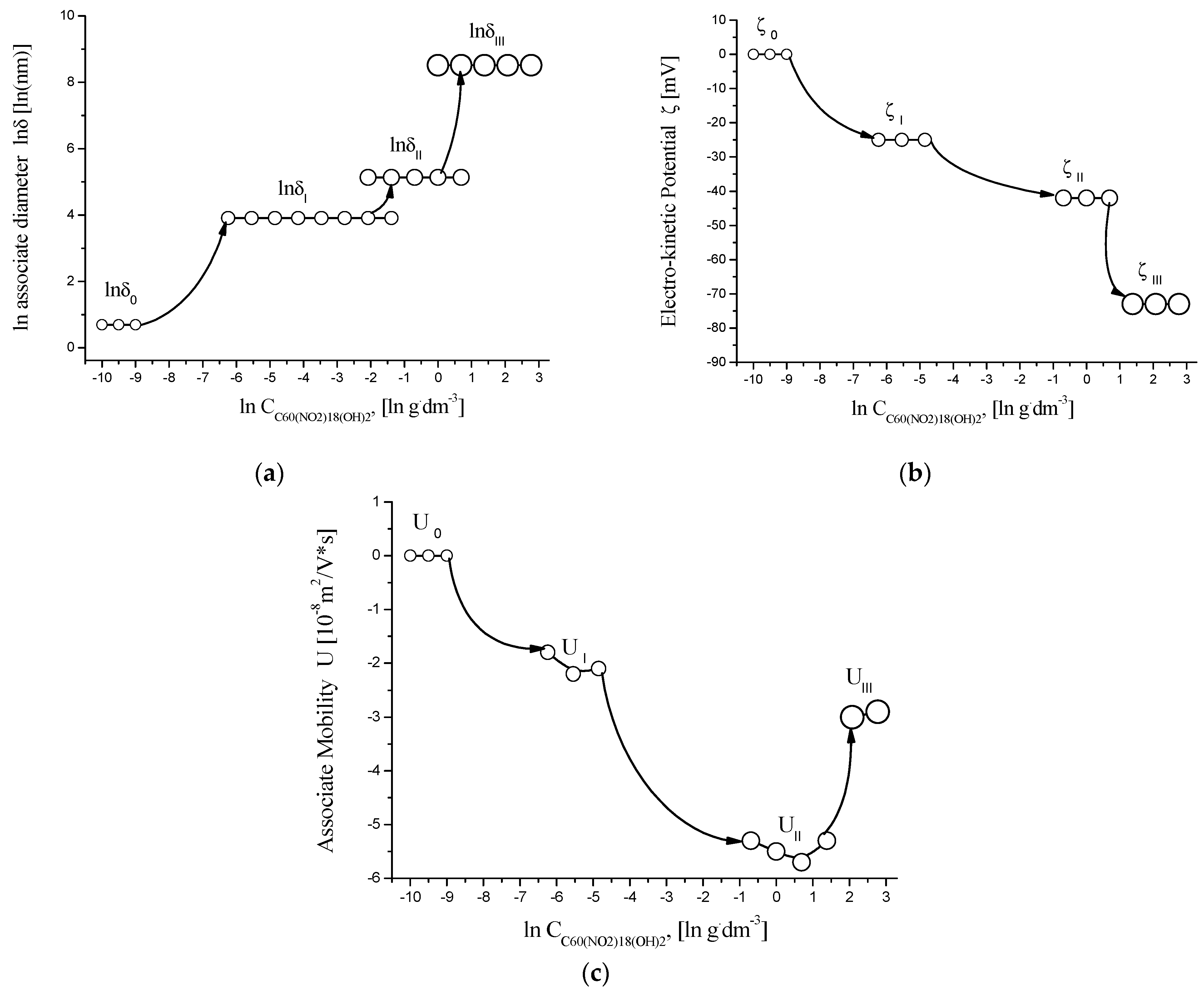Abstract
A direct non-catalytic synthesis of a new water-soluble polynitro-hydroxylated fullerene derivative, C60(NO2)18(OH)2, was carried out using a mixture of concentrated nitric and sulfuric acids. The resulting poly-nitro adduct was comprehensively characterized by elemental C-H-N analysis, energy-dispersive X-ray spectroscopy, infrared (IR) and electron spectroscopy, nuclear magnetic resonance (NMR), high-performance liquid chromatography (HPLC), and thermogravimetric analysis (TGA). A detailed investigation of the physicochemical properties of aqueous solutions of C60(NO2)18(OH)2 demonstrated that the synthesized compound is a previously undescribed mixed polynitro-hydroxyl adduct of light fullerene C60, featuring a high degree of nitration (18 nitro groups per fullerene core). The composition and structure of the adduct were confirmed by spectroscopic and refractometric analyses. In terms of redox behavior, the compound exhibits significant reducing and antioxidant properties. These physicochemical characteristics suggest the potential of C60(NO2)18(OH)2 for further development as a biocompatible nanomaterial suitable for medical applications.
1. Introduction
Fullerenes have been intensively studied for almost four decades, and interest in these carbon nanostructures continues to grow. Owing to their high chemical reactivity and unique cage geometry, carbon-based nanoparticles display outstanding tribological, mechanical, and electrical properties across a wide range of morphologies and sizes [1,2,3,4]. Among them, the low-density fullerene C60 is especially promising for biomedical applications [5,6,7].
Recent work shows that incorporating C60 into nanocomposite coatings markedly improves anticorrosion performance and mechanical integrity relative to the corresponding neat polymers [1]. A comprehensive review of fullerene-based nanocomposites highlights their broad biomedical potential, including corrosion-resistant polymers and antimicrobial materials [8,9]. In addition, C60 exhibits neuroprotective activity by scavenging superoxide and hydroxyl radicals [10] and possesses pronounced antioxidant properties [11,12]. These effects are observed not only for pristine C60 but also for low-molecular-weight derivatives such as fullerenols and for polymeric conjugates [13,14].
The possibility of exploiting fullerene coatings in joint endoprostheses is therefore attracting considerable attention [15]. A key challenge in implantology, however, is peri-implant infection, which may necessitate revision surgery and threaten patient survival [16]. Fullerene-modified biopolymers offer a promising avenue for antimicrobial surfaces and medical devices [16]. Moreover, C60 coatings have been shown to enhance cell adhesion and viability on complex dental implants, indicating anti-inflammatory potential and improved biocompatibility [17].
Although pristine C60 is essentially water-insoluble, its solubility can be tailored by chemical functionalization with hydrophilic groups [6,18,19]. Water-soluble, nitrogen-containing derivatives are of special interest for nanomedical diagnostics and therapy [6]. As early as 1996, the high reactivity of C60 toward nitrogen-dioxide radicals was reported, leading to polyhydroxylated fullerenes (fullerenols) [20].
Selective nitration of C60 remains a challenging topic; relatively few studies address it, and the available data are not always consistent [21,22,23]. Reported nitration methods employ reagents such as concentrated HNO3/NaNO2, N2O4, fuming HNO3, or aqueous NaNO2/FeSO4/H2SO4 under air [22]. Polynitrofullerenes subsequently undergo slow hydrolysis in water, yielding mixed poly(hydroxynitro) derivatives.
A recent survey discusses the major classes of multifunctionalized fullerenes, emphasizing structure–property relationships [24]. In general, organic chemistry, nitration of hydrocarbons yields two principal product families: nitroadducts R(NO2)ₙ and nitro-esters R–O–NO2. Classic nitrating agents include HNO3 itself, its protonated form NO2+, various nitrogen oxides (NO2•, NO•, N2O5, N2O3), and mixed systems containing additional oxidants or Lewis-acid catalysts such as AlCl3, ZnCl2, or BF3 [24,25]. These nitro compounds are indispensable intermediates in chemical manufacturing, pharmacology, and materials science (e.g., nitrobenzene for aniline synthesis).
Fullerenes (C60, C70, etc.) are formally polyolefins, and their nitration parallels that of unsaturated hydrocarbons [25]. When C60 is treated with nitric acid, nitrates, or NO2•, followed by alkaline hydrolysis, polyhydroxylated fullerenes C60(OH)ₙ (n ≈ 16–20) are obtained [20,26]. Consequently, nitration pathways that would normally generate poly-nitroadducts often converge to fullerenols after base treatment.
Nitric acid is the most accessible nitrating reagent; NO2+ generated in situ acts as the electrophile, while other active species arise as secondary products. For unsaturated systems, six mechanistic routes are possible:
- Electrophilic H–NO2 substitution (infeasible for hydrogen-free C60)
- H-substitution via N2O5 (also impossible for C60)
- Addition of HNO3 across C═C to give nitro-alcohols
- Addition of pure HNO3 to yield nitro-ethers
- Radical addition of NO2•, N2O3, or N2O5 affording mono- or di-nitroadducts, nitro-alcohols, nitro-esters, or their mixtures [18]
- Multi-step sequences combining these elementary reactions
The present work aims to synthesize a water-soluble polynitro derivative of C60 and to elucidate its structure and physicochemical properties by a suite of analytical techniques. The resulting material is intended for selective etching and surface modification of titanium medical implants, with the goals of enhancing hardness, tribological performance, antioxidant capacity, electrical conductivity, and corrosion resistance. Because the grafted adduct displays limited yet significant solubility in physiological fluids, beneficial modifications of the surrounding biological milieu (blood, lymph, cerebrospinal fluid, etc.) are anticipated. We therefore hypothesize that the C60 polynitro-adduct obtained herein is a promising biocompatible nanomaterial for medical applications [27].
Selective nitration of fullerenes and their derivatives by novel synthetic approaches and reagents is an important yet challenging topic. The number of papers devoted to the study of nitration of the lightest fullerene, C60, is relatively small [11,28,29,30], and the data presented by the authors do not seem to be fully consistent.
- (A)
- Let us first focus on the article [28], which most fully corresponds to the thematics of our article, i.e., the direct nitration of fullerene C60. Nitration of fullerene molecules has been carried out under different conditions and nitration reagents, including a mixture of conc. HNO3 and sodium nitrite, dinitrogen tetroxide, fuming nitric acid, and a mixture of aqueous sodium nitrite, FeSO4, and H2SO4 in the presence of air. Hexaanilino [60]fullerenes (), as the predominant nitration product, have been described [28]. In an aqueous alkaline solution, rapid and complete hydrolysis of poly-nitrofullerenes was observed to produce fullerenol molecules containing at least 16 hydroxy- groups per C60 core [28]. The latter, in our opinion, indirectly indicates a deeper course of fullerene nitration up to 16 nitro groups, at least for a part of the mixture of products obtained in this work. The mixed composition of poly-nitroadducts during nitration is also indirectly confirmed by the results of HPLC studies, where the yield of a mixture of products from the column is characterized by a mixture of 4–7 chromatographic peaks [28] with a mixture output time of several minutes. At the same time, with an increase in the synthesis time from 24 h to 144 h of HPLC, the picture is significantly simplified. From the latter, it can be assumed that either longer synthesis times, more intensive mixing, or a higher temperature could have been used to complete the nitration process.
- (B)
- Article [11] is also directly devoted to the direct fullerene nitration. The nitration of fullerene molecules has been carried out under different conditions and nitration reagents (very close to [28]), including a mixture of conc. HNO3 and sodium nitrite (7), dinitrogen tetroxide, fuming nitric acid, and a mixture of aqueous sodium nitrite, FeSO4, and H2SO4 in the presence of air. However, polynitrofullerenes react slowly with H2O to yield partially hydroxylated products of poly(hydroxynitro)fullerenes. In the presence of an aqueous alkaline solution, rapid and complete hydrolysis of poly-nitrofullerenes was observed to produce fullerenol molecules containing at least 16 hydroxy groups per C60 core. The result of nitration in this work is also very close to the article [28], the formation of two main poly-nitroadducts, tetra- and hexa-derivatives, was found. However, the authors of the review [11] come to more cautious conclusions, noting that in some studies reported, C60 was nitrated by the multiple addition of NO2 and the product isomerized partly to the nitrito form with subsequent hydrolysis by atmospheric moisture to yield nitrofullerols consisting of 6–8 nitro and 7–12 hydroxy groups per C60.
- (C)
- Authors [28] summarize the recent progress of functionalized fullerene materials (i.e., fullerene derivatives) that have been applied in PSCs, focusing on chemical functionalization strategies. Despite the completeness and undoubted value of the presented review, the last one is generalized and non-specific and has no direct relation to the topic of our article related to the direct nitration of fullerene C60. The goal of the authors [28] was to propose an outlook on the future development of fullerene derivatives in realizing high-performance PSC devices.
- (D)
- Article [30] is devoted to the consideration of the major types of multi-functionalized fullerenes through selected examples with a link to the structural assignments. At the same time, the structural features of fullerene derivatives are the main subject of this review. At the same time, this aspect of the review is not the main and essential determinant in our work.
When comparing our results with the above works, we can say that as a result of the synthesis, the authors were able to obtain and isolate a poly-nitroadduct of fullerene C60 with a very high degree of addition of nitro groups (18 instead of a maximum of 6 [11,28,29,30]). The authors attribute this fact to the simultaneous use of strong mineral acid as a catalyst, the duration of synthesis at elevated temperatures, and, most importantly, the prevention of hydrolysis by maintaining low pH values of the medium.
2. Results
2.1. Synthesis of the Polynitro-Hydroxylated Adduct C60(NO2)18(OH)2
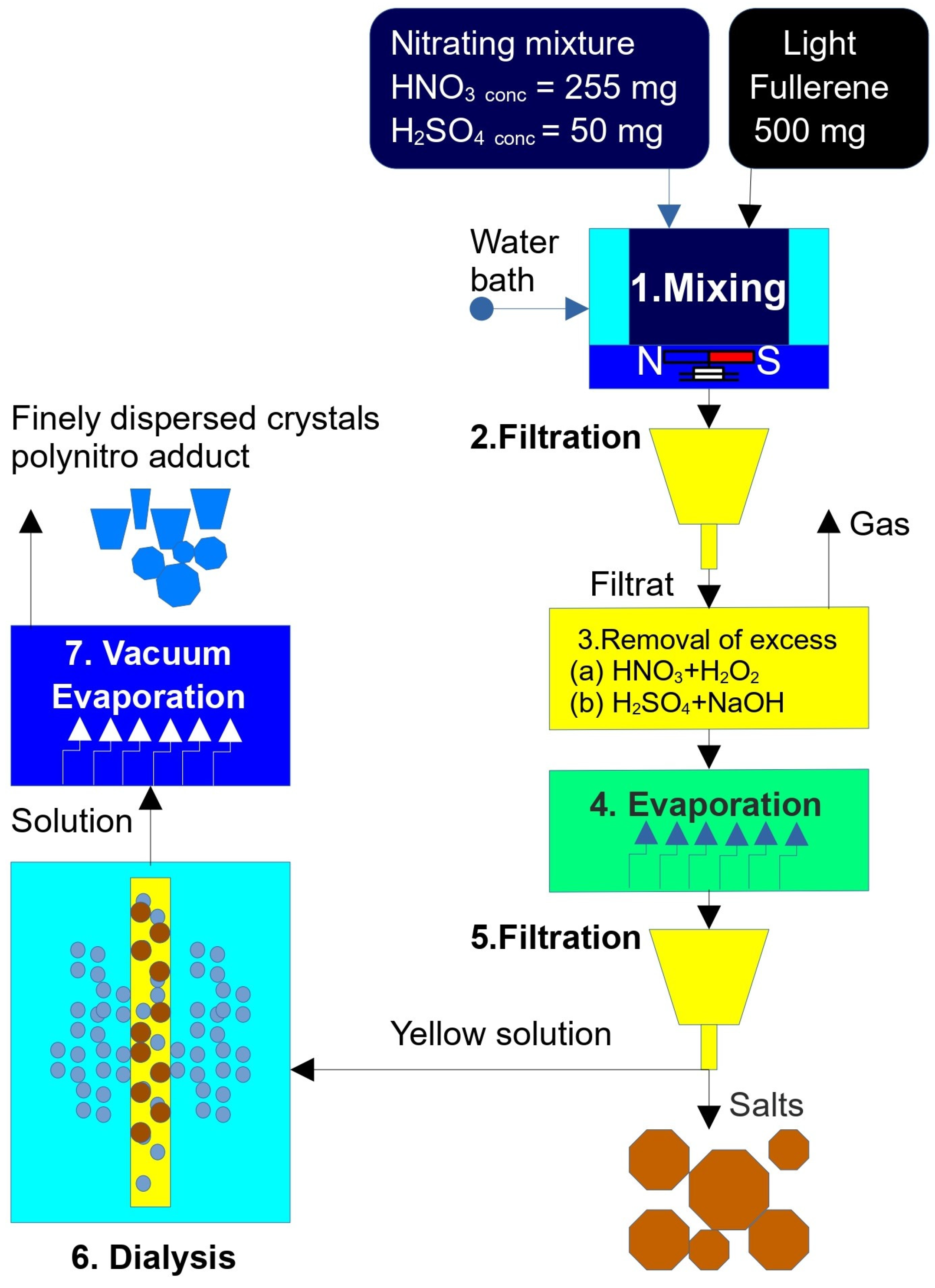
A direct, one-pot nitration–hydroxylation of pristine fullerene C60 was performed in mixed fuming nitric and sulfuric acids (20:1 v/v; ρ ≈ 1.50 g cm−3) under vigorous stirring at 50 °C for 7 days. Trace SO3 in the medium acted as a Brønsted super-acid catalyst, generating NO2+ electrophile in situ. The overall stoichiometry can be summarized as:
After reaction completion, the dark green solution was repeatedly diluted with ice water, neutralized to pH ≈ 6, and dialyzed (MW 1000) to remove acid residues. Rotary evaporation afforded a brown microcrystalline solid in 62% isolated yield.
The control of the reaction was indeed carried out by the authors using standard methods: electron spectroscopy, thin-layer chromatography, and turbodimetry (the latter for tracking the formation of associates in solution). The analysis was performed once a day. Experience has shown that there were no qualitative changes in the composition of the solution, and the quantitative reaction was completed after about 4–5 days. We did not observe the destruction of the fullerene core, including according to the results of mass spectrometric analysis, which in this case would show molecular weights less than M/z < 720 (even if the nitro groups were destroyed under experimental conditions). Nevertheless, the authors undoubtedly recognize the possibility and high probability of the formation of isomeric and closely related forms during synthesis.
2.2. Structural Characterization
To streamline the presentation, all analytical data are grouped into a single subsection; Figure 1 gives a schematic overview of the workflow.
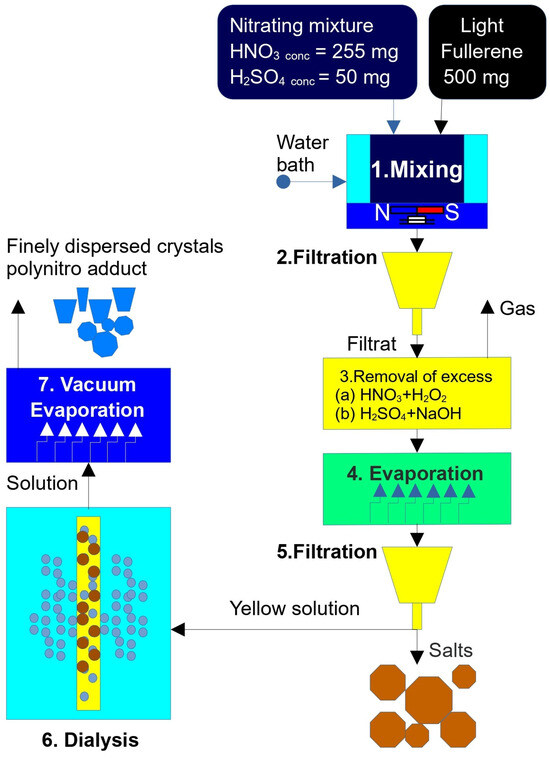
Figure 1.
Scheme of synthesis of water-soluble nitro derivatives .
Elemental and Spectroscopic Evidence
The results of elemental analysis of poly-nitroadduct are summarised in Table 1.

Table 1.
Elemental analysis of synthesized poly-nitroadduct .
Based on the results of the elemental analysis, it was found that the composition in atomic ratio (per 1 fullerene core : C = 60; N= ; H = 2 O = 38 . Formula:
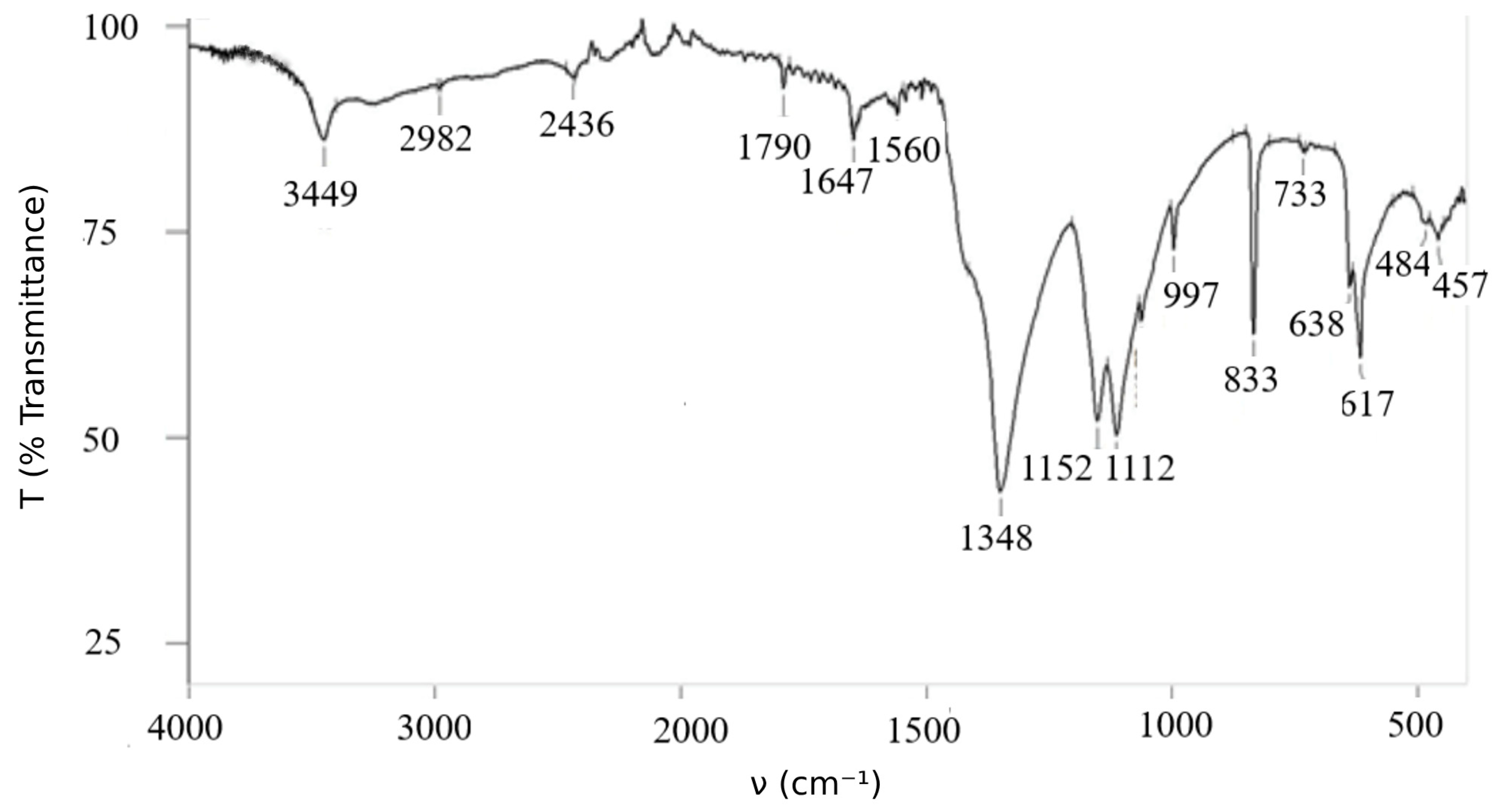
The obtained data show the correspondence between calculated and experimental data. Infrared spectrum poly-nitroadduct shown in Figure 2.
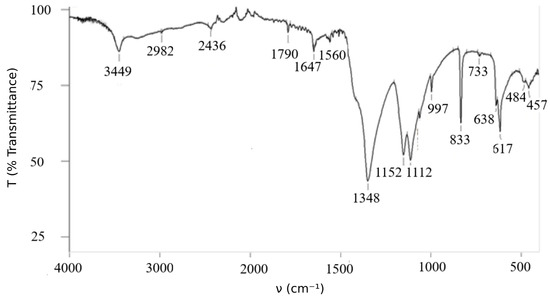
Figure 2.
Infrared spectrum of .
The infrared (IR) spectrum displays characteristic features: a prominent absorption band corresponding to the O–H stretching vibration is observed at 3449 cm−1. Fullerenols synthesized by various methods exhibit similar IR patterns, typically showing strong hydroxyl group absorption near 3400 cm−1 [21].
The identification of key absorption bands in the IR spectrum is as follows (see Figure 2; ṽ in cm−1):
- ν(O–H): 3449
- ν(NO2): 1647, 1348, 1560
- δ(NO2) (“scissoring”): 733, 638, 617
- ν(C–O): 1790, 1647, 1152, 1063
- ν(C–N): 1113 (strong), 833
- ν(C–O–N): 2982
- Fullerene core vibrations: 997, 733, 484, 457 [9]
Notably, the typical cage-related vibrational bands of pristine C60 at 528, 578, and 1183 cm−1 are absent, which indicates significant functionalization of the fullerene surface.
It should be emphasized that the IR spectrum of C60(NO2)18(OH)2 is highly informative and can be reliably used for compound identification.
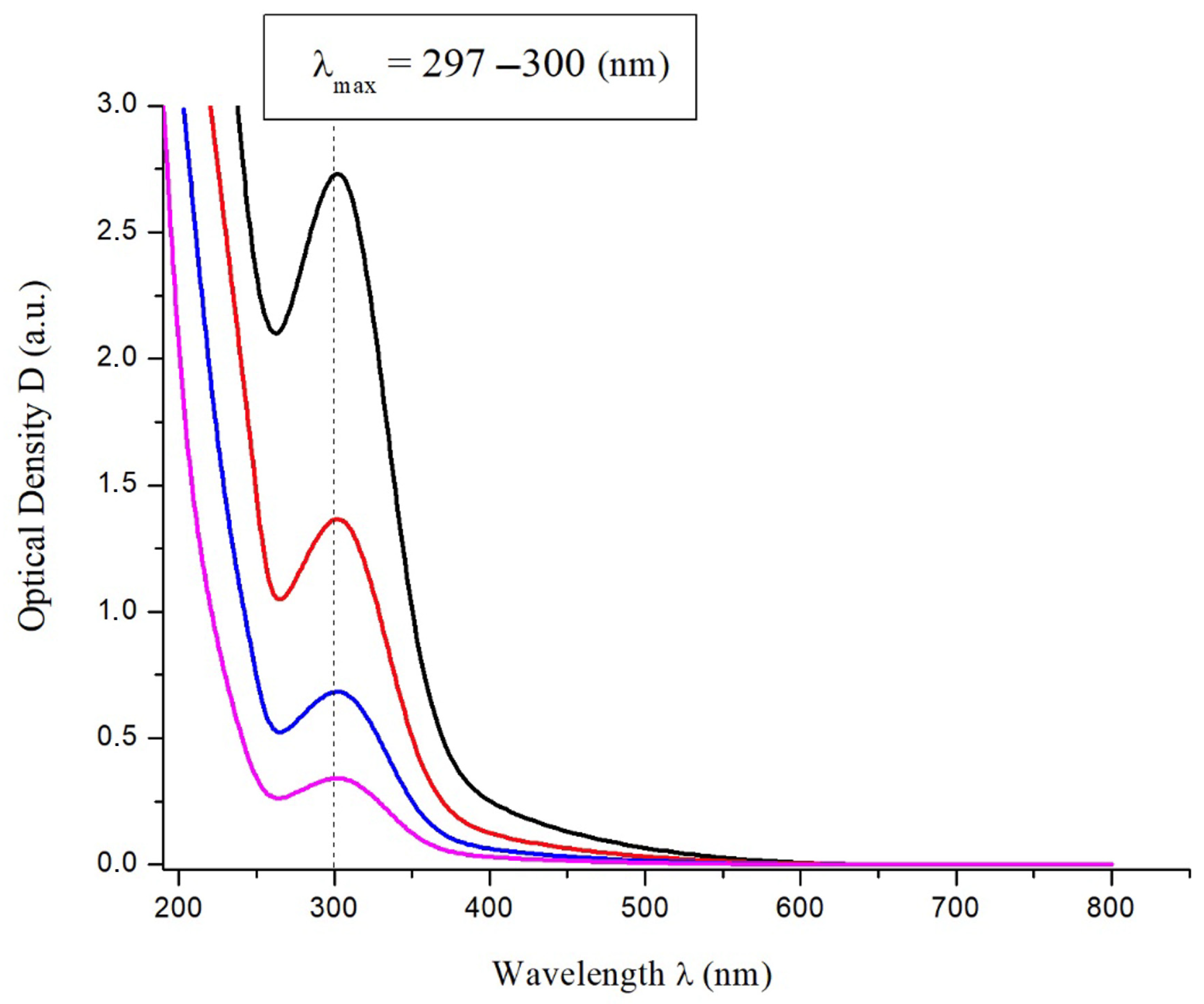
The electronic absorption spectrum of C60(NO2)18(OH)2 in aqueous solution was recorded in the UV–Vis–NIR range to investigate the optical properties of the synthesized compound. The spectra at various concentrations are presented in Figure 3. A distinct absorption maximum is observed at λₘₐₓ = 297–300 nm, which becomes more intense with increasing concentration. This peak is characteristic of π–π* electronic transitions associated with the conjugated fullerene structure modified by nitro and hydroxyl groups.
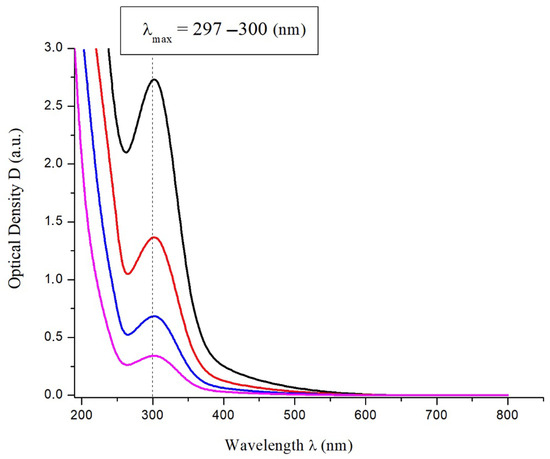
Figure 3.
UV–Vis absorption spectra of aqueous solutions of C60(NO2)18(OH)2 at various concentrations.
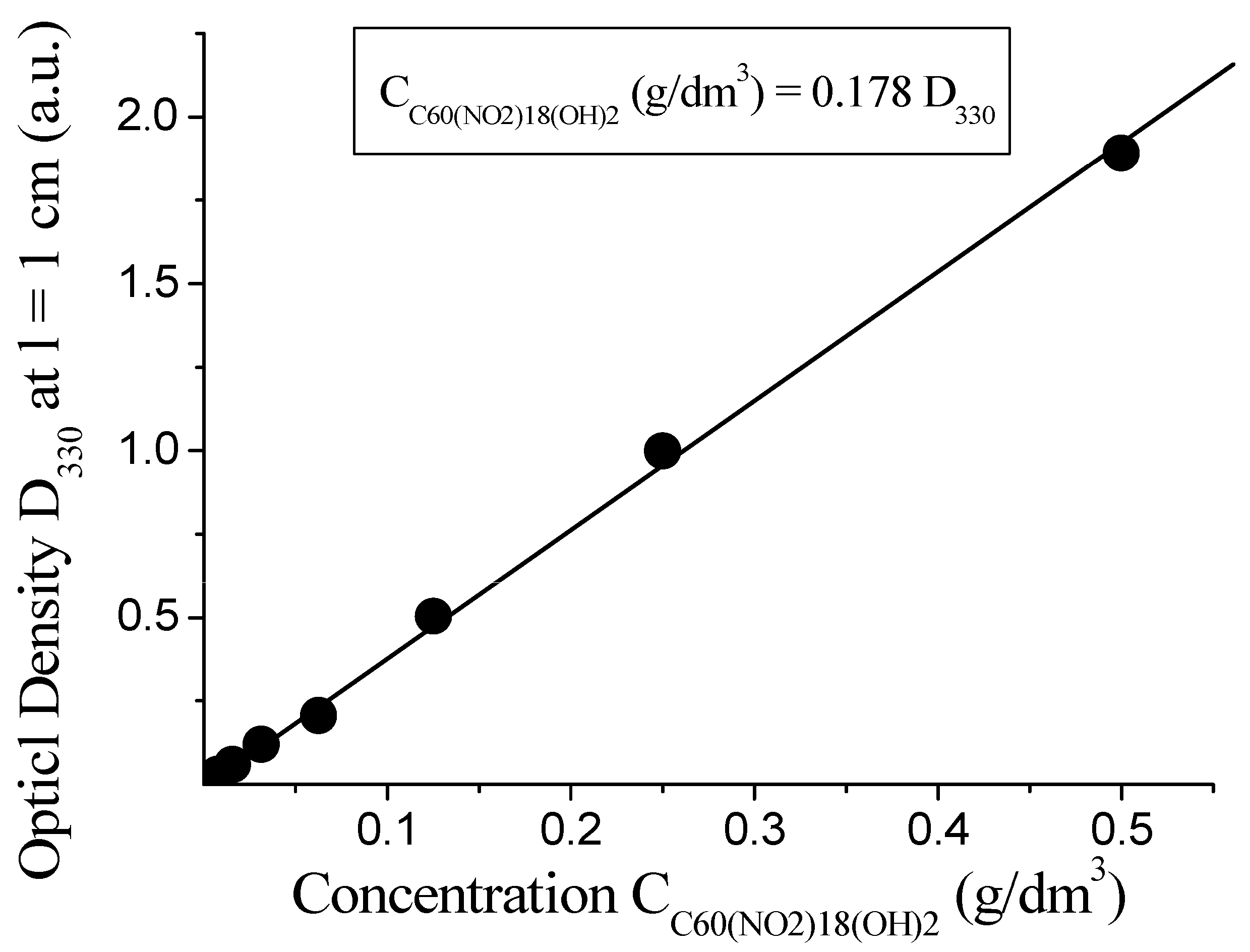
To verify the applicability of the Bouguer–Lambert–Beer law, the optical density at 300 nm was plotted against the concentration of the compound. As shown in Figure 4, a clear linear relationship is observed within the concentration range of 0.06 to 0.48 g/dm3, confirming the validity of the law in this system. The slope of the linear fit corresponds to an empirical coefficient of 0.178 g/dm3 per unit of D300, allowing for reliable spectrophotometric quantification of C60(NO2)18(OH)2 in aqueous media.
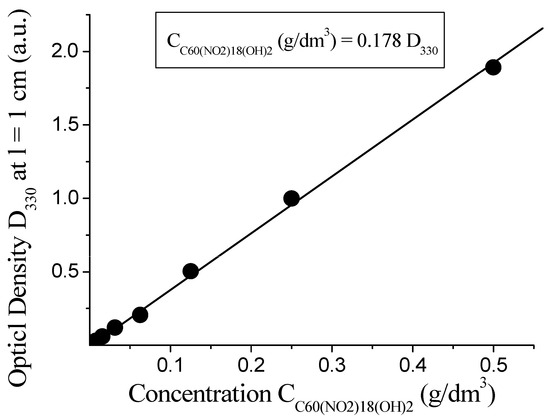
Figure 4.
Linear relationship between optical density and concentration of C60(NO2)18(OH)2 at 300 nm.
A single, well-defined absorption peak is observed in the visible and near-UV region at λ = 300 ± 3 nm. The absorbance at this wavelength follows the Bouguer–Lambert–Beer law, allowing for precise quantitative analysis. The relationship between concentration and absorbance at 300 nm is described by the following empirical equation:
This linear relationship holds in the concentration range of 0.06 to 0.48 g/dm3. At shorter wavelengths, an increase in absorbance is observed, indicative of higher energy π–π* transitions. The observed spectral behavior confirms the utility of UV–Vis spectrophotometry for determining the concentration of C60(NO2)18(OH)2 in aqueous media with high sensitivity.
To further evaluate the chemical purity of the synthesized compound, high-performance liquid chromatography (HPLC) was performed. The analysis was carried out under the following conditions:
- Column—Agilent Zorbax SB-C18;
- Eluent—CH3CN/H2O (1:20, v/v);
- Detection—spectrophotometric monitoring at λ = 330 nm.
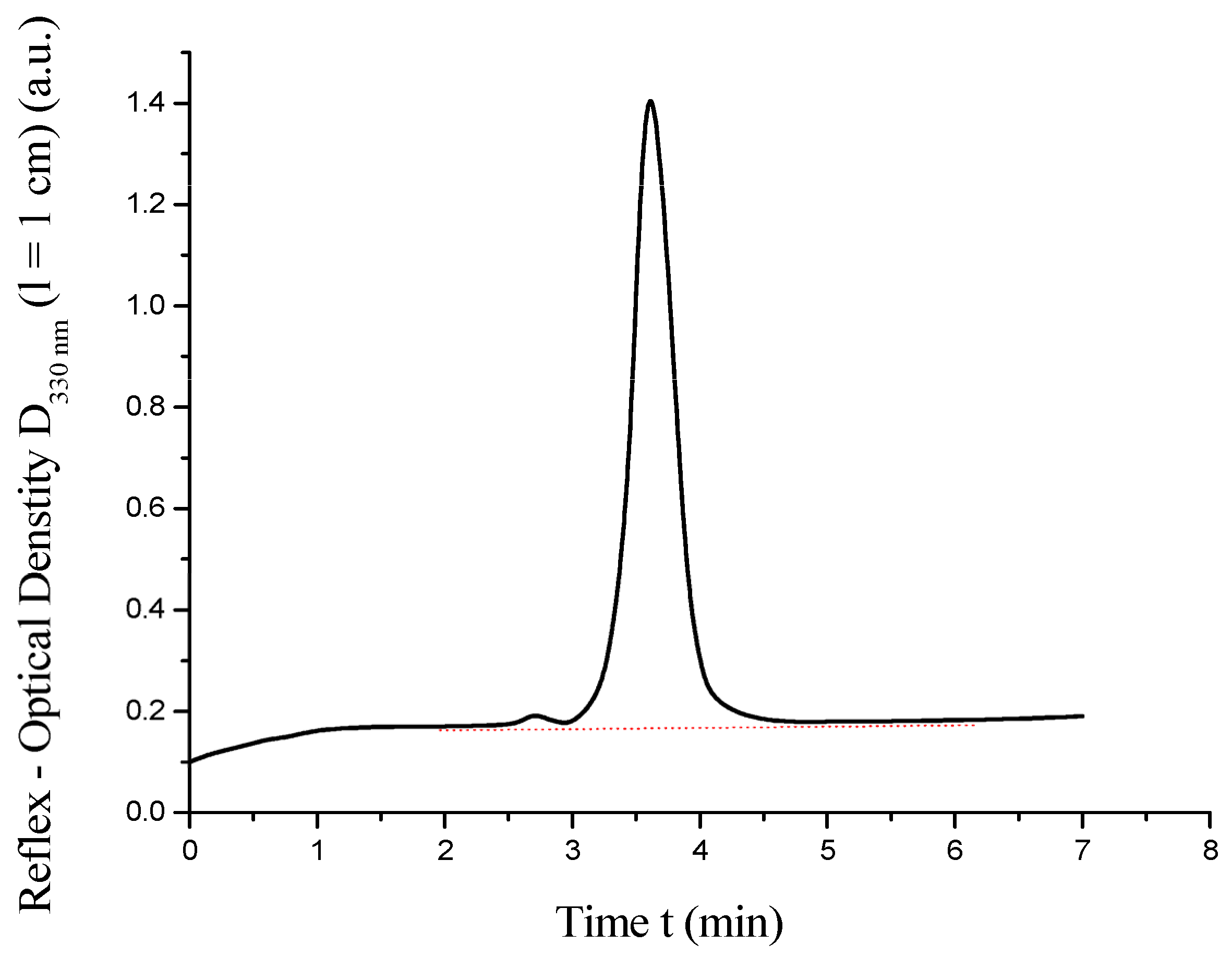
The resulting chromatogram is presented in Figure 5.
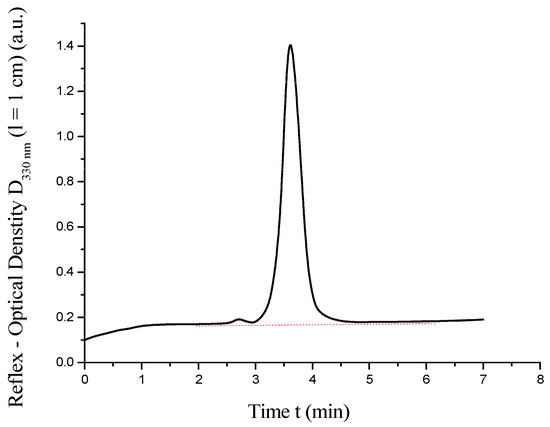
Figure 5.
High-performance liquid chromatography of .
As shown in Figure 5, the HPLC chromatogram of the synthesized C60(NO2)18(OH)2 reveals a single sharp peak at t = 3.6 min, corresponding to the main product. A minor impurity peak, constituting approximately 3% of the total signal area, is detected at t = 2.7 min. The half-width of the primary peak (d1/2 ≈ 0.45 min) and its symmetrical shape indicate high chromatographic purity. The authors realize that the chromatographic purity of the poly-nitroadduct, estimated at 97–98 wt%, is rather arbitrary. It is clear that the peak of impurity yield is very diffuse and wide. It probably corresponds to a mixture of closely related and partially isomeric products, which are also probably lower in molecular weight than poly-nitroadduct. We did not isolate this impurity individually. Nevertheless, we believe that the quantities are small enough not to significantly affect the physicochemical properties of the target product.
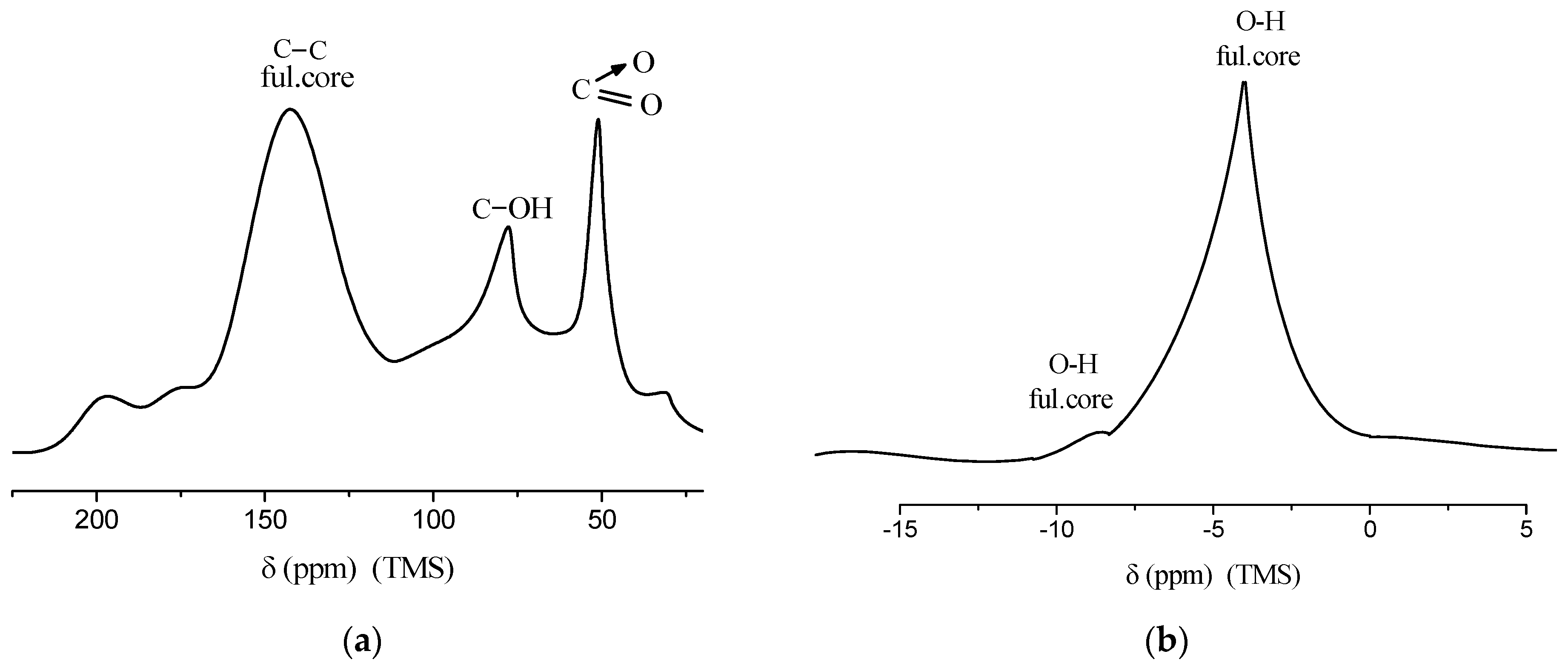
To further confirm the chemical structure, nuclear magnetic resonance (NMR) spectroscopy was performed. The 13C and 1H NMR spectra of the product, recorded at a frequency of 100.4 MHz and a temperature of 25 °C, are presented in Figure 6. These spectra confirm the presence of the expected functional groups: nitro (–NO2), hydroxyl (–OH), and sp2 carbon–carbon bonds characteristic of the fullerene core.
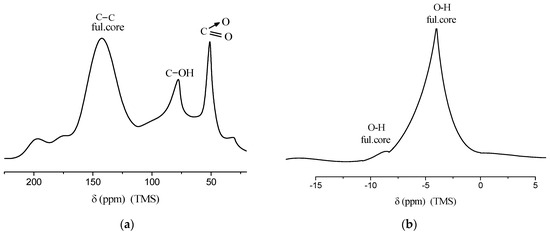
Figure 6.
(a) 13C NMR spectrum of C60(NO2)18(OH)2 recorded in CDCl3, showing signals corresponding to the fullerene core (C=C), hydroxylated carbon atoms (C–OH), and nitro-substituted carbon atoms (C–NO2); (b) 1H NMR spectrum of C60(NO2)18(OH)2 indicating a broad signal associated with hydroxyl protons (O–H) linked to the fullerene structure. Spectra were recorded at 100.4 MHz and 25 °C using TMS as an internal standard.
In the 13C NMR spectrum, distinct signals are observed at δ = 142–143 ppm, corresponding to the unmodified cage carbon atoms (C=C); at δ ≈ 76 ppm, attributed to hydroxylated carbon atoms (C–OH); and at δ ≈ 53 ppm, assigned to carbon atoms bonded to nitro groups (C–NO2). The 1H NMR spectrum shows a weak signal at δ ≈ 5 ppm, with a very faint broad peak at δ ≈ 8 ppm, likely arising from exchangeable hydroxyl protons.
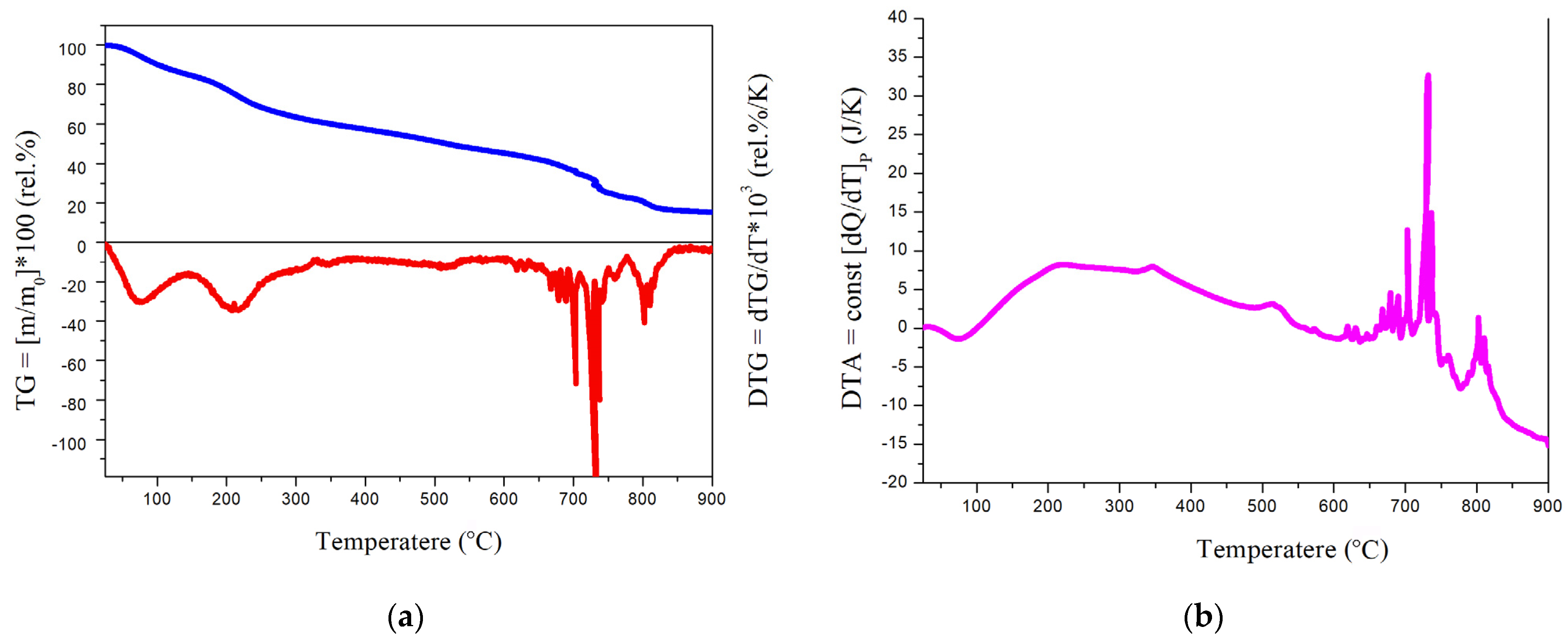
The thermal stability of the polynitro adduct was assessed using thermogravimetric and differential thermal analysis under atmospheric conditions. The results are presented in Figure 7.
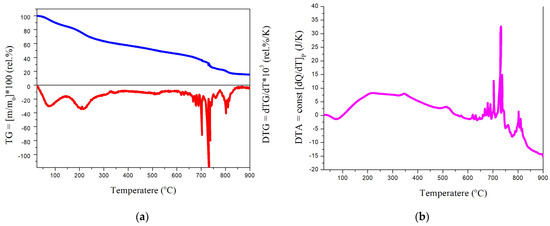
Figure 7.
(a) Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of C60(NO2)18(OH)2 recorded in air over the temperature range 0–900 °C. TG is shown in blue, DTG in red. Heating rate: 5 K/min; pressure: 1 atm; (b) differential thermal analysis (DTA) curve of the same sample under identical conditions, showing endothermic and exothermic transitions as a function of temperature.
The thermal behavior of C60(NO2)18(OH)2 was investigated using complex thermal analysis, including thermogravimetric (TG), derivative thermogravimetric (DTG), and differential thermal analysis (DTA), as shown in Figure 7. The TG/DTG curves reveal a multistage decomposition profile under an air atmosphere from 0 to 900 °C.
According to the data, the compound undergoes:
- 60–100 °C: decomposition of crystallohydrates due to external dehydration;
- 170–250 °C: breakdown of hydroxyl groups, corresponding to internal dehydration;
- 400–650 °C: gradual thermal degradation of relatively stable nitro groups;
- 680–740 °C: active decomposition of residual nitro substituents;
- 800 °C and above: explosive destruction of remaining nitro groups and oxidative combustion of the fullerene core.
These results confirm the moderate thermal stability and reactive nature of the polynitro adduct, with a clear transition from dehydration to progressive denitration and eventual carbon skeleton oxidation.
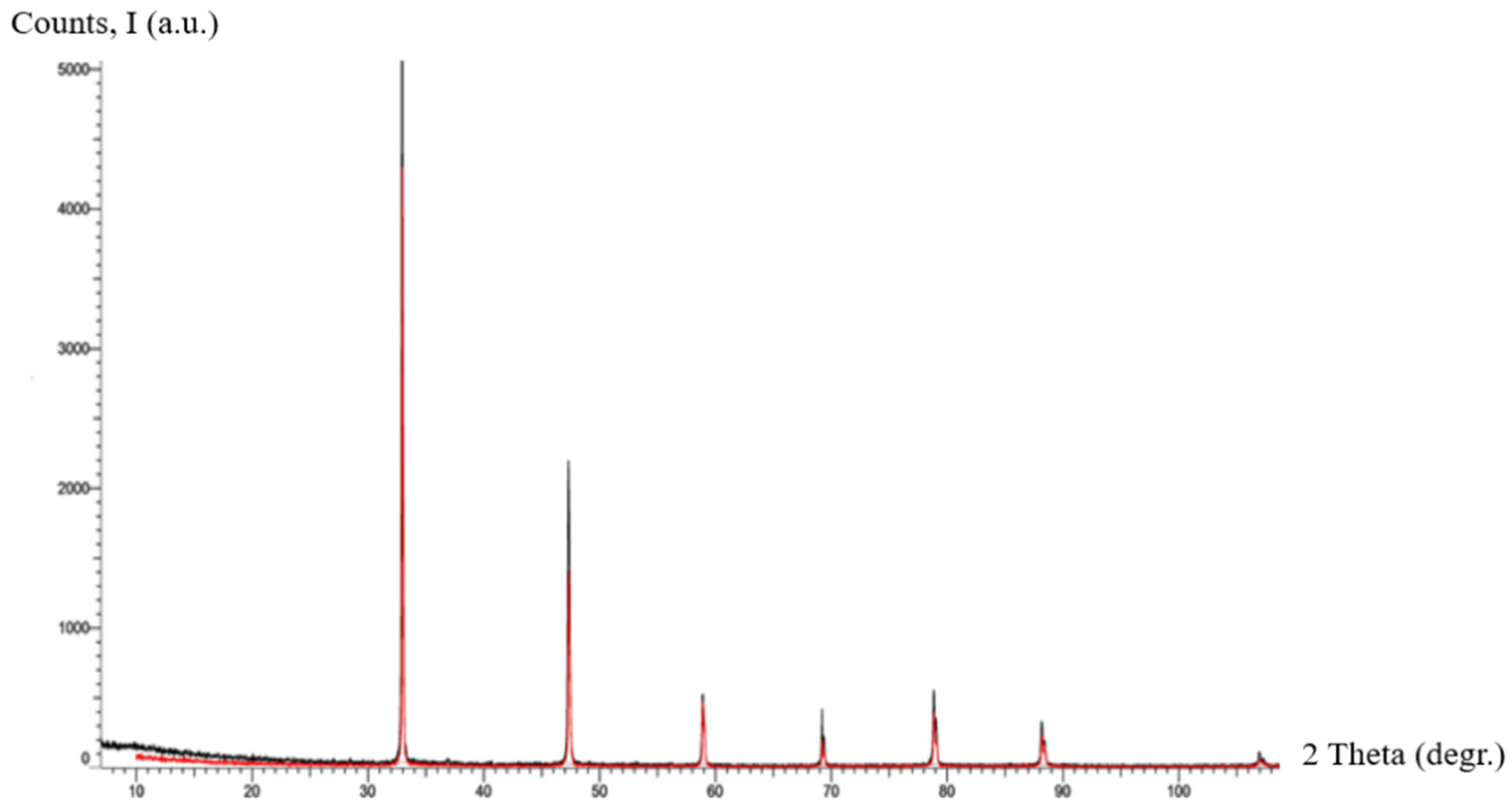
To assess the solid-state structural features of the synthesized material, X-ray diffraction analysis was performed. The diffraction pattern obtained under standard conditions (Co Kα radiation, 30 kV, 10 mA, 0.2 s exposure, 2θ range 10–80°) is presented in Figure 8.
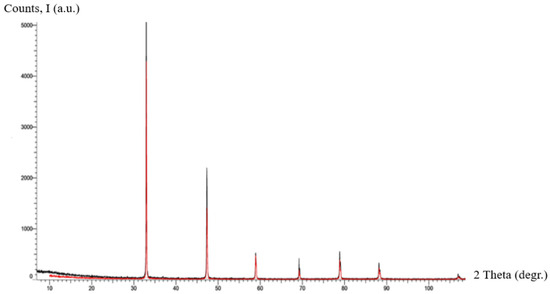
Figure 8.
X-ray diffraction pattern for the sample of . The red and black lines correspond to the different dispersions of the poly-nitroadduct powder.
As illustrated in Figure 8, the X-ray diffractogram of C60(NO2)18(OH)2 demonstrates a unique fingerprint distinctly different from those of pure C60, fullerenols (C60(OH)n, where n = 8–40), fullerene oxides (C60On), and other known derivatives in existing diffraction databases. The difference in peak positions and intensities confirms that the synthesized compound represents a structurally unique form. Although no direct reference patterns for polynitro-hydroxylated fullerenes have been reported in the literature to date, the observed diffractogram provides strong indirect evidence supporting the structural individuality of the new adduct.
Here and everywhere after, authors insert all the numerical data on the properties of the poly-nitroadduct and its solutions into Supplement (1) in the form of 13 tables.
Building on the established molecular structure and thermal properties, the next stage of the study focused on the physicochemical behavior of C60(NO2)18(OH)2 in aqueous solution, which is of central importance for its prospective biomedical applications. Specifically, information regarding solubility, colloidal state, aggregation, and volumetric properties is crucial, as these parameters significantly affect biological activity, delivery, and interaction with physiological media.
The concentration-dependent behavior of C60(NO2)18(OH)2 in aqueous solution was studied by analyzing the size distribution of molecular associates, zeta (ζ) potentials, and electrokinetic mobility at 25 °C. These characteristics were evaluated across a concentration range of 0.001–10 g/dm3, with the results summarized in Tables S1–S3 (Supplementary Materials) and graphically presented in Figure 9.
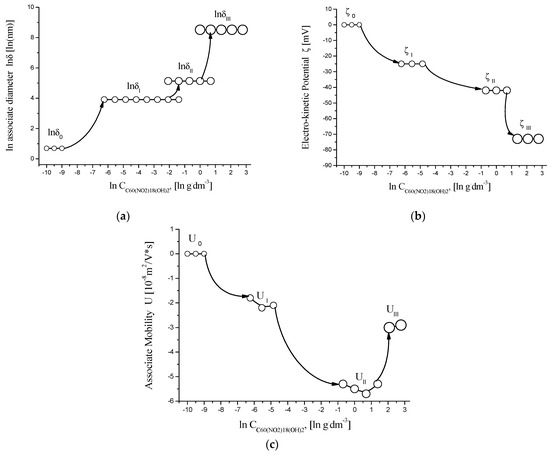
Figure 9.
Concentration dependence of physicochemical parameters in aqueous solutions of C60(NO2)18(OH)2 at 25 °C, shown in semi-logarithmic coordinates: (a) natural logarithm of associated hydrodynamic diameter (ln δ) vs. ln C; (b) zeta potential (ζ, mV) vs. ln C; (c) electrokinetic mobility (U, ×10−8 m2/V·s) vs. ln C. The plots illustrate the transition between molecular, nano-, and micro-associated states with increasing concentration.
The analysis of the obtained data allows us to draw the following conclusions:
- Monomeric molecules of C60(NO2)18(OH)2, with an estimated hydrodynamic diameter of δ ≈ 2 nm, are dominant at concentrations C ≤ 0.00195 g/dm3.
- In the range of 0.00195–0.50 g/dm3, first-order associates form, with sizes around δ ≈ 50 ± 10 nm.
- Between 0.0624 and 2.0 g/dm3, second-order associates (nanocolloidal particles) are observed, with sizes approximately δ ≈ 170 ± 50 nm.
- At concentrations C ≥ 1.0 g/dm3, third-order associates (microcolloids) with sizes up to δ ≈ 5 ± 0.6 µm coexist with second-order aggregates.
In the concentration range C ≥ 0.125 g/dm3, the zeta potential (ζ) values range from –42 to –73 mV, indicating strong electrostatic stabilization and high colloidal stability of the aqueous system.
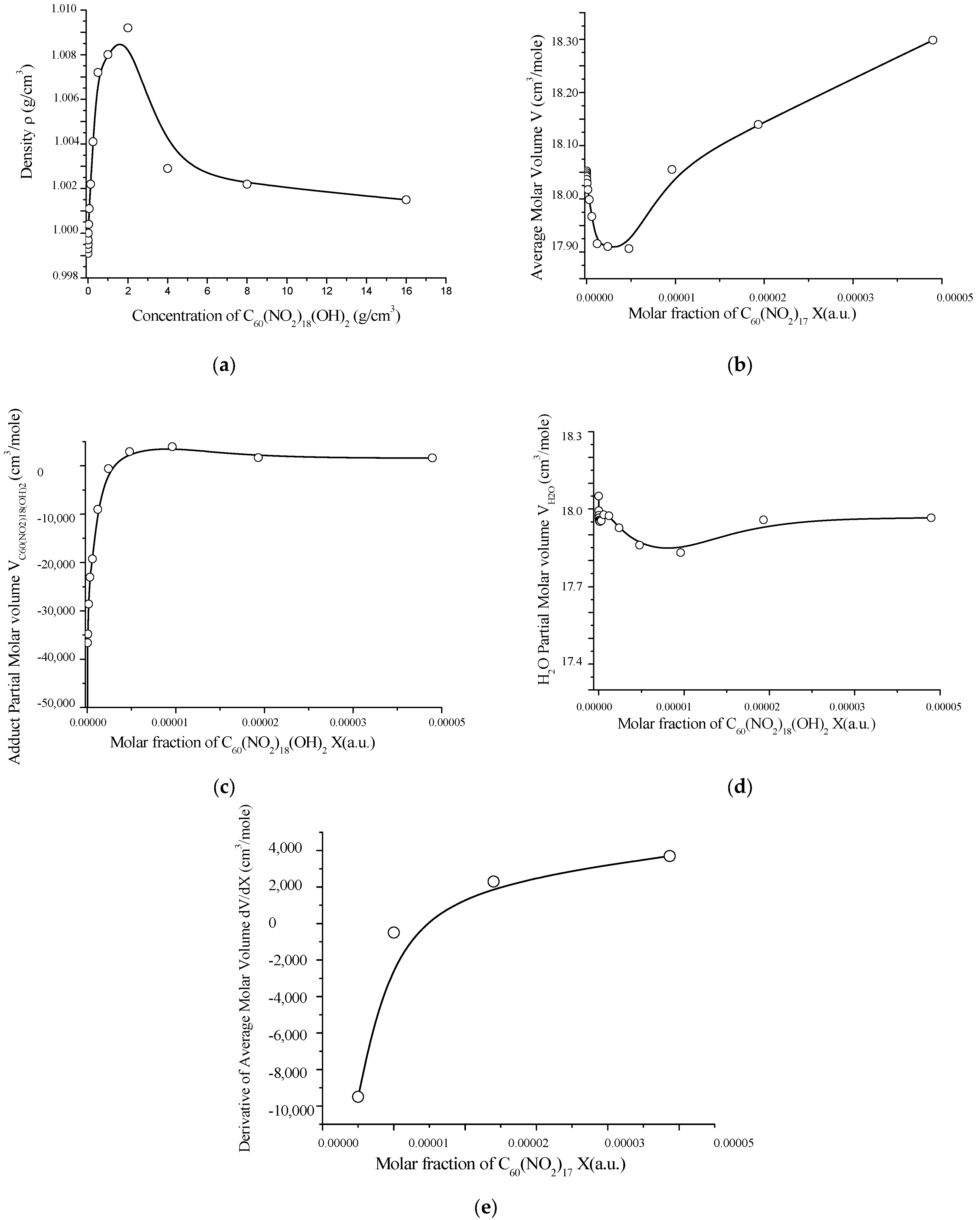
The volumetric behavior of aqueous solutions of C60(NO2)18(OH)2 was also investigated. The data, obtained at 25 °C in the concentration range 1.95 × 10−3 to 16.0 g/dm3, are presented in Table S4 (Supplementary Materials) and visualized in Figure 9.
As shown in Figure 10, the density of the solution increases with concentration, reaching a maximum at C ≈ 2.0 g/dm3. The average molar volume exhibits a shallow minimum in the range C = 0.0624–1.0 g/dm3, while the partial molar volume of the polynitro adduct passes through a broad maximum, and the partial molar volume of water shows a corresponding minimum near C ≈ 4.0 g/dm3.
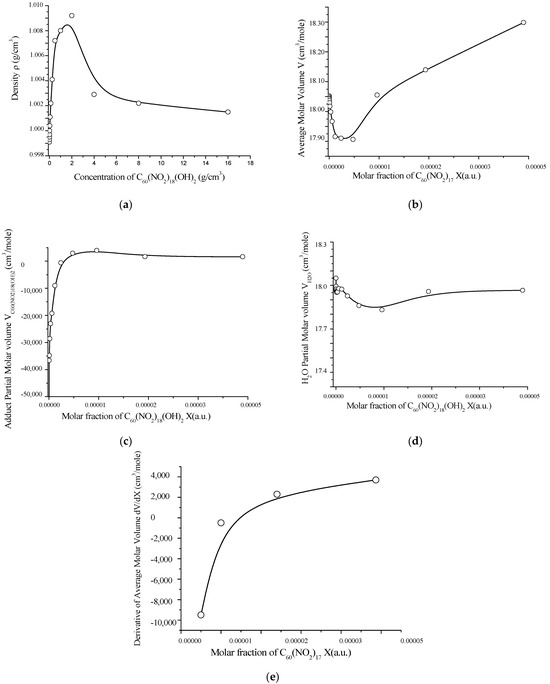
Figure 10.
Concentration-dependent volumetric properties of aqueous solutions of C60(NO2)18(OH)2 at 25 °C: (a) solution density vs. molar fraction; (b) average molar volume of the solution; (c) partial molar volume of C60(NO2)18(OH)2; (d) partial molar volume of water; (e) first derivative of the average molar volume with respect to concentration. These plots illustrate the structural reorganization of the solution with increasing concentration of the polynitro adduct.
At low concentrations (C ≤ 0.0156 g/dm3), the partial molar volume of the polynitro adduct is significantly negative, indicating a pronounced structuring or compaction of the solvent matrix around the solute. As concentration increases, the partial molar volume becomes positive and rises steadily. This behavior clearly reflects a shift from monomeric and small associative states to the formation of larger aggregates (associates of order II and III), with corresponding changes in the solution’s physicochemical environment.
The activity and activity coefficients of the polynitro adduct C60(NO2)18(OH)2 in aqueous solution were determined using cryometric analysis in the near-freezing temperature range T = 272–273 K. This method is based on the depression of the freezing point of water upon the addition of solutes, which reflects non-ideal solution behavior and allows estimation of thermodynamic parameters.
The measurements were conducted in the binary system C60(NO2)18(OH)2–H2O, with the mole fraction of the polynitro adduct varying from 0 to 1.82 × 10−4. The onset of ice crystallization was recorded to determine the freezing point depression ΔT (K), and the corresponding values of water activity (ln aₜ) and activity coefficients for both water and the solute were calculated using classical cryoscopic equations.
The results of the cryometric study are presented in Table 2.
where ∆HfW = 5990 J/mole, ∆CP = −38.893 J/mole∙K, and Tf0 = 273.15 K are the heat of fusion, isobar heat capacity change at crystallization (in the temperature range T = 272 ÷ 273 K, the value is practically constant), and H2O fusion temperature, correspondingly. High positive deviations from the ideality are observed. The results show strong positive deviations from ideality, indicating the presence of significant solute–solvent interactions and non-linear behavior typical of structured or aggregated systems.
[−∆HfW∆T − ∆CP∆T2]/[R (Tf0 − ∆T)Tf0] = lnaW

Table 2.
Cryometric analysis of aqueous solutions of C60(NO2)18(OH)2 in the temperature range T = 272–273 K.
To model the non-ideality more accurately, the Virial Decomposition Asymmetric Model (VD-AS) was applied. This model accounts for higher-order interactions in asymmetric binary systems and allows quantification of non-ideality parameters Λ2, Λ3, and Λ4. These values, as well as the concentration range of diffusional instability (corresponding to spinodal decomposition boundaries), are presented in Table 3.

Table 3.
VD-AS model parameters (Λ2, Λ3, Λ4) and concentration range of stability region (Xdiff-instab) in the binary system: H2O (in temperature range ).
Using the Virial Decomposition Asymmetric Model (VD-AS), the non-ideality parameters were determined (Table 3):
where 1 is the poly-nitroadduct number and 2 represents the solvent number (w).
lnγ1ass ≈ 2Λ2 X1 + 3Λ3 X12 + 4Λ4 X13
lnγ2ass ≈ −Λ2 X12 − 2Λ3 X13 − 3Λ4 X14
Concentration region of solution diffusional instability was calculated for the system (the region of instability are determined by inequality):
12Λ4 X13 + 6Λ3 X12 + 2Λ2 X1 +1 > 0
The model parameters allowed us to calculate from water activity, activity, and activity coefficients of polynitro addcuts, as well as the diffusion stability regions of liquid solutions (i.e., the boundaries of delamination regions—the so-called spinodals). The latter coincide with the concentration boundaries of the existence of III-order associates with linear dimensions δ several μm.
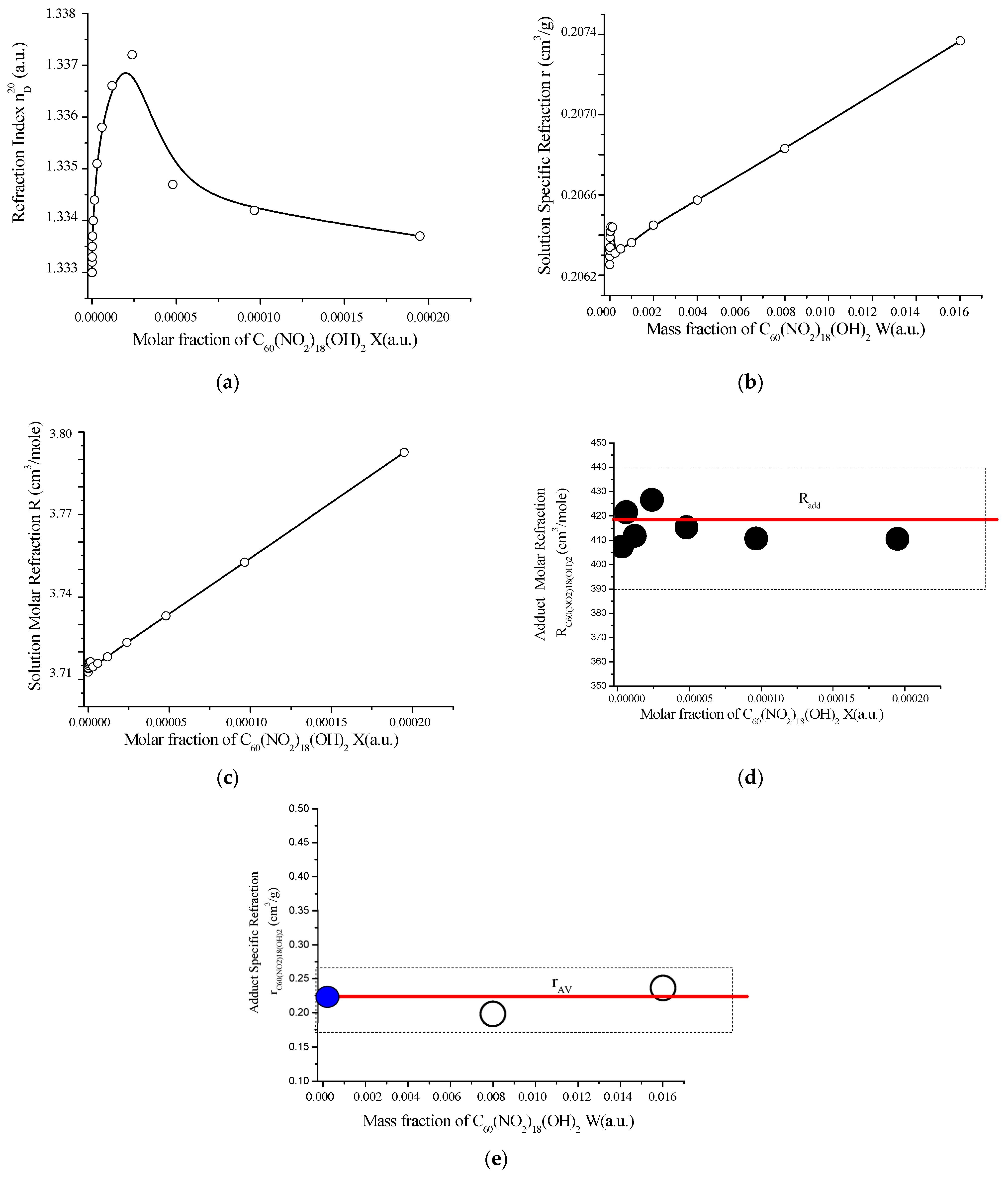
The dependence of refractive indices of aqueous solutions of poly-nitroadduct in (nD20) was determined by refractometry under the following conditions: atmospheric pressure 748 mm. Hg; relative humidity of air—81%).
The experimental values of the refractive indices of aqueous solutions, the dependence of the refractive index on concentration, and the specific and molar refractive indices of the poly-nitro-adduct solution H2O are given in Table S5 (in Supplement Materials) and Figure 11.
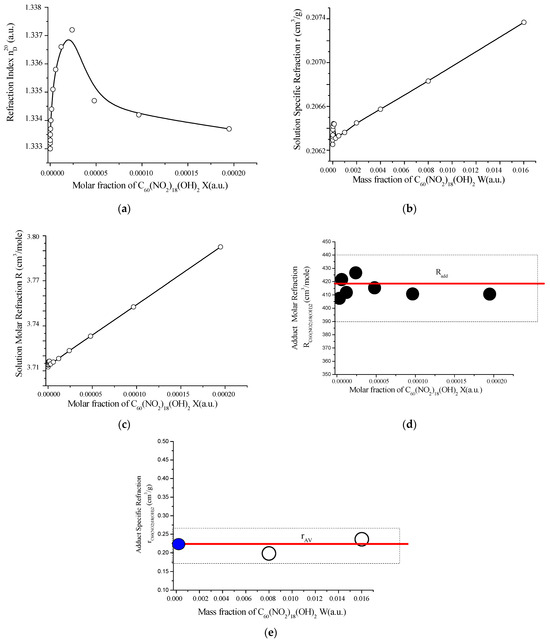
Figure 11.
Concentration-dependent optical properties of the C60(NO2)18(OH)2–H2O system at 21 ± 2 °C and atmospheric pressure (748 mmHg): (a) refractive index (nᴰ20) vs. molar fraction of C60(NO2)18(OH)2; (b) specific refraction of the solution vs. mass fraction; (c) molar refraction of the solution; (d) molar refraction of the adduct, compared with theoretical additive value (red line); (e) specific refraction of C60(NO2)18(OH)2 as a function of volume concentration, compared with the average (red line) and solvent (blue circle, H2O) values. These graphs confirm the optical additivity of the system and validate the calculated structure of the polynitro adduct based on its refractive response.
As shown in Figure 11, the refractive index of aqueous solutions of C60(NO2)18(OH)2 exhibits a distinct maximum at a concentration of approximately C ≈ 2.0 g/dm3. Both the specific refraction and molar refraction of the solution demonstrate significant changes at and above this concentration, indicating nonlinear behavior related to structural transformations in the system.
The molar refraction of the polynitro adduct itself aligns well with the theoretical value calculated by the additivity rule:
This calculation assumes that the total molar refraction of a compound equals the sum of the atomic and bond contributions within the molecule. The experimentally determined molar refraction matches the average values reported in Table S5 (Supplementary Materials), thereby reinforcing the validity of the proposed molecular structure and elemental composition.
In addition, the specific refraction data provide a useful tool for determining the concentration of binary aqueous solutions. According to the additivity rule for specific refraction, the specific refraction of a solution is equal to the weighted sum of the specific refractions of its components, each multiplied by its corresponding mass fraction. This relationship enhances the utility of refractometry as a rapid and non-invasive method for concentration monitoring in fullerene-based nanomaterials.
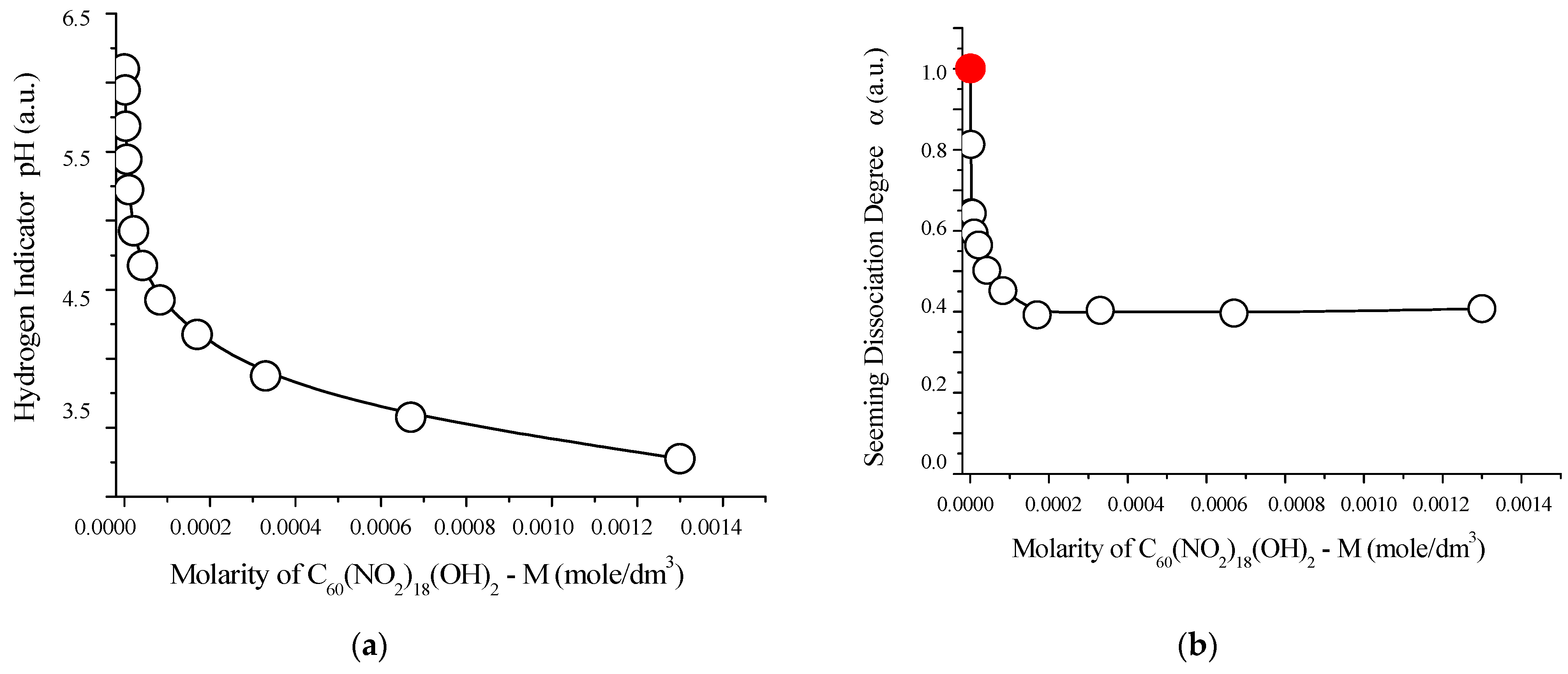
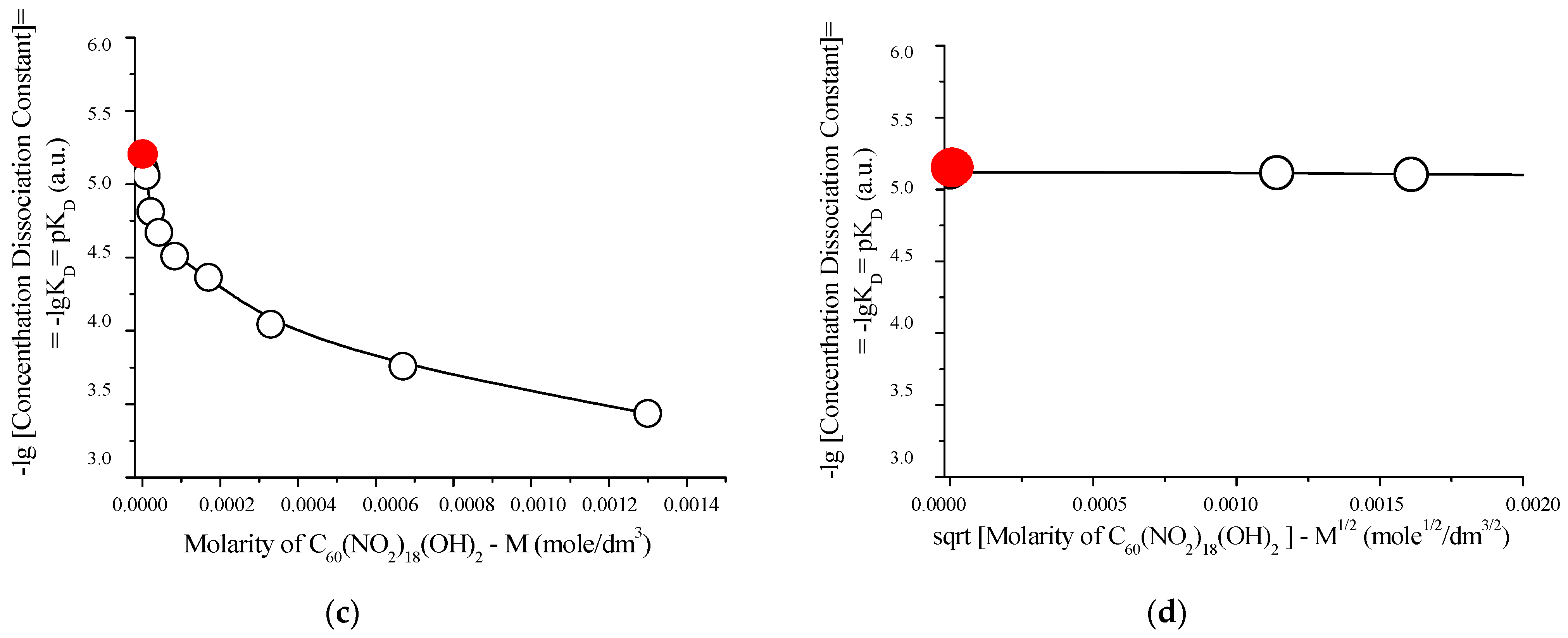
The acid–base properties of the synthesized compound were further investigated at 25 °C, and the results are summarized in Figure 12. These findings offer additional insights into the dissociation behavior and potential buffering capacity of the polynitro adduct in aqueous environments.
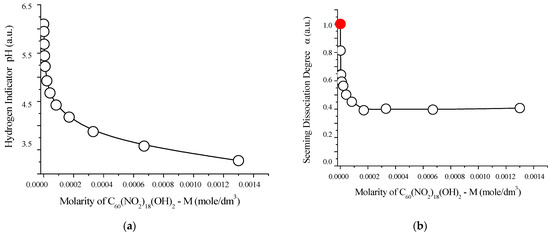
Figure 12.
Acid–base properties of aqueous solutions of C60(NO2)18(OH)2 at 25 °C: (a) pH values (hydrogen ion indicator) as a function of molar concentration; (b) apparent degree of dissociation (α) vs. molarity; (c) negative logarithm of the concentration dissociation constant −log vs. molarity; (d) extrapolation of −log to infinite dilution (red circle), based on the square root of molarity. These graphs confirm the weakly acidic behavior of the polynitro adduct, with a thermodynamic dissociation constant of approximately , indicative of partial proton release in aqueous media.
As shown in Figure 12, an evident monotonic acidification of the solution is observed with increasing concentration of C60(NO2)18(OH)2, with pH values decreasing from approximately 5.1 to 4.3. This trend indicates progressive dissociation of acidic functional groups in the polynitro adduct.
Extrapolation of the dissociation constant to infinite dilution yields a thermodynamic dissociation constant of:
This value confirms that the compound behaves as a weak monoprotic acid in aqueous media. The corresponding dissociation equilibrium can be described by the following reaction:
This partial release of protons supports the presence of ionizable hydroxyl groups in the structure, consistent with prior NMR and IR spectral analyses.
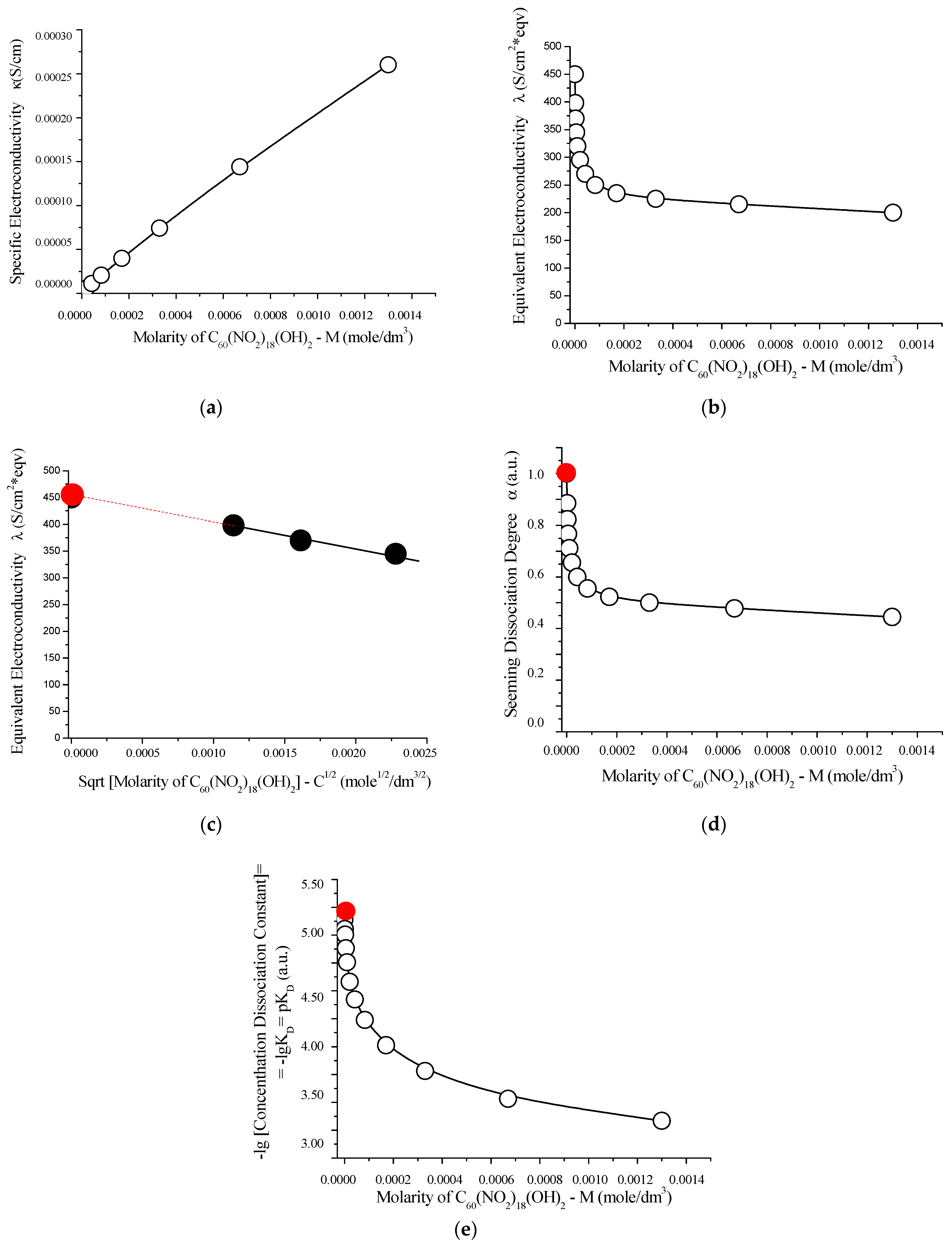
To complement the acid–base study, the electrical conductivity properties of C60(NO2)18(OH)2 solutions were evaluated at 25 °C using a quartz conductivity cell equipped with platinum electrodes. Sodium chloride in water (NaCl–H2O) was used as the reference system. The resulting conductivity data, including specific and equivalent conductivities as well as their extrapolated values at infinite dilution, are presented in Table S6 (Supplementary Materials) and graphically summarized in Figure 13.
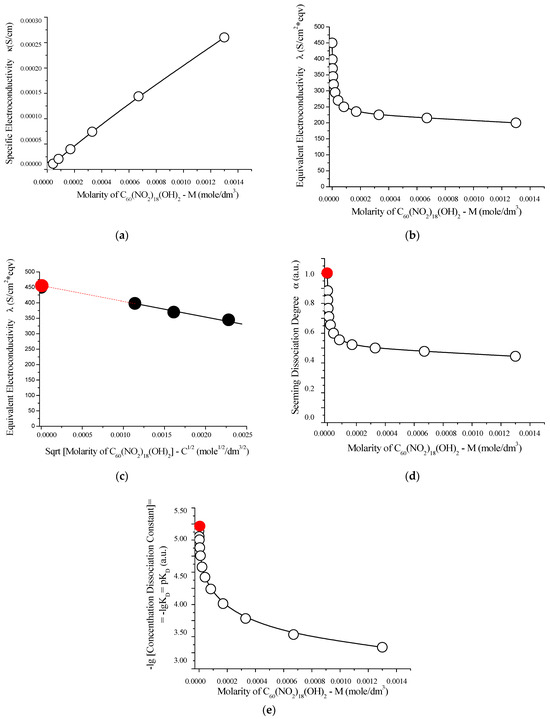
Figure 13.
Electrochemical characterization of aqueous solutions of C60(NO2)18(OH)2 at 25 °C: (a) specific conductivity (κ) as a function of molarity; (b) equivalent conductivity (Λ) versus molarity; (c) extrapolation of equivalent conductivity (Λ) to infinite dilution (red circle), plotted against the square root of molarity; (d) apparent degree of dissociation (α) as a function of molarity; (e) negative logarithm of the concentration dissociation constant −log vs. molarity. These results confirm the weak electrolyte nature of C60(NO2)18(OH)2 in water. The limiting molar conductivity and the extrapolated dissociation constant (≈ 5.1) are consistent with its behavior as a weakly dissociating acid. The red circles indicate extrapolated values at infinite dilution.
As the concentration of C60(NO2)18(OH)2 increases, a nearly linear increase in specific electrical conductivity is observed. Simultaneously, a sharp decline in equivalent conductivity occurs, reflecting the typical behavior of weak electrolytes. Extrapolation of the concentration dissociation constant to infinite dilution yields a thermodynamic dissociation constant:
This value indicates that C60(NO2)18(OH)2 behaves as a weak acid, comparable in strength to acetic acid (CH3COOH), and supports the interpretation of partial dissociation in aqueous solution.
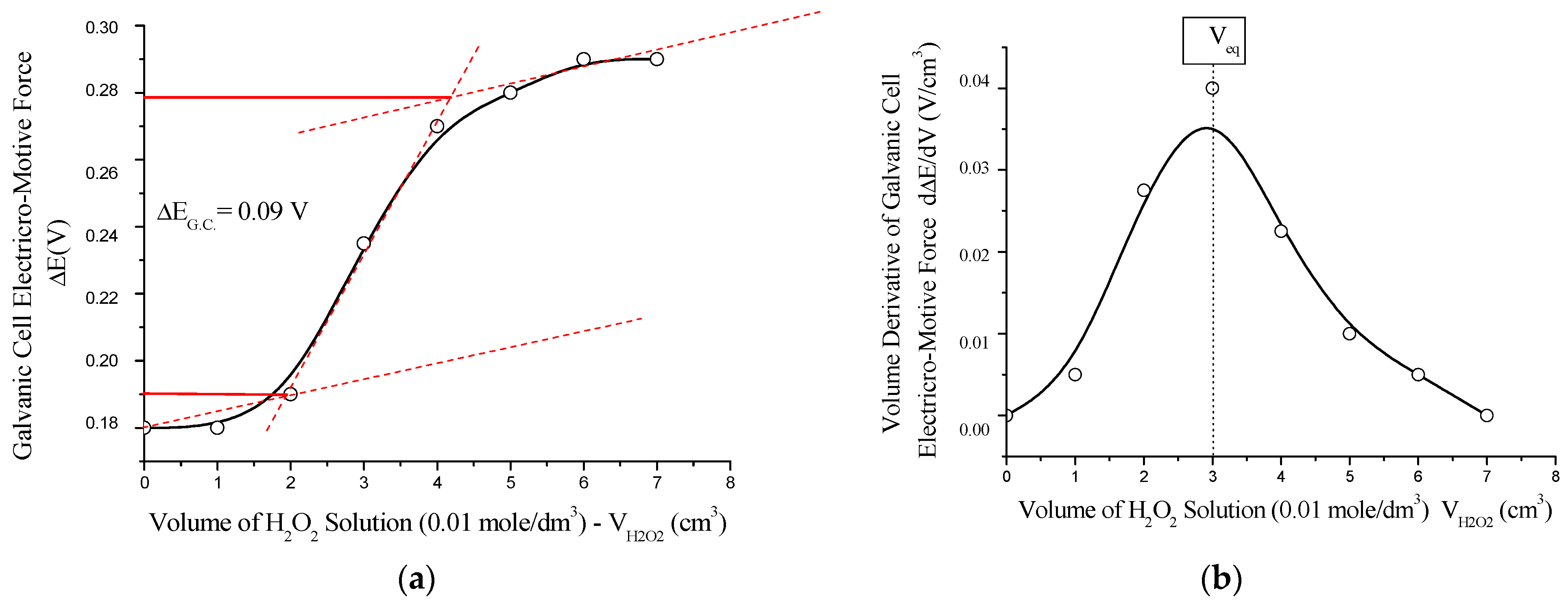
The antioxidant capacity of the polynitro adduct was evaluated by potentiometric redox titration, using hydrogen peroxide (H2O2) as the oxidizing agent. The experiments were conducted using a platinum working electrode in H2O2/NaOH medium, with a reference calomel electrode (Hg2Cl2/Cl−, saturated KCl, E0 = +0.28 V), and the standard potential of the H2O2/2OH− system set at E0 = +0.40 V. Solutions of 0.01 mol/dm3 C60(NO2)18(OH)2 and 0.01 mol/dm3 H2O2 were used for mutual titration (see Figure 14).
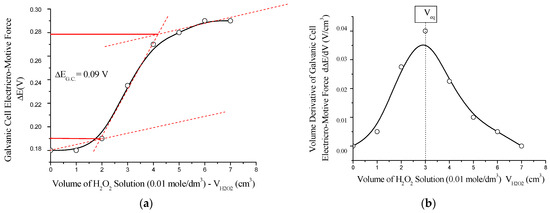
Figure 14.
Potentiometric titration of C60(NO2)18(OH)2 with H2O2 solution (0.01 mol/dm3) using a platinum working electrode (Pt) in the H2O2/OH− system: (a) integral titration curve showing the electromotive force (ΔE) of the galvanic cell as a function of the added volume of H2O2; (b) differential curve representing the first derivative of ΔE with respect to volume (dE/dV), indicating the equivalence point (V_eq). The titration reveals a sigmoidal ΔE profile with a narrow inflection range and a well-defined maximum in the dE/dV plot, confirming quasi-equilibrium redox behavior and effective antioxidant capacity of the polynitro adduct.
This redox transformation (Equations (8)–(10)) occurs in quasi-equilibrium mode and reflects the ability of the polynitro adduct to accept electrons and reduce oxidizing agents. These findings confirm that the synthesized nanomaterial possesses notable antioxidant properties, which may be of practical significance in biomedical applications where oxidative stress is a concern.
The redox reaction proceeds through the following electrochemical equilibria:
- At the cathode:
H2O2 → H2O + ½O2 + 2e− → 2OH−
- At the anode:
C60(NO2)18(OH)2 + 2OH− − 2e− → C60(NO2)18(OH)4
- Overall cell reaction:
C60(NO2)18(OH)2 + H2O2 → C60(NO2)18(OH)4
The potentiometric titration curve exhibits a characteristic sigmoidal (S-shaped) profile, indicating a single-step redox transition. The titration was performed in an acetate buffer (CH3COOH/CH3COONa, 1:1) at pH ≈ 4.8. The observed jump in the electromotive force of the galvanic cell was relatively small, |ΔE_G.C.| ≈ 0.091 V, which is indicative of a quasi-equilibrium redox process. The standard Gibbs free energy change for the reaction was estimated to be ΔG0 ≈ –17 kJ/mol, confirming the mild antioxidant potential of the polynitro compound through a reversible electron transfer pathway.
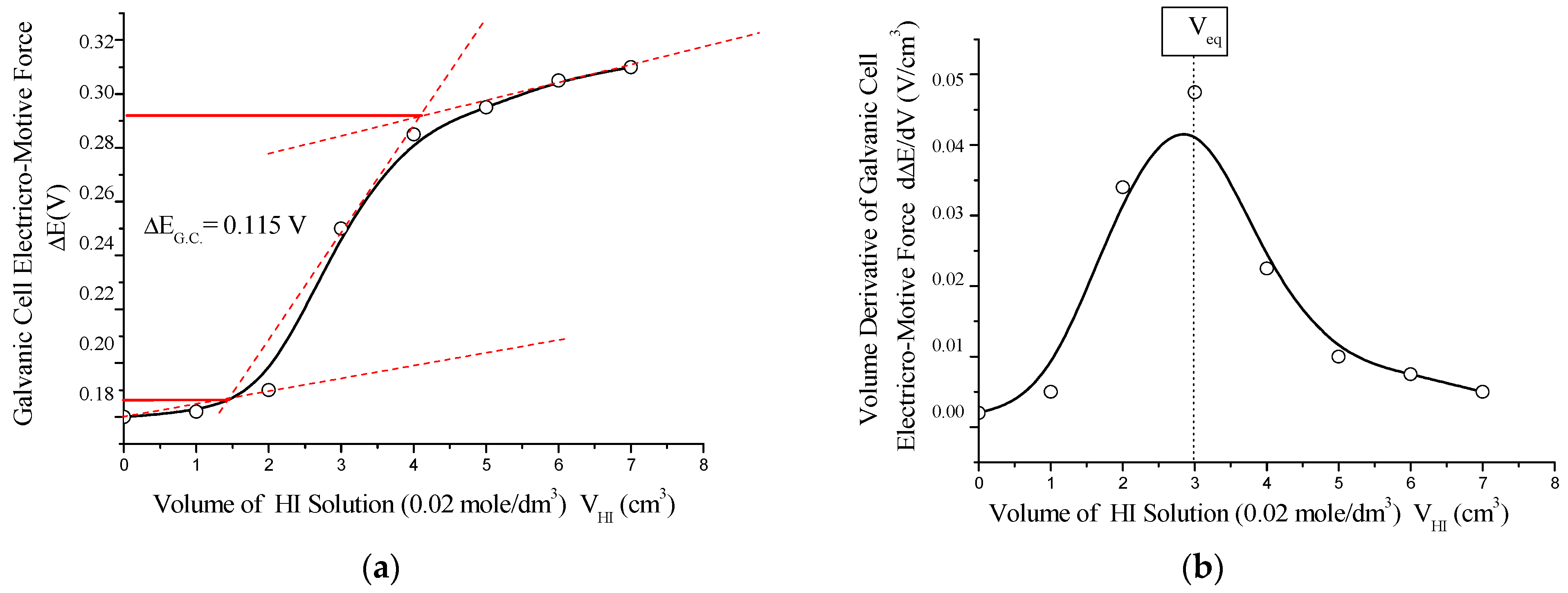
To further evaluate the redox behavior, a second potentiometric titration was conducted using iodine (I2/I−) as the redox couple. The system employed a platinum working electrode (Pt) with a standard potential of E0 = +0.54 V and a saturated calomel reference electrode (Hg2Cl2/Cl− in 1 mol/dm3 KCl, E0 = +0.28 V). A 0.01 mol/dm3 aqueous solution of C60(NO2)18(OH)2 was titrated with 0.01 mol/dm3 iodine solution in potassium iodide (I2/KI), and vice versa (see Figure 15).
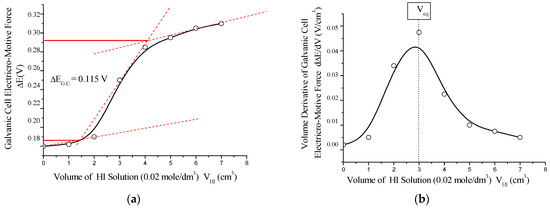
Figure 15.
(a) Integral potentiometric titration curve and (b) its differential form for the redox system involving the working electrode I2/I− (Pt), obtained during titration of C60(NO2)18(OH)2 solution with 0.02 mol/dm3 HI solution. The sharp potential jump (ΔE_G.C. = 0.115 V) and corresponding equivalence point (V_eq) confirm a single-stage quasi-equilibrium redox process between the polynitrofullerene and iodine/iodide couple.
This redox transformation (Equations (11)–(13)) reflects a one-step electron transfer involving iodine as the oxidant, further supporting the antioxidant character of the synthesized polynitro fullerenol derivative. The shape and amplitude of the titration curve correspond to a low electrochemical driving force, with the reaction proceeding under near-equilibrium conditions.
The redox equilibria can be described as follows:
- Cathodic half-reaction:
I2 + 2e− → 2I−
- Anodic half-reaction:
C60(NO2)18(OH)2 + 2I− + 2H2O − 2e− → C60(NO2)18(OH)4 + 2HI−
- Overall cell reaction:
C60(NO2)18(OH)2 + I2 + 2H2O → C60(NO2)18(OH)4 + 2HI
The potentiometric titration curve exhibits a characteristic sigmoidal (S-shaped) form. The titration was conducted in an acetate buffer solution with a 1:1 molar ratio of CH3COOH to CH3COONa at a pH of approximately 4.8. The redox reaction proceeded in a single stage and resulted in a relatively small jump in the electromotive force of the galvanic cell, ΔE_G.C. ≈ 0.115 V. This behavior is indicative of a quasi-equilibrium process corresponding to the electrochemical reaction (7), with an associated standard Gibbs free energy change of approximately ΔG0 ≈ –22 kJ/mol.
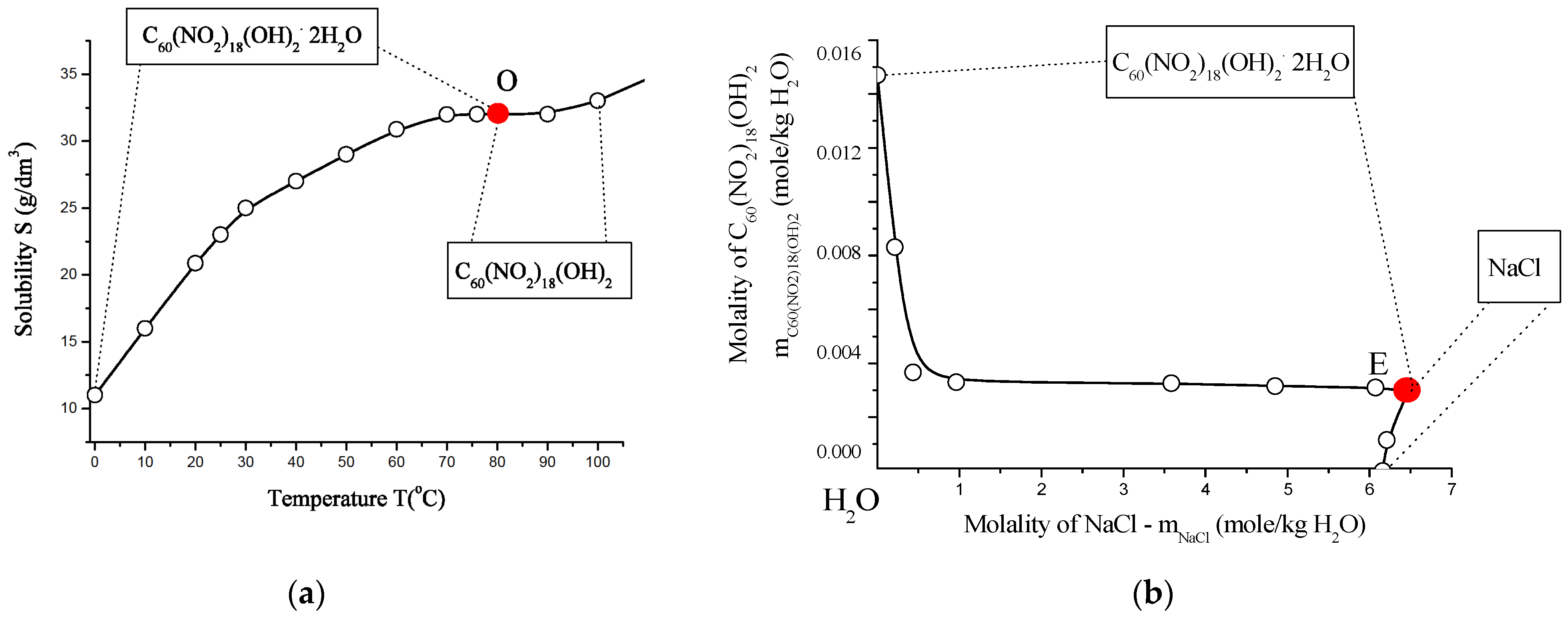
The solubility behavior of the poly-nitrofullerene C60(NO2)18(OH)2 was examined in both binary (water) and ternary (C60(NO2)18(OH)2–NaCl–H2O) systems. Experimental data are presented in Tables S7 and S8 (see Supplementary Materials) and illustrated in Figure 16. Saturation was performed isothermally over 8 h in a shaker-thermostat operating at ~2 Hz, with temperature control precision of ±0.2 K at 0 °C, ±0.05 K at 25 °C, and ±0.5 K at 100 °C. Concentrations of the polynitro adduct in solution were determined spectrophotometrically using UV-visible absorption at λ = 300 nm.
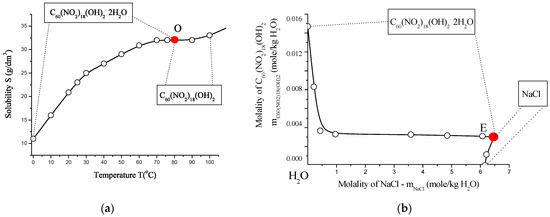
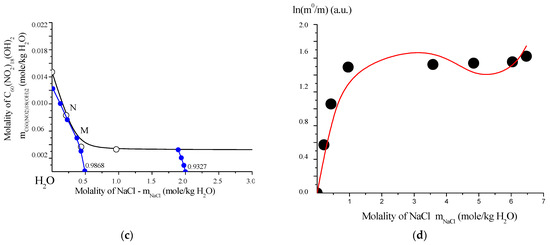
Figure 16.
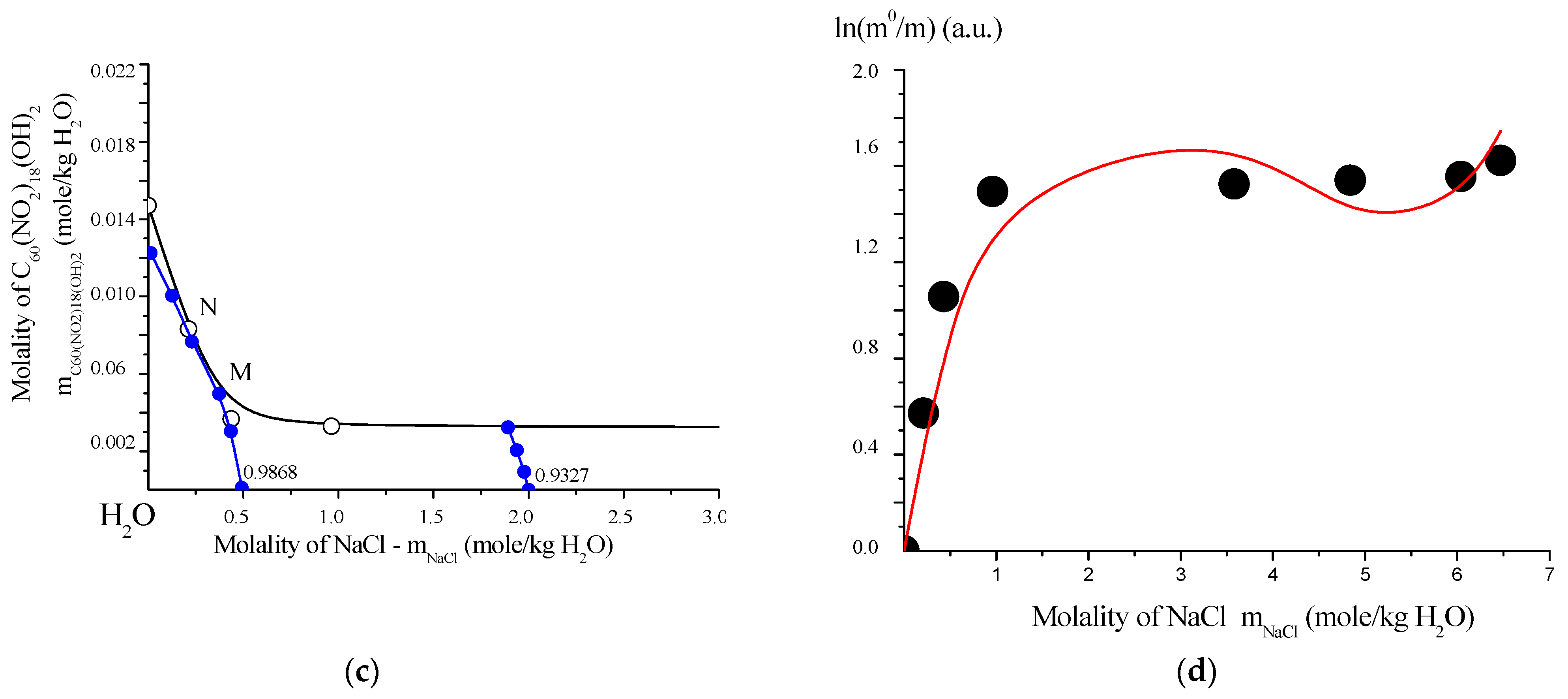
Solubility behavior of C60(NO2)18(OH)2 in aqueous systems: (a) polythermal solubility diagram for the binary system C60(NO2)18(OH)2–H2O in the temperature range T = 0–100 °C. The red point “O” indicates the non-variant point where both crystal hydrates are saturated simultaneously. (b) Isothermal solubility curve for the ternary system C60(NO2)18(OH)2–NaCl–H2O at 25 °C. The red point “E” marks the eutonic point corresponding to saturation by both the dihydrate and NaCl. (c) Fragment of the solubility isotherm with water iso-activity contours (iso-activates), represented by blue lines and values. (d) Fitting of C60(NO2)18(OH)2 solubility data using the extended four-parameter Sechenov model (SEM-4): red line—model fit; black circles—experimental data; m0 and m—solubility values in saturated binary and ternary systems, respectively. This comprehensive diagram set characterizes both the temperature and salinity dependence of C60(NO2)18(OH)2 solubility in water, emphasizing phase boundaries, hydrate formation, and the predictive power of the SEM-4 model in complex electrolyte environments.
To model the solubility behavior, an extended four-parameter version of the Sechenov equation (SEM-4 model) was employed. This model is particularly suited for describing the solubility of weak or non-electrolytes in solutions of strong electrolytes within polar solvents such as water, acetonitrile (CH3CN), methanol (CH3OH), and trifluoroacetic acid (CF3COOH), among others. The SEM-4 model allows for accurate fitting of experimental data and accounts for complex association effects and ionic strength contributions within multicomponent aqueous systems.
The solubility of C60(NO2)18(OH)2 was initially modeled using the classical mono-parameter Sechenov model (SM), which is expressed as:
where m0 and m represent the concentrations of C60(NO2)18(OH)2 in saturated binary (water only) and ternary (NaCl–water) systems, respectively. However, this model proved to be inadequate for accurately describing the solubility behavior of systems with complex, hierarchical, and multistage association mechanisms, such as those observed in fullerene derivatives [23,25].
To address this limitation, an extended four-parameter version of the Sechenov model (SEM-4) was employed:
This expanded model significantly improved the fit to the experimental data and provided a more reliable representation of solubility in the ternary system C60(NO2)18(OH)2–NaCl–H2O, accounting for the complex molecular associations and salt effects present in the solution.
3. Discussion
The synthesized compound, C60(NO2)18(OH)2, represents a novel polynitro-hydroxyl adduct of light fullerene C60, characterized by an exceptionally high degree of nitration—18 nitro groups per fullerene core. To the best of our knowledge, such a structure has not been previously reported. Its molecular composition and unique structure were thoroughly confirmed using a combination of analytical techniques, including elemental analysis, UV-visible and infrared spectroscopy, NMR spectroscopy, and refractometric analysis.
The adduct exhibits significant thermal instability, which limits the application of mass spectrometry, even under gentle ionization conditions such as soft sublimation. Moreover, the compound shows pronounced chemical lability in alkaline media: irreversible alkaline hydrolysis occurs with the transformation of nitro groups into hydroxyls, forming fullerenol derivatives. It follows from the results of complex thermal analysis (pp. 9–10) that in the natural temperature range for humans, the poly-nitroadduct is quite stable both in the solid phase and in aqueous solutions. Under conditions of normal values of the hydrogen index of physiological fluids (close to neutral, as well as pronounced acidic media)—blood, cerebrospinal fluid, lymph, and gastric juice—the polynitro-adduct is quite resistant to hydrolysis. In alkaline physiological fluids, such as in the oral cavity or, for example, intestinal juice, with increased secretion, more or less irreversible hydrolysis may occur with the substitution of nitro groups for hydroxyl groups.
Such substitution will not lead to the formation of solid-phase precipitates, since with an increase in such substitution, the solubility of the substituted adducts only increases, and for fully substituted fullerenols (C60(OH)20 in our case), it is several tens of g/dm3. Salting of the poly-nitroadduct in aqueous solutions of electrolytes (sodium chloride, first of all) undoubtedly occurs (see, for example, p. 21); however, it is also insignificant if low concentrations of poly-nitroadduct (up to units of g/dm3) are used (the phrase is inserted in p. 22).
In aqueous solutions, the adduct demonstrates a pronounced hierarchical self-association behavior, forming aggregates of increasing complexity—monomeric units, first-order (nanocluster) associates, second-order (nanocolloidal) aggregates, and ultimately third-order (microcolloidal) structures. These higher-order associations correspond to a microheterogeneous phase state. The observed negative zeta potential across all aggregate types imparts relative colloidal stability, especially at low to moderate concentrations. However, at concentrations exceeding several grams per liter, the system progressively loses diffusion and sedimentation stability due to the formation of large third-order associates.
Electrokinetic measurements indicate that the primary charge carriers in solution are hydrated protons and small- to medium-sized associates. This interpretation is further supported by volumetric data, including concentration-dependent average and partial molar volumes, which confirm structural transitions and aggregation behavior in solution.
From an acid-base perspective, C60(NO2)18(OH)2 behaves as a weak acid in aqueous media, with a dissociation constant (pK_D ≈ 5.1) comparable to that of acetic acid (CH3COOH). In terms of redox behavior, the adduct displays notable antioxidant and reducing properties, though slightly weaker than those of ascorbic acid. Importantly, the redox activity is multi-step and reversible, depending sensitively on the pH and redox potential of the surrounding medium.
C60(NO2)18(OH)2 demonstrates high water solubility, reaching tens of grams per liter, and this solubility increases markedly with temperature. The compound is also highly compatible with physiological fluids such as saline, blood plasma, lymph, cerebrospinal fluid, and gastric juice, highlighting its potential for biomedical applications. However, the introduction of strong electrolytes (e.g., NaCl, HCl) into solution leads to a pronounced decrease in solubility due to the well-known salting-out effect.
4. Materials and Methods
The synthesis of the mixed polynitro-hydroxylated adduct C60(NO2)18(OH)2 (see Figure 1) was performed using a classical nitration procedure with a strongly oxidizing nitrating mixture composed of concentrated nitric acid (HNO3) and sulfuric acid (H2SO4). The acid volumes were as follows: HNO3—approximately 250 cm3 (65 wt.%, chemically pure, GOST 4461-77, manufacturer: Magna, Magna International, Orora, ON, Canada); H2SO4—approximately 50 cm3 (94 wt.%, extra pure, GOST 14262-78, manufacturer: ChemExpress, Saint-Petersburg, Russia).
A quantity of 500 mg of high-purity C60 fullerene (≥99.9 wt.%, ILIP Ltd., St. Petersburg, Russia) was combined with the nitrating mixture. The reaction mixture was stirred magnetically (rotation speed ~2 Hz) for 7 days at a temperature of 80 ± 5 °C using silicone oil as the heat-transfer medium. During the reaction, the initial black-blue opaque suspension gradually turned into a translucent green-brown viscous solution.
The reaction mixture was then filtered through a Schott glass filter to obtain a clear, light-yellow filtrate.
To remove excess nitric acid, concentrated hydrogen peroxide (H2O2, 37 wt.%, chemically pure, GOST 177-88, manufacturer: Lenreactive, Saint-Petersburg, Russia) was added dropwise at room temperature. This resulted in the evolution of brown nitrogen dioxide gas according to the reaction: 2 HNO3 + H2O2 → 2 NO2 + 2 H2O + O2↑.
Subsequently, colorless oxygen was slowly released. An additional 10 cm3 of H2O2 was introduced to ensure the complete decomposition of residual HNO3, and the solution was left to stand for 24 h.
The remaining sulfuric acid was neutralized using an aqueous sodium hydroxide solution (30 wt.%, analytical grade, GOST 4328-77, Chem. Express., Saint-Petersburg, Russia) until the pH reached approximately 7 ± 1.
The resulting solution was evaporated using a rotary vacuum evaporator at 80 ± 5 °C. During evaporation, colorless sodium sulfate (Na2SO4) crystals precipitated and were removed by filtration. The remaining transparent yellow solution had a final volume of approximately 100 ± 10 cm3.
Residual inorganic salts were removed by dialysis using regenerated cellulose dialysis tubing (MD25, molecular weight cut-off ~1000, 77 mm diameter, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The dialyzed solution was then evaporated to dryness in a vacuum drying oven at 50 ± 5 °C and a residual pressure of 18 ± 3 mm Hg.
This procedure yielded approximately 475 mg of brown, finely dispersed crystalline polynitro adduct. Based on the assumed molecular formula C60(NO2)18(OH)2, the reaction yield was estimated at ~44% of the theoretical maximum. Authors tried to obtain reproducible mass spectra, using soft ionization methods in mass spectrometry (which is usually used for nanocluster investigation): MALDI Express, electrospray ionization, and chemical ionization at atmospheric pressure. However, unfortunately, the result was irreproducible; during sublimation-ionization, a large number of uninterpretable fragments were formed. The authors realize that in the absence of reliable mass spectrometric data, the established formula of poly-nitroadduct is not justified, and the established output is relatively conditional.
Grapher software package ORIGIN (Version OriginPro 7.0 with subprogram package Nonlinear curve fit) was used (Electronic Arts, Redwood City, CA, USA).
Main characteristics of used research methods in the present investigation are represented below in Table 4.

Table 4.
Research methods and instruments used in the identification of
The structure and composition of the synthesized C60(NO2)18(OH)2 were confirmed using elemental analysis, infrared (IR) spectroscopy, electron (UV–Vis) spectroscopy, high-performance liquid chromatography (HPLC), thermogravimetric analysis (TGA), nuclear magnetic resonance (NMR) spectroscopy, and X-ray powder diffraction (XRD).
Methods and devices used in the study of physicochemical properties of poly-nitroadduct are shown in Table 5.

Table 5.
Methods and devices used in the study of physicochemical properties of poly-nitroadduct
Authors have not conducted detailed preliminary biocompatibility or antimicrobial tests to support these claims. The authors conducted only a few primary tests on protozoa: Biotesting on chlorella vulgaris growth and toxicity of poly-nitroadduct C60 in this case, Paramecium caudatum. Data are represented in the Supplement to the article. The primary study of antioxidant activity is described on pp. 19–21 of the main text. The authors believe that in the standard conditions of existence of living organisms, it can be argued that super-low and low concentrations of poly-nitroaddct are non-toxic or low-toxic. Undoubtedly, a detailed and thorough study of the possible biochemical and medical aspects of the use of poly-nitroadducts will need to be carried out in the future. However, this was not the direct purpose of this article, which is mainly chemical-oriented.
5. Conclusions
A direct, non-catalytic synthesis of a novel water-soluble polynitro-hydroxylated adduct based on light fullerene C60 was successfully carried out. The resulting compound, C60(NO2)18(OH)2, represents a previously undescribed molecular structure with a high degree of nitration—18 nitro groups per C60 core.
The identity and composition of the adduct were confirmed using a range of analytical techniques, including elemental (C-H-N) analysis, energy-dispersive X-ray microanalysis, infrared (IR) and electron spectroscopy, nuclear magnetic resonance (NMR), high-performance liquid chromatography (HPLC), and thermogravimetric analysis (TGA). Refractive, volumetric, cryometric, electrochemical, acid–base, and antioxidant properties were also studied in detail.
Cryometric measurements, interpreted via the Virial Decomposition Asymmetric Model (VD-AS), allowed for the calculation of activity coefficients and identification of diffusion instability zones in binary C60(NO2)18(OH)2–H2O solutions.
The physicochemical data collected are mutually consistent and support the proposed molecular formula. The adduct is thermally unstable, which prevents reliable mass spectrometric analysis, even under mild ionization conditions. In alkaline media, the compound undergoes irreversible hydrolysis, leading to the formation of fullerenols.
In aqueous solution, C60(NO2)18(OH)2 exhibits hierarchical self-association. Monomeric molecules form primary (I-order), secondary (II-order), and ternary (III-order) associates, with the latter corresponding to a microheterogeneous phase. These associations are accompanied by uniformly negative electrokinetic potentials, which provide colloidal stability at low to moderate concentrations. However, at higher concentrations (>several g/dm3), the solutions lose both diffusion and sedimentation stability. The formation of associates of the I and II orders (with associate sizes in the tens and first hundreds of nanometers and nanocluster concentrations in hundredths and tenths of g/dm3, respectively) should not significantly limit the biomedical use of poly-nitroadducts. Moreover, as the nanocluster solutions are naturally diluted with physiological fluids, the nanoclusters are crushed with a decrease in their order and size. However, the formation of micro-heterogenic associates of the III order of several μm in size (in solutions with a concentration of several g/dm3) can be dangerous from a medical point of view. They can fundamentally block micro-vessels in living organisms, injure them, serve as centers of subsequent crystallization—plaque growth, etc. The effectiveness of such large associates when used may be very low. That is why the authors believe that for further biomedical applications, the upper limit of the concentration of poly-nitroadducts is a conditional concentration of 1 g/dm3. However, in the future, if medical research establishes the fact of concentration and secondary accumulation of nanoclusters in any tissues, our assessment may change.
Electrochemical mobility data suggest that the primary charge carriers in solution are hydrated protons and I- and II-order associates. These findings are reinforced by volumetric studies showing concentration-dependent variations in average and partial molar volumes.
From an acid–base standpoint, the adduct behaves as a weak acid, similar in strength to acetic acid (CH3COOH). In redox behavior, the compound exhibits significant antioxidant and reducing activity—though somewhat weaker than ascorbic acid—and can undergo multistep, reversible redox transitions in response to changes in pH or redox potential.
The compound demonstrates high solubility in water (tens of grams per liter), with solubility increasing with temperature. It also dissolves well in physiological fluids, including saline, blood plasma, lymph, cerebrospinal fluid, and gastric juice. However, the introduction of strong electrolytes such as NaCl or HCl causes a marked reduction in solubility due to salting-out effects.
Overall, the comprehensive physicochemical characterization of C60(NO2)18(OH)2 suggests strong potential for its application as a medical-grade nanomaterial. These results provide a foundation for further research into its biological compatibility, therapeutic activity, and functional performance in biomedical systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13070212/s1, Table S1: Linear dimensions of associates in the system H2O at 25 °C; Table S2: Average monomers number in Associates in the system H2O при 25 °C; Table S3: Electro-kinetic ξ- potential and associative mobility in the system H2O при 25 °C; Table S4: Volume properties, concentration dependence of density of water solutions, average and partial components volumes in the system H2O at 25 °C; Table S5: Refraction properties, concentration dependence of refraction index, specific and molar refraction of the solution and poly-nitroadduct in the system H2O at 21 ± 1 °C; Table S6: Electric properties, concentration dependence of specific, equivalent electric conductivity, seeming dissociation degree, concentration dissociation constant in the system H2O at 25 °C; Table S7: Base-Acid properties, concentration dependence of hydrogen indicator, seeming dissociation degree, concentration dissociation constant in the system H2O при 25 °C; Table S8: Potentiometric titration of 10 cm3 of H2O with volume concentration by water solution of NaOH with concentration (Seven Compact pH-meter S220) при 25 °C; Table S9: Potentiometric titration of 10 cm3 of H2O with volume concentration by water solution of with concentration and water solution of with concentration (Ion-meter-Voltmeter 781 pH/Ion Meter) at 25 °C. Titration: Working Pt electrode, auxiliary electrode is normal calomel: . —measured potential of the galvanic cell, and —potentials of working and standard calomel electrodes; Table S10: Solubility in the system H2O (in temperature range ); Table S11: Solubility in ternary system H2O at ; Table S12: Vapor-liquid phase equilibrium data (isopiestic measurements) in ternary system H2O at ; Table S13: Parameters of modified I.Sechenov model—SEM in ternary system H2O at Figure S1: The dependencies of light absorption activity in the differ-ent processes of photosynthesis and chemosynthesis (blue—chlorophyll synthesis, green—photosynthesis, red—photo-morphogenesis) on the wavelength in visional region of light wavelength [24]; Figure S2: The dependencies of growth of chlorella vulgaris bijer population (a.u.) on the time of cultivation at the different dilutions: blue—Control, violet—dilution 1/100, green—dilution 1/10, red—dilution 1/1; Figure S3: Toxicity index against –lg of the dilution of basic poly-nitroadduct C60 solution with the concentration 0.35 g/dm3.
Author Contributions
Conceptualization, N.K. and M.S.; methodology, N.K. and N.B.; software, B.M.; validation, N.K., M.S. and N.C.; formal analysis, B.A., M.A. and N.B.; investigation, N.K., M.S., B.A. and N.B.; resources, N.B.; data curation, B.M., N.C. and N.B.; writing—original draft preparation, N.K., M.S., N.C. and N.B.; writing—review and editing, N.K., N.C. and N.B.; visualization, N.K., B.A. and M.A.; supervision, M.S. and N.C.; project administration, N.K. and B.A.; funding acquisition, M.S. and B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR24992786 “Development of technology for manufacturing samples of domestic medical instruments and medical products”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable. This study did not involve human participants, human data, or human tissue.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IR | Infrared Spectroscopy |
| UV-Vis | Ultraviolet–Visible Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| HPLC | High-Performance Liquid Chromatography |
| TG-DTA | Thermogravimetric and Differential Thermal Analysis |
| XRD | X-ray Diffraction |
| SEM-4 | Sechenov Extended Model (four-parameter) |
| VD-AS | Virial Decomposition Asymmetric Model |
| Dissociation Constant (Acid-Base) | |
| ζ-Potential | Zeta Potential (Electrokinetic Potential) |
| C60 | Fullerene C60 |
| NO2 | Nitro Group |
| OH | Hydroxyl Group |
| NaCl | Sodium Chloride |
| HNO3 | Nitric Acid |
| H2SO4 | Sulfuric Acid |
| H2O2 | Hydrogen Peroxide |
References
- Ganjoo, R.; Assad, H.; Sharma, P.K.; Kaya, S.; Kumar, A. Fullerenes as Anticorrosive Coating Materials. In Smart Anticorrosive Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 91–107. [Google Scholar] [CrossRef]
- Hirsch, A. Fullerene Derivatives for Medical Applications. In Proceedings of the AIP Conference Proceedings, Salt Lake City, UT, USA, 10–11 August 2005. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Lu, F.-J.; Lin, J.-T. Medical Applications of Water-Soluble Polyhydroxylated Fullerene Derivatives. MRS Proc. 1994, 359, 327. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Troshin, P.A. A Review on Fullerene Derivatives with Reduced Electron Affinity as Acceptor Materials for Organic Solar Cells. Energies 2023, 16, 1924. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-Ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal Applications of Fullerenes. PubMed 2007, 2, 639–649. [Google Scholar]
- Moztarzadeh, O.; Jamshidi, M.; Taherpour, A.A.; Babuska, V. Molecular Modelling of Fullerene C60 Functionalized by Nitric Oxide for Use in Biological Environment. Sci. Rep. 2024, 14, 2565. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-T.; Lee, P.-H.; Chen, C.-F.; Ma, M.-C.; Lai, M.-K.; Hsu, S.-M. De Novo Demonstration and Co-Localization of Free-Radical Production and Apoptosis Formation in Rat Kidney Subjected to Ischemia/Reperfusion. J. Am. Soc. Nephrol. 2001, 12, 973–982. [Google Scholar] [CrossRef]
- Kausar, A. Potential of Polymer/Fullerene Nanocomposites for Anticorrosion Applications in the Biomedical Field. J. Compos. Sci. 2022, 6, 394. [Google Scholar] [CrossRef]
- Mashino, T.; Okuda, K.; Hirota, T.; Hirobe, M.; Nagano, T.; Mochizuki, M. Inhibition of E. Coli Growth by Fullerene Derivatives and Inhibition Mechanism. Bioorganic Med. Chem. Lett. 1999, 9, 2959–2962. [Google Scholar] [CrossRef]
- Bhakta, P.; Barthunia, B. Fullerene and Its Applications: A Review. J. Indian Acad. Oral Med. Radiol. 2020, 32, 159. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Ding, R.G.; Lu, G.Q.; Yan, Z.F.; Wilson, M.A. Recent Advances in the Preparation and Utilization of Carbon Nanotubes for Hydrogen Storage. J. Nanosci. Nanotechnol. 2001, 1, 7–29. [Google Scholar] [CrossRef]
- Acquah, S.F.A.; Penkova, A.V.; Markelov, D.A.; Semisalova, A.S.; Leonhardt, B.E.; Magi, J.M. Review—The Beautiful Molecule: 30 Years of C60and Its Derivatives. ECS J. Solid State Sci. Technol. 2017, 6, M3155–M3162. [Google Scholar] [CrossRef]
- Biazar, E.; Majdi, N.; Zafari, M.; Avar, M.; Aminifard, S.; Zaeifi, D.; Ai, N.; Jafarpour, N.; Montazeri, N.; Gh, N. Nanotoxicology and Nanoparticle Safety in Biomedical Designs. Int. J. Nanomed. 2011, 6, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Lashneva, V.V.; Tkachenko, Y.G.; Dubok, V.A.; Schur, D.V.; Sychev, V.V.; Matveeva, L.A. Prospects of Usage of Materials with Fullerene Coatings for Endoprosthesis of Joints. In Nanostructured Materials and Coatings for Biomedical and Sensor Applications; Springer: Berlin/Heidelberg, Germany, 2003; pp. 103–109. [Google Scholar]
- Saulėnienė, G.; Kirsnyte-Snioke, M.; Stirkė, A.; Jasulaitiene, V.; Straksys, A.; Dobilaitis, S.; Melo, W.C.M.A. Development of Photoactive Biomaterial Using Modified Fullerene Nanoparticles. Front. Chem. 2024, 12, 1432624. [Google Scholar] [CrossRef]
- Dorner-Reisel, A.; Wang, T.; Freiberger, E.; Ritter, U.; Moje, J.; Zhao, M.; Scharff, P. Fullerene C60 Films on Dental Implants: Durability Study after in Vitro Short-Term Exposure. Diam. Relat. Mater. 2023, 135, 109886. [Google Scholar] [CrossRef]
- Grebowski, J.; Kazmierska, P.; Krokosz, A. Fullerenols as a New Therapeutic Approach in Nanomedicine. BioMed Res. Int. 2013, 2013, 751913. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Fu, S.; Zhang, A. Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants. Nanomaterials 2019, 9, 1647. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.Y.; Bhonsle, J.B.; Wang, L.; Shu, S.F.; Chang, T.M.; Hwu, J.R. Efficient One-Flask Synthesis of Water-Soluble [60]Fullerenols. Tetrahedron 1996, 52, 4963–4972. [Google Scholar] [CrossRef]
- Sharoyko, V.V.; Ageev, S.V.; Meshcheriakov, A.A.; Podolsky, N.E.; Vallejo, J.P.; Lugo, L.; Rakipov, I.T.; Petrov, A.V.; Ivanova, A.V.; Charykov, N.A.; et al. Physicochemical Investigation of Water-Soluble C60(C2NH4O2)4H4 (C60-Gly) Adduct. J. Mol. Liq. 2021, 344, 117658. [Google Scholar] [CrossRef]
- Kovel, E.S.; Kicheeva, A.G.; Vnukova, N.G.; Churilov, G.N.; Stepin, E.A.; Kudryasheva, N.S. Toxicity and Antioxidant Activity of Fullerenol C60,70 with Low Number of Oxygen Substituents. Int. J. Mol. Sci. 2021, 22, 6382. [Google Scholar] [CrossRef]
- Charykov, N.A.; Semenov, K.N.; López, E.R.; Fernández, J.; Serebryakov, E.B.; Keskinov, V.A.; Murin, I.V. Excess Thermodynamic Functions in Aqueous Systems Containing Soluble Fullerene Derivatives. J. Mol. Liq. 2018, 256, 305–311. [Google Scholar] [CrossRef]
- Ravelo-Nieto, E.; Duarte-Ruiz, A.; Reyes, L.H.; Cruz, J.C. Synthesis and Characterization of a Fullerenol Derivative for Potential Biological Applications. Mater. Proc. 2020, 4, 15. [Google Scholar] [CrossRef]
- Liu, B.; Jin, J.; Liu, M. Mapping Structure-Property Relationships in Fullerene Systems: A Computational Study from C20 to C60. Npj Comput. Mater. 2024, 10, 227. [Google Scholar] [CrossRef]
- Tomilin, F.N.; Artyushenko, P.V.; Shchugoreva, I.A.; Rogova, A.V.; Vnukova, N.G.; Churilov, G.N.; Shestakov, N.P.; Tchaikovskaya, O.N.; Ovchinnikov, S.G.; Avramov, P.V. Structure and Vibrational Spectroscopy of C82 Fullerenol Valent Isomers: An Experimental and Theoretical Joint Study. Molecules 2023, 28, 1569. [Google Scholar] [CrossRef]
- Shershakova, N.; Baraboshkina, E.; Andreev, S.; Purgina, D.; Struchkova, I.; Kamyshnikov, O.; Nikonova, A.; Khaitov, M. Anti-Inflammatory Effect of Fullerene C60 in a Mice Model of Atopic Dermatitis. J. Nanobiotechnol. 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-X. Review on the Nitration of [60]Fullerene. Propellants Explos. Pyrotech. 2001, 26, 109–111. [Google Scholar] [CrossRef]
- Anantharaj, V.; Bhonsle, J.; Canteenwala, T.; Chiang, L.Y. Synthesis and characterization of nitrated [60]fullerene derivatives. J. Chem. Soc. Perkin Trans. 1999, 1, 31–36. [Google Scholar] [CrossRef]
- Yan, W.; Seifermann, S.M.; Pierra, P.; Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).