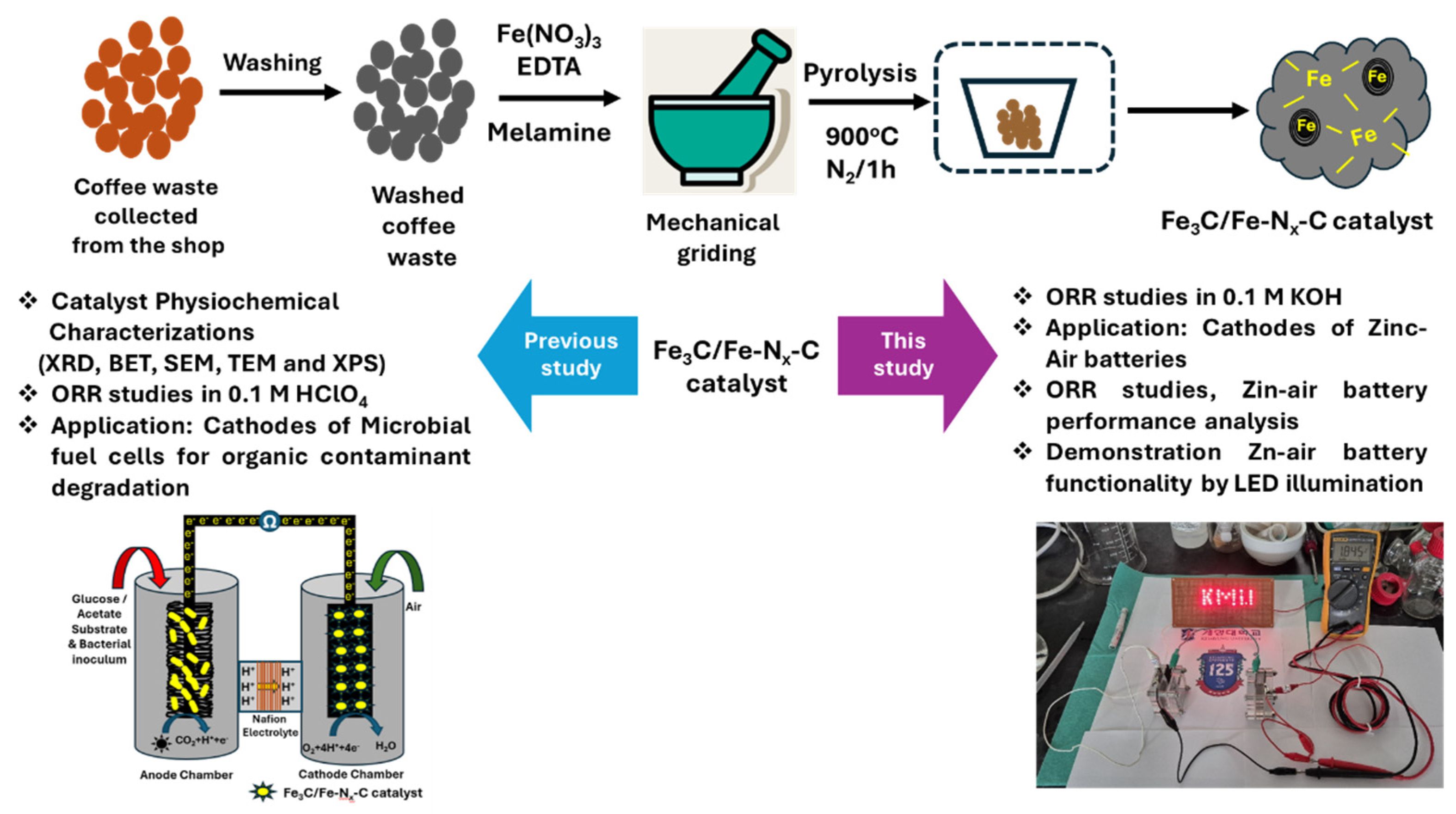
Sustainable Fe3C/Fe-Nx-C Cathode Catalyst from Biomass for an Oxygen Reduction Reaction in Alkaline Electrolytes and Zinc–Air Battery Application
Abstract
1. Introduction
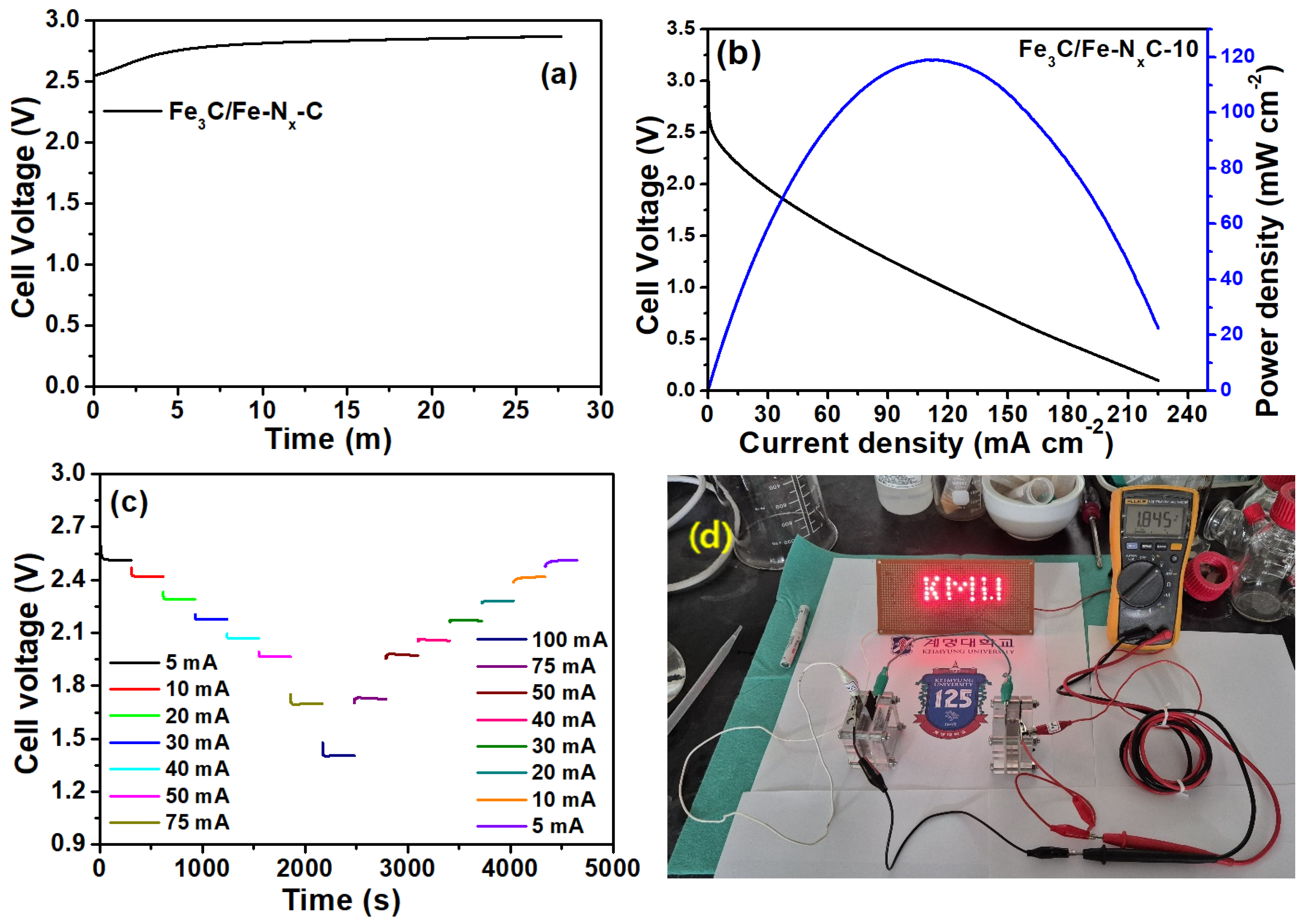
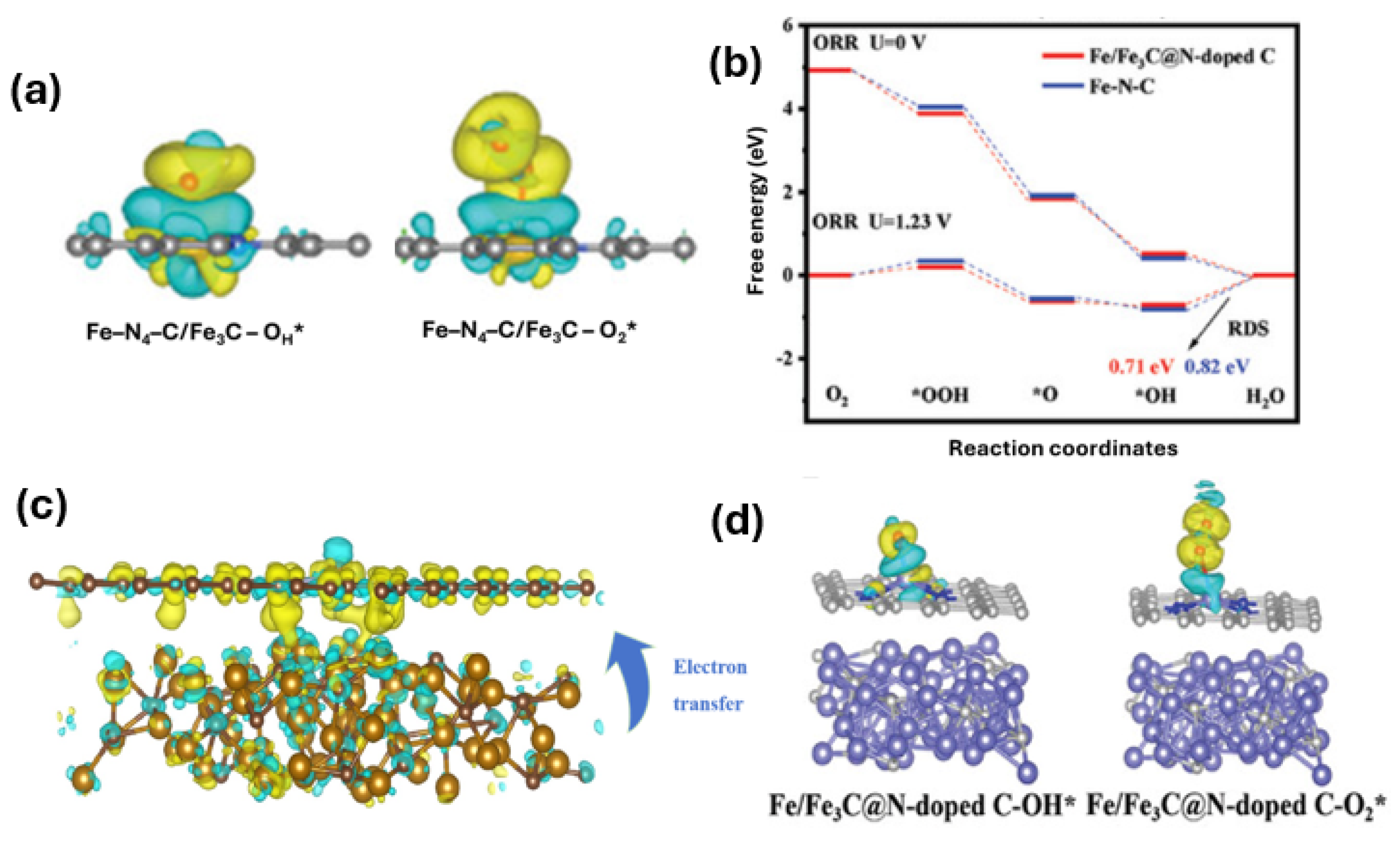
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.R.; Vishnuram, P.; Alagarsamy, S.; Bajaj, M.; Blazek, V.; Damaj, I.; Rathore, R.S.; Al-Wesabi, F.N.; Othman, K.M. Electric vehicle charging technologies, infrastructure expansion, grid integration strategies, and their role in promoting sustainable e-mobility. Alex. Eng. J. 2024, 105, 300–330. [Google Scholar] [CrossRef]
- Gao, L.; Hu, H.; Zhang, C.; Cao, M. Gallium regulated MnO2 toward high performance Zn ion batteries. Vacuum 2024, 219, 112671. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y. Unsupervised dynamic prognostics for abnormal degradation of lithium-ion battery. Appl. Energy 2024, 365, 123280. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; Zhou, M.; Huang, X.; Gao, Y.; Li, K.; Chen, Z.; Zhou, C.; Zhou, Z.; Zheng, C.; et al. Short process recovery of silver and purification mechanism of crystalline silicon deep etching from end-of-life photovoltaic cells. Chem. Eng. J. 2025, 510, 161651. [Google Scholar] [CrossRef]
- Ali, A.O.; Elgohr, A.T.; El-Mahdy, M.H.; Zohir, H.M.; Emam, A.Z.; Mostafa, M.G.; Al-Razgan, M.; Kasem, H.M.; Elhadidy, M.S. Advancements in photovoltaic technology: A comprehensive review of recent advances and future prospects. Energy Convers. Manag. X 2025, 26, 100952. [Google Scholar] [CrossRef]
- Liu, X.; Shang, J.; Li, J.; Liu, H.; Zhang, F.; Pan, Q.; Tang, Y. Insight into Robust Anion Coordination Behavior of Organic Cathode with Dual Elongated π-Conjugated Motifs. Angew. Chem. 2025, 137, e202420160. [Google Scholar] [CrossRef]
- Fu, L.; Wang, J.; Fu, X.; Zhao, G. Finite-time Pade-based adaptive FNN controller implementation for microbial fuel cell with delay and multi-disturbance. Int. J. Hydrogen Energy 2025, 98, 1034–1043. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Lee, J.-H.; Kim, C.-H.; Gautam, J.; Heo, K.; Hussain, S.; Ikram, M.; AlObaid, A.A.; Lee, S.-Y.; et al. A review of rechargeable zinc–air batteries: Recent progress and future perspectives. Nano-Micro Lett. 2024, 16, 138. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced architectures and relatives of air electrodes in Zn–air batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Fu, H. Research progress on the construction of synergistic electrocatalytic ORR/OER self-supporting cathodes for zinc–air batteries. J. Mater. Chem. A 2023, 11, 4400–4427. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, W.; Chen, Y.; Xu, Z.; Cai, D.; Xu, M.; Tong, R. Cobalt-containing ZIF-derived catalysts for Zn–air batteries. Mater. Chem. Front. 2024, 8, 2394–2419. [Google Scholar] [CrossRef]
- Zhao, H. Recent Advances in Rechargeable Zn-Air Batteries. Molecules 2024, 29, 5313. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Tran, P.K.L.; Malhotra, D.; Nguyen, T.H.; Nguyen, T.T.A.; Duong, N.T.A.; Kim, N.H.; Lee, J.H. Current status of developed electrocatalysts for water splitting technologies: From experimental to industrial perspective. Nano Converg. 2025, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, Q.; Dong, S.; Wang, M.; Song, Y.; Diao, H.; Yuan, D. Building synergistic multiple active sites in branch-leaf nanostructured carbon nanofiber derived from MOF/COF hybrid for flexible wearable Zn-air battery. J. Colloid Interface Sci. 2024, 666, 35–46. [Google Scholar]
- Zhang, Q.; Li, W.; Peng, J.; Xue, L.; He, G. Cold plasma activated Ni 0/Ni 2+ interface catalysts for efficient electrocatalytic methane oxidation to low-carbon alcohols. Green Chem. 2024, 26, 7091–7100. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Xiong, H.; Jiang, S.; Li, W.; Fu, X.; Shang, S.; Xu, J.; He, G. Cold plasma-activated Ni-Co tandem catalysts with CN vacancies for enhancing CH4 electrocatalytic to methyl formate. Appl. Catal. B Environ. Energy 2025, 362, 124759. [Google Scholar] [CrossRef]
- Sun, X.; Wei, P.; Gu, S.; Zhang, J.; Jiang, Z.; Wan, J.; Chen, Z.; Huang, L.; Xu, Y.; Fang, C.; et al. Atomic-level Fe-N-C coupled with Fe3C-Fe nanocomposites in carbon matrixes as high-efficiency bifunctional oxygen catalysts. Small 2020, 16, 1906057. [Google Scholar] [CrossRef]
- Han, J.; Bao, H.; Wang, J.-Q.; Zheng, L.; Sun, S.; Wang, Z.L.; Sun, C. 3D N-doped ordered mesoporous carbon supported single-atom Fe-NC catalysts with superior performance for oxygen reduction reaction and zinc-air battery. Appl. Catal. B Environ. 2021, 280, 119411. [Google Scholar] [CrossRef]
- Lian, Y.; Xu, J.; Zhou, W.; Lin, Y.; Bai, J. Research Progress on Atomically Dispersed Fe-N-C Catalysts for the Oxygen Reduction Reaction. Molecules 2024, 29, 771. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.-J.; Wang, J.-Q.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Understanding the high activity of Fe–N–C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe–Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar]
- Yang, Q.; Liu, R.; Pan, Y.; Cao, Z.; Zuo, J.; Qiu, F.; Yu, J.; Song, H.; Ye, Z.; Zhang, S. Ultrahigh-Loaded Fe Single Atoms and Fe3C Nanoparticle Catalysts as Air Cathodes for High-Performance Zn–Air Batteries. ACS Appl. Mater. Interfaces 2023, 15, 5720–5731. [Google Scholar] [CrossRef] [PubMed]
- Peera, S.G.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Sustainable synthesis of Co@ NC core shell nanostructures from metal organic frameworks via mechanochemical coordination self-assembly: An efficient electrocatalyst for oxygen reduction reaction. Small 2018, 14, 1800441. [Google Scholar] [CrossRef]
- Cui, X.; Gao, L.; Lei, S.; Liang, S.; Zhang, J.; Sewell, C.D.; Xue, W.; Liu, Q.; Lin, Z.; Yang, Y. Simultaneously crafting single-atomic Fe sites and graphitic layer-wrapped Fe3C nanoparticles encapsulated within mesoporous carbon tubes for oxygen reduction. Adv. Funct. Mater. 2021, 31, 2009197. [Google Scholar] [CrossRef]
- Ye, Q.; Li, M.; Xiao, T.; Hou, S.; Deng, Y.; Luo, J.; Tian, X. Ultra-fine Fe3C nanoparticles decorated in the Fe–N co-doped carbon networks with hierarchical nanoarchitectonics towards efficient oxygen reduction electrocatalysis. Int. J. Hydrogen Energy 2024, 49, 1014–1021. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Xie, Y.; Tong, M.; Tian, C.; Fu, H. The Fe3C–N x Site Assists the Fe–N x Site to Promote Activity of the Fe–N–C Electrocatalyst for Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2022, 10, 3346–3354. [Google Scholar] [CrossRef]
- Li, G.; Liu, J.; Xu, C.; Chen, H.; Hu, H.; Jin, R.; Sun, L.; Chen, H.; Guo, C.; Li, H.; et al. Regulating the Fe-spin state by Fe/Fe3C neighbored single Fe-N4 sites in defective carbon promotes the oxygen reduction activity. Energy Storage Mater. 2023, 56, 394–402. [Google Scholar] [CrossRef]
- Zong, L.; Chen, X.; Liu, S.; Fan, K.; Dou, S.; Xu, J.; Zhao, X.; Zhang, W.; Zhang, Y.; Wu, W.; et al. Ultrafine Fe/Fe3C decorated on Fe-Nx-C as bifunctional oxygen electrocatalysts for efficient Zn-air batteries. J. Energy Chem. 2021, 56, 72–79. [Google Scholar] [CrossRef]
- Tong, M.; Yu, P.; Xie, Y.; Wang, L.; Wang, Y.; Fu, H. Atomically dispersed fe–n3c sites induce asymmetric electron structures to afford superior oxygen reduction activity. Small 2022, 18, 2201255. [Google Scholar] [CrossRef]
- Xu, C.; Guo, C.; Liu, J.; Hu, B.; Dai, J.; Wang, M.; Jin, R.; Luo, Z.; Li, H.; Chen, C. Accelerating the oxygen adsorption kinetics to regulate the oxygen reduction catalysis via Fe3C nanoparticles coupled with single Fe-N4 sites. Energy Storage Mater. 2022, 51, 149–158. [Google Scholar] [CrossRef]
- Murugesan, R.; Yesupatham, M.S.; Agamendran, N.; Sekar, K.; Neethinathan, C.S.S.; Maruthapillai, A. Recent Advances in Biomass-Derived Carbon-Based Nanostructures for Electrocatalytic Reduction Reactions: Properties–Performance Correlations. Energy Technol. 2024, 12, 2400882. [Google Scholar]
- Nguyen, V.-T.; Cho, K.; Choi, Y.; Hwang, B.; Park, Y.-K.; Nam, H.; Lee, D. Biomass-derived materials for energy storage and electrocatalysis: Recent advances and future perspectives. Biochar 2024, 6, 96. [Google Scholar] [CrossRef]
- Liu, T.; Yabu, H. Biomass-Derived Electrocatalysts: Low-Cost, Robust Materials for Sustainable Electrochemical Energy Conversion. Adv. Energy Sustain. Res. 2024, 5, 2300168. [Google Scholar] [CrossRef]
- Pagett, M.; Teng, K.S.; Sullivan, G.; Zhang, W. Reusing waste coffee grounds as electrode materials: Recent advances and future opportunities. Glob. Chall. 2023, 7, 2200093. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guo, C.; Xiang, J.; Qi, Y.; Yu, J.; Li, K.; Tao, Z.; Wu, J.; Lv, Y. Synthesis and applications of biomass-derived electrocatalysts in water electrolysis. Int. J. Hydrogen Energy 2024, 60, 845–866. [Google Scholar] [CrossRef]
- Alemu, M.A.; Assegie, A.A.; Ilbas, M.; Al Afif, R.; Getie, M.Z. Biomass-Derived Metal-Free Nanostructured Carbon Electrocatalysts for High-Performance Rechargeable Zinc–Air Batteries. Adv. Energy Sustain. Res. 2025, 2400414. [Google Scholar] [CrossRef]
- Zhu, K.; Liang, X.; Chen, Y.; Huang, Z.; Tang, Y.; Qiu, R.; Yan, K. Recent progress in biomass-derived single-atom catalysts for environmental remediation. Coord. Chem. Rev. 2024, 519, 216110. [Google Scholar] [CrossRef]
- Ashmath, S.; Rajeshkhanna, G.; Koyyada, G.; Pandiaraj, S.; Liu, C.; Peera, S.G.; Lee, T.-G. Biomass derived Fe3C/Fe-Nx-C as cathode catalyst for oxygen reduction reaction in dual chamber microbial fuel cells. Int. J. Hydrogen Energy 2024, in press. [Google Scholar] [CrossRef]
- Xu, H.-M.; Zhu, H.-R.; Huang, C.-J.; Zhang, Z.-J.; Shuai, T.-Y.; Zhan, Q.-N.; Li, G.-R. Recent advances in Fe-NC-and Co-NC-based materials as bifunctional electrocatalysts for oxygen reduction and oxygen evolution. Sci. China Chem. 2024, 67, 1137–1160. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, Y.; Wang, P.; Bai, J.; Guan, X.; Wang, S.; Lan, C.; Wu, H.; Shi, Z.; Zhu, S.; et al. Unraveling the potential-dependent degradation mechanism in Fe-NC catalysts for oxygen reduction reaction. Sci. China Chem. 2025, 68, 1541–1549. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, Y.; Jiang, Z.; Jiang, D.; Wei, W.; Hu, Z. Nitrogen-Doped Bimetallic Carbide-Graphite Composite as Highly Active and Extremely Stable Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media. Adv. Funct. Mater. 2022, 32, 2204031. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.-X.; Liu, J.-N.; Ren, D.; Li, B.-Q.; Huang, J.-Q.; Zhang, Q. Quantitative kinetic analysis on oxygen reduction reaction: A perspective. Nano Mater. Sci. 2021, 3, 313–318. [Google Scholar] [CrossRef]
- Holewinski, A.; Linic, S. Elementary mechanisms in electrocatalysis: Revisiting the ORR Tafel slope. J. Electrochem. Soc. 2012, 159, H864. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.-Z. Determination of the electron transfer number for the oxygen reduction reaction: From theory to experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Liu, M.; Ke, S.; Sun, C.; Zhang, C.; Liao, S. Hf Doping Boosts the Excellent Activity and Durability of Fe-N-C Catalysts for Oxygen Reduction Reaction and Li-O2 Batteries. Nanomaterials 2024, 14, 2003. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Reddy, P.S.P.; Koyyada, G.; Alodhayb, A.N.; Pandiaraj, S.; Kim, S.W.; Tamtam, M.R. Effect of N-Doped Carbon on the Morphology and Oxygen Reduction Reaction (ORR) Activity of a Xerogel-Derived Mn(II)O Electrocatalyst. Catalysts 2024, 14, 792. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Akula, S.; Asokan, A.; Moni, P.; Selvaraj, M.; Balamurugan, J.; Kim, S.O.; Liu, C.; Sahu, A.K. Carbon nanofibers as potential catalyst support for fuel cell cathodes: A review. Energy Fuels 2021, 35, 11761–11799. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, Q.; Yu, J.; Jing, W.; Wang, S.; Yin, G.; Waterhouse, G.I.N.; Lu, S. A Fe Single Atom Seed-Mediated Strategy Toward Fe3C/Fe-N-C Catalysts with Outstanding Bifunctional ORR/OER Activities. Adv. Sci. 2023, 10, 2301656. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Ling, G.; Guo, J.; Lv, Y.; Ren, R.-P. Prussian Blue-Derived Atomic Fe/Fe3C@ N-Doped C Catalysts Supported by Carbon Cloth as Integrated Air Cathode for Flexible Zn-Air Batteries. Adv. Sci. 2025, 12, 2407631. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Q.; Zhang, L. Bio-assisted atomically dispersed Fe–N–C electrocatalyst with ultra-low Fe loading toward ph-universal oxygen reduction reaction and neutral Zn-air battery. ACS Sustain. Chem. Eng. 2023, 11, 8131–8139. [Google Scholar] [CrossRef]
- Lin, K.; Chen, M.; Zhou, Z.; Huang, H.; Zhang, J.; Peng, S.; Sun, M.; Yu, L. Biomass-Derived Carbon-Coated FeCo Alloys as Highly Efficient Bifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries. ACS Appl. Energy Mater. 2024, 7, 11172–11183. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, B.; Zhou, Y. Atomically dispersed iron catalyst supported on nitrogen doped biomass aerogels for high-performance rechargeable zinc-air batteries. Fuel 2024, 371, 132119. [Google Scholar] [CrossRef]
- Hao, R.; Gu, S.; Chen, J.; Wang, Z.; Gan, Q.; Wang, Z.; Huang, Y.; Liu, P.; Zhang, K.; Liu, K.; et al. Microporous Fe–N4 cataysts derived from biomass aerogel for a high-performance Zn–air battery. Mater. Today Energy 2021, 21, 100826. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Wang, H.; Li, X.; Wang, Q.; Si, W.; Lan, W.; Wang, F.; Han, N. Fundamental understanding of nitrogen in biomass electrocatalysts for oxygen reduction and zinc-air batteries. iScience 2024, 27, 108913. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Jiang, C.; Zheng, M.; Peng, X.; Liu, T.; Xi, S.; Chi, X.; Zhang, Q.; Gu, L.; Zhang, S.; et al. Wood carbon based single-atom catalyst for rechargeable Zn–air batteries. ACS Energy Lett. 2021, 6, 3624–3633. [Google Scholar] [CrossRef]
- Jiao, C.; Xu, Z.; Shao, J.; Xia, Y.; Tseng, J.; Ren, G.; Zhang, N.; Liu, P.; Liu, C.; Li, G.; et al. High-density atomic Fe–N4/C in tubular, biomass-derived, nitrogen-rich porous carbon as air-electrodes for flexible Zn–air batteries. Adv. Funct. Mater. 2023, 33, 2213897. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.; Han, G.; Cao, M.; Han, L.; Zhou, B.; Mehdi, S.; Wu, X.; Li, B.; Jiang, J. Wood-Derived Integral Air Electrode for Enhanced Interfacial Electrocatalysis in Rechargeable Zinc–Air Battery. Small 2021, 17, 2101607. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Kong, Z.; Zhang, L.; Xin, Z.; She, X.; Sun, J.; Yang, D.; Li, D. Electrostatic interaction in amino protonated chitosan–metal complex anion hydrogels: A simple approach to porous metal carbides/N-doped carbon aerogels for energy conversion. ACS Appl. Mater. Interfaces 2022, 14, 22151–22160. [Google Scholar] [CrossRef]
- Han, S.; Peng, S.; Gao, Z.; Sun, M.; Cheng, G.; Zhang, H.; Su, X.; Chen, M.; Yu, L. Green bridge between waste and energy: Conversion the rotten wood into cathode for functional Zn-air battery. Electrochim. Acta 2022, 424, 140667. [Google Scholar] [CrossRef]
- Shi, K.; Li, Y.; Zhang, Y.; Li, X.; Zhu, Z.; Xu, H.; Zheng, L.; Gao, J. N-doped 3D hierarchical carbon from water hyacinth for high-performance Zn-air batteries. Diam. Relat. Mater. 2023, 135, 109841. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, K.; Cui, X.; Li, B.; Jiang, J. Defect-rich, graphenelike carbon sheets derived from biomass as efficient electrocatalysts for rechargeable zinc–air batteries. ACS Sustain. Chem. Eng. 2020, 8, 2981–2989. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Wang, J.; Li, J.; Yu, L.; Zhou, F.; Li, J.; Shen, X.; Wang, X.; Wang, S.; et al. Biomass-derived Fe2N@ NCNTs from bioaccumulation as an efficient electrocatalyst for oxygen reduction and Zn–Air battery. ACS Sustain. Chem. Eng. 2022, 10, 9105–9112. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Wang, S.; Zhou, L.; Liu, T.; Sun, K.; Cao, H.; Jiang, J.; Wu, X.; Li, B. Wood-Derived Monolithic Catalysts With the Ability of Activating Water Molecules for Oxygen Electrocatalysis. Small 2022, 18, 2202725. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liu, Y.; Sun, K.; Li, H.; Lin, X.; Zhang, P.; Zhou, L.; Wang, A.; Mehdi, S.; Wu, X.; et al. Coupling Fe3C Nanoparticles and N-Doping on Wood-Derived Carbon to Construct Reversible Cathode for Zn–Air Batteries. Small 2022, 18, 2202014. [Google Scholar] [CrossRef] [PubMed]
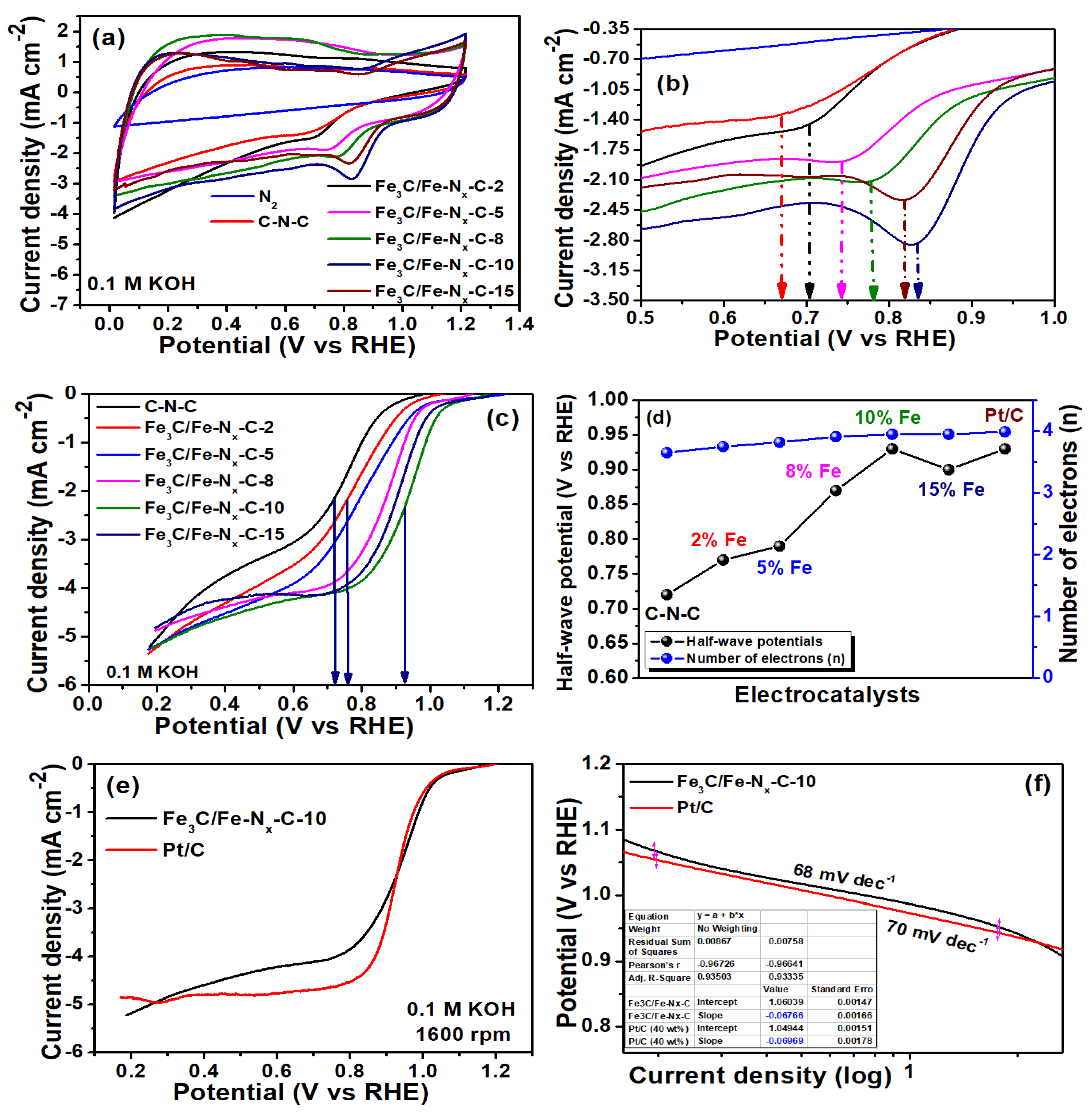

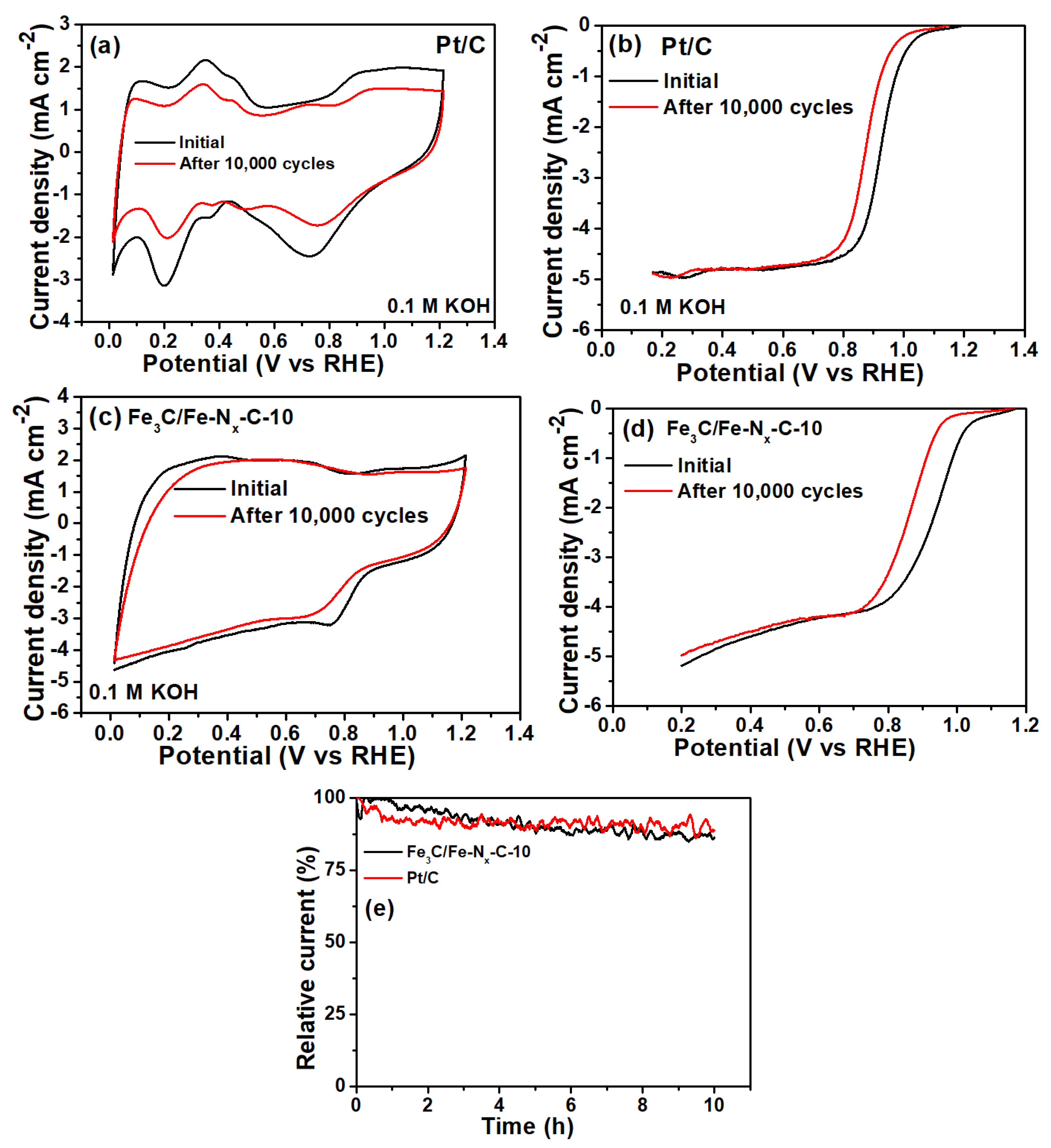
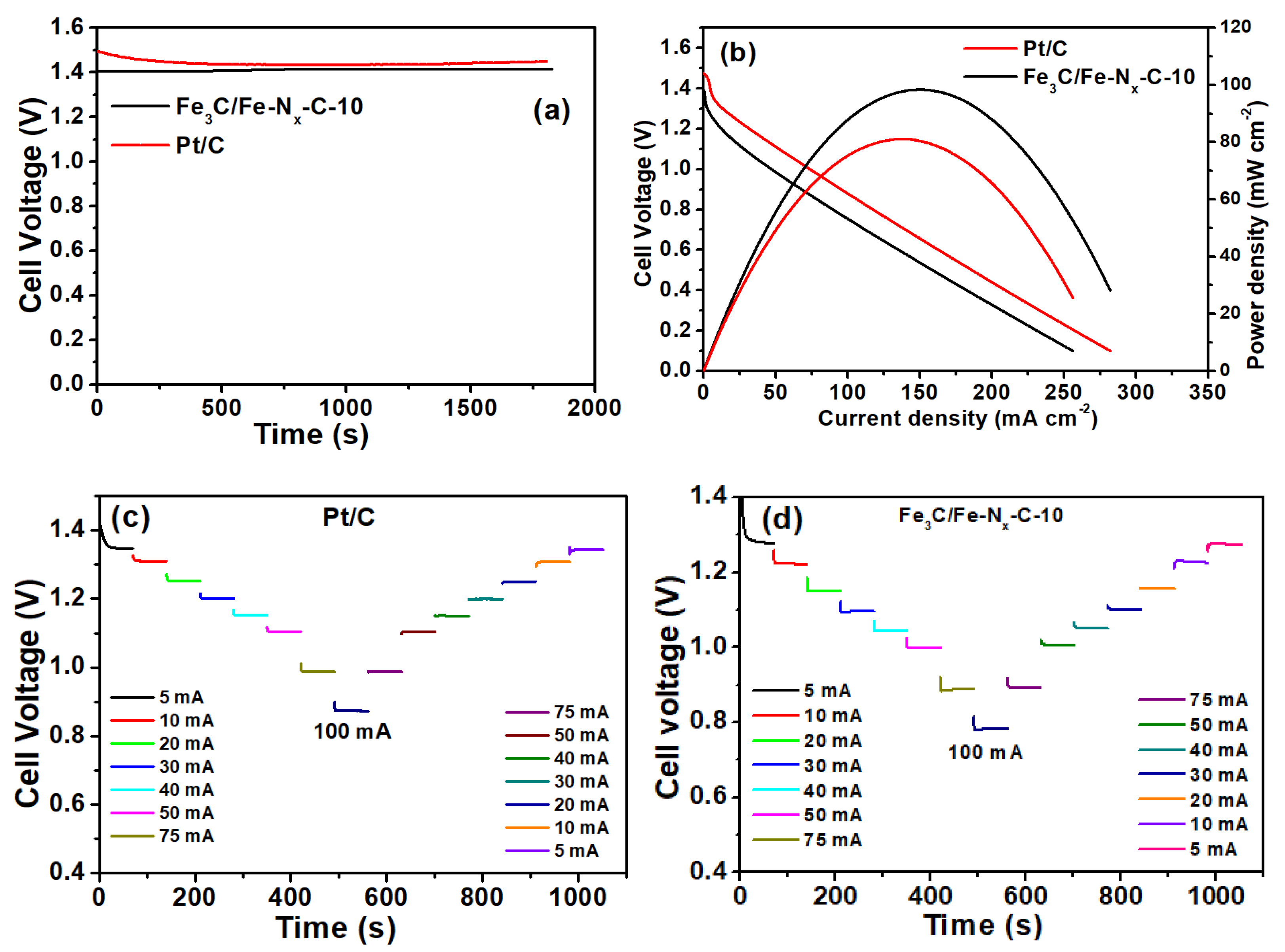
| Catalyst | Eredox Potentials (V vs. RHE) | E1/2 (V vs. RHE) | ‘n’ | ESCA (cm−2) | Loss in E1/2 Potential (10,000 Cycles mV vs. RHE) | Loss of Relative Current (%) | Zn–Air Battery Performance | |
|---|---|---|---|---|---|---|---|---|
| OCV (V) | Power Density (mW cm−2) | |||||||
| C–N–C | 0.68 | 0.72 | 3.65 | 0.00777 | - | - | - | |
| Fe3C/Fe-Nx-C-2 | 0.70 | 0.77 | 3.75 | 0.01016 | - | - | - | |
| Fe3C/Fe-Nx-C-5 | 0.75 | 0.79 | 3.82 | 0.01797 | - | - | - | |
| Fe3C/Fe-Nx-C-8 | 0.78 | 0.87 | 3.91 | 0.03406 | - | - | - | |
| Fe3C/Fe-Nx-C-10 | 0.83 | 0.93 | 3.95 | 0.07861 | 75 | 13 | 1.41 | 81 |
| Fe3C/Fe-Nx-C-15 | 0.82 | 0.90 | 3.95 | 0.07614 | ||||
| Pt/C | - | 0.93 | 4.0 | - | 60 | 14 | 1.46 | 98 |
| Biomass Source | Catalysts | E1/2 (V vs. RHE) | Tafel Slope (mV dec−1) | Number of Electrons (n) | Zn–Air Battery Performance (Aqueous) | Ref. | |
|---|---|---|---|---|---|---|---|
| OCV (V) | Power Density (mW cm−2) | ||||||
| Garlic biomass | Fe@G-800/100 | 0.91 | 69 | 3.89 | 1.48 | 20 | [49] |
| Loofah Sponge | FeCo@NC-900 | 0.81 | 71 | 3.44–3.64 | 1.49 | 103 | [50] |
| Eucalyptus pulp | Fe-N-C-1000 | 0.84 | NR | ~4 | 1.49 | 125 | [51] |
| Sodium alginate | FeNC-900-8 | 0.88 | 63 | 3.87 | 1.67 | 125 | [52] |
| Soybeans | Fe-NC-800 | 0.91 | 74 | 3.99 | 1.53 | 220 | [53] |
| Natural wood | SAC-FeN-WPC | 0.85 | 83 | ~4 | 1.53 | 152 | [54] |
| Corn silk | Fe SA/NCZ | 0.80 | 70 | 3.9 | 1.44 | 101 | [55] |
| Wood | Co/CoO@NWC | 0.85 | 96 | 3.90–3.95 | 1.38 | 28 | [56] |
| Chitosan | Fe3C/NCA-1000 | 0.83 | 79 | 3.6 | NR | 253 | [57] |
| Rotten wood | NRW-1000 | 0.87 | 70 | 3.8 | 1.53 | 118 | [58] |
| Water hyacinths | WHNC-A | 0.84 | 92 | 2.89 | 1.43 | 80 | [59] |
| fruits of glossy privet | GPNCS | 0.92 | NR | 3.8–3.9 | 1.43 | 68 | [60] |
| Eichhornia crassipes | Fe2N@NCNTs | 0.86 | 67 | 3.87–3.9 | 1.53 | 135 | [61] |
| Wood | FeP-NWC | 0.86 | 74 | 3.77–3.90 | 1.50 | 144 | [62] |
| Wood | Fe3C@NPW | 0.87 | 98 | 3.78–3.89 | 1.48 | 125 | [63] |
| Waste coffee grounds | Fe3C/Fe-Nx-C-10 | 0.93 | 3.95 | 1.41 | 81 | This work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peera, S.G.; Kim, S.-W.; Ashmath, S.; Lee, T.-G. Sustainable Fe3C/Fe-Nx-C Cathode Catalyst from Biomass for an Oxygen Reduction Reaction in Alkaline Electrolytes and Zinc–Air Battery Application. Inorganics 2025, 13, 143. https://doi.org/10.3390/inorganics13050143
Peera SG, Kim S-W, Ashmath S, Lee T-G. Sustainable Fe3C/Fe-Nx-C Cathode Catalyst from Biomass for an Oxygen Reduction Reaction in Alkaline Electrolytes and Zinc–Air Battery Application. Inorganics. 2025; 13(5):143. https://doi.org/10.3390/inorganics13050143
Chicago/Turabian StylePeera, Shaik Gouse, Seung-Won Kim, Shaik Ashmath, and Tae-Gwan Lee. 2025. "Sustainable Fe3C/Fe-Nx-C Cathode Catalyst from Biomass for an Oxygen Reduction Reaction in Alkaline Electrolytes and Zinc–Air Battery Application" Inorganics 13, no. 5: 143. https://doi.org/10.3390/inorganics13050143
APA StylePeera, S. G., Kim, S.-W., Ashmath, S., & Lee, T.-G. (2025). Sustainable Fe3C/Fe-Nx-C Cathode Catalyst from Biomass for an Oxygen Reduction Reaction in Alkaline Electrolytes and Zinc–Air Battery Application. Inorganics, 13(5), 143. https://doi.org/10.3390/inorganics13050143








