Photoelectrochemical and Photocatalytic Properties of SnS/TiO2 Heterostructure Thin Films Prepared by Magnetron Sputtering Method
Abstract
1. Introduction
2. Results and Discussion
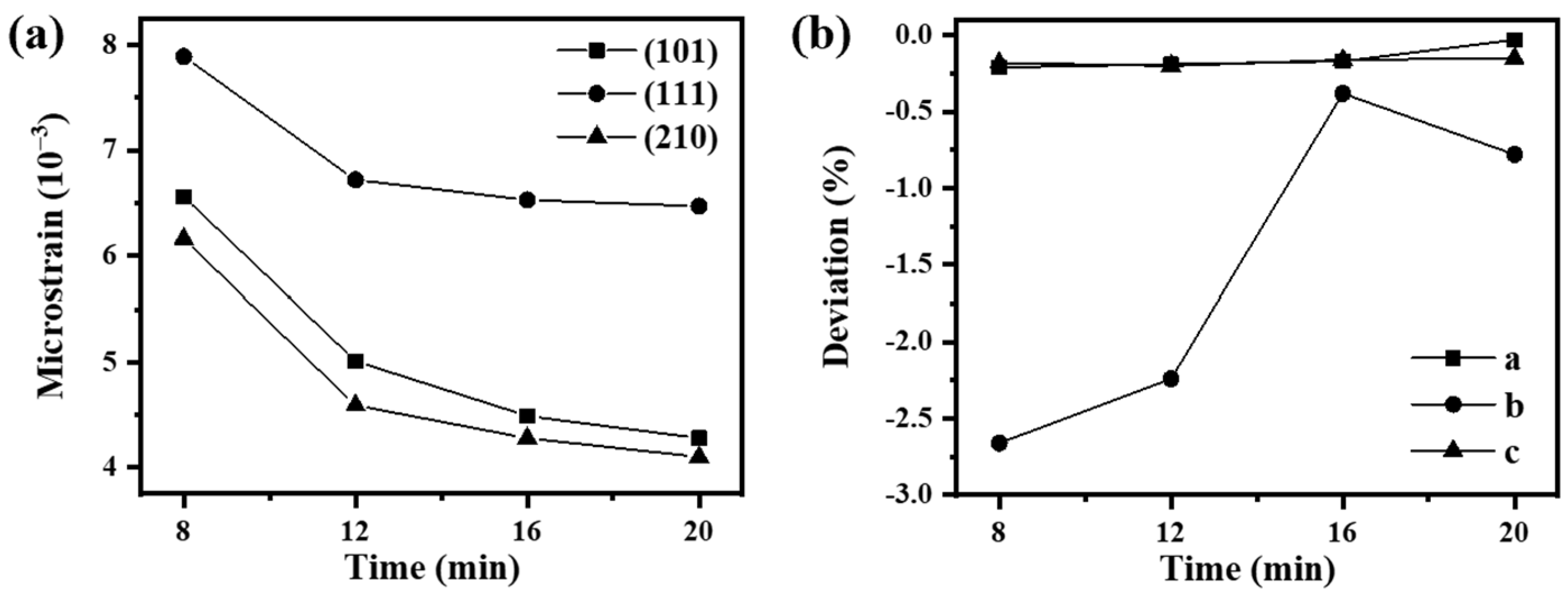
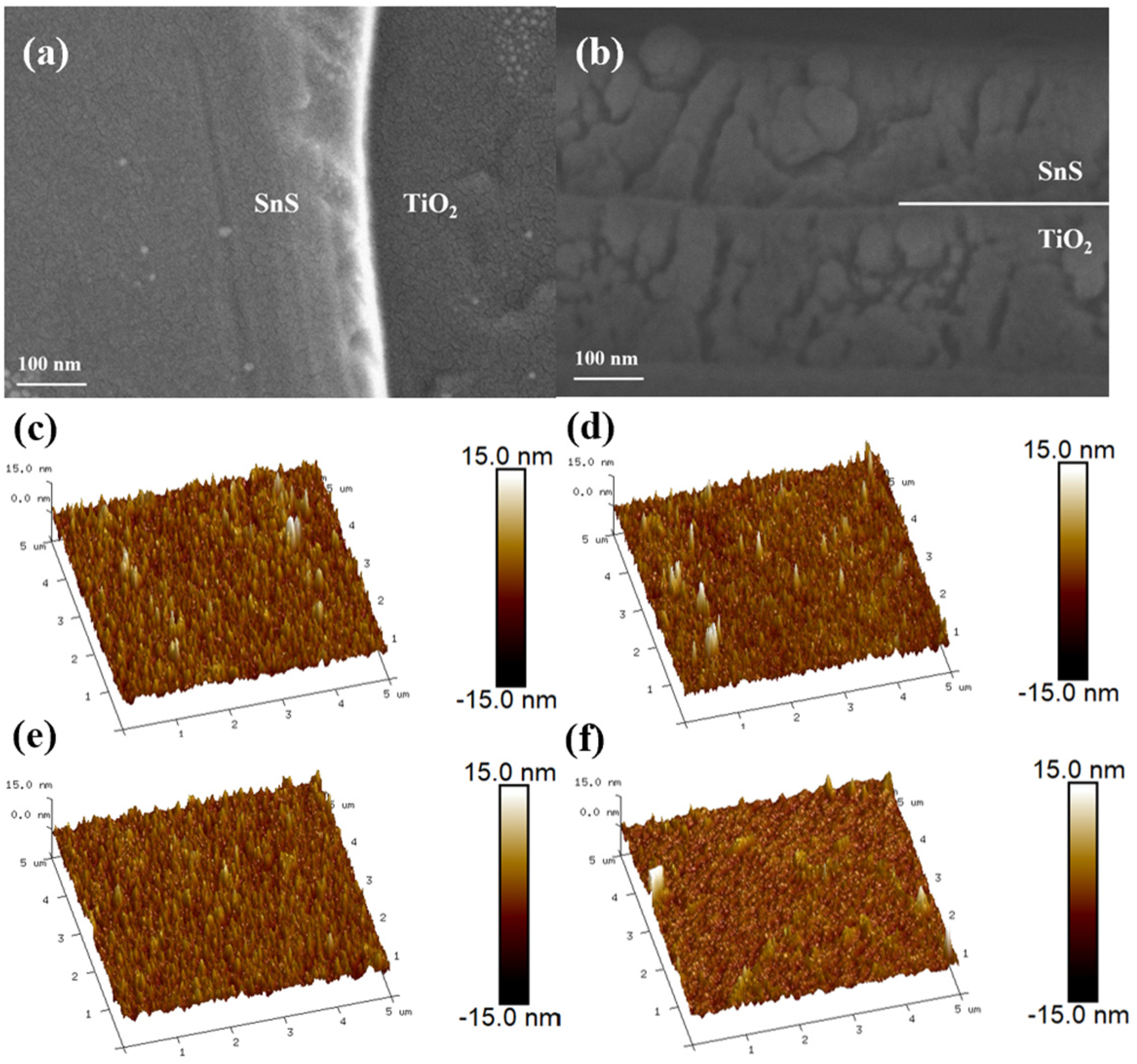
2.1. Structural Characterization
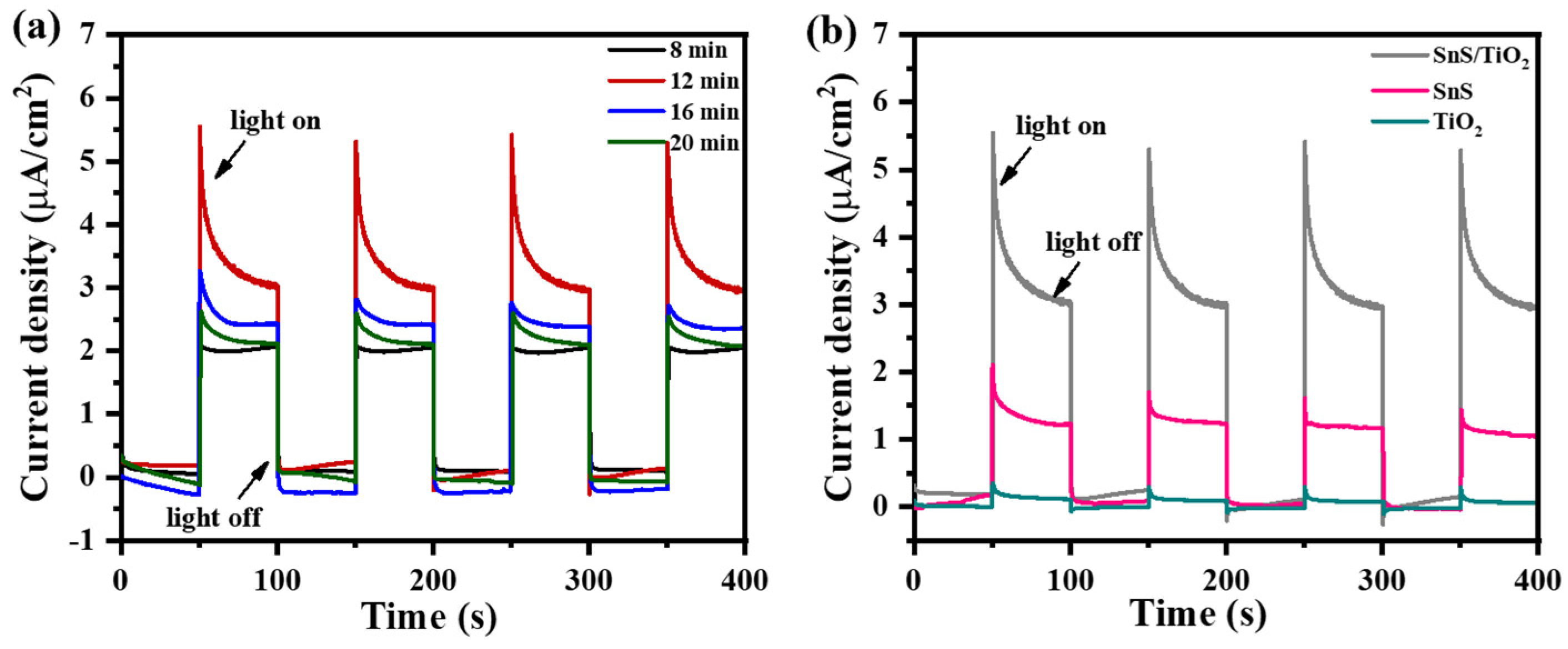
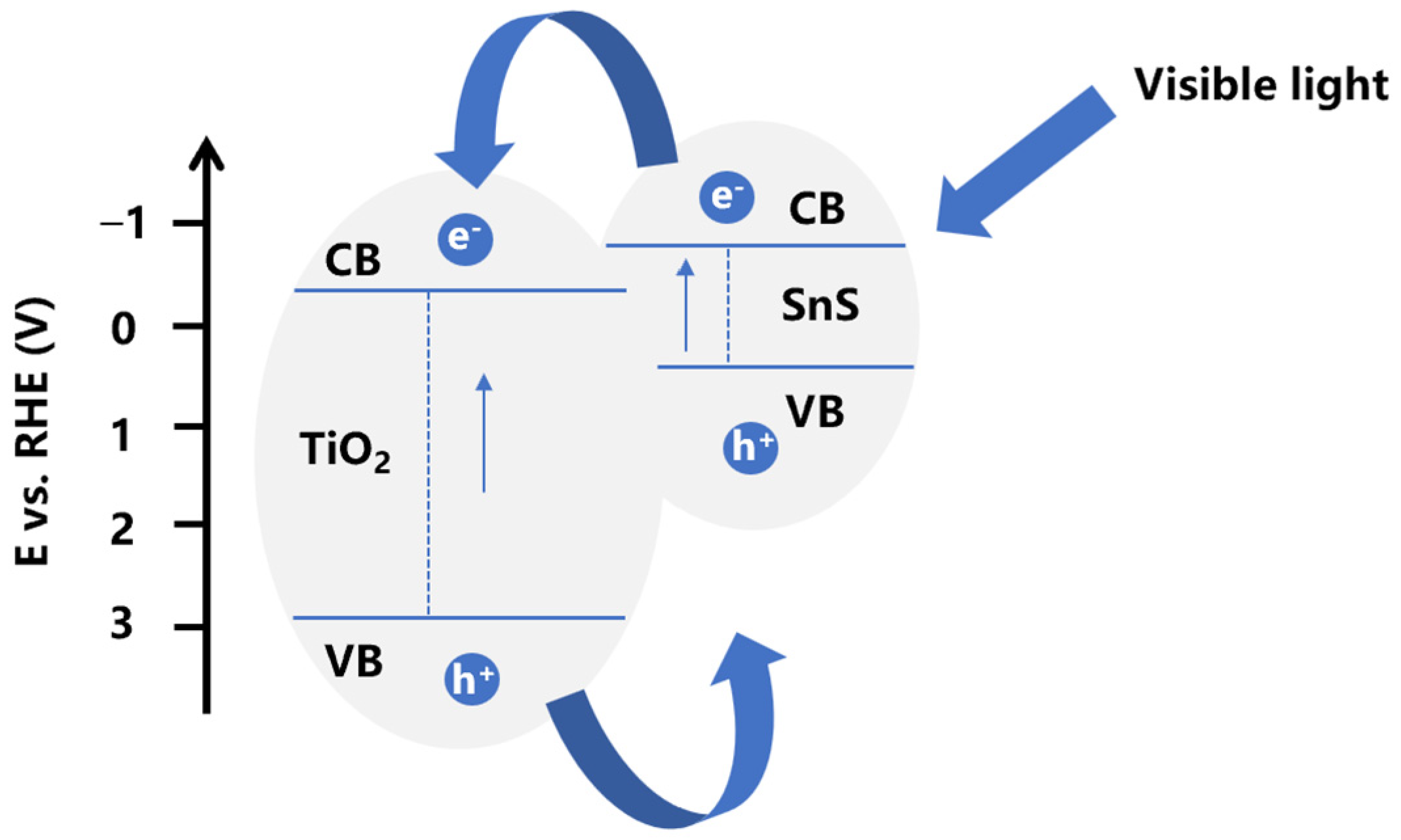
2.2. Photoelectrochemical Characterization
3. Materials and Methods
3.1. Preparation of SnS/TiO2 Heterostructure Thin Films
3.2. Characterization and Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, deconta ination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Green, M.; Just, M.; Li, Y.Y.; Chen, X.B. Titanium dioxide nanomaterials for photocatalysis. J. Phys. D Appl. Phys. 2017, 50, 193003. [Google Scholar] [CrossRef]
- Chen, D.J.; Cheng, Y.L.; Zhou, N.; Chen, P.; Wang, Y.P.; Li, K.; Huo, S.H.; Cheng, P.F.; Peng, P.; Zhang, R.C. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Shu, Z.X.; Jiao, X.L.; Chen, D.R. Synthesis and photocatalytic properties of flower-like zirconia nanostructures. Cryst. Eng. Comm. 2012, 14, 1122–1127. [Google Scholar] [CrossRef]
- Chu, M.N.; Hu, K.; Wang, J.S.; Liu, Y.D.; Ali, S.; Qin, C.L.; Jing, L.Q. Synthesis of g-C3N4-based photocatalysts with recyclable feature for efficient 2,4-dichlorophenol degradation and mechanisms. Appl. Catal. B 2019, 243, 57–65. [Google Scholar] [CrossRef]
- Hu, J.S.; Ren, L.L.; Guo, Y.G.; Liang, H.P.; Cao, A.M.; Wan, L.J.; Bai, C.L. Mass production and high photocatalytic activity of ZnS nanoporous nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 1269–1273. [Google Scholar] [CrossRef]
- Wu, G.L.; Li, P.; Xu, D.B.; Luo, B.F.; Hong, Y.Z.; Shi, W.D.; Liu, C.B. Hydrothermal synthesis and visible-light-driven photocatalytic degradation for tetracycline of Mn-doped SrTiO3 nanocubes. Appl. Surf. Sci. 2015, 333, 39–47. [Google Scholar] [CrossRef]
- Gopidas, K.R.; Bohorquez, M.; Kamat, P.V. Photophysical and photochemical aspects of coupled semiconductors: Charge-transfer processes in colloidal cadmium sulfide-titania and cadmium sulfide-silver(I) iodide systems. J. Phys. Chem. 1990, 94, 6435–6440. [Google Scholar] [CrossRef]
- Khanchandani, S.; Kundu, S.; Patra, A.; Ganguli, A.K. Band gap tuning of ZnO/In2S3 core/shell nanorod arrays for enhanced visible-light-driven photocatalysis. J. Phys. Chem. C 2013, 117, 5558–5567. [Google Scholar] [CrossRef]
- He, X.H.; Kai, T.H.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Jiang, L.B.; Liang, J.; Pan, Y.; Zhang, J.; Wang, H.; Leng, L.J.; Wu, Z.B.; Guan, R.P.; Zeng, G.M.; et al. In-situ synthesis of 3D microsphere-like In2S3/InVO4 heterojunction with efficient photocatalytic activity for tetracycline degradation under visible light irradiation. Chem. Eng. J. 2019, 356, 371–381. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Ahmad, M.; Ghafoor, A.; Haoran, S.; Ali, U.; Meng, X.L.; Kamara, A.; Nazir, A.; Jie, Z.; Liu, Z.; Yan, Z.F. Acquiescent tailoring of TiO2 band gap via surged NiSe NPs decoration for accelerated photocatalytic degradation of methyl orange. J. Water Process Eng. 2025, 70, 107001. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Gilani, M.R.H.S.; Maaz, M.; Akhter, P.; Hussain, M.; Jeong, K.; Kwon, E.E.; Bae, S.; Park, Y. Advancements in TiO2-based photocatalysis for environmental remediation: Strategies for enhancing visible-light-driven activity. Chemosphere 2024, 349, 140703. [Google Scholar] [CrossRef]
- Cui, F.M.; Hua, Z.L.; Wei, C.Y.; Li, J.Q.; Gao, Z.; Shi, J.L. Highly dispersed Au nanoparticles incorporated mesoporous TiO2 thin films with ultrahigh Au content. J. Mater. Chem. 2009, 19, 7632–7637. [Google Scholar] [CrossRef]
- Naseri, N.; Kim, H.; Choi, W.; Moshfegh, A.Z. Optimal Ag concentration for H2 production via Ag:TiO2 nanocomposite thin film photoanode. Int. J. Hydrogen Energy 2012, 37, 3056–3065. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhao, J.L.; Kang, Y.R.; Li, L.L.; Xu, X.R. Photoelectrochemical properties of Fe-doped TiO2 nanotube arrays fabricated by anodization. J. Appl. Electrochem. 2014, 44, 1–4. [Google Scholar] [CrossRef]
- Guo, W.; Shen, Y.H.; Wu, L.Q.; Gao, Y.R.; Ma, T.L. Effect of N dopant amount on the performance of dye-Sensitized solar cells based on N-doped TiO2 electrodes. J. Phys. Chem. C 2011, 115, 21494–21499. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Daikopoulos, C.; Deligiannakis, Y.; Konstantinou, I. Characterization and catalytic performance of B-doped, B–N co-doped and B–N–F tri-doped TiO2 towards simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl. Catal. B Environ. 2016, 184, 44–54. [Google Scholar] [CrossRef]
- Zhao, W.X.; Bai, Z.P.; Ren, A.L.; Guo, B.; Wu, C. Sunlight photocatalytic activity of CdS modified TiO2 loaded on activated carbon fibers. Appl. Surf. Sci. 2010, 256, 3493–3498. [Google Scholar] [CrossRef]
- Han, H.; Riboni, F.; Karlicky, F.; Kment, S.; Goswami, A.; Sudhagar, P.; Yoo, J.; Wang, L.; Tomanec, O.; Petr, M.; et al. α-Fe2O3/TiO2 3D hierarchical nanostructures for enhanced photoelectrochemical water splitting. Nanoscale 2017, 9, 134–142. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhan, Y.; Wang, Z.Y. TiO2, MoS2, and TiO2/MoS2 heterostructures for use in organic dyes degradation. Chem. Sel. 2018, 3, 1713–1718. [Google Scholar] [CrossRef]
- Wang, G.M.; Wang, H.Y.; Ling, Y.C.; Tang, Y.C.; Yang, X.Y.; Fitzmorris, R.C.; Wang, C.C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Yan, M.; Li, G.L.; Guo, C.S.; Guo, W.; Ding, D.D.; Zhang, S.H.; Liu, S.Q. WO3-x sensitized TiO2 spheres with full-spectrum-driven photocatalytic activities from UV to near infrared. Nanoscale 2016, 8, 17828–17835. [Google Scholar] [CrossRef]
- Adamu, H.; McCue, A.J.; Taylor, R.S.F.; Manyar, H.G.; Anderson, J.A. Simultaneous photocatalytic removal of nitrate and oxalic acid over Cu2O/TiO2 and Cu2O/TiO2-AC composites. Appl. Catal. B Environ. 2017, 217, 181–191. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, W.J.; Chen, X.J.; Wang, J.; Zhu, Y.F. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Kumar, V.; Goswami, Y.C. Ultrasound-assisted facile synthesis of tin sulfide (SnS) nanostructures and their structural, optical, and morphological studies. Phosphorus Sulfur Silicon Relat. Elem. 2025, 200, 405–412. [Google Scholar] [CrossRef]
- Kawamura, F.; Song, Y.; Murata, H.; Tampo, H.; Nagai, T.; Koida, T.; Imura, M.; Yamada, N. Tunability of the bandgap of SnS by variation of the cell volume by alloying with AE elements. Sci. Rep. 2022, 12, 7434. [Google Scholar] [CrossRef]
- Reddy, N.K.; Devika, M.; Gopal, E.S.R. Review on tin (II) sulfide (SnS) material: Synthesis, properties, and applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 359–398. [Google Scholar] [CrossRef]
- Norton, K.J.; Alam, F.; Lewis, D.J. A review of the synthesis, properties, and applications of bulk and two-dimensional tin (II) sulfide(SnS). Appl. Sci. 2021, 11, 2062. [Google Scholar] [CrossRef]
- Nengzi, L.C.; Yang, H.; Hu, J.Z.; Zhang, W.M.; Jiang, D.A. Fabrication of SnS/TiO2 NRs/NSs photoelectrode as photoactivator of peroxymonosulfate for organic pollutants elimination. Sep. Purif. Technol. 2020, 249, 117172. [Google Scholar] [CrossRef]
- He, H.Y.; Lu, J.; Cao, L.Y.; Li, M. Photodegradation of methyl orange from wastewater on TiO2/SnS combined powders. Res. Chem. Intermed. 2012, 38, 537–547. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gao, Y.J.; Nengzi, L.C.; Li, B.; Gou, J.F.; Cheng, X.W. Synthesis of SnS/TiO2 nano-tube arrays photoelectrode and its high photoelectrocatalytic performance for elimination of 2,4,6-trichlorophenol. Sep. Purif. Technol. 2019, 228, 115742. [Google Scholar] [CrossRef]
- Xu, F.; Scaramuzza, N.; Versace, C. Decorating TiO2 nanoparticle thin film with SnSx(x<1): Preparation, characterization, and photocatalytic activity. Coatings 2024, 14, 1185. [Google Scholar]
- Jia, Y.F.; Yang, F.; Cai, F.G.; Cheng, C.H.; Zhao, Y. Photoelectrochemical and charge transfer properties of SnS/TiO2 heterostructure nanotube arrays. Electron. Mater. Lett. 2013, 9, 287–291. [Google Scholar] [CrossRef]
- Mittal, A.; Sharma, S.; Kumar, T.; Chauhan, N.S.; Kumari, K.; Maken, S.; Kumar, N. Surfactant-assisted hydrothermally synthesized novel TiO2/SnS@Pd nano-composite: Structural, morphological and photocatalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 2010–2021. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, H.; Fan, B.H.; Hu, G.X. Photovoltaic behavior of nanocrystalline SnS/TiO2. J. Phys. Chem. C 2010, 114, 3256–3259. [Google Scholar] [CrossRef]
- Al Ahmed, S.R. Investigation on the performance enhancement of heterojunction SnS thin-film solar cell with a Zn3P2 hole transport layer and a TiO2 electron transport layer. Energy Fuels 2024, 38, 1462–1476. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Maleki, R.; Ghoreishian, S.M.; Mohammadi, A.; Raju, G.S.R.; Huh, Y.S.; Han, Y.K. Defective phase engineering of S-scheme TiO2-SnS/SnS2 core-shell photocatalytic nanofibers for elevated visible light responsive H2 generation and nitrogen fixation. J. Mater. Chem. A 2024, 12, 33818–33833. [Google Scholar] [CrossRef]
- Qiu, P.L.; Qin, Y.X.; Xia, Q. Ultrasensitive gas sensor developed from SnS/TiO2-based memristor for dilute methanol detection at room temperature. Sens. Actuators B Chem. 2023, 392, 134038. [Google Scholar] [CrossRef]
- Burton, L.A.; Walsh, A. Phase stability of the earth-abundant tin sulfides SnS, SnS2, and Sn2S3. J. Phys. Chem. C 2012, 116, 24262–24267. [Google Scholar] [CrossRef]
- Lim, D.; Suh, H.; Suryawanshi, M.; Song, G.Y.; Cho, J.Y.; Kim, J.H.; Jang, J.H.; Jeon, C.W.; Cho, A.; Ahn, S.; et al. Kinetically controlled growth of phase-pure SnS absorbers for thin film solar cells: Achieving efficiency near 3% with long-term stability using an SnS/CdS heterojunction. Adv. Energy Mater. 2018, 8, 1702605. [Google Scholar] [CrossRef]
- Guneri, E.; Ulutas, C.; Kirmizigul, F.; Altindemir, G.; Gode, F.; Gumus, C. Effect of deposition time on structural, electrical, and optical properties of SnS thin films deposited by chemical bath deposition. Appl. Surf. Sci. 2010, 257, 1189–1195. [Google Scholar] [CrossRef]
- Hase, Y.; Sharma, V.; Doiphode, V.; Waghmare, A.; Punde, A.; Shinde, P.; Shah, S.; Rahane, S.; Vairale, P.; Bade, B.; et al. Humidity sensor properties of hydrothermally grown rutile-TiO2 microspheres on interdigital electrodes (IDEs). J. Mater. Sci. Mater. Electron. 2022, 33, 11825–11840. [Google Scholar] [CrossRef]
- Kul, M. Electrodeposited SnS film for photovoltaic applications. Vacuum 2014, 107, 213–218. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Voznyi, A.; Kosyak, V.; Grase, L.; Vecstaudza, J.; Onufrijevs, P.; Yeromenko, Y.; Medvid’, A.; Opanasyuk, A. Formation of SnS phase obtained by thermal vacuum annealing of SnS2 thin films and its application in solar cells. Mater. Sci. Semicond. Process. 2018, 79, 32–39. [Google Scholar] [CrossRef]
- Kumari, T.P.; Raja, M.M.; Kumar, A.; Srinath, S.; Kamat, S.V. Effect of thickness on structure, microstructure, residual stress and soft magnetic properties of DC sputtered Fe65Co35 soft magnetic thin films. J. Magn. Magn. Mater. 2014, 365, 93–99. [Google Scholar] [CrossRef]
- Bashkirov, S.A.; Gremenok, V.F.; Ivanov, V.A.; Shevtsova, V.V.; Gladyshev, P.P. Influence of substrate material on the microstructure and optical properties of hot wall deposited SnS thin films. Thin Solid Film. 2015, 585, 40–44. [Google Scholar] [CrossRef]
- Baby, B.H.; Mohan, D.B. The effect of in-situ and post deposition annealing towards the structural optimization studies of RF sputtered SnS and Sn2S3 thin films for solar cell application. Sol. Energy 2019, 189, 207–218. [Google Scholar] [CrossRef]
- Reddy, V.R.M.; Gedi, S.; Park, C.; Miles, R.W.; Reddy, K.T.R. Development of sulphurized SnS thin film solar cells. Curr. Appl. Phys. 2015, 15, 588–598. [Google Scholar] [CrossRef]
- Deng, J.L.; Gao, J.; Liu, M.; Wang, Y.F.; Zheng, L.C.; Li, Y.; Liu, Y. SnS nanoparticles and MoS2 nanosheets co-decorated TiO2 nanorod film with remarkable photocatalytic and photoelectrochemical properties. J. Electrochem. Soc. 2022, 169, 056513. [Google Scholar] [CrossRef]
- Xue, D.N.; Luo, J.; Li, Z.; Yin, Y.L.; Shen, J. Enhanced photoelectrochemical properties from Mo-doped TiO2 nanotube arrays film. Coatings 2020, 10, 75. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorov, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627. [Google Scholar] [CrossRef]
- Coulter, J.B.; Birnie, D.P. Assessing Tauc plot slope quantification: ZnO thin films as a model system. Phys. Status Solidi B 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Araújo, M.M.; Silva, L.K.R.; Sczancoski, J.C.; Orlandi, M.O.; Longo, E.; Santos, A.G.D.; Sá, J.L.S.; Santos, R.S.; Luz, G.E.; Cavalcante, L.S. Anatase TiO2 nanocrystals anchored at inside of SBA-15 mesopores and their optical behavior. Appl. Surf. Sci. 2016, 389, 1137–1147. [Google Scholar] [CrossRef]
- Sharma, P.K.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Han, Y.S.; Byrne, J.A.; Nolan, M. Surface modification of TiO2 with copper clusters for band gap narrowing. Catal. Today 2019, 321, 9–17. [Google Scholar] [CrossRef]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc plot method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Liu, W.J.; Liu, M.L.; Wang, X.T.; Shen, T.; Chang, G.Q.; Lei, M.; Deng, H.X.; Wei, Z.M.; Wei, Z.Y. Thickness-dependent ultrafast photonics of SnS2 nanolayers for optimizing fiber lasers. ACS Appl. Nano Mater. 2019, 2, 2697–2705. [Google Scholar] [CrossRef]
- Javed, A.; Khan, N.; Bashir, S.; Ahmad, M.; Bashir, M. Thickness dependent structural, electrical and optical properties of cubic SnS thin films. Mater. Chem. Phys. 2020, 246, 122831. [Google Scholar] [CrossRef]
- Huang, L.Y.; Meng, L. Effects of film thickness on microstructure and electrical properties of the pyrite films. Mater. Sci. Eng. B 2007, 137, 310–314. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, D.; Biradar, A.M.; Rajesh. Electrochemical impedance spectroscopy characterization of mercaptopropionic acid capped ZnS nanocrystal based bioelectrode for the detection of the cardiac biomarker-myoglobin. Bioelectrochemistry 2012, 88, 118–126. [Google Scholar] [CrossRef]
- Li, P.J.; Zhang, Y.G.; Yang, X.G.; Gao, Y.H.; Ge, S.X. Alloyed PtNi counter electrodes for high-performance dye-sensitized solar cell applications. J. Alloys Compd. 2017, 725, 1272–1281. [Google Scholar] [CrossRef]
- Cheraghizade, M.; Jamali-Sheini, F.; Shabani, P. Charge transportation mechanisms in TiO2/SnS/Ag solar cells. Mater. Res. Bull. 2020, 124, 110727. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization and Mott-Schottky analysis of corrosion of welded X70 pipeline steel in carbonate/bicarbonate solution. Electrochim. Acta 2009, 55, 316–324. [Google Scholar] [CrossRef]
- Ellis, A.B.; Kaiser, S.W.; Bolts, J.M.; Wrighton, M.S. Study of n-type semiconducting cadmium chalcogenide-based photoelectrochemical cells employing polychalcogenide electrolytes. J. Am. Chem. Soc. 1977, 99, 2839–2848. [Google Scholar] [CrossRef]
- Liao, X.L.; Li, T.T.; Ren, H.T.; Mao, Z.Y.; Zhang, X.F.; Lin, J.H.; Lou, C.W. Enhanced photocatalytic performance through the ferroelectric synergistic effect of p-n heterojunction BiFeO3/TiO2 under visible-light irradiation. Ceram. Int. 2021, 47, 10786–10795. [Google Scholar] [CrossRef]
- Li, S.N.; Meng, L.X.; Tian, W.; Li, L. Engineering interfacial band bending over ZnIn2S4/SnS2 by interface chemical bond for efficient solar-driven photoelectrochemical water splitting. Adv. Energy Mater. 2022, 12, 2200629. [Google Scholar] [CrossRef]
- Jamali-Sheini, F.; Yousefi, R.; Bakrc, N.A.; Cheraghizade, M.; Sookhakian, M.; Huang, N.M. Highly efficient photo-degradation of methyl blue and band gap shift of SnS nanoparticles under different sonication frequencies. Mater. Sci. Semicond. Process. 2015, 32, 172–178. [Google Scholar] [CrossRef]
- Liu, H.H.; Gong, H.; Zou, M.M.; Jiang, H.; Abolaji, R.S.; Tareen, A.K.; Hakala, B.V.; Yang, M.H. Mo-N-co-doped mesoporous TiO2 microspheres with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2017, 96, 10–17. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Han, M.E.; Lyu, L.; Li, T.; Gao, Y.W.; Zeng, Q.Y.; Hu, C. Efficient inhibition of photogenerated electron-hole recombination through persulfate activation and dual-pathway degradation of micropollutants over iron molybdate. Appl. Catal. B Environ. 2019, 257, 117904. [Google Scholar] [CrossRef]
- Yue, T.C.; Yin, L.; Huang, J.B.; Wang, L.L.; Wang, D.Z. Assembly of a three-dimensional Cu-MOF for efficient Fenton-like degradation of dyes and iodine capture. Appl. Organomet. Chem. 2024, 38, e7629. [Google Scholar] [CrossRef]
- Song, Y.Y.; Jiang, J.J.; Ma, Y.H.; Li, T.R.; Dong, S.S. Visible-light activation of peroxymonosulfate by NiCo2O4/Bi24O31Br10 to accelerate tetracycline degradation. Catal. Sci. Technol. 2021, 11, 2110–2118. [Google Scholar] [CrossRef]
- Qorbani, M.; Sabbah, A.; Lai, Y.R.; Kholimatussadiah, S.; Quadir, S.; Huang, C.Y.; Shown, I.; Huang, Y.F.; Hayashi, M.; Chen, K.H.; et al. Atomistic insights into highly active reconstructed edges of monolayer 2H-WSe2 photocatalyst. Nat. Commun. 2022, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.T.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.H.; Wen, W.; Yu, S.S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Li, D.Z.; Calebe, V.C.; Li, Y.Q.; Liu, H.M.; Lei, Y.M. Interstitial N-doped TiO2 for photocatalytic methylene blue degradation under visible light irradiation. Catalysts 2024, 14, 681. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Yang, M.J.; Du, J.Y.; Ke, G.L.; Zhong, X.H.; Zhou, Y.; Dong, F.Q.; Bian, L.; He, H.C. MoO3/BiVO4 heterojunction film with oxygen vacancies for efficient and stable photoelectrochemical water oxidation. J. Mater. Sci. 2019, 54, 671–682. [Google Scholar] [CrossRef]
- Ding, Y.X.; Xue, D.N.; Yu, H.Z.; Shen, J. Preparation and photoelectrochemical properties of Mo/N co-doped TiO2 nanotube array films. Coatings 2023, 13, 1230. [Google Scholar] [CrossRef]
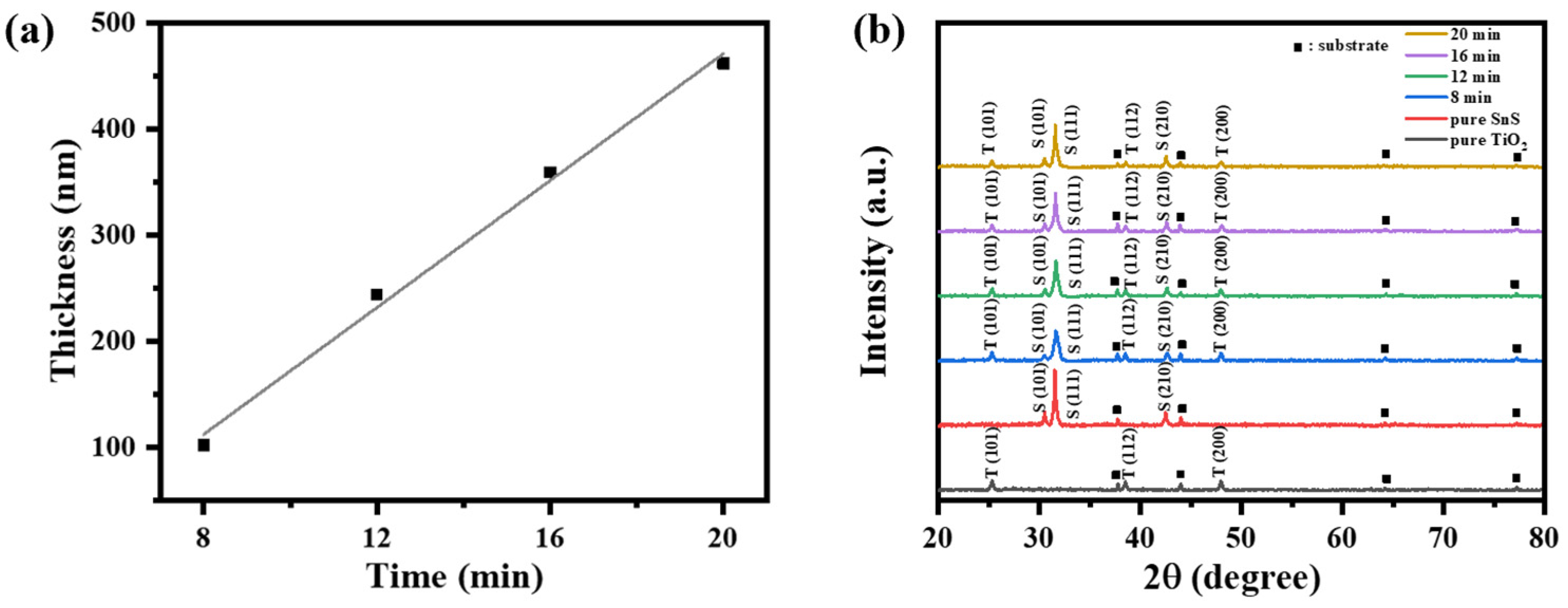



| Sputtering Time of SnS (min) | Thicknesses of SnS (nm, ±4) | Thicknesses of TiO2 (nm, ±4) |
|---|---|---|
| 8 | 102 | 220 |
| 12 | 244 | 225 |
| 16 | 359 | 217 |
| 20 | 462 | 231 |
| Sputtering Time of SnS (min) | SnS | TiO2 | ||||||
|---|---|---|---|---|---|---|---|---|
| D ± 0.8 (nm) | ε ± 0.09 (×10−3) | a (Å) | b (Å) | c (Å) | D ± 0.8 (nm) | a (Å) | c (Å) | |
| 8 | 18.5 | 6.87 | 4.3199 | 10.8950 | 3.9765 | 27.0 | 3.7900 | 9.4453 |
| 12 | 22.6 | 5.44 | 4.3210 | 10.9415 | 3.9757 | 27.3 | 3.7928 | 9.4292 |
| 16 | 23.8 | 5.10 | 4.3219 | 11.1500 | 3.9775 | 27.2 | 3.7848 | 9.5549 |
| 20 | 24.6 | 4.95 | 4.3277 | 11.1054 | 3.9777 | 27.9 | 3.7900 | 9.4725 |
| pure SnS | 27.5 | 4.29 | 4.3279 | 11.1735 | 3.9771 | / | / | / |
| pure TiO2 | / | / | / | / | / | 27.5 | 3.7899 | 9.4736 |
| Sputtering Time of SnS (min) | RMS (nm) |
|---|---|
| 8 | 1.93 |
| 12 | 1.74 |
| 16 | 1.63 |
| 20 | 1.60 |
| Sputtering Time of SnS (min) | Ti (At%) | O (At%) | Sn (At%) | S (At%) |
|---|---|---|---|---|
| 8 | 21.08 | 45.85 | 18.24 | 14.83 |
| 12 | 15.24 | 32.18 | 29.01 | 23.57 |
| 16 | 7.06 | 14.97 | 43.18 | 34.79 |
| 20 | 4.63 | 9.69 | 47.36 | 38.32 |
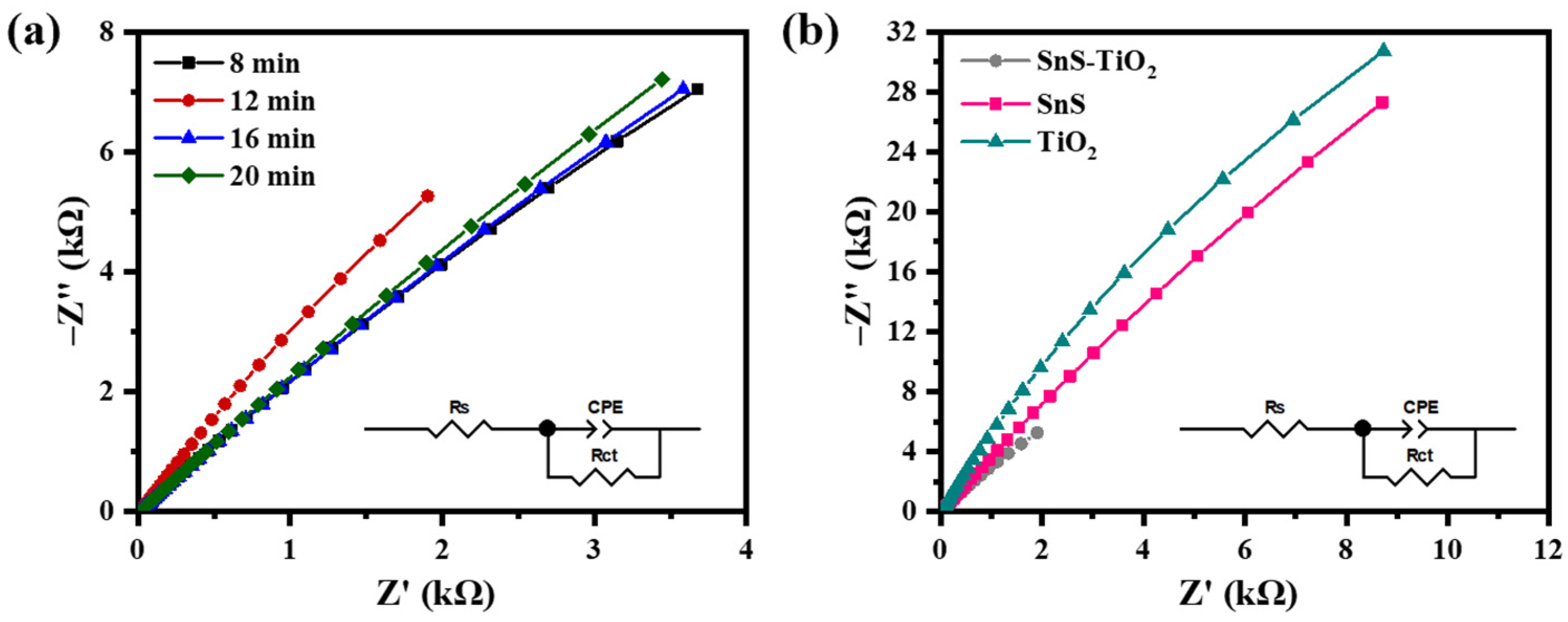
| Sputtering Time of SnS (min) | Rct (kΩ) | Rs (Ω) | CPEdl (µF) | n | k0 (×10−9 m/s) | J0 (×10−8 A/cm2) |
|---|---|---|---|---|---|---|
| 8 | 99.85 | 10.55 | 47.70 | 0.74 | 13.11 | 12.65 |
| 12 | 79.96 | 15.65 | 14.94 | 0.82 | 16.37 | 15.80 |
| 16 | 123.32 | 10.52 | 48.49 | 0.73 | 10.61 | 10.24 |
| 20 | 168.33 | 14.02 | 31.22 | 0.74 | 7.78 | 7.50 |
| SnS | 523.53 | 25.13 | 26.71 | 0.84 | 2.50 | 2.41 |
| TiO2 | 289.52 | 13.47 | 16.54 | 0.89 | 4.52 | 4.36 |
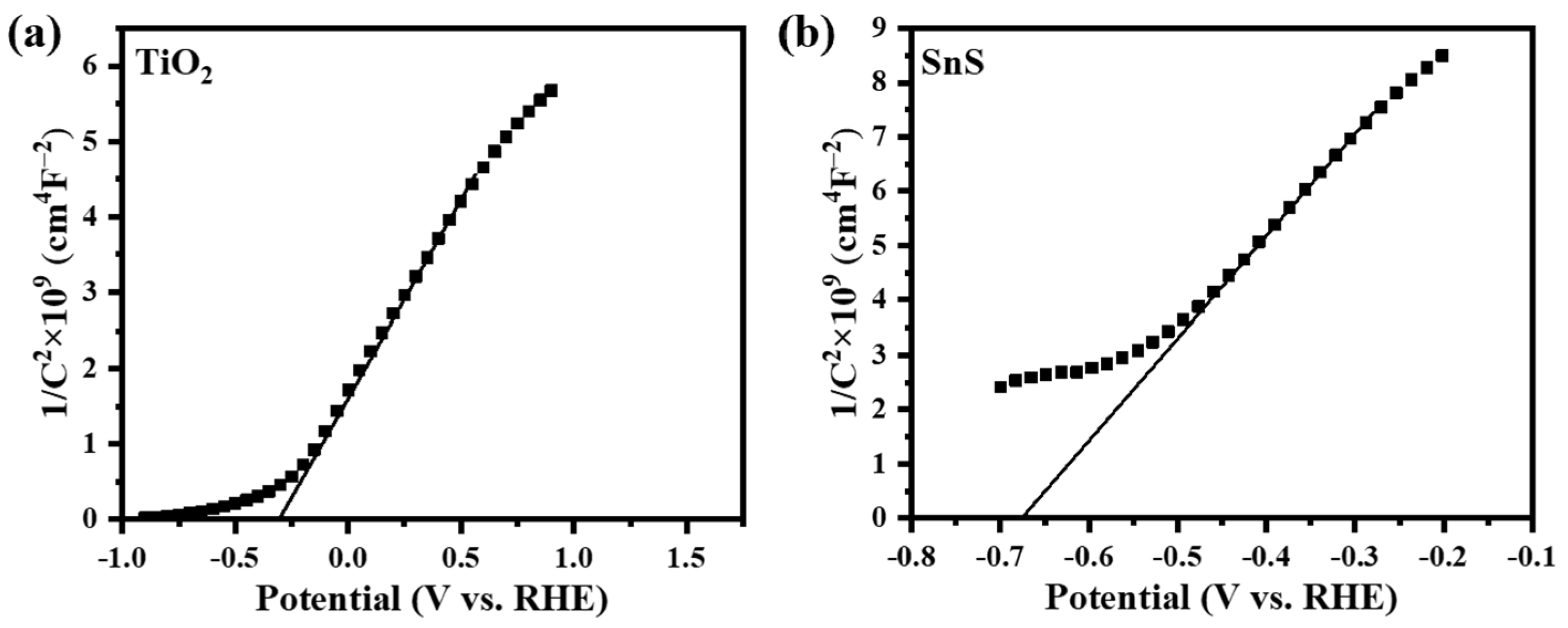
| Samples | k | R2 |
|---|---|---|
| SnS/TiO2 | 0.0170 | 0.9967 |
| SnS | 0.0121 | 0.9932 |
| TiO2 | 0.0104 | 0.9910 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Leng, J.; Zhang, M.; Shen, J. Photoelectrochemical and Photocatalytic Properties of SnS/TiO2 Heterostructure Thin Films Prepared by Magnetron Sputtering Method. Inorganics 2025, 13, 208. https://doi.org/10.3390/inorganics13070208
Ding Y, Leng J, Zhang M, Shen J. Photoelectrochemical and Photocatalytic Properties of SnS/TiO2 Heterostructure Thin Films Prepared by Magnetron Sputtering Method. Inorganics. 2025; 13(7):208. https://doi.org/10.3390/inorganics13070208
Chicago/Turabian StyleDing, Yaoxin, Jiahao Leng, Mingyang Zhang, and Jie Shen. 2025. "Photoelectrochemical and Photocatalytic Properties of SnS/TiO2 Heterostructure Thin Films Prepared by Magnetron Sputtering Method" Inorganics 13, no. 7: 208. https://doi.org/10.3390/inorganics13070208
APA StyleDing, Y., Leng, J., Zhang, M., & Shen, J. (2025). Photoelectrochemical and Photocatalytic Properties of SnS/TiO2 Heterostructure Thin Films Prepared by Magnetron Sputtering Method. Inorganics, 13(7), 208. https://doi.org/10.3390/inorganics13070208





