A Unique Trinuclear, Triangular Ni(II) Complex Composed of Two tri-Anionic bis-Oxamates and Capping Nitroxyl Radicals
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
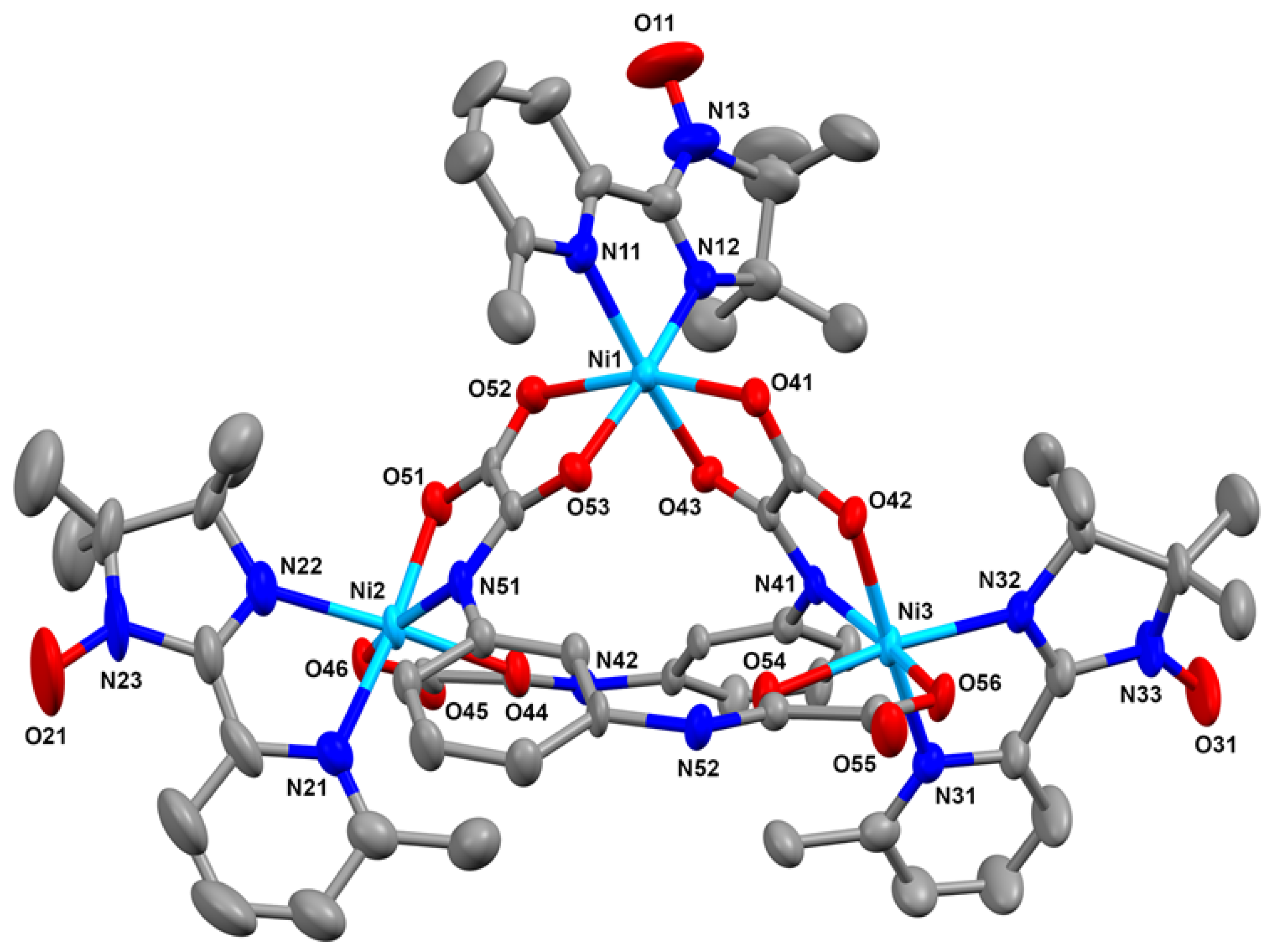
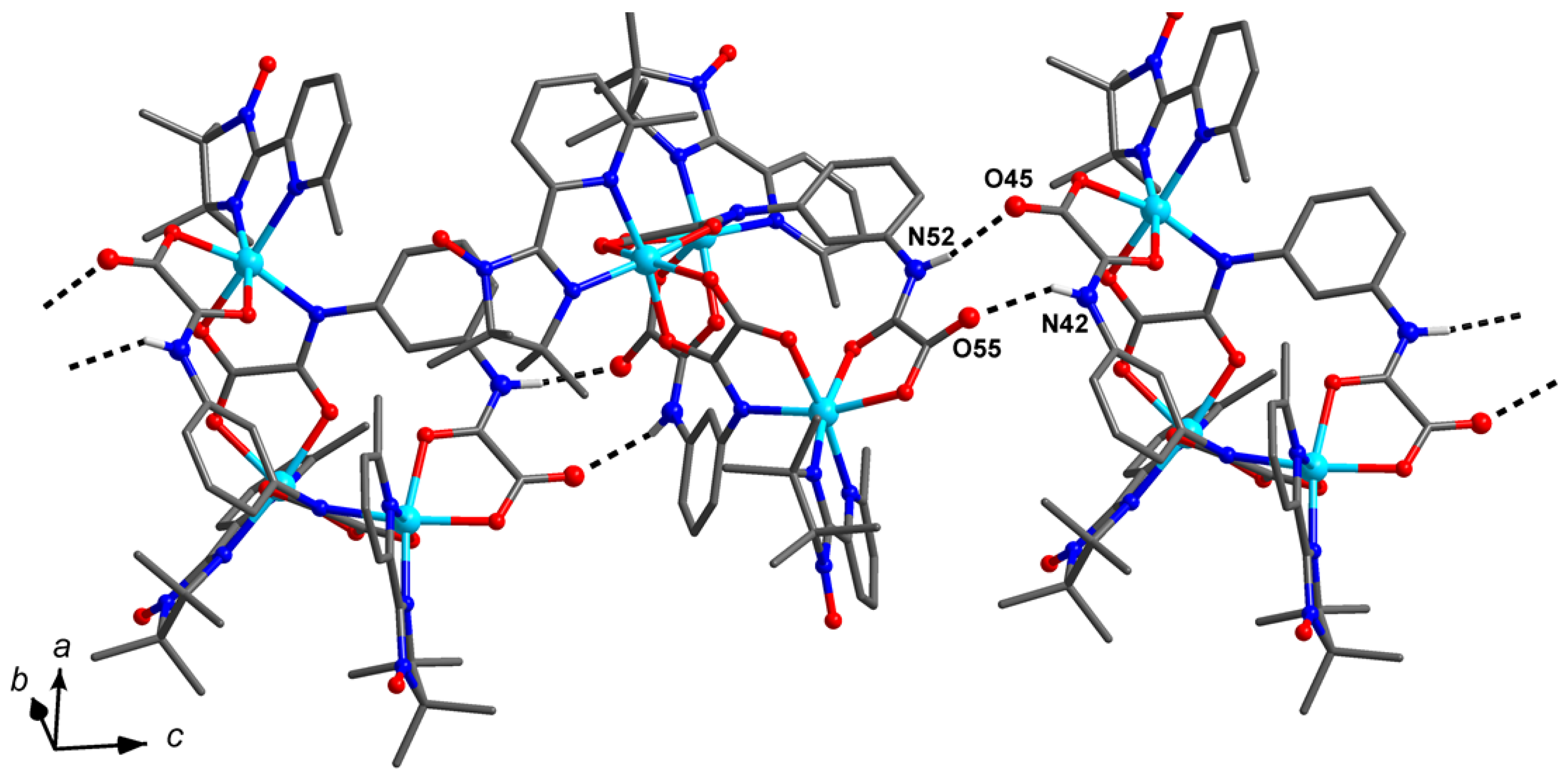
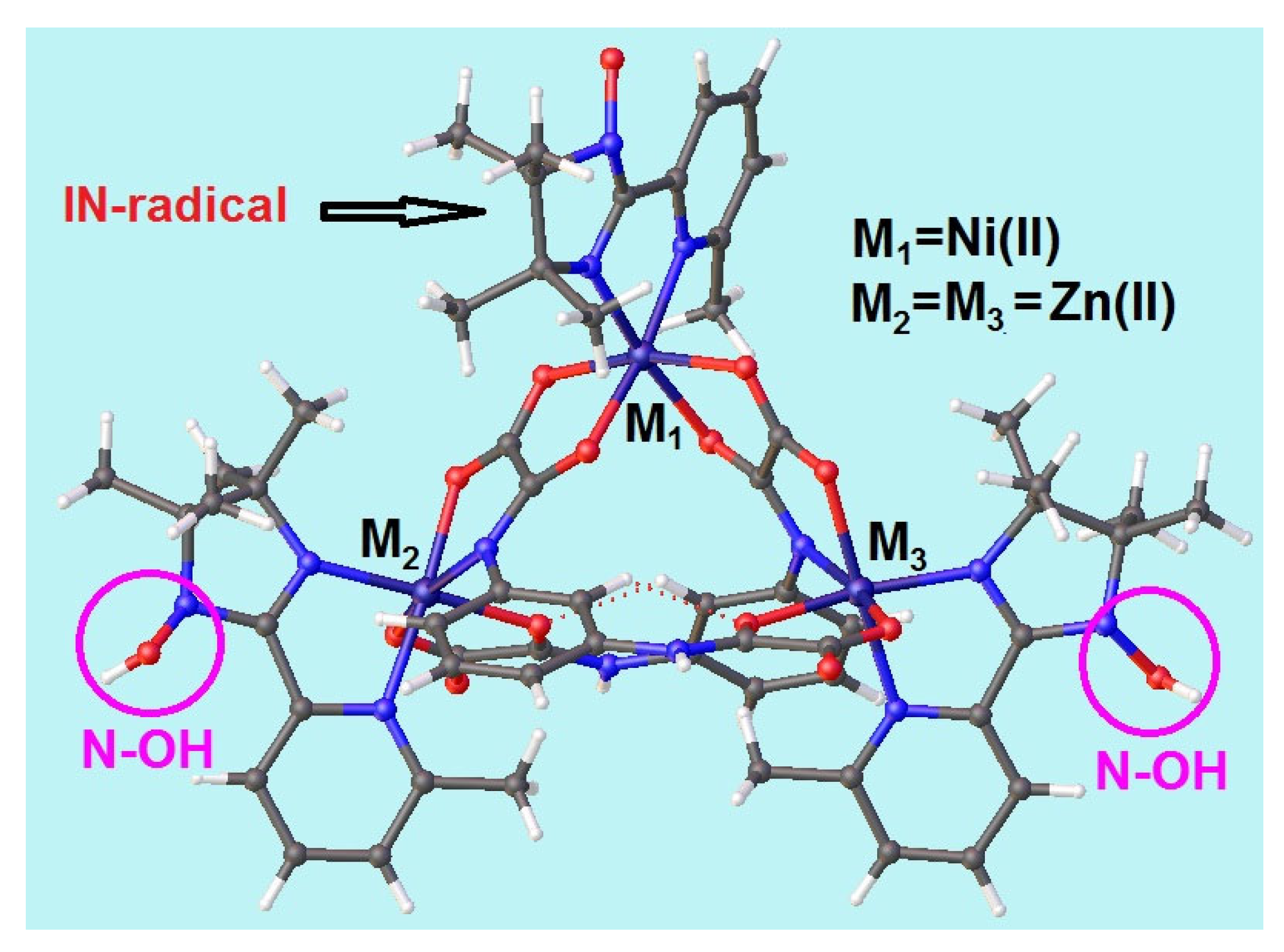
2.2. Crystal and Molecular Structure
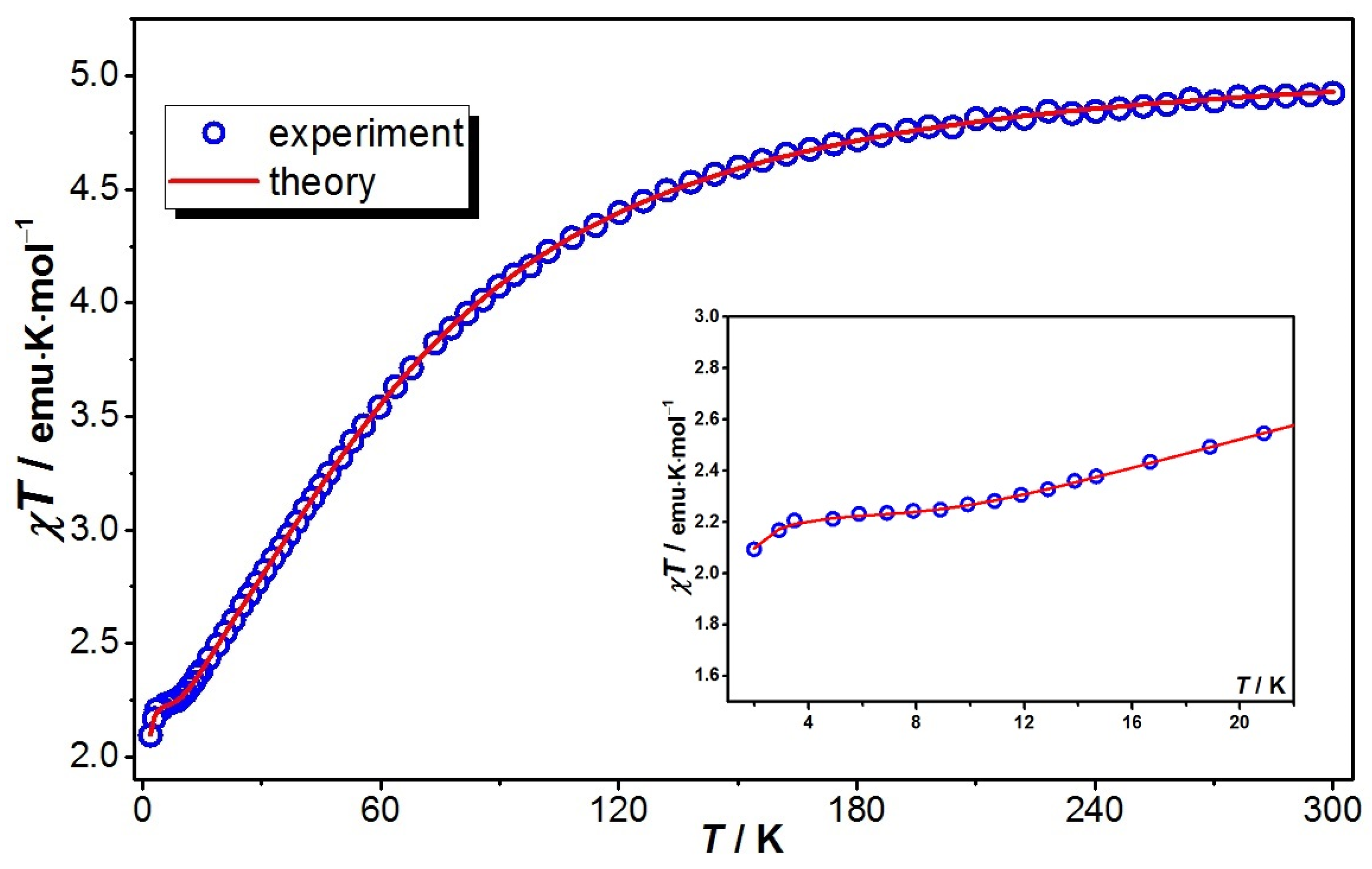
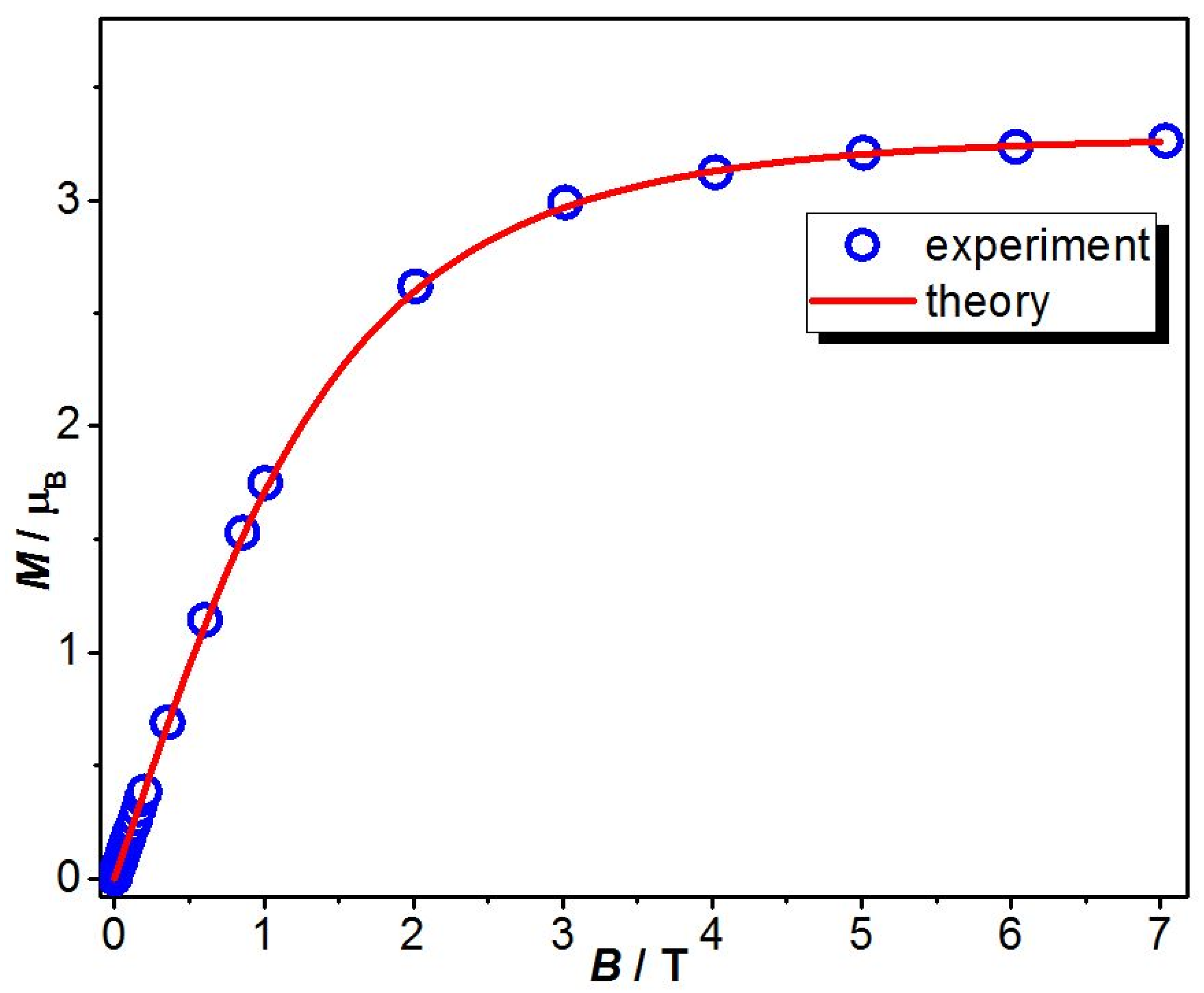
2.3. Cryomagnetic Measurements
2.4. Theoretical Model and Exhaustive Parameter Set Required for Magnetic Data Simulation
3. Materials and Methods
3.1. Instrumental and Physical Measurements
3.2. Theoretical Calculations
3.3. Preparations
Synthesis of [Ni3(L3−)2(IN)3]∙5CH3OH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatt, V.; Ram, S. The Role of Ligands, Polytopic Ligands and Metal Organic Ligands (Mols) in Coordination Chemistry. Chem. Sci. Rev. Lett. 2015, 4, 414–428. [Google Scholar]
- Ruiz, R.; Faus, J.; Lloret, F.; Julve, M.; Journaux, Y. Coordination Chemistry of N,N′-Bis(Coordinating Group Substituted)Oxamides: A Rational Design of Nuclearity Tailored Polynuclear Complexes. Coord. Chem. Rev. 1999, 193–195, 1069–1117. [Google Scholar] [CrossRef]
- Pardo, E.; Ruiz-García, R.; Cano, J.; Ottenwaelder, X.; Lescouëzec, R.; Journaux, Y.; Lloret, F.; Julve, M. Ligand Design for Multidimensional Magnetic Materials: A Metallosupramolecular Perspective. Dalt. Trans. 2008, 2008, 2780–2805. [Google Scholar] [CrossRef]
- Dul, M.-C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussu, D.; et al. Supramolecular Coordination Chemistry of Aromatic Polyoxalamide Ligands: A Metallosupramolecular Approach toward Functional Magnetic Materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [Google Scholar] [CrossRef]
- Journaux, Y.; Ferrando-Soria, J.; Pardo, E.; Ruiz-Garcia, R.; Julve, M.; Lloret, F.; Cano, J.; Li, Y.; Lisnard, L.; Yu, P.; et al. Design of Magnetic Coordination Polymers Built from Polyoxalamide Ligands: A Thirty Year Story. Eur. J. Inorg. Chem. 2018, 2018, 228–247. [Google Scholar] [CrossRef]
- Mariano, L.d.S.; Rosa, I.M.L.; De Campos, N.R.; Doriguetto, A.C.; Dias, D.F.; do Pim, W.D.; Valdo, A.K.S.M.; Martins, F.T.; Ribeiro, M.A.; De Paula, E.E.B.; et al. Polymorphic Derivatives of NiII and CoII Mesocates with 3D Networks and “Brick and Mortar” Structures: Preparation, Structural Characterization, and Cryomagnetic Investigation of New Single-Molecule Magnets. Cryst. Growth Des. 2020, 20, 2462–2476. [Google Scholar] [CrossRef]
- Simões, T.R.G.; do Pim, W.D.; Silva, I.F.; Oliveira, W.X.C.; Pinheiro, C.B.; Pereira, C.L.M.; Lloret, F.; Julve, M.; Stumpf, H.O. Solvent-Driven Dimensionality Control in Molecular Systems Containing CuII, 2,2′-Bipyridine and an Oxamato-Based Ligand. CrystEngComm 2013, 15, 10165–10170. [Google Scholar] [CrossRef]
- Lisnard, L.; Chamoreau, L.-M.; Li, Y.; Journaux, Y. Solvothermal Synthesis of Oxamate-Based Helicate: Temperature Dependence of the Hydrogen Bond Structuring in the Solid. Cryst. Growth Des. 2012, 12, 4955–4962. [Google Scholar] [CrossRef]
- de Campos, N.R.; Simosono, C.A.; Landre Rosa, I.M.; da Silva, R.M.R.; Doriguetto, A.C.; do Pim, W.D.; Gomes Simões, T.R.; Valdo, A.K.S.M.; Martins, F.T.; Sarmiento, C.V.; et al. Building-up Host–Guest Helicate Motifs and Chains: A Magneto-Structural Study of New Field-Induced Cobalt-Based Single-Ion Magnets. Dalt. Trans. 2021, 50, 10707–10728. [Google Scholar] [CrossRef]
- Francescon, J.E.; de J Pfau, S.C.; Borth, K.W.; Marinho, M.V.; Pedroso, E.F.; Giese, S.O.K.; Pereira, C.L.M.; Miorim, A.J.F.; Maciel, G.M.; Hughes, D.L.; et al. Isomorphic Oxamate Derivatives Triple Mesocates: From the Synthesis to Antibacterial Activities. J. Mol. Struct. 2025, 1328, 141272. [Google Scholar] [CrossRef]
- Fernández, I.; Ruiz, R.; Faus, J.; Julve, M.; Lloret, F.; Cano, J.; Ottenwaelder, X.; Journaux, Y.; Muñoz, M.C. Ferromagnetic Coupling through Spin Polarization in a Dinuclear Copper(II) Metallacyclophane. Angew. Chem. Int. Ed. 2001, 40, 3039–3042. [Google Scholar] [CrossRef]
- Pardo, E.; Morales-Osorio, I.; Julve, M.; Lloret, F.; Cano, J.; Ruiz-García, R.; Pasán, J.; Ruiz-Pérez, C.; Ottenwaelder, X.; Journaux, Y. Magnetic Anisotropy of a High-Spin Octanuclear Nickel(II) Complex with a Meso-Helicate Core. Inorg. Chem. 2004, 43, 7594–7596. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Ruiz-García, R.; Lloret, F.; Julve, M.; Cano, J.; Pasán, J.; Ruiz-Pérez, C.; Filali, Y.; Chamoreau, L.-M.; Journaux, Y. Molecular-Programmed Self-Assembly of Homo- and Heterometallic Penta- and Hexanuclear Coordination Compounds: Synthesis, Crystal Structures, and Magnetic Properties of Ladder-type CuII2MIIx (M = Cu, Ni; x = 3,4) Oxamato Complexes with CuII2 Metall. Inorg. Chem. 2007, 46, 4504–4514. [Google Scholar] [CrossRef]
- Cangussu, D.; Pardo, E.; Dul, M.-C.; Lescouëzec, R.; Herson, P.; Journaux, Y.; Pedroso, E.F.; Pereira, C.L.M.; Stumpf, H.O.; Carmen Muñoz, M.; et al. Rational Design of a New Class of Heterobimetallic Molecule-Based Magnets: Synthesis, Crystal Structures, and Magnetic Properties of Oxamato-Bridged (M′ = LiI and MnII; M = NiII and CoII) Open-Frameworks with a Three-Dimensional Honeycomb Architecture. Inorg. Chim. Acta 2008, 361, 3394–3402. [Google Scholar] [CrossRef]
- Pardo, E.; Cangussu, D.; Lescouëzec, R.; Journaux, Y.; Pasán, J.; Delgado, F.S.; Ruiz-Pérez, C.; Ruiz-García, R.; Cano, J.; Julve, M.; et al. Molecular-Programmed Self-Assembly of Homo- and Heterometallic Tetranuclear Coordination Compounds: Synthesis, Crystal Structures, and Magnetic Properties of Rack-Type Cu II 2 M II 2 Complexes (M = Cu and Ni) with Tetranucleating Phenylenedioxamato Bridgi. Inorg. Chem. 2009, 48, 4661–4673. [Google Scholar] [CrossRef] [PubMed]
- Dul, M.-C.; Ottenwaelder, X.; Pardo, E.; Lescoueëzec, R.; Journaux, Y.; Chamoreau, L.-M.; Ruiz-Garciía, R.; Cano, J.; Julve, M.; Lloret, F. Ferromagnetic Coupling by Spin Polarization in a Trinuclear Copper(II) Metallacyclophane with a Triangular Cage-Like Structure. Inorg. Chem. 2009, 48, 5244–5249. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Ferrando-Soria, J.; Dul, M.; Lescouëzec, R.; Journaux, Y.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cañadillas-Delgado, L.; et al. Oligo m-phenyleneoxalamide Copper(II) Mesocates as Electro-Switchable Ferromagnetic Metal–Organic Wires. Chem.—A Eur. J. 2010, 16, 12838–12851. [Google Scholar] [CrossRef]
- Dul, M.-C.; Lescouëzec, R.; Chamoreau, L.-M.; Journaux, Y.; Carrasco, R.; Castellano, M.; Ruiz-García, R.; Cano, J.; Lloret, F.; Julve, M.; et al. Self-Assembly, Metal Binding Ability, and Magnetic Properties of Dinickel(II) and Dicobalt(Ii) Triple Mesocates. CrystEngComm 2012, 14, 5639–5648. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. Palladium(II)–Copper(II) Assembling with Bis(2-pyridylcarbonyl)amidate and Bis(oxamate) Type Ligands. Cryst. Growth Des. 2015, 15, 1325–1335. [Google Scholar] [CrossRef]
- da Cunha, T.T.; Oliveira, W.X.C.; Pedroso, E.F.; Lloret, F.; Julve, M.; Pereira, C.L.M. Heterobimetallic One-Dimensional Coordination Polymers MICuII (M = Li and K) Based on Ferromagnetically Coupled Di- and Tetracopper(II) Metallacyclophanes. Magnetochemistry 2018, 4, 38. [Google Scholar] [CrossRef]
- Wojnar, M.K.; Laorenza, D.W.; Schaller, R.D.; Freedman, D.E. Nickel(II) Metal Complexes as Optically Addressable Qubit Candidates. J. Am. Chem. Soc. 2020, 142, 14826–14830. [Google Scholar] [CrossRef] [PubMed]
- Tlemsani, I.; Lambert, F.; Suaud, N.; Herrero, C.; Guillot, R.; Barra, A.-L.; Gambarelli, S.; Mallah, T. Assessing the Robustness of the Clock Transition in a Mononuclear S = 1 Ni(II) Complex Spin Qubit. J. Am. Chem. Soc. 2025, 147, 4685–4688. [Google Scholar] [CrossRef]
- Vostrikova, K.E.; Luneau, D.; Wernsdorfer, W.; Rey, P.; Verdaguer, M. A S = 7 Ground Spin-State Cluster Built from Three Shells of Different Spin Carriers Ferromagnetically Coupled, Transition-Metal Ions and Nitroxide Free Radicals. J. Am. Chem. Soc. 2000, 122, 718–719. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Suzuki, T.; Kaizaki, S. Syntheses, Structures, Magnetic, and Spectroscopic Properties of Cobalt(Ii), Nickel(Ii) and Zinc(Ii) Complexes Containing 2-(6-Methyl)Pyridyl-Substituted Nitronyl and Imino Nitroxide†. J. Chem. Soc. Dalt. Trans. 2001, 2001, 2943–2950. [Google Scholar] [CrossRef]
- Higashikawa, H.; Inoue, K.; Maryunina, K.Y.; Romanenko, G.V.; Bogomyakov, A.S.; Kuznetsova, O.V.; Fursova, E.Y.; Ovcharenko, V.I. Synthesis, Structure and Magnetic Properties of the Pentanuclear Complex [Fe2(CN)12Ni3L6]·27H2O, where L is Nitronyl Nitroxide. J. Struct. Chem. 2009, 50, 1155–1158. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Romanenko, G.V.; Letyagin, G.A.; Bogomyakov, A.S. Binuclear Co(II), Ni(II), and Cu(II) Hexafluoroacetylacetonates with Spin-Labeled Nitrophenols. J. Struct. Chem. 2023, 64, 1470–1481. [Google Scholar] [CrossRef]
- Ovcharenko, V.; Kuznetsova, O.; Fursova, E.; Letyagin, G.; Romanenko, G.; Bogomyakov, A.; Zueva, E. Simultaneous Introduction of Two Nitroxides in the Reaction: A New Approach to the Synthesis of Heterospin Complexes. Inorg. Chem. 2017, 56, 14567–14576. [Google Scholar] [CrossRef]
- Luneau, D.; Rey, P.; Laugier, J.; Belorizky, E.; Cogne, A. Ferromagnetic Behavior of Nickel(II)-Imino Nitroxide Derivatives. Inorg. Chem. 1992, 31, 3578–3584. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Kamatani, T.; Iino, A.; Suzuki, T.; Kaizaki, S. Synthesis, Magnetic Properties, and Electronic Spectra of Bis(β-Diketonato)Chromium(III) and Nickel(II) Complexes with a Chelated Imino Nitroxide Radical: X-Ray Structures of [Cr(AcaMe)2(IM2py)]PF6 and [Ni(acac)2(IM2Py)]. Inorg. Chem. 2002, 41, 4363–4370. [Google Scholar] [CrossRef]
- Babailov, S.P.; Peresypkina, E.V.; Journaux, Y.; Vostrikova, K.E. Nickel(II) Complex of a Biradical: Structure, Magnetic Properties, High NMR Temperature Sensitivity and Moderately Fast Molecular Dynamics. Sens. Actuators B Chem. 2017, 239, 405–412. [Google Scholar] [CrossRef]
- Hayakawa, K.; Shiomi, D.; Ise, T.; Sato, K.; Takui, T. Stable Iminonitroxide Biradical in the Triplet Ground State. Chem. Lett. 2004, 33, 1494–1495. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Li, L.-C.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P. Exchange Interaction of a Novel Heterospin Polynuclear Complex Containing Transition Metals and Imino Nitroxide Radicals: {[(CuL)Nid(IM-2Py)2](C1O4)2}2∙2H2O. Chin. J. Chem. 2003, 21, 133–138. [Google Scholar] [CrossRef]
- Oshio, H.; Yamamoto, M.; Ito, T.; Kawauchi, H.; Koga, N.; Ikoma, T.; Tero-Kubota, S. Experimental and Theoretical Studies on Ferromagnetically Coupled Metal Complexes with Imino Nitroxides. Inorg. Chem. 2001, 40, 5518–5525. [Google Scholar] [CrossRef]
- Petrov, P.A.; Romanenko, G.V.; Shvedenkov, Y.G.; Ikorskii, V.N.; Ovcharenko, V.I.; Reznikov, V.A.; Sagdeev, R.Z. Complexes of CuII, NiII, and CoII with 2-cyano-2-(1-oxyl-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-2-yl)-1-R-Ethylenolates. Russ. Chem. Bull. 2004, 53, 99–108. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Suzuki, T.; Kaizaki, S. Crystal Structures, Magnetic and Spectroscopic Properties of Manganese(II), Cobalt(II), Nickel(II) and Zinc(II) Dichloro Complexes Bearing Two 2-Pyridyl-Substituted Imino Nitroxides. J. Chem. Soc. Dalt. Trans. 2001, 2001, 1566–1572. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Romanenko, G.V.; Bogomyakov, A.S.; Ovcharenko, V.I. Synthesis, Structure, and Magnetic Properties of Heterospin Polymers MI[MII(Hfac)L2]. Russ. J. Coord. Chem. 2020, 46, 521–527. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Vostrikova, K.E.; Romanenko, G.V.; Ikorski, V.N.; Podberezskaya, N.V.; Larionov, S.V. Synthesis, Crystal Structure and Magnetic Properties of Di(Methanol) and Di(Ethanol)-Bis 2,2,5,5-Tetramethyl-1-Oxyl-3-Imidazoline-4-(3′,3′,3′-Trifluoromethyl-1-Propenyl-2′-Oxyato Nickel(II)—A New Type of Low Temperature Ferromagnetics. Dokl. Akad. Nauk SSSR 1989, 306, 115–118. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/1152963 (accessed on 24 June 2025).
- Spek, A.L. CheckCIF Validation ALERTS: What They Mean and How to Respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.B.; Estrader, M.; Ghosh, A. Coordination-Driven Self-Assembly of a Novel Carbonato-Bridged Heteromolecular Neutral Nickel(II) Triangle by Atmospheric CO2 Fixation. Inorg. Chem. 2008, 47, 7784–7791. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Shoji, M.; Koizumi, K.; Kitagawa, Y.; Kawakami, T.; Yamanaka, S.; Okumura, M.; Yamaguchi, K. A General Algorithm for Calculation of Heisenberg Exchange Integrals J in Multispin Systems. Chem. Phys. Lett. 2006, 432, 343–347. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-coupled Polynuclear d- and f-block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.B.; Hall, C.M.; Johnson, H.G. N,N’-(Phenylene)Dioxamic Acids and Their Esters as Antiallergy Agents. J. Med. Chem. 1978, 21, 930–935. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Pedro, J.R.; Ruiz-García, R.; Muñoz, M.C.; Cano, J.; Carrasco, R. A Hydrogen-Bonded Supramolecular Meso-Helix. Eur. J. Org. Chem. 2003, 2003, 1627–1630. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Bruker Advanced X-Ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2004.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]

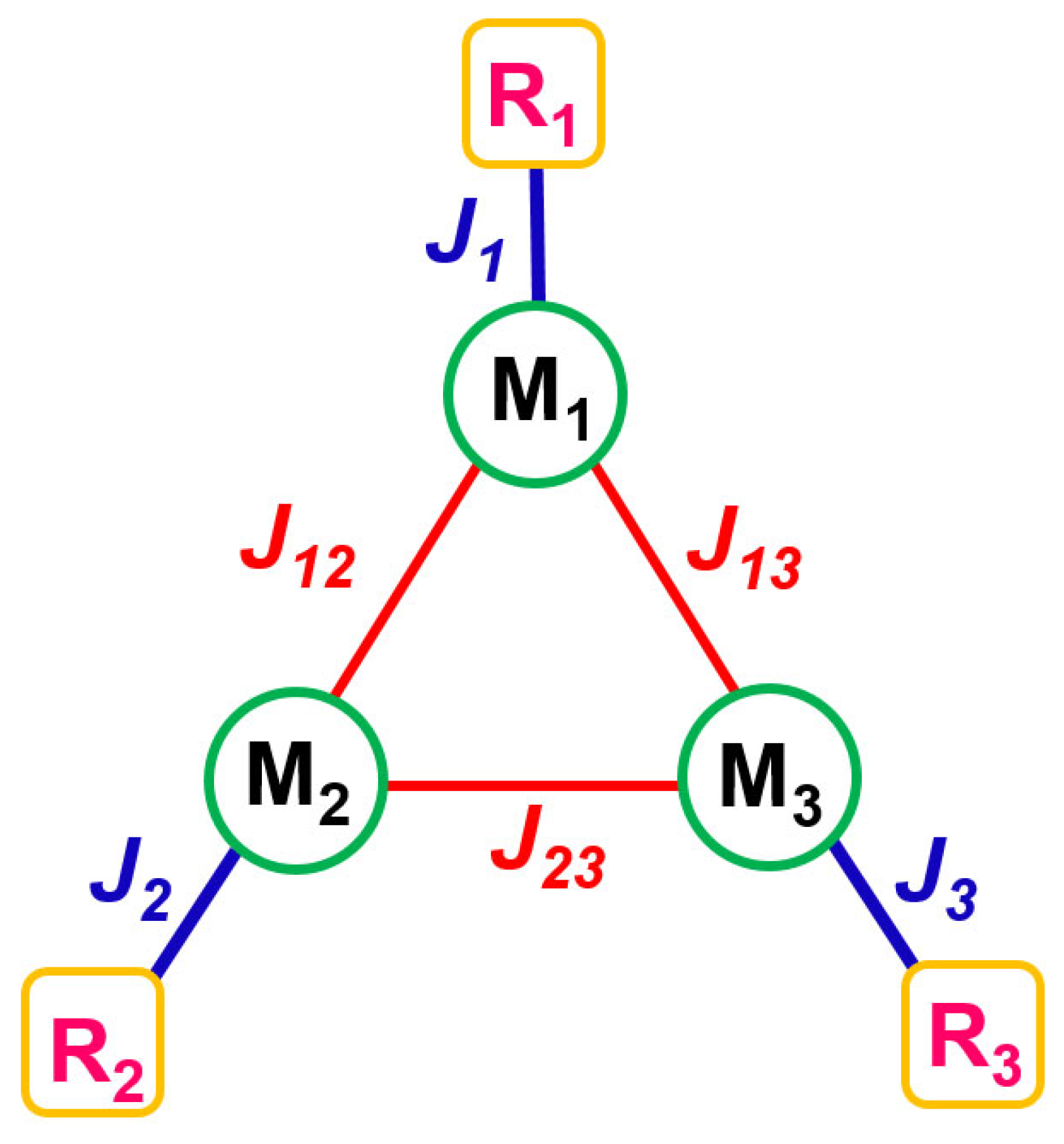

| DFT Level | J1 | J2 | J3 | J12 | J13 |
|---|---|---|---|---|---|
| B3LYP | 157.4 1 | 179.5 | 146.1 | −26.6 1 | −26.3 |
| TPSSh | 209.6 | 231.4 | 188.4 | −37.5 | −37.3 |
| cam-B3LYP | 116.3 | 137.9 | 112.3 | −17.8 | −17.4 |
| LC-BLYP | 131.4 | 155.0 | 125.6 | −25.7 | −25.6 |
| wB97m-v | 98.2 | 116.5 | 95.9 | −14.6 | −14.4 |
| CAS(n,m) Roots(Q,D) | E(Q) − E(D) CASSCF | J1 CASSCF | E(Q) − E(D) NEVPT2 | J1 NEVPT2 |
|---|---|---|---|---|
| (13,9)/(2,2) | −95.9 | 32.0 | −108.6 | 36.2 |
| CAS(n,m) Roots(Q,T,S) | E (Singlet) | E (Triplet) | E (Quintet) | J12 |
|---|---|---|---|---|
| CAS(10,9)/(2,2,2)) | 0 | 3.5 | 10.6 | −1.75 |
| NEVPT2 | 0 | 7.7 | 21.3 | −3.85 |
| SH Parameter 4 | Method | ||||||
|---|---|---|---|---|---|---|---|
| CAS(8,5) 1 | NEVPT2 2 | B3LYP 3 | TPSSh 3 | cam-B3LYP 3 | LC-BLYP 3 | wB97m-v 3 | |
| g | 2.29 | 2.22 | 2.17 | 2.12 | 2.18 | 2.15 | 2.14 |
| D | 7.43 | 4.92 | 2.49 | 1.83 | 2.01 | 8.07 | 3.20 |
| E/D | 0.069 | 0.087 | 0.328 | 0.183 | 0.297 | 0.243 | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozov, V.A.; Samsonenko, D.G.; Vostrikova, K.E. A Unique Trinuclear, Triangular Ni(II) Complex Composed of Two tri-Anionic bis-Oxamates and Capping Nitroxyl Radicals. Inorganics 2025, 13, 214. https://doi.org/10.3390/inorganics13070214
Morozov VA, Samsonenko DG, Vostrikova KE. A Unique Trinuclear, Triangular Ni(II) Complex Composed of Two tri-Anionic bis-Oxamates and Capping Nitroxyl Radicals. Inorganics. 2025; 13(7):214. https://doi.org/10.3390/inorganics13070214
Chicago/Turabian StyleMorozov, Vitaly A., Denis G. Samsonenko, and Kira E. Vostrikova. 2025. "A Unique Trinuclear, Triangular Ni(II) Complex Composed of Two tri-Anionic bis-Oxamates and Capping Nitroxyl Radicals" Inorganics 13, no. 7: 214. https://doi.org/10.3390/inorganics13070214
APA StyleMorozov, V. A., Samsonenko, D. G., & Vostrikova, K. E. (2025). A Unique Trinuclear, Triangular Ni(II) Complex Composed of Two tri-Anionic bis-Oxamates and Capping Nitroxyl Radicals. Inorganics, 13(7), 214. https://doi.org/10.3390/inorganics13070214








