Plasma-Assisted Regeneration of Activated Carbon: Current Status and Prospects
Abstract
1. Introduction
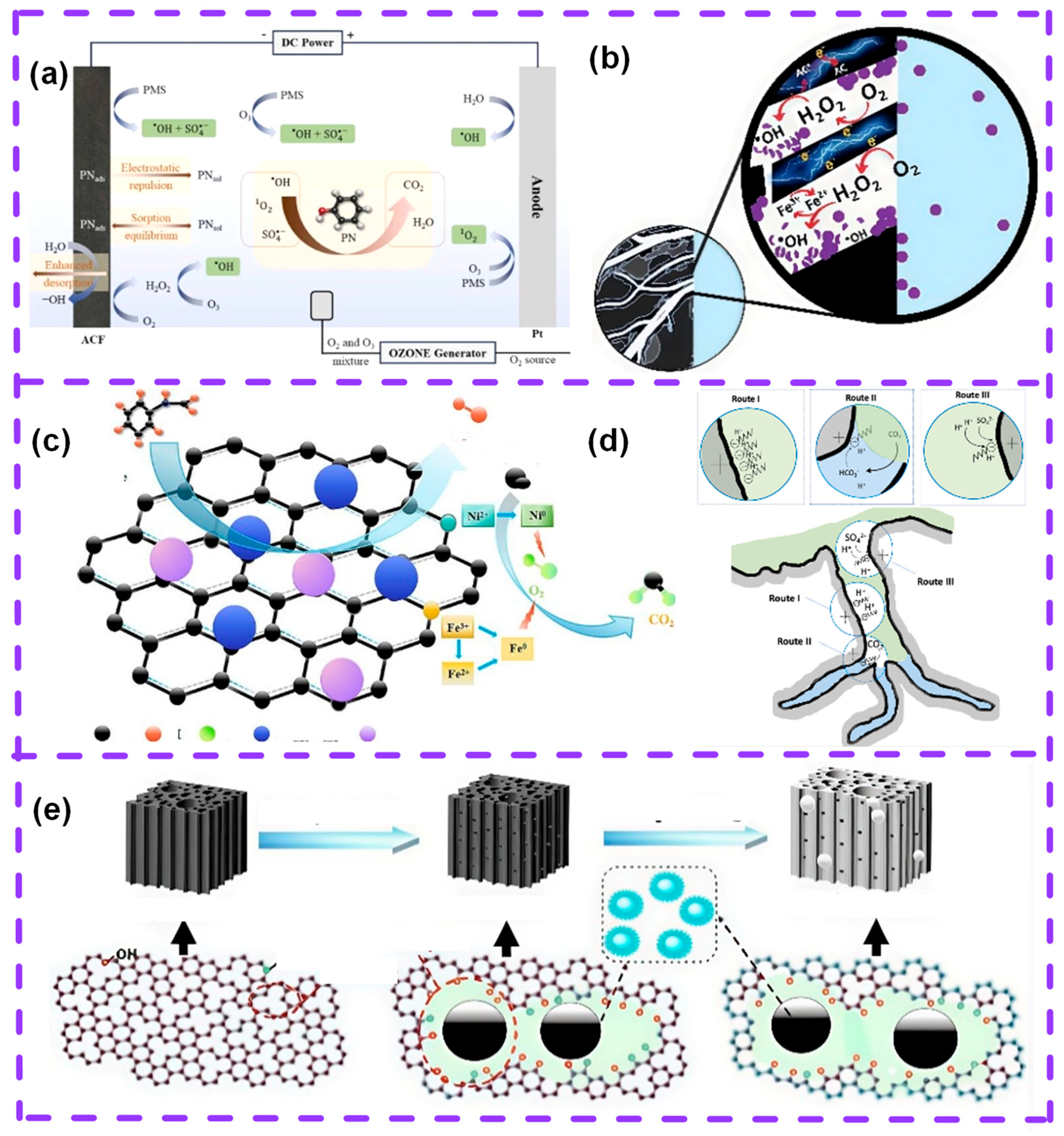
2. Mechanism of AC Regeneration by Plasma
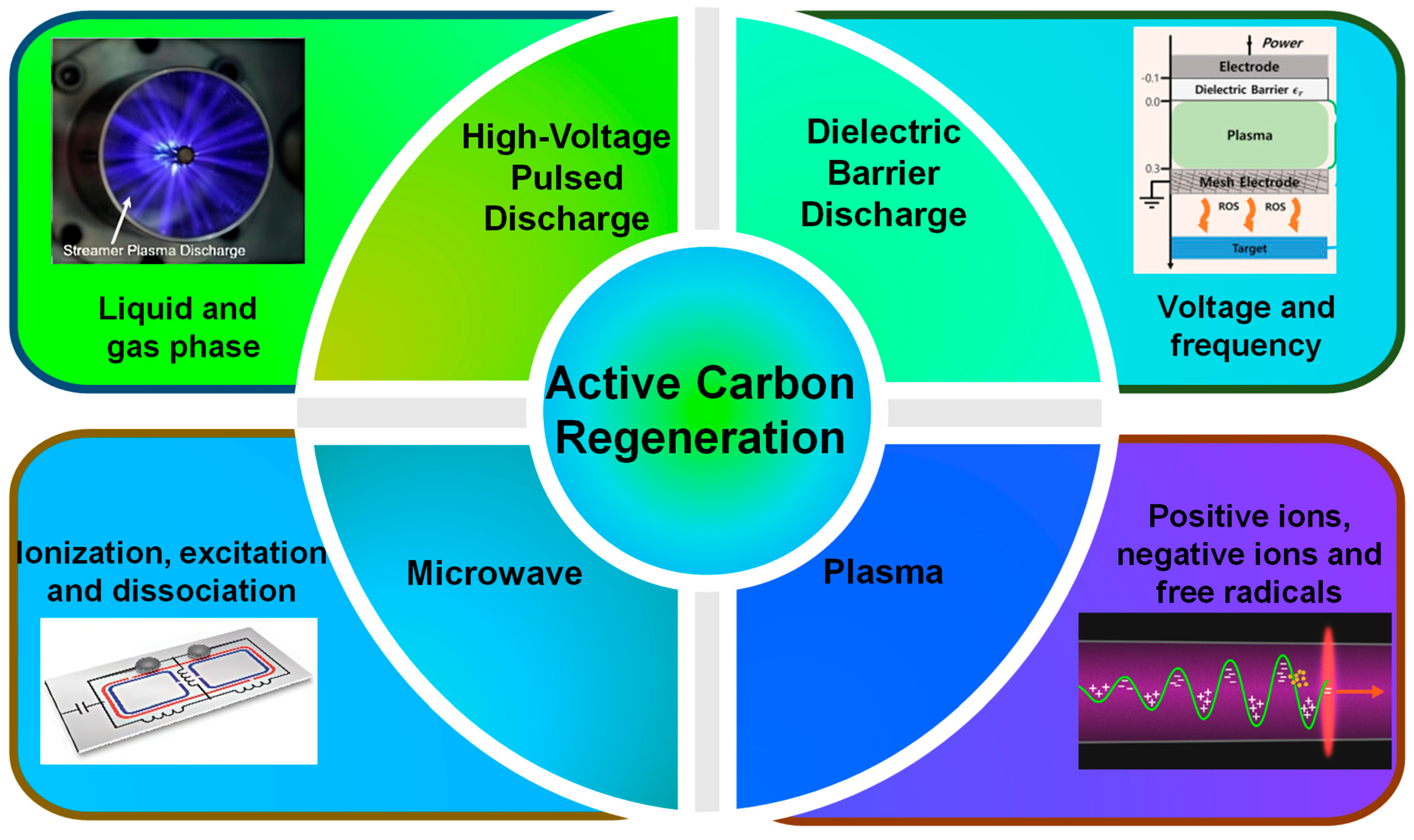
3. Different Methods for Producing Plasma-Regenerated AC Technologies
3.1. AC Regenerated by High-Voltage Pulsed Discharge Plasma
3.1.1. Gas-Phase Pulse Discharge
3.1.2. Liquid-Phase Pulse Discharge
3.1.3. Gas–Liquid Two-Phase Mixed Pulse Discharge
3.1.4. Experimental Results and Discussion of High-Voltage Pulsed Discharge Plasma
3.2. DBD Plasma-Regenerated AC
3.2.1. Mechanism of DBD Plasma
3.2.2. Experimental Results and Discussion of DBD Plasma
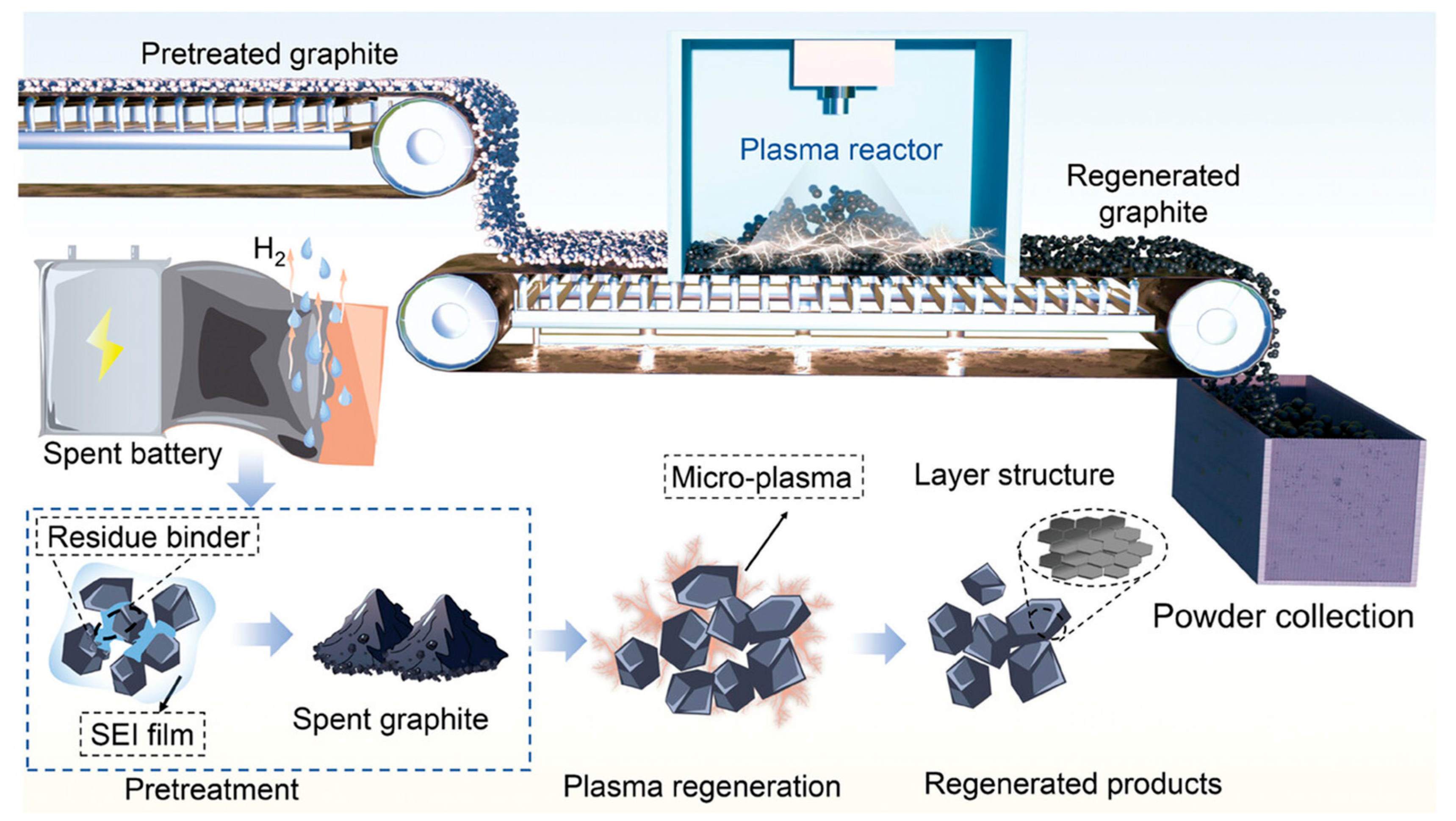
3.3. Microwave Plasma-Regenerated AC
Experimental Results and Discussion of Microwave Plasma
4. Summary and Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Réty, B.; Yiin, H.Y.; Ghimbeu, C.M. Quantification of activated carbon functional groups and active surface area by TPD-MS and their impact on supercapacitor performance. Energy Storage Mater. 2025, 74, 103963. [Google Scholar] [CrossRef]
- Xue, Q.; Yu, B.; Dagnew, M.; Li, W.; Ding, H.; Zhang, J.; Sun, Z.; Wang, P.; Zhao, C. Rapid in situ regeneration of phenol-saturated AC fiber by an electro-permonosulfate-ozone process: Performance. Oper. Mech. 2024, 12, 111932. [Google Scholar]
- Mattson, J.S.; Mark, H.B. Activated Carbon: Surface Chemistry and Ad-Sorption from Solution; M. Dekker: New York, NY, USA, 1971. [Google Scholar]
- Pré, P.; Delage, F.; Le Cloirec, P. A Model to predict the adsorber thermal behavior during treatment of volatile organic compounds onto wet activated carbon. Environ. Sci. Technol. 2002, 36, 4681–4688. [Google Scholar] [CrossRef] [PubMed]
- Lépinay, L.M.; Damskägg, E.; Ockeloen-Korppi, C.F.; Sillanpää, M.A. Realization of directional amplification in a microwave optomechanical device. Phys. Rev. Appl. 2019, 11, 034027. [Google Scholar] [CrossRef]
- Yang, S.; Aravind, I.; Zhang, B.; Weng, S.; Zhao, B.; Thomas, M.; Umstattd, R.; Singleton, D.; Sanders, J.; Cronin, S.B. Plasma-enhanced electrostatic precipitation of diesel exhaust using high voltage nanosecond pulse discharge. J. Environ. Chem. Eng. 2021, 9, 106565. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Chen, H.; Li, X.; Zhang, A.; Li, X.; Chen, C.; He, Y.; Xue, G. Achieving multi-cycle regeneration of AC and Cr(VI) removal over a wide pH range by hydrothermal converting quinonimine dye into difunctional pyrrolic-N: Implication for carbon capture in printing and dyeing wastewater treatment. Chem. Eng. J. 2023, 459, 141646. [Google Scholar] [CrossRef]
- Chen, X.J.; Guo, Y.X.; Zhang, H.R.; Cheng, F.; Jiao, Z. Coke powder improving the performance of desulfurized activated carbon from the cyclic thermal regeneration. Chem. Eng. J. 2022, 448, 137459. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J. Insights into chemical regeneration of activated carbon for water treatment. J. Environ. Chem. Eng. 2021, 9, 105555. [Google Scholar] [CrossRef]
- Berenbaum, M.R. Proceedings of the national academy of sciences-its evolution and adaptation. Proc. Natl. Acad. Sci. USA 2019, 116, 704–706. [Google Scholar] [CrossRef]
- Russell, A.J.B. 75th Anniversary of ‘Existence of Electromagnetic–Hydrodynamic Waves’. Sol. Phys. 2018, 293, 83. [Google Scholar] [CrossRef]
- Grüner, F. Shooting ahead with wakefield acceleration. Phys. Viewp. 2019, 12, 19. [Google Scholar] [CrossRef]
- Na, J.H.; Lee, J.G.; Hong, S.C.; Seo, J.; Lee, J.P.; Lee, Y.; Kim, J.H.; Na, Y.S.; Lee, S.; Park, J.U. Availability of indirect atmospheric plasma from a dielectric barrier discharge device on biofilm-forming bacteria. Curr. Appl. Phys. 2020, 20, 1307–1313. [Google Scholar] [CrossRef]
- Jung, A.; Lee, H.; Kim, H.; Jeon, H.J.; Park, S.; Gweon, B. Impact of plasma discharge pressure on implant surface properties and osteoblast activities in vacuum-assisted plasma treatment. Sci. Rep. 2024, 14, 31757. [Google Scholar] [CrossRef] [PubMed]
- Murari, A.; Rossi, R.; Craciunescu, T.; Vega, J.; Contributors, J.; Gelfusa, M. A control oriented strategy of disruption prediction to avoid the configuration collapse of tokamak reactors. Nat. Commun. 2024, 15, 2424. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Guo, R.L.; Dunn, W.R.; Yao, Z.H.; Zhang, H.S.; Hao, Y.X.; Shi, Q.Q.; Rong, Z.J.; Liu, J.; Tian, A.M.; et al. Plasmapause surface wave oscillates the magnetosphere and diffuse aurora. Nat. Commun. 2020, 11, 1668. [Google Scholar] [CrossRef]
- Ma, H.J.; Kim, S.; Kim, H.N.; Kim, M.J.; Ko, J.W.; Lee, J.W.; Kim, J.H.; Lee, H.C.; Park, Y.J. Microstructural characterization and inductively coupled plasma-reactive ion etching resistance of Y2O3–Y4Al2O9 composite under CF4/Ar/O2 mixed gas conditions. Sci. Rep. 2024, 14, 7008. [Google Scholar] [CrossRef]
- Muto, R.; Hayashi, N. Sterilization characteristics of narrow tubing by nitrogen oxides generated in atmospheric pressure air plasma. Sci. Rep. 2023, 13, 6947. [Google Scholar] [CrossRef]
- Kostyushin, V.A.; Poznyak, I.M.; Toporkov, D.A.; Burmistrov, D.A.; Zhuravlev, K.V.; Lidzhigoryaev, S.D.; Usmanov, R.R.; Tsybenko, V.Y.; Nemchinov, V.S. MK-200 Plasma Gun Facility. Instrum. Exp. Tech. 2023, 66, 920–925. [Google Scholar] [CrossRef]
- Kuzenov, V.V.; Ryzhkov, S.V. Plasma Dynamics Modeling of the Interaction of Pulsed Plasma Jets. Phys. At. Nucl. 2018, 81, 1460–1464. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G.; Si, W.; Liu, Y.; Zhang, G. Novel methodologies for addressing regeneration challenges in styrene-saturated activated carbon for styrene removal. Sep. Purif. Technol. 2024, 340, 126749. [Google Scholar] [CrossRef]
- Qin, W.; Dong, Y.; Jiang, H.; Loh, W.H.; Imbrogno, J.; Swenson, T.M.; Garcia-Rodriguez, O.; Lefebvre, O. A new approach of simultaneous adsorption and regeneration of activated carbon to address the bottlenecks of pharmaceutical wastewater treatment. Water Res. 2024, 252, 121180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, R.; Qi, M.; Zhao, X.; Zhang, J.; Xu, D.; Yang, Y. Studies on a sinusoidally driven gas–liquid two-phase plasma discharge and its application to sterilization. AIP Adv. 2022, 12, 115218. [Google Scholar] [CrossRef]
- Sanito, R.C.; You, S.J.; Wang, Y.F. Degradation of contaminants in plasma technology: An overview. J. Hazard. Mater. 2022, 424, 127390. [Google Scholar] [CrossRef] [PubMed]
- Guclu, C.; Alper, K.; Erdem, M.; Tekin, K.; Karagoz, S. Activated carbons from co-carbonization of waste truck tires and spent tea leaves. Sustain. Chem. Pharm. 2021, 21, 100410. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Chen, L.; Liu, Z.; Qin, J. Utilization of bark waste of Acacia mangium: The preparation of activated carbon and adsorption of phenolic wastewater. Ind. Crop. Prod. 2021, 160, 113157. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Bazaka, K.; Ostrikov, K.K. Ostrikov, Continuous flow removal of acid fuchsine by dielectric barrier discharge plasma water bed enhanced by activated carbon adsorption. Front. Chem. Sci. Eng. 2019, 13, 340–349. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, N.; Li, J.; Wu, Y. Synergistic degradation of bisphenol A by pulsed discharge plasma with granular AC: Effect of operating parameters, synergistic mechanism and possible degradation pathway. Vacuum 2018, 156, 402–410. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Ayala-Cortés, A.; María, A.D.; Longoria, A.; Cuentas-Gallegos, A.K.; Sebastian, P.J.; Okoye, P.U. Advances in activated carbon modification, surface heteroatom configuration, reactor strategies, and regeneration methods for enhanced wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105626. [Google Scholar] [CrossRef]
- Didenko, T.; Lau, A.; Purohit, A.L.; Feng, J.; Pinkard, B.; Ateia, M.; Novosselov, I.V. Regeneration of PFAS-laden granular activated carbon by modified supercritical CO2 extraction. Chemosphere 2024, 370, 143986. [Google Scholar] [CrossRef]
- Zhang, Q.; Mian, M.M.; Zhang, A.; Zhou, L.; Du, R.; Ao, W.; Yu, G.; Deng, S. Catalytic degradation of hexafluoropropylene oxide trimeric acid during the hydrothermal regeneration of spent activated carbon. ACS EST Eng. 2024, 4, 1391–1400. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Yang, Y.; Zheng, Y. Nitrogen/Oxygen Co-Doped Porous Carbon Derived from Biomass for Low-Pressure CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 14055–14063. [Google Scholar] [CrossRef]
- Kang, H.; Choi, S.; Lee, J.H.; Kim, K.T.; Song, Y.H.; Lee, D.H. Plasma jet assisted carbonization and activation of coffee ground waste. Environ. Int. 2020, 145, 106113. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qiao, Z.; Wang, B.; Li, Z.; Zhang, M. A dual-strategy for the valorization and regeneration of spent activated carbon. Diam. Relat. Mater. 2025, 151, 111800. [Google Scholar] [CrossRef]
- Kim, M.; Biswas, S.; Alvarez, I.B.; Christopher, P.; Wong, B.M.; Mangolini, L. Nonthermal Plasma Activation of Adsorbates: The Case of CO on Pt. JACS Au 2024, 4, 2979–2988. [Google Scholar] [CrossRef]
- Shao, T.; Wang, R.; Zhang, C.; Yan, P. Atmospheric-Pressure Pulsed Discharges and Plasmas: Mechanism, Characteristics and Applications. High Volt. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, J.L.; Li, X.S.; Zhu, X.; Zhu, A.M. In Situ Regeneration of Au Nanocatalysts by Atmospheric-Pressure Air Plasma: Regeneration Characteristics of Square-Wave Pulsed Plasma. Top. Catal. 2017, 60, 914–924. [Google Scholar] [CrossRef]
- Wang, T.; Qu, G.; Ren, J.; Sun, Q.; Liang, D.; Hu, S. Organic acids enhanced decoloration of azo dye in gas phase surface discharge plasma system. J. Hazard. Mater. 2016, 302, 65–71. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, D.; Rao, Y.; Zhang, J.; Qu, Y.; Gu, J. Evaluation of antibiotic oxytetracycline removal in water using a gas phase dielectric barrier discharge plasma. J. Environ. Manag. 2018, 226, 22–29. [Google Scholar] [CrossRef]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on discharge Plasma for water treatment: Mechanism, reactor geometries, active species and combined processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- Qu, G.Z.; Li, J.; Li, G.F.; Wu, Y.; Lu, N. DBD regeneration of GAC loaded with acid orange 7. Asia-Pac. J. Chem. Eng. 2009, 4, 649–653. [Google Scholar] [CrossRef]
- Wandell, R.J.; Wang, H.; Tachibana, K.; Makled, B.; Locke, B.R. Nanosecond pulsed plasma discharge over a flowing water film: Characterization of hydrodynamics, electrical, and plasma properties and their effect on hydrogen peroxide generation. Plasma Process Polym. 2018, 15, 1800008. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, H.; Liu, Z.; Zhang, L.; Li, Y.; Zhang, Q.; Zou, X.; Wang, X. Nanosecond pulsed discharge in a gas–liquid mixture produced by hydrodynamic cavitation using Venturi tube. AIP Adv. 2023, 13, 025150. [Google Scholar]
- Qazi, H.I.A.; Xin, Y.Y.; Zhou, L.; Huang, J.J. Description of the physicochemical properties of a gas–liquid phase discharge under the Ar-N2 environment. AIP Adv. 2020, 10, 095207. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, X.; Xu, P.; Chen, J.; Xu, Y.; Luo, G.; Xu, M.; Yao, H. Using the novel method of nonthermal plasma to add Cl active sites on activated carbon for removal of mercury from flue gas. Environ. Sci. Technol. 2016, 50, 11837–11843. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yuan, D.; Li, N.; Qi, J.; Gu, J.; Huang, H. Hydrogen peroxide generation during regeneration of granular AC by bipolar pulse dielectric barrier discharge plasma. J. Taiwan Inst. Chem. Eng. 2017, 78, 178–184. [Google Scholar] [CrossRef]
- Wang, K.P.; Bhuiyan, S.I.; Hil Baky, M.A.; Kraus, J.; Campbell, C.; Jemison, H.; Staack, D. Relative breakdown voltage and energy deposition in the liquid and gas phase of multiphase hydrocarbon plasmas. J. Appl. Phys. 2021, 129, 123301. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takada, N.; Wahyudiono; Kanda, H.; Goto, M. Hydrogen Peroxide Formation by Electric Discharge with Fine Bubbles. Plasma Chem. Plasma Process. 2016, 37, 125–135. [Google Scholar] [CrossRef]
- Yamada, M.; Wahyudiono; Machmudah, S.; Kanda, H.; Goto, M. Nonthermal Atmospheric Pressure Plasma for Methylene Blue Dye Decolorization by Using Slug Flow Reactor System. Plasma Chem. Plasma Process. 2020, 40, 985–1000. [Google Scholar] [CrossRef]
- Sasakawa, T.; Serizawa, A.; Kawara, Z. Fluid-elastic vibration in two-phase cross flow. Exp. Therm. Fluid Sci. 2005, 29, 403–413. [Google Scholar] [CrossRef]
- Song, W.; Yu, L.; Xie, X.; Hao, Z.; Sun, M.; Wen, H.; Li, Y. Effect of textual features and surface properties of AC on the production of hydrogen peroxide from hydroxylamine oxidation. RSC Adv. 2017, 7, 25305–25313. [Google Scholar] [CrossRef]
- Park, Y.W.; Choi, H.J.; Choi, J.H.; Park, T.H.; Jeong, J.W.; Song, E.H.; Ju, B.K. Enhanced Power Efficiency of Organic Light-Emitting Diodes using Pentacene on CF4-Plasma-Treated Indium Tin Oxide Anodes. IEEE Electron Device Lett. 2012, 33, 1156–1158. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Li, X.; Cheng, C. RuP nanoparticles on ordered macroporous hollow nitrogen-doped carbon spheres for efficient hydrogen evolution reactio. Nanotechnol. 2020, 31, 295401. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lu, N.; Li, J.; Wu, Y. Design and application of an up-scaled dielectric barrier discharge plasma reactor for regeneration of phenol-saturated granular AC. Sep. Purif. Technol. 2012, 95, 73–79. [Google Scholar] [CrossRef]
- De Meyer, R.; Verbeeck, J.; Bals, S.; Bogaerts, A. Contamination in Dielectric Barrier Discharge Plasmas by Electrode Erosion. ACS Mater. Lett. 2025, 7, 52–58. [Google Scholar] [CrossRef]
- Eliasson, B.; Kogelschatz, U. Modeling and applications of silent discharge plasmas. IEEE Trans. Plasma Sci. 1991, 19, 309–323. [Google Scholar] [CrossRef]
- Pipa, A.V.; Hoder, T.; Koskulics, J.; Schmidt, M.; Brandenburg, R. Experimental determination of dielectric barrier discharge capacitance. Reveiw Sci. Instrum. 2012, 83, 075111. [Google Scholar] [CrossRef]
- Hao, X.L.; Zhang, X.W.; Lei, L.C. Degradation characteristics of toxic contaminant with modified ACs in aqueous pulsed discharge plasma process. Carbon 2009, 47, 153–161. [Google Scholar] [CrossRef]
- Sima, J.; Wang, J.; Song, J.; Du, X.; Lou, F.; Zhu, Y.; Lei, J.; Huang, Q. Efficient degradation of polystyrene microplastic pollutants in soil by dielectric barrier discharge plasma. J. Hazard. Mater. 2024, 468, 133754. [Google Scholar] [CrossRef]
- Chen, J.; Pan, X.; Chen, J. Regeneration of AC saturated with odors by non-thermal plasma. Chemosphere 2013, 92, 725–730. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic ozonation of sulfonated aromatic compounds in the presence of AC. Appl. Catal. B. Environ. 2008, 83, 150–159. [Google Scholar] [CrossRef]
- Kim, D.W.; Shin, D.C.; Seo, D.K.; Kim, T.W.; Kim, J.P.; Moon, B.Y. Protuberant arrays of carbon nanotubes grown on substrate irradiated with MeV-energy protons. Surf. Coat. Technol. 2014, 259, 647–653. [Google Scholar] [CrossRef]
- Buczek, B.; Czepirski, L.; Zietkiewicz, J. Improvement of Hydrogen Storage Capacity for Active Carbon. Manuf. Neth. 2005, 11, 877–880. [Google Scholar] [CrossRef]
- Kljajevic, L.; Jovanovic, V.; Stevanovic, S.; Bogdanov, Z.; Kaludjerovic, B. Influence of chemical agents on the surface area and porosity of active carbon hollow fibers. J. Serbian Chem. Soc. 2011, 76, 1283–1294. [Google Scholar] [CrossRef]
- Qu, G.Z.; Li, J.; Wu, Y.; Li, G.F.; Li, D. Regeneration of acid orange 7-exhausted granular activated carbon with dielectric barrier discharge plasma. Chem. Eng. J. 2009, 146, 168–173. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Li, X.S.; Liu, J.L.; Wen, X.Q.; Zhu, A.M. Plasma Nitrogen Fixation: NOx Synthesis in MnOx/Al2O3 Packed-Bed Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2023, 43, 1907–1919. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, O.; Villot, A.; Olvera-Vargas, H.; Gerente, C.; Andres, Y.; Lefebvre, O. Impact of the saturation level on the electrochemical regeneration of activated carbon in a single sequential reactor. Carbon 2020, 163, 265–275. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, C.; Wu, M.; Chen, X.; Liu, D.; Ma, J. High-efficient microwave plasma discharging initiated conversion of waste plastics into hydrogen and carbon nanotubes. Energy Convers. Manag. 2022, 268, 116017. [Google Scholar] [CrossRef]
- Zamri, A.A.; Ong, M.Y.; Nomanbhay, S.; Show, P.L. Microwave plasma technology for sustainable energy production and the electromagnetic interaction within the plasma system: A review. Environ. Res. 2021, 197, 111204. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Microwave-assisted regeneration of activated carbon. Bioresour. Technol. 2012, 119, 234–240. [Google Scholar] [CrossRef]
- Xin, C.; Hu, W.; Xia, H.; Zhang, Q.; Yan, H. Regeneration of anthraquinone dye-loaded waste AC by microwave heating and its reuse to adsorb dye-containing wastewater. Desalination Water Treat. 2021, 243, 275–286. [Google Scholar] [CrossRef]
- Kumar N, S.; Grekov, D.; Pré, P.; Alappat, B.J. Microwave mode of heating in the preparation of porous carbon materials for adsorption and energy storage applications—An overview. Renew. Sustain. Energy Rev. 2020, 124, 109743. [Google Scholar] [CrossRef]
- Cherbański, R. Regeneration of granular activated carbon loaded with toluene -Comparison of microwave and conductive heating at the same active powers. Chem. Eng. Process.-Process Intensif. 2018, 123, 148–157. [Google Scholar] [CrossRef]
- Shan, M.; Xu, S.; Cao, Y.; Han, B.; Zhu, X.; Zhang, T.; Dang, C.; Zhu, J.; Zhou, Q.; Xue, Z.; et al. Rapid Regeneration of Graphite Anodes via Self-Induced Microwave Plasma. Adv. Funct. Mater. 2024, 34, 2411834. [Google Scholar] [CrossRef]
- Jayasinghe, S.; Siriwardena, D.P.; Munaweera, I.; Perera, C.; Kottegoda, N. Sustainable Synthesis of Highly Functionalized Activated Carbon using Plasma Technology. ChemPlusChem 2022, 87, e202200202. [Google Scholar] [CrossRef] [PubMed]



| Method | Efficiency | Advantages | Limitations | Key Parameters |
|---|---|---|---|---|
| High-voltage pulsed discharge | 55–94% | Multi-phase compatibility Effective organics removal | Electrode degradation Structural damage Precise parameter control | Voltage: 18–28 kV Moisture: 10–31% Flow rate: 0.45 m3/h |
| Dielectric barrier discharge | 65–90% | Electrode protection Multi-cycle durability | Significant power demand Potential pore occlusion | Field strength: 18–28 kV O2: 5–21% |
| Microwave plasma | ~81% | Microstructure conservation Green process | Material thermal limits Cyclic structural changes | Power: 600 W Duration: 3–5 min N2: 300 cm3/min |
| Thermal regeneration | 50–70% | Proven technology Broad applicability | Carbon attrition Energy intensive Capacity reduction | Temperature: 400–600 °C |
| Chemical regeneration | Partial restoration | Mild conditions Low energy input | Pore fouling Secondary contamination | Reagent selection Concentration optimization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Meng, J.; Tan, S.; Liang, L.; Wang, F.; Liu, H.; Guo, C.; Bao, W.; Zhang, G.; Yu, F. Plasma-Assisted Regeneration of Activated Carbon: Current Status and Prospects. Inorganics 2025, 13, 209. https://doi.org/10.3390/inorganics13070209
Chen R, Meng J, Tan S, Liang L, Wang F, Liu H, Guo C, Bao W, Zhang G, Yu F. Plasma-Assisted Regeneration of Activated Carbon: Current Status and Prospects. Inorganics. 2025; 13(7):209. https://doi.org/10.3390/inorganics13070209
Chicago/Turabian StyleChen, Routong, Jiaxin Meng, Shiyi Tan, Litao Liang, Faxing Wang, He Liu, Cong Guo, Weizhai Bao, Guozhen Zhang, and Feng Yu. 2025. "Plasma-Assisted Regeneration of Activated Carbon: Current Status and Prospects" Inorganics 13, no. 7: 209. https://doi.org/10.3390/inorganics13070209
APA StyleChen, R., Meng, J., Tan, S., Liang, L., Wang, F., Liu, H., Guo, C., Bao, W., Zhang, G., & Yu, F. (2025). Plasma-Assisted Regeneration of Activated Carbon: Current Status and Prospects. Inorganics, 13(7), 209. https://doi.org/10.3390/inorganics13070209











