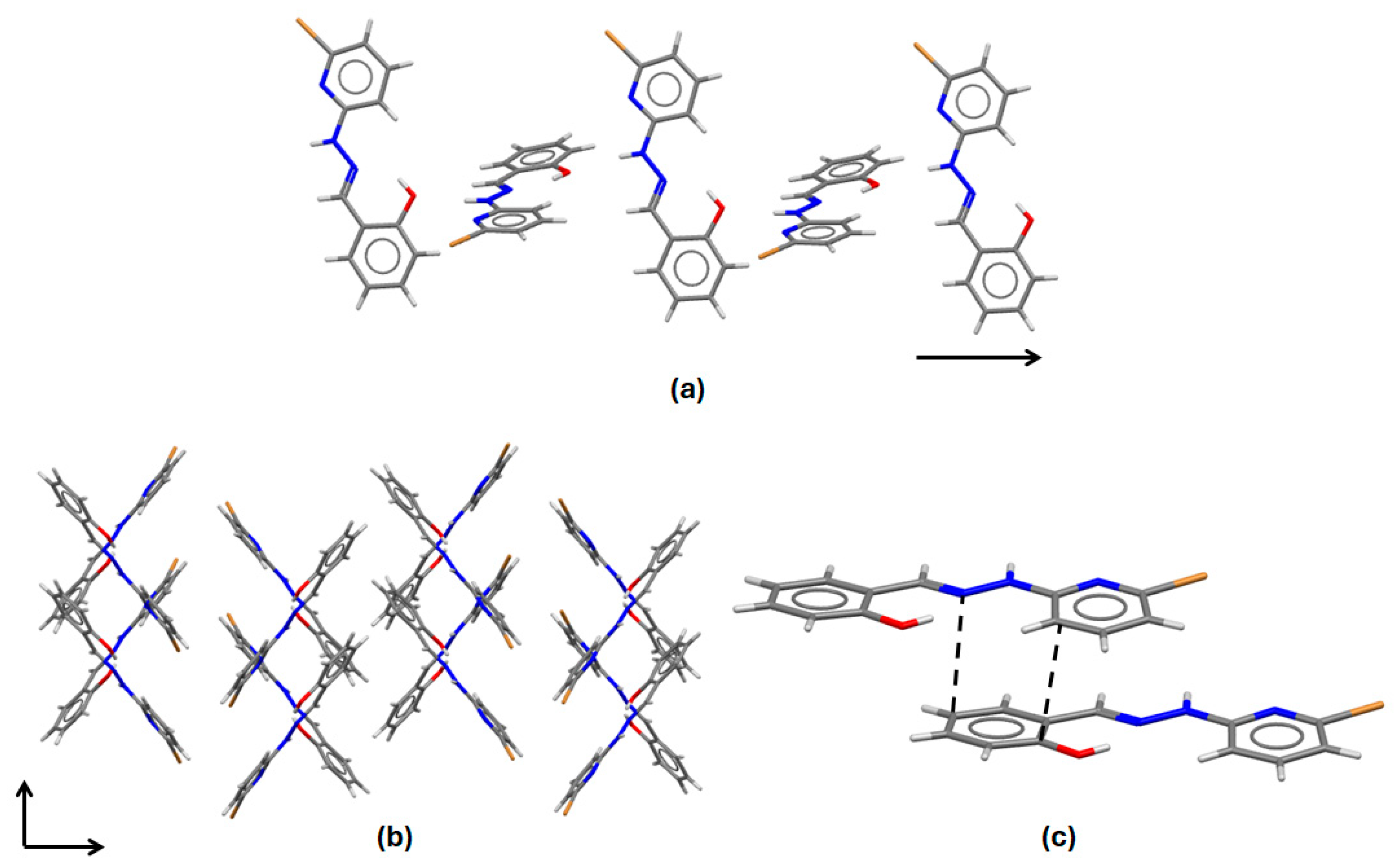
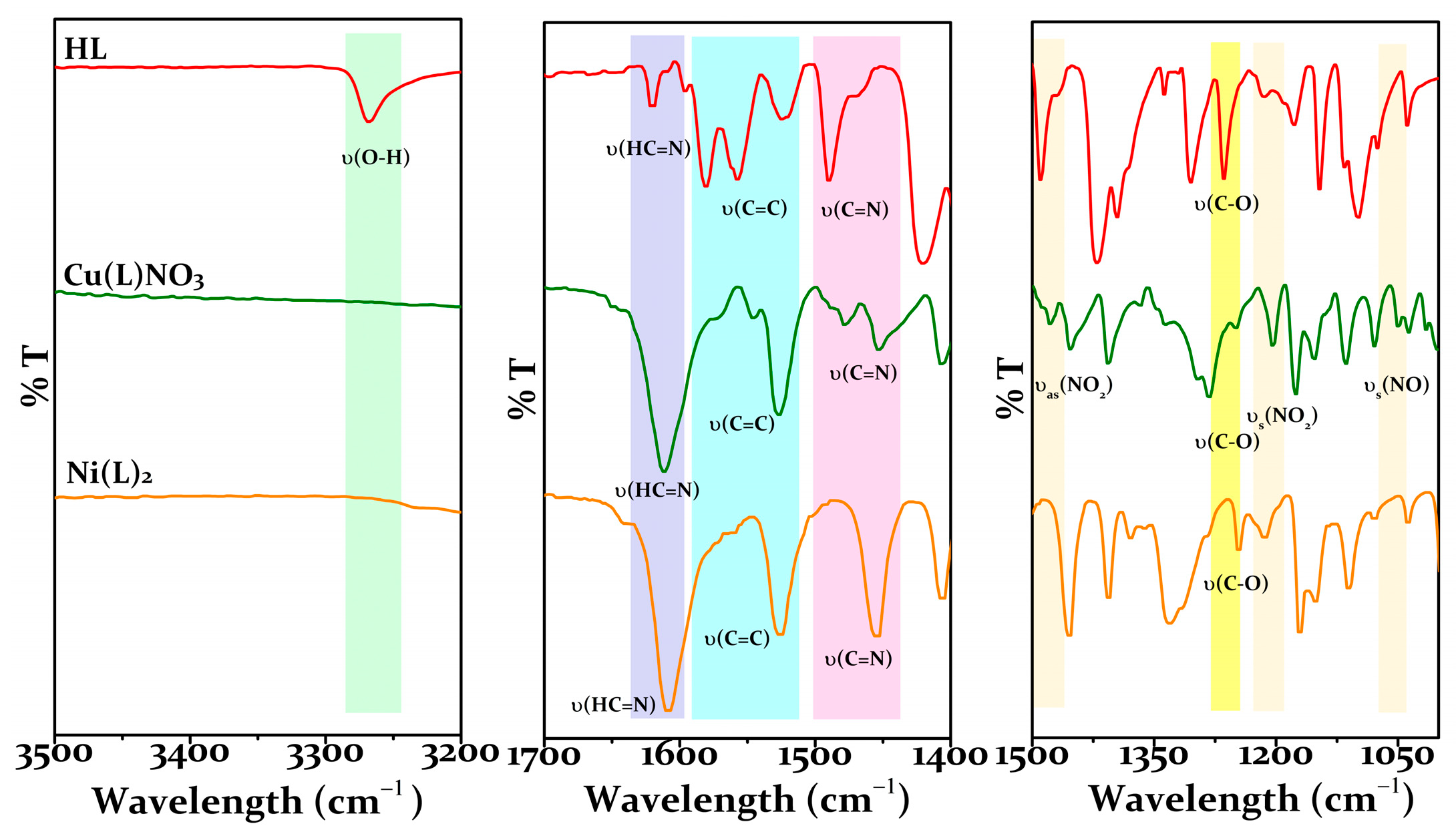
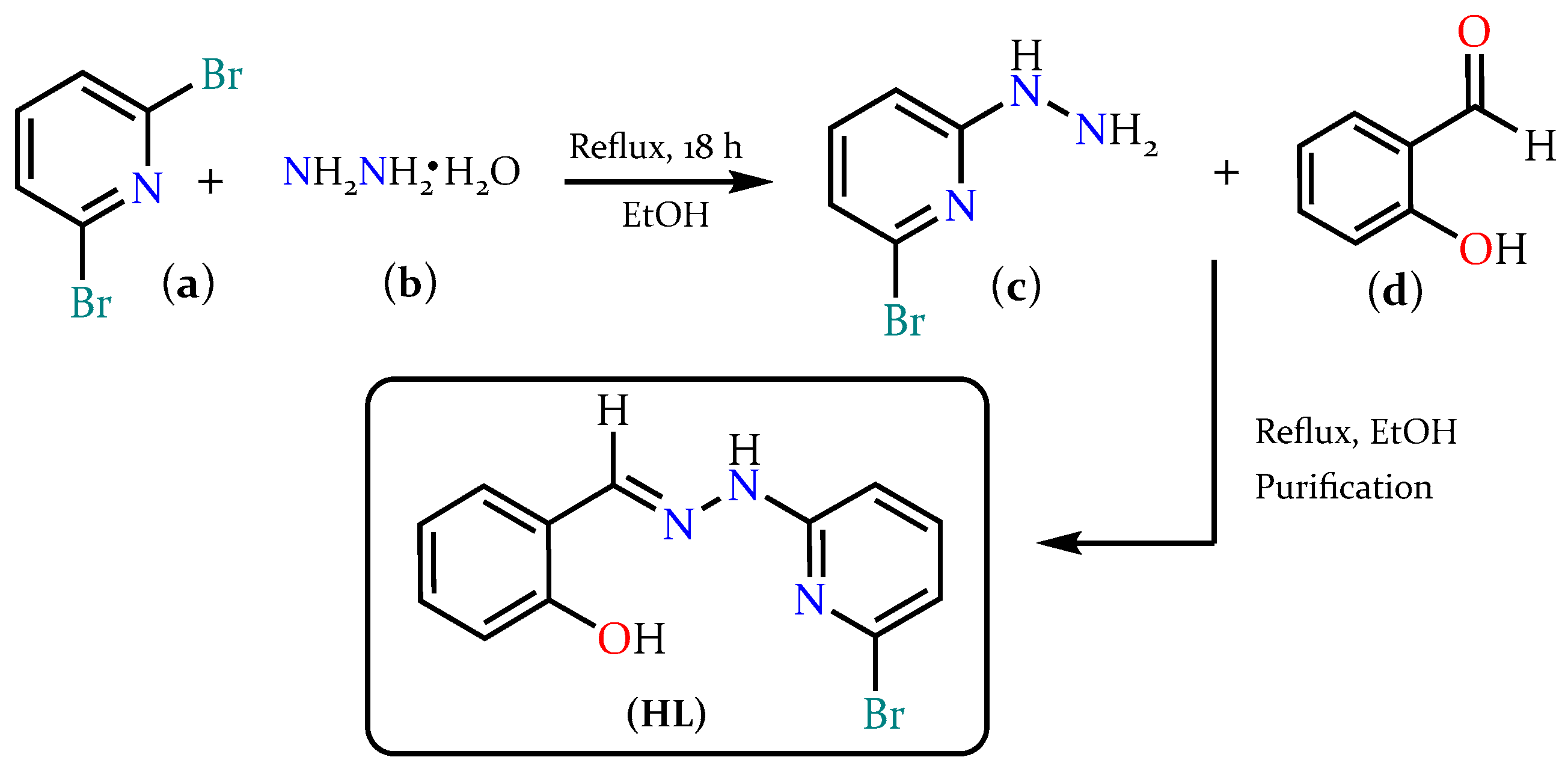
2.1. Synthesis and Characterization of HL
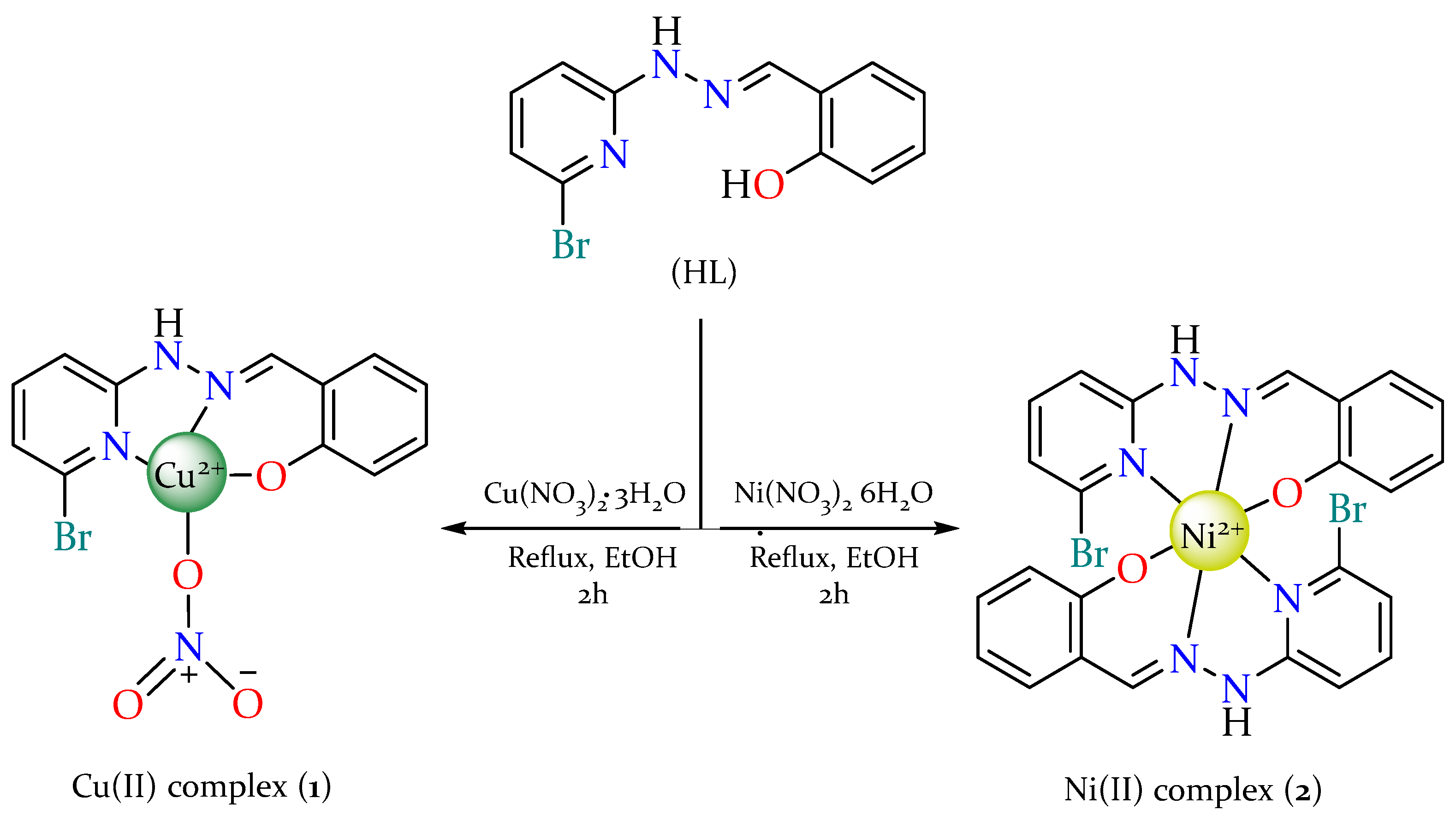
2.2. Synthesis and Characterization of Metal Complexes
2.3. Biological Studies
2.3.1. Antibacterial Activity
2.3.2. DNA Interactions
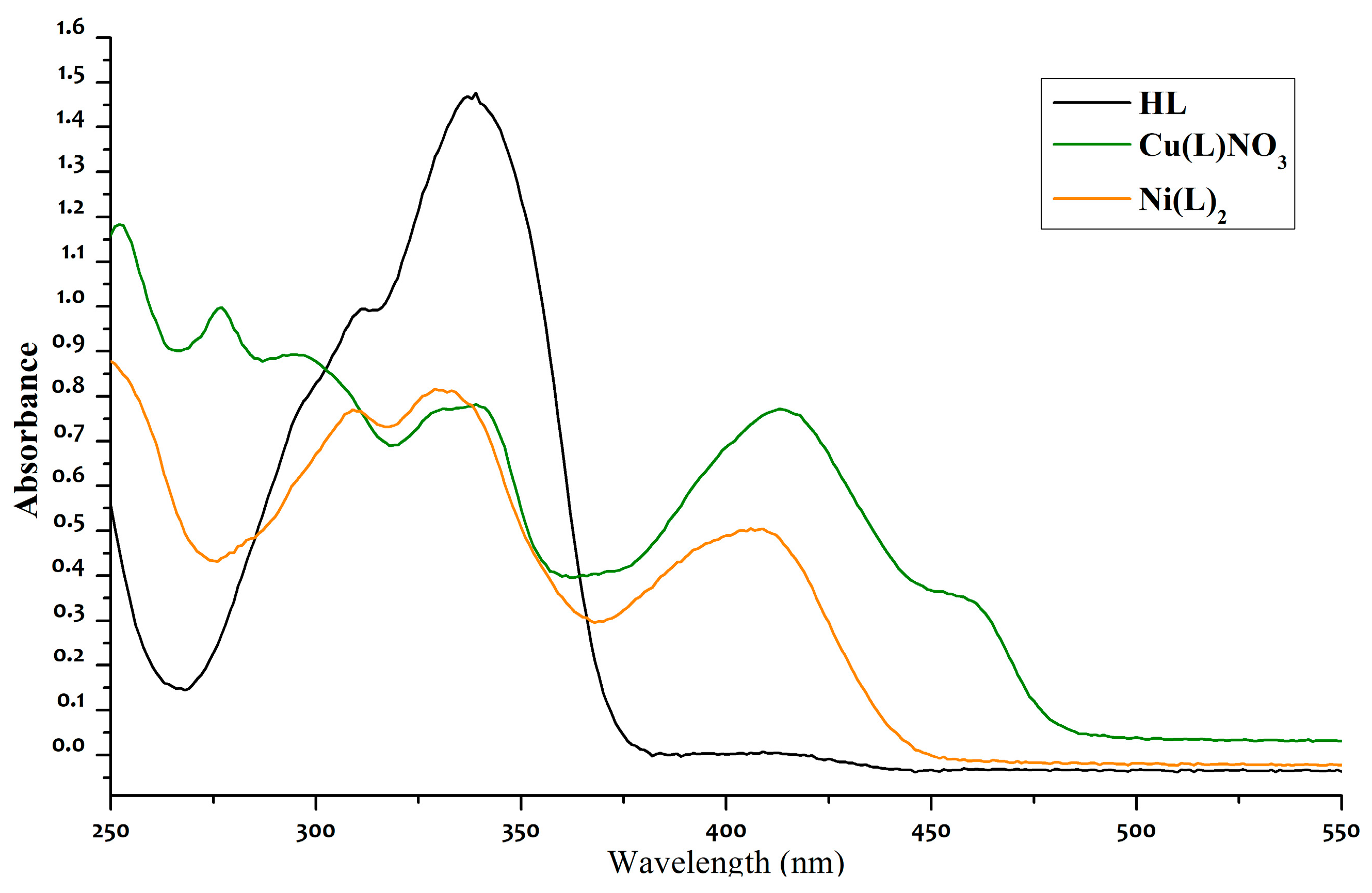
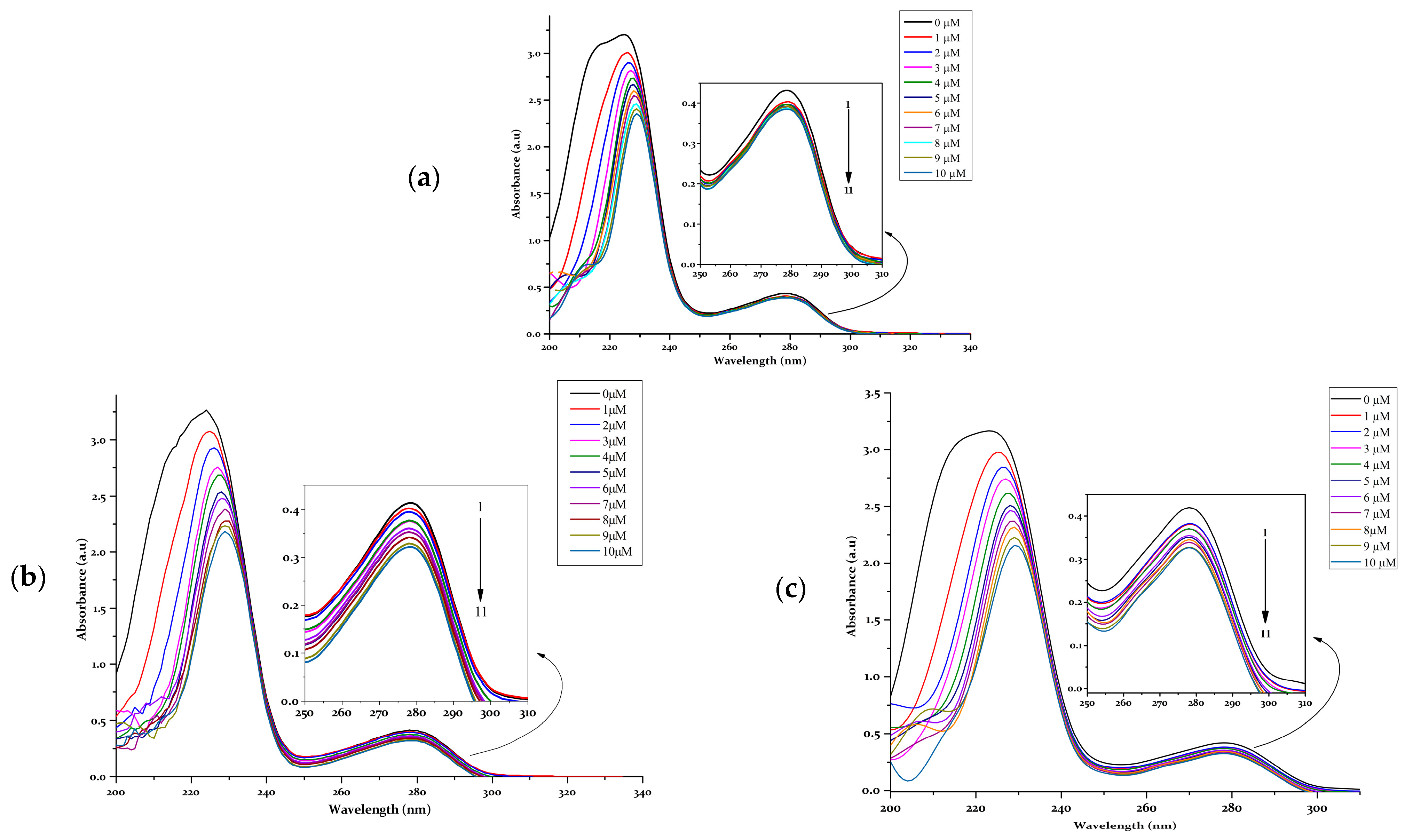
- UV-vis studies
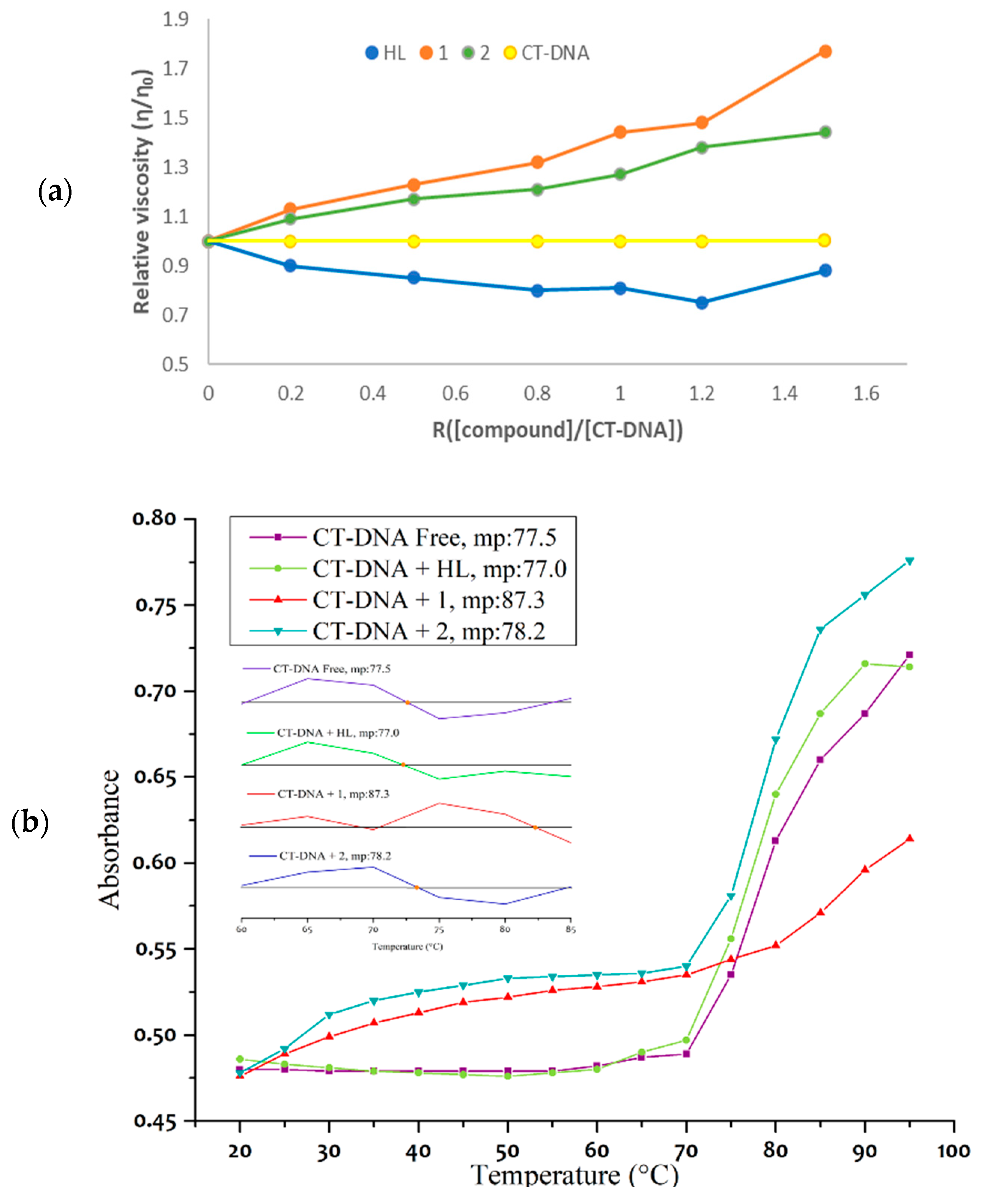
- Viscosity measurements and DNA melting
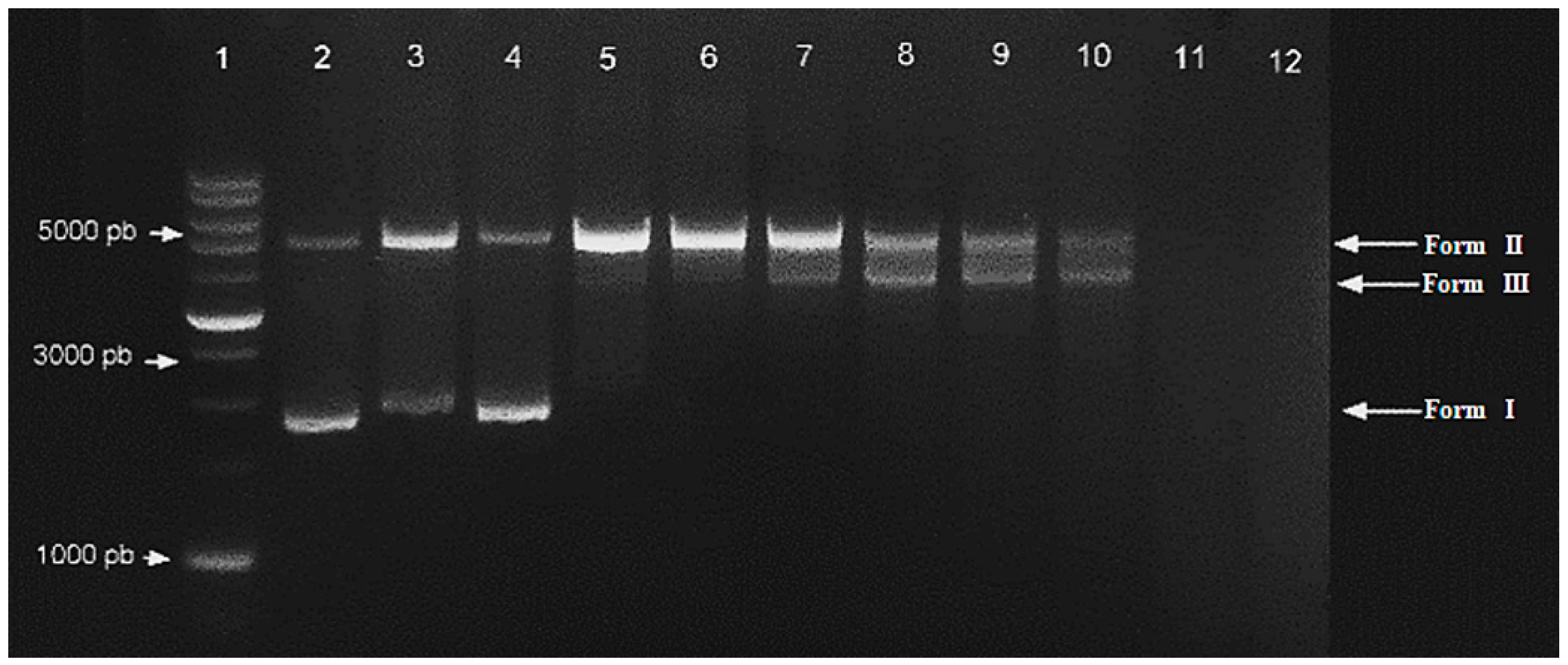
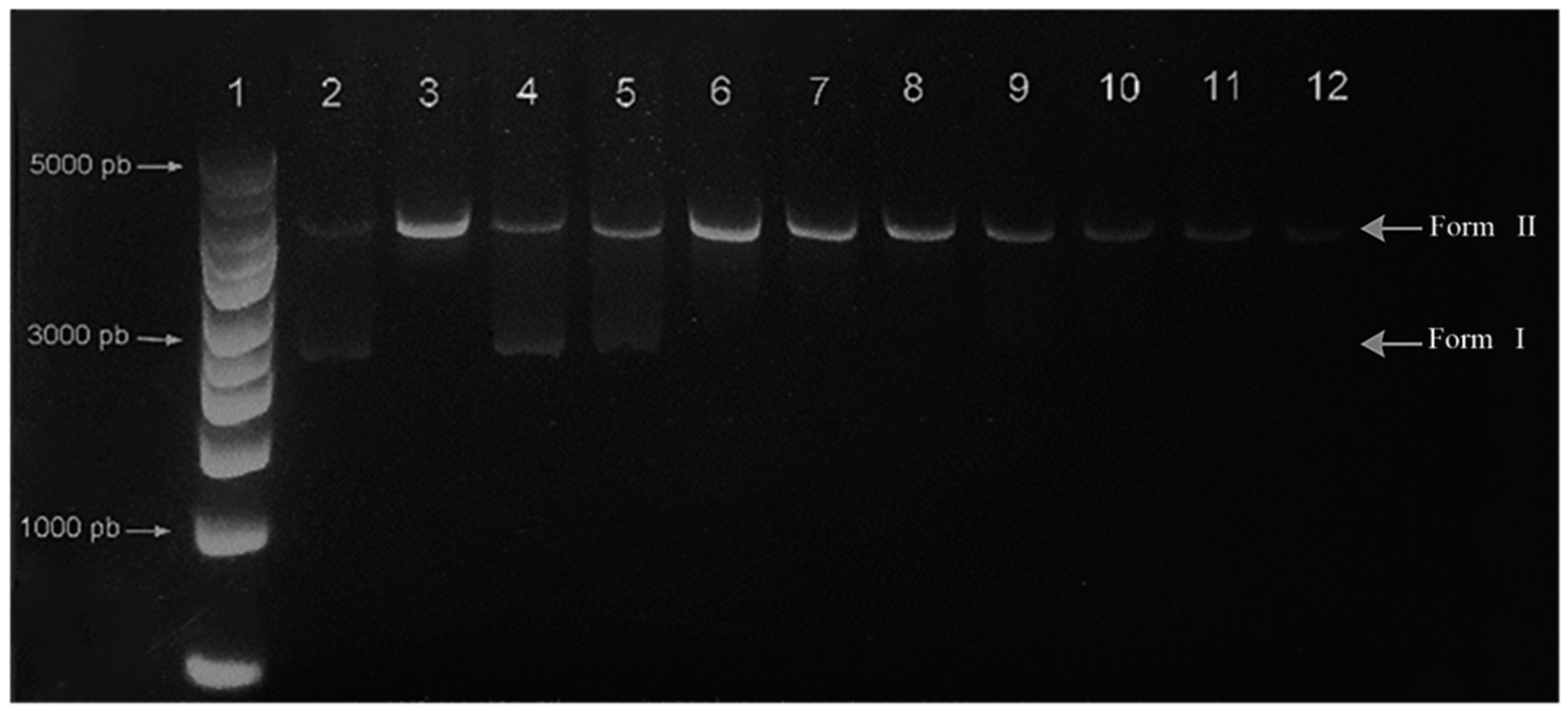
- DNA cleavage
2.3.3. BSA Interaction
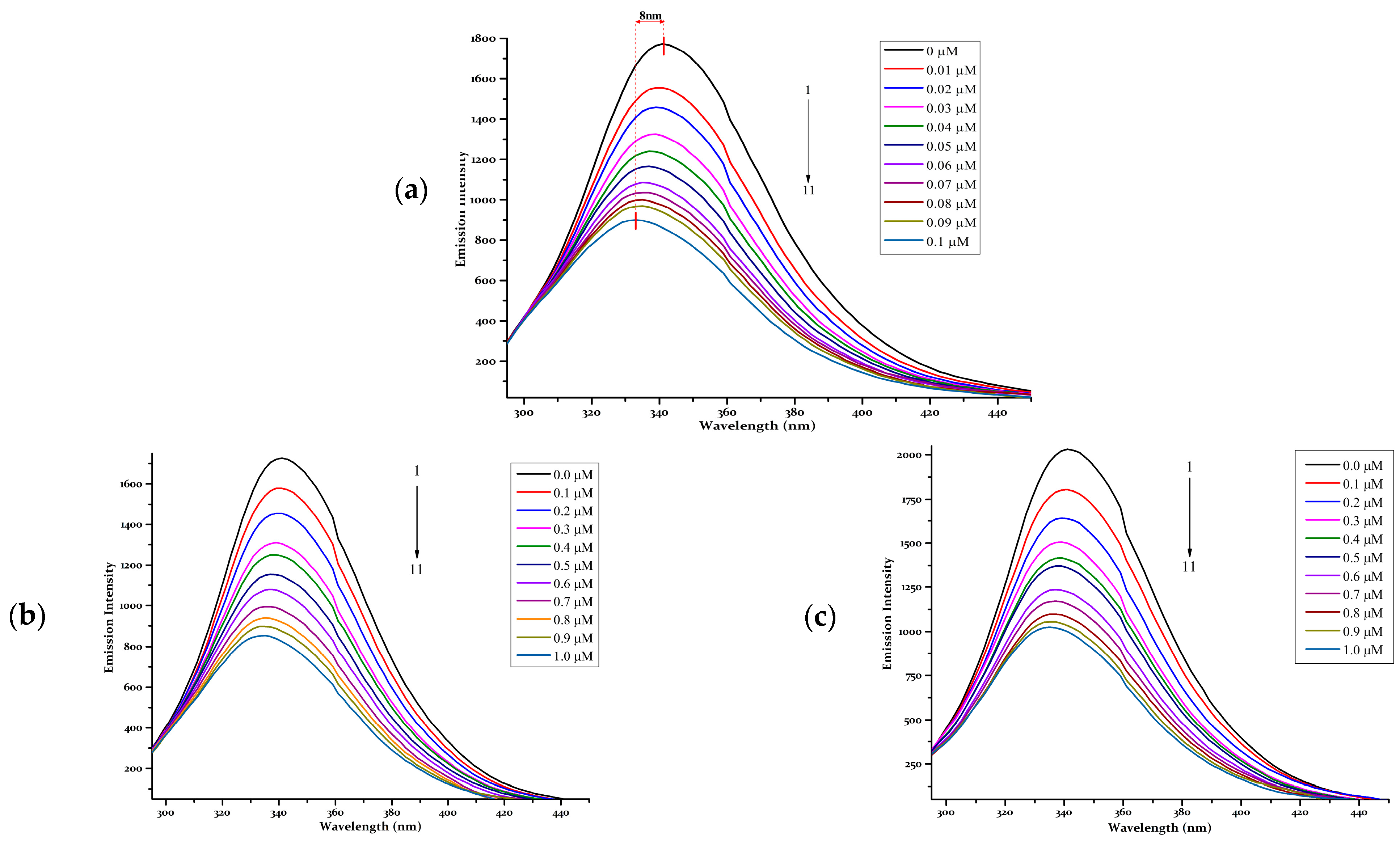
- BSA fluorescence-quenching assays
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ligand (HL)
3.3. Synthesis of Metal(II) Complexes
3.3.1. Synthesis of [Cu(L)(NO3)] (1)
3.3.2. Synthesis of [Ni(L)2] (2)
3.4. Single-Crystal Structure Determination of HL
3.5. Antibacterial Activity Test
3.6. DNA Interaction
3.7. BSA Interaction
3.8. Stability Studies of the Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The Silent Pandemic: Emergent Antibiotic Resistances Following the Global Response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.; Li, Z.; Zhang, X.; Elnagdy, S.; Mansour, M.S.; ElSherif, A.; Ma, N.; Abd-El-Aziz, A.S. Advances in Coordination Chemistry of Schiff Base Complexes: A Journey from Nanoarchitectonic Design to Biomedical Applications. Top. Curr. Chem. 2025, 383, 8. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Reyes-Márquez, V.; Cañavera-Buelvas, F.; Parra-Unda, J.R.; Cuenú-Cabezas, F.; Polo-Cerón, D.; Colorado-Peralta, R.; Suárez-Moreno, G.V.; Aguilar-Castillo, B.A.; Morales-Morales, D. Pincer Complexes Derived from Tridentate Schiff Bases for Their Use as Antimicrobial Metallopharmaceuticals. Inorganics 2022, 10, 134. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal Complexes with Schiff Bases: Data Collection and Recent Studies on Biological Activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef] [PubMed]
- Czyżewska, I.; Mazur, L.; Popiołek, Ł. Transition Metal Complexes of Hydrazones as Potential Antimicrobial and Anticancer Agents: A Short Review. Chem. Biol. Drug Des. 2024, 104, e14590. [Google Scholar] [CrossRef]
- Shaabani, B.; Khandar, A.A.; Ramazani, N.; Fleck, M.; Mobaiyen, H.; Cunha-Silva, L. Chromium(III), Manganese(II) and Iron(III) Complexes Based on Hydrazone Schiff-Base and Azide Ligands: Synthesis, Crystal Structure and Antimicrobial Activity. J. Coord. Chem. 2017, 70, 696–708. [Google Scholar] [CrossRef]
- Jabeen, M. A Comprehensive Review on Analytical Applications of Hydrazone Derivatives. J. Turk. Chem. Soc. A Chem. 2022, 9, 663–698. [Google Scholar] [CrossRef]
- Cukierman, D.S.; Accardo, E.; Gomes, R.G.; De Falco, A.; Miotto, M.C.; Freitas, M.C.R.; Lanznaster, M.; Fernández, C.O.; Rey, N.A. Aroylhydrazones Constitute a Promising Class of ‘Metal-Protein Attenuating Compounds’ for the Treatment of Alzheimer’s Disease: A Proof-of-Concept Based on the Study of the Interactions between Zinc(II) and Pyridine-2-Carboxaldehyde Isonicotinoyl Hydrazone. J. Biol. Inorg. Chem. 2018, 23, 1227–1241. [Google Scholar] [CrossRef]
- Cukierman, D.S.; Rey, N.A. Tridentate N-Acylhydrazones as Moderate Ligands for the Potential Management of Cognitive Decline Associated with Metal-Enhanced Neuroaggregopathies. Front. Neurol. 2022, 13, 828654. [Google Scholar] [CrossRef]
- Mandewale, M.C.; Kokate, S.; Thorat, B.; Sawant, S.; Yamgar, R. Zinc Complexes of Hydrazone Derivatives Bearing 3,4-Dihydroquinolin-2(1H)-One Nucleus as New Anti-Tubercular Agents. Arab. J. Chem. 2019, 12, 4479–4489. [Google Scholar] [CrossRef]
- Balsa, L.M.; Ferraresi-Curotto, V.; Lavecchia, M.J.; Echeverría, G.A.; Piro, O.E.; García-Tojal, J.; Pis-Diez, R.; González-Baró, A.C.; León, I.E. Anticancer Activity of a New Copper(ii) Complex with a Hydrazone Ligand. Structural and Spectroscopic Characterization, Computational Simulations and Cell Mechanistic Studies on 2D and 3D Breast Cancer Cell Models. Dalton Trans. 2021, 50, 9812–9826. [Google Scholar] [CrossRef] [PubMed]
- Shakdofa, M.M.E.; Shtaiwi, M.H.; Morsy, N.; Abdel-rassel, T.M.A. Metal Complexes of Hydrazones and Their Biological, Analytical and Catalytic Applications: A Review. Main Group Met. Chem. 2014, 13, 187–218. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Brischigliaro, M.; Fernández-Vizarra, E. Emerging Mechanisms in the Redox Regulation of Mitochondrial Cytochrome c Oxidase Assembly and Function. Biochem. Soc. Trans. 2024, 52, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Narváez, M.E.; González-Sebastián, L.; Colorado-Peralta, R.; Reyes-Márquez, V.; Franco-Sandoval, L.O.; Romo-Pérez, A.; Cruz-Navarro, J.A.; Mañozca-Dosman, I.V.; Aragón-Muriel, A.; Morales-Morales, D. Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review. Inorganics 2025, 13, 38. [Google Scholar] [CrossRef]
- Sigel, H.; Sigel, A. Metal Ions in Biological Systems; CRC Press: Boca Raton, FL, USA, 2024; ISBN 9781003573685. [Google Scholar]
- Qiu, X.-Y.; Liu, S.-J. Synthesis, Characterization and Crystal Structures of Nickel(II) Complexes Derived from Schiff Bases with Antimicrobial Activity. Polyhedron 2024, 247, 116708. [Google Scholar] [CrossRef]
- Alqasaimeh, M.M.; Abu-Yamin, A.-A.M.; Matar, S.A.; Sarairah, I.A.; Salman, M.M.; Al-As’ad, R.M. Synthesis and Characterization of a New Schiff-Base Derivative as an Optical Nickel (II) Chemosensor and Its Antimicrobial Activity. J. Photochem. Photobiol. A Chem. 2024, 447, 115277. [Google Scholar] [CrossRef]
- Samir, S. Human DNA Mutations and Their Impact on Genetic Disorders. Recent Pat. Biotechnol. 2024, 18, 288–315. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Pal, U.; Roy, P.; Samanta, T.; Chattopadhyay, N.; Sen, K.; Bose, A. Effect of a Metal Ion in Modulating the Binding Interaction of a Dietary Flavonoid with Bovine Serum Albumin and DNA: A Spectroscopic and Theoretical Approach. ACS Food Sci. Technol. 2022, 2, 114–124. [Google Scholar] [CrossRef]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Riding the Metal Wave: A Review of the Latest Developments in Metal-Based Anticancer Agents. Coord. Chem. Rev. 2024, 501, 215579. [Google Scholar] [CrossRef]
- Alagesan, M.; Bhuvanesh, N.S.P.; Dharmaraj, N. Potentially Cytotoxic New Copper(Ii) Hydrazone Complexes: Synthesis, Crystal Structure and Biological Properties. Dalton Trans. 2013, 42, 7210. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Saleem, K. Empirical Formulae to Molecular Structures of Metal Complexes by Molar Conductance. Synth. React. Inorg. Met. Org. Chem. 2013, 43, 1162–1170. [Google Scholar] [CrossRef]
- Polo-Cerón, D. Cu(II) and Ni(II) Complexes with New Tridentate NNS Thiosemicarbazones: Synthesis, Characterisation, DNA Interaction, and Antibacterial Activity. Bioinorg. Chem. Appl. 2019, 2019, 3520837. [Google Scholar] [CrossRef]
- Gómez-Saiz, P.; García-Tojal, J.; Maestro, M.A.; Mahía, J.; Arnaiz, F.J.; Lezama, L.; Rojo, T. New 1,3,4-Oxadiazolecopper(ii) Derivatives Obtained from Thiosemicarbazone Complexes. Eur. J. Inorg. Chem. 2003, 2003, 2639–2650. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared Spectra of Surface Nitrates: Revision of the Current Opinions Based on the Case Study of Ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- El-Sherif, A.A.; Fetoh, A.; Abdulhamed, Y.K.; Abu El-Reash, G.M. Synthesis, Structural Characterization, DFT Studies and Biological Activity of Cu(II) and Ni(II) Complexes of Novel Hydrazone. Inorganica Chim. Acta 2018, 480, 1–15. [Google Scholar] [CrossRef]
- Zülfikaroğlu, A.; Yüksektepe Ataol, Ç.; Çelikoğlu, E.; Çelikoğlu, U.; İdil, Ö. New Cu(II), Co(III) and Ni(II) Metal Complexes Based on ONO Donor Tridentate Hydrazone: Synthesis, Structural Characterization, and Investigation of Some Biological Properties. J. Mol. Struct. 2020, 1199, 127012. [Google Scholar] [CrossRef]
- Patil, S.S.; Tadavi, S.K.; Dikundwar, A.; Bendre, R.S. The Transition Metal Complexes of Fe(II), Ni(II) and Cu(II) Derived from Phthalazine Based Ligands: Synthesis, Crystal Structures and Biological Activities. J. Mol. Struct. 2022, 1247, 131293. [Google Scholar] [CrossRef]
- Panja, S.K.; Dwivedi, N.; Saha, S. Tuning the Intramolecular Charge Transfer (ICT) Process in Push–Pull Systems: Effect of Nitro Groups. RSC Adv. 2016, 6, 105786–105794. [Google Scholar] [CrossRef]
- Daravath, S.; Vamsikrishna, N.; Ganji, N.; Venkateswarlu, K.; Shivaraj. Synthesis, Characterization, DNA Binding Ability, Nuclease Efficacy and Biological Evaluation Studies of Co(II), Ni(II) and Cu(II) Complexes with Benzothiazole Schiff Base. Chem. Data Collect. 2018, 17–18, 159–168. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano, Y.; Upegui, Y.; Robledo, S.M.; Ramírez-Apan, M.T.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. In Vitro Evaluation of the Potential Pharmacological Activity and Molecular Targets of New Benzimidazole-Based Schiff Base Metal Complexes. Antibiotics 2021, 10, 728. [Google Scholar] [CrossRef]
- Howsaui, H.B.; Sharfalddin, A.A.; Abdellattif, M.H.; Basaleh, A.S.; Hussien, M.A. Synthesis, Spectroscopic Characterization and Biological Studies of Mn(II), Cu(II), Ni(II), Co(II) and Zn(II) Complexes with New Schiff Base of 2-((Pyrazine-2-Ylimino)Methyl)Phenol. Appl. Sci. 2021, 11, 9067. [Google Scholar] [CrossRef]
- Kellett, A.; Molphy, Z.; Slator, C.; McKee, V.; Farrell, N.P. Molecular Methods for Assessment of Non-Covalent Metallodrug–DNA Interactions. Chem. Soc. Rev. 2019, 48, 971–988. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug–DNA Interactions and Their Study by UV–Visible, Fluorescence Spectroscopies and Cyclic Voltametry. J. Photochem. Photobiol. B 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Saygıdeğer Demir, B.; İnce, S.; Yilmaz, M.K.; Sezan, A.; Derinöz, E.; Taskin-Tok, T.; Saygideger, Y. DNA Binding and Anticancer Properties of New Pd(II)-Phosphorus Schiff Base Metal Complexes. Pharmaceutics 2022, 14, 2409. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.N.; Parmar, P.A.; Gandhi, D.S.; Patidar, A.P. DNA Interactions and Cytotoxic Studies of Cis-Platin Analogues of Substituted 2,2′-Bipyridines. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 54–59. [Google Scholar] [CrossRef]
- Alsaedi, S.; Babgi, B.A.; Abdellattif, M.H.; Arshad, M.N.; Emwas, A.-H.M.; Jaremko, M.; Humphrey, M.G.; Asiri, A.M.; Hussien, M.A. DNA-Binding and Cytotoxicity of Copper(I) Complexes Containing Functionalized Dipyridylphenazine Ligands. Pharmaceutics 2021, 13, 764. [Google Scholar] [CrossRef]
- Shinde, R.G.; Khan, A.A.; Barik, A. Exploring the Interaction of Copper-Esculetin Complex with Ct-DNA: Insight from Spectroscopic and Docking Studies. J. Mol. Struct. 2020, 1208, 127901. [Google Scholar] [CrossRef]
- Gewirtz, D. A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Alam, A.; Agrawal, G.P.; Khan, S.; Khalilullah, H.; Saifullah, M.K.; Arshad, M.F. Towards the Discovery of Potential RdRp Inhibitors for the Treatment of COVID-19: Structure Guided Virtual Screening, Computational ADME and Molecular Dynamics Study. Struct. Chem. 2022, 33, 1569–1583. [Google Scholar] [CrossRef]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Med. Pharm. Rep. 2014, 87, 215–219. [Google Scholar] [CrossRef]
- Samari, F.; Hemmateenejad, B.; Shamsipur, M.; Rashidi, M.; Samouei, H. Affinity of Two Novel Five-Coordinated Anticancer Pt(II) Complexes to Human and Bovine Serum Albumins: A Spectroscopic Approach. Inorg. Chem. 2012, 51, 3454–3464. [Google Scholar] [CrossRef]
- Szymańska, M.; Pospieszna-Markiewicz, I.; Mańka, M.; Insińska-Rak, M.; Dutkiewicz, G.; Patroniak, V.; Fik-Jaskółka, M.A. Synthesis and Spectroscopic Investigations of Schiff Base Ligand and Its Bimetallic Ag(I) Complex as DNA and BSA Binders. Biomolecules 2021, 11, 1449. [Google Scholar] [CrossRef] [PubMed]
- Lyndem, S.; Sarmah, S.; Das, S.; Singha Roy, A. Coumarin Derivatives: Biomedicinal Properties and Interactions with Carrier Proteins. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 173–220. [Google Scholar]
- Long, X.; Zeng, Y.-F.; Liu, Y.; Liu, Y.; Li, T.; Liao, L.; Guo, Y. Synthesis of Novel Genistein Amino Acid Derivatives and Investigation on Their Interactions with Bovine Serum Albumin by Spectroscopy and Molecular Docking. RSC Adv. 2018, 8, 31201–31212. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1997; pp. 307–326. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Londoño-Mosquera, J.-D.; Aragón-Muriel, A.; Polo-Cerón, D. Synthesis, Antibacterial Activity and DNA Interactions of Lanthanide(III) Complexes of N(4)-Substituted Thiosemicarbazones. Univ. Sci. 2018, 23, 141–169. [Google Scholar] [CrossRef]
- Alroba, A.A.N.; Aazam, E.S.; Zaki, M. Synthesis and Characterization of Metal Complexes [M(L)(Im)]NO3, In Vitro DNA/BSA Binding Behavior and Cytotoxicity on HepG2 Cancer Cells. ChemistrySelect 2024, 9, e202400737. [Google Scholar] [CrossRef]
- Churchill, C.M.; Brake, T.; Cuss, C.W. Effect of PH and Metal Ions on Protein–Metal Complexation Modeled by Fluorescence Quenching. J. Chem. Educ. 2025, 102, 316–323. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano-Martínez, Y.; Rufino-Felipe, E.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. Synthesis, Biological Evaluation and Model Membrane Studies on Metal Complexes Containing Aromatic N,O-Chelate Ligands. Heliyon 2020, 6, e04126. [Google Scholar] [CrossRef] [PubMed]
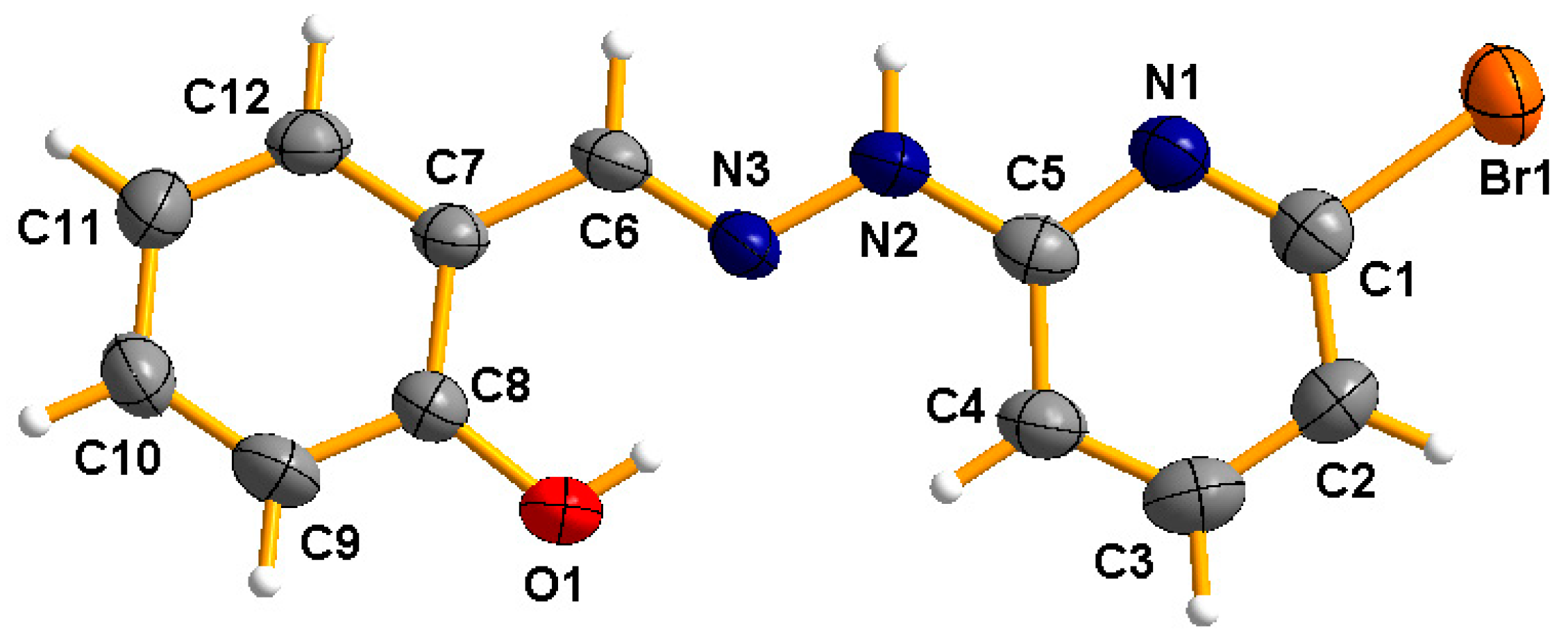

| Compound | (E)-2-((2-(6-Bromopyridin-2-yl)hydrazono)methyl)phenol |
|---|---|
| Emp. Formula | C12H10BrN3O |
| FW (g/mol) | 292.13 |
| Temp. (K) | 293 |
| λ (Å) | MoKα = 0.71073 |
| Crystal system | Orthorhombic |
| Space group | Pbca |
| a (Å) | 5.7564(1) |
| b (Å) | 12.4294(4) |
| c (Å) | 32.2384(10) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| Volume (Å3) | 2306.61(11) |
| Z | 8 |
| ρ calcd (mg/m3) | 1.683 |
| Abs.Coeff (mm−1) | 3.550 |
| F(000) | 1168 |
| θ range (°) | 3.3 to 26.4 |
| Reflections collected/unique [R(int)] | 18,864/2345 [0.124] |
| Completeness (%) | 99.5 |
| Data/restraints/parameters | 2345/0/156 |
| Gof on F2 | 0.956 |
| R1 [I > 2σ(I)] | 0.0476 |
| wR2 [I > 2σ(I)] | 0.1342 |
| Compound | S. aureus ATCC25923 | L. monocytogenes ATCC19115 | B. cereus ATCC10876 | E. coli ATCC25922 | S. typhimurium ATCC14028 | P. aeruginosa ATCC 27853 |
|---|---|---|---|---|---|---|
| HL | >1000 | >1000 | >250 | >500 | >1000 | >2000 |
| 1 | >250 | >500 | >62.5 | >250 | >250 | 500 |
| 2 | 125 | >250 | 62.5 | 250 | 62.5 | 250 |
| Cp * | <3.9 | <3.9 | <3.9 | <3.9 | <3.9 | <3.9 |
| AgNO3 * | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 | <5.0 |
| n | ||||
|---|---|---|---|---|
| BSA-HL | 8.92 | 8.92 | 6.71 | 0.89 |
| BSA-1 | 11.30 | 11.30 | 12.20 | 1.07 |
| BSA-2 | 9.90 | 9.90 | 7.30 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triviño-Rojas, M.K.; Jiménez-Lopez, S.J.; D’Vries, R.; Aragón-Muriel, A.; Polo-Cerón, D. In Vitro Antibacterial Activities and Calf Thymus DNA–Bovine Serum Albumin Interactions of Tridentate NNO Hydrazone Schiff Base–Metal Complexes. Inorganics 2025, 13, 213. https://doi.org/10.3390/inorganics13070213
Triviño-Rojas MK, Jiménez-Lopez SJ, D’Vries R, Aragón-Muriel A, Polo-Cerón D. In Vitro Antibacterial Activities and Calf Thymus DNA–Bovine Serum Albumin Interactions of Tridentate NNO Hydrazone Schiff Base–Metal Complexes. Inorganics. 2025; 13(7):213. https://doi.org/10.3390/inorganics13070213
Chicago/Turabian StyleTriviño-Rojas, Maida Katherine, Santiago José Jiménez-Lopez, Richard D’Vries, Alberto Aragón-Muriel, and Dorian Polo-Cerón. 2025. "In Vitro Antibacterial Activities and Calf Thymus DNA–Bovine Serum Albumin Interactions of Tridentate NNO Hydrazone Schiff Base–Metal Complexes" Inorganics 13, no. 7: 213. https://doi.org/10.3390/inorganics13070213
APA StyleTriviño-Rojas, M. K., Jiménez-Lopez, S. J., D’Vries, R., Aragón-Muriel, A., & Polo-Cerón, D. (2025). In Vitro Antibacterial Activities and Calf Thymus DNA–Bovine Serum Albumin Interactions of Tridentate NNO Hydrazone Schiff Base–Metal Complexes. Inorganics, 13(7), 213. https://doi.org/10.3390/inorganics13070213









