Bimetallic Ir-Sn Non-Carbon Supported Anode Catalysts for PEM Water Electrolysis
Abstract
1. Introduction
2. Results and Discussion
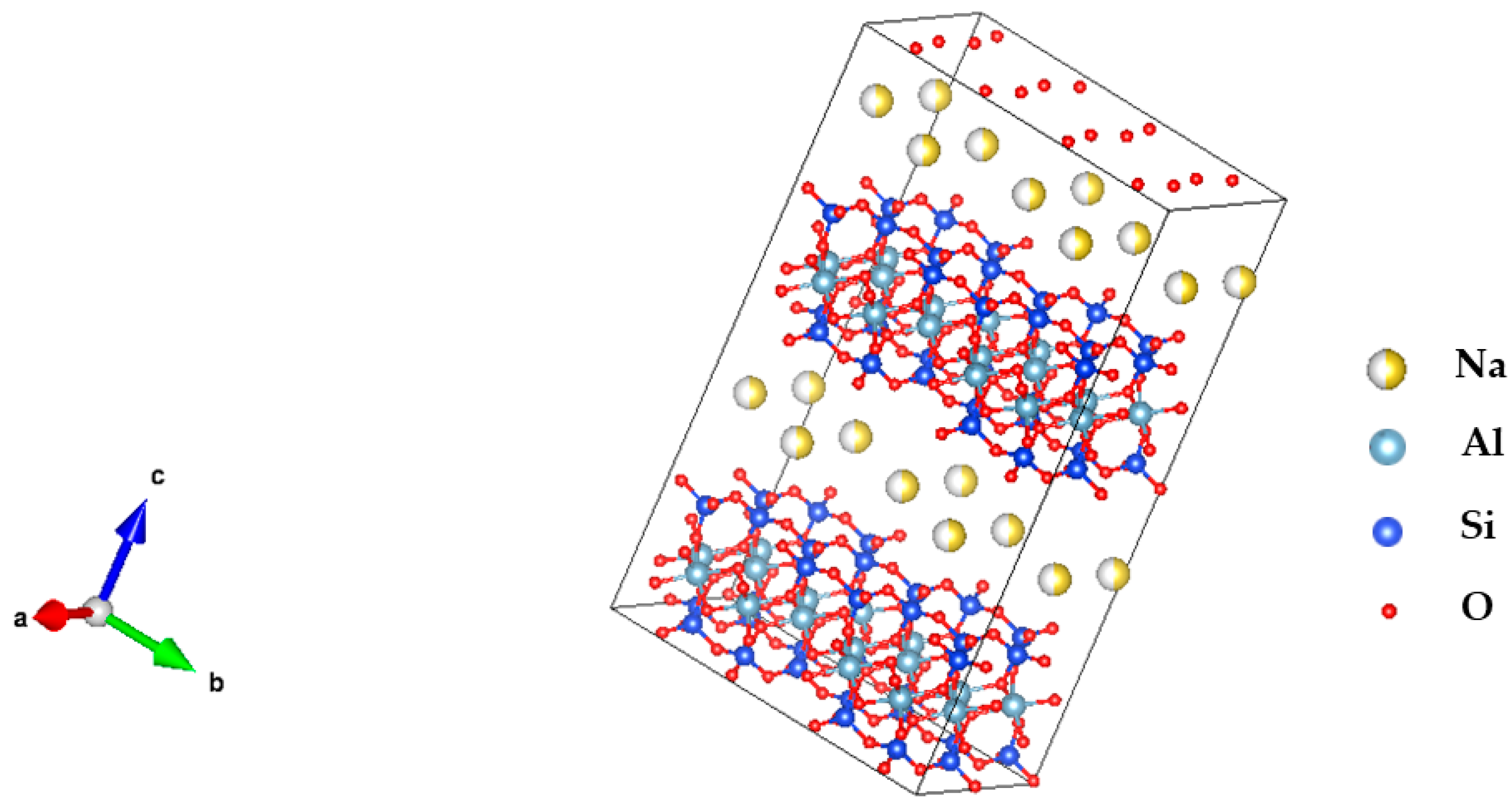
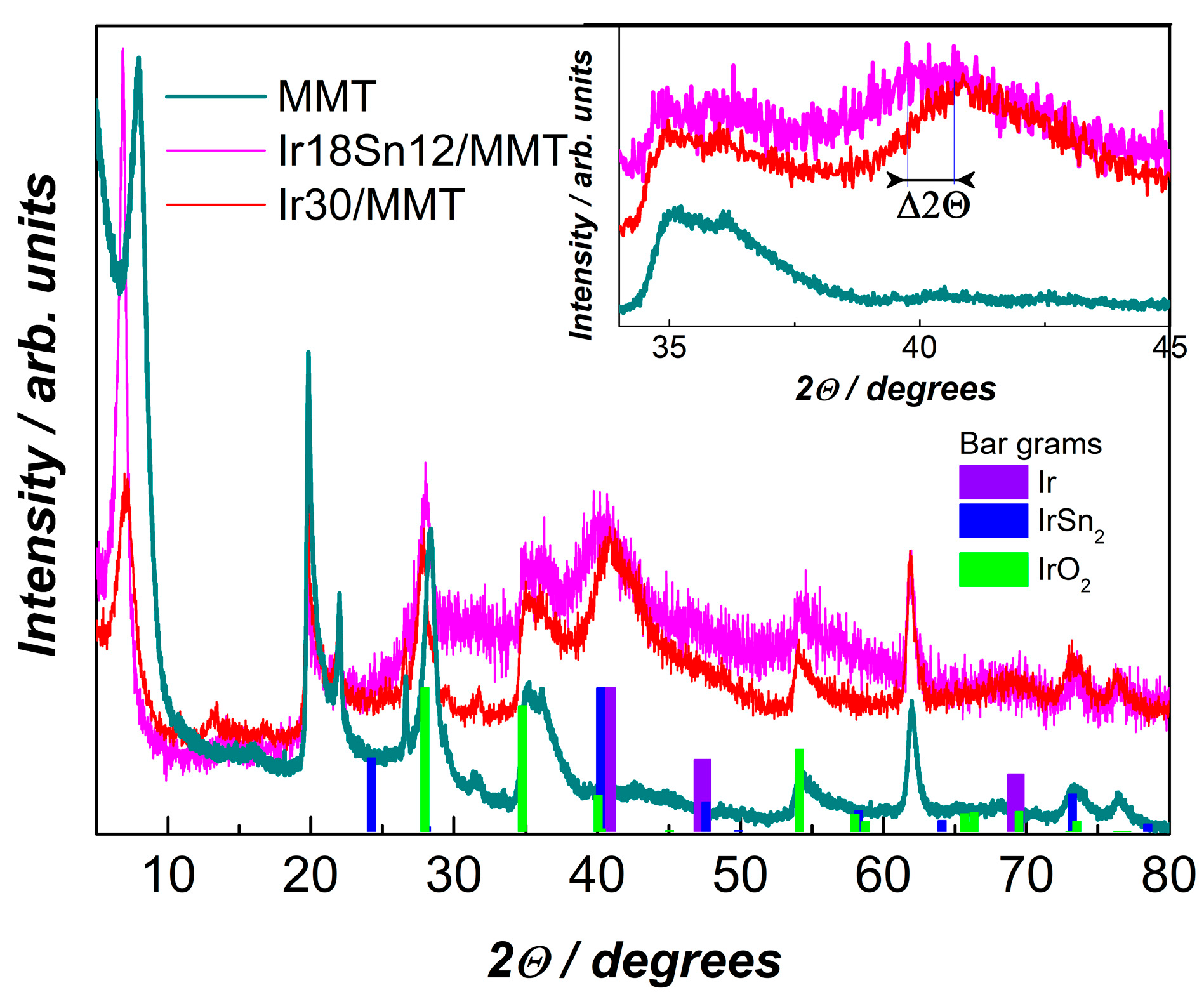
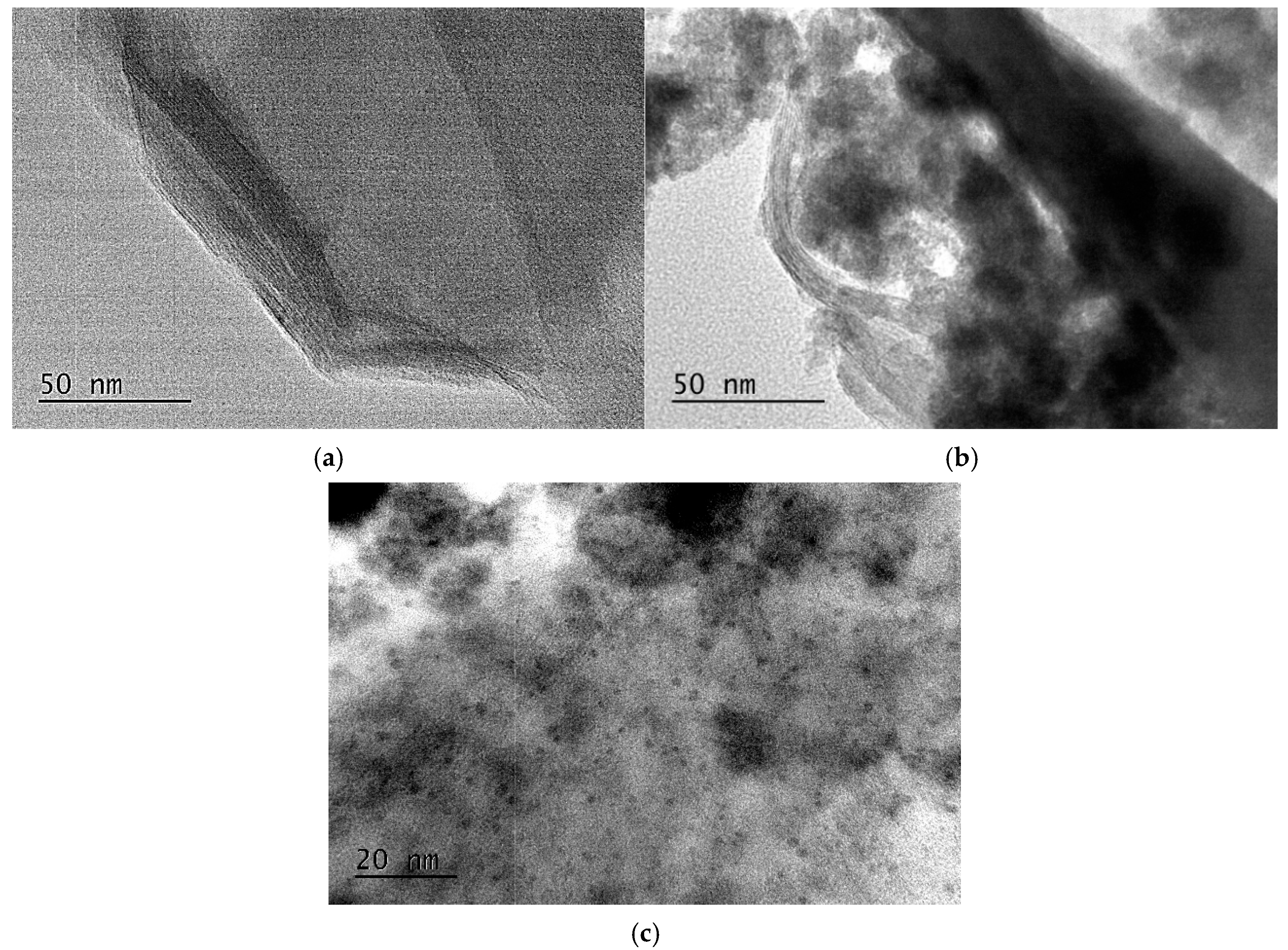
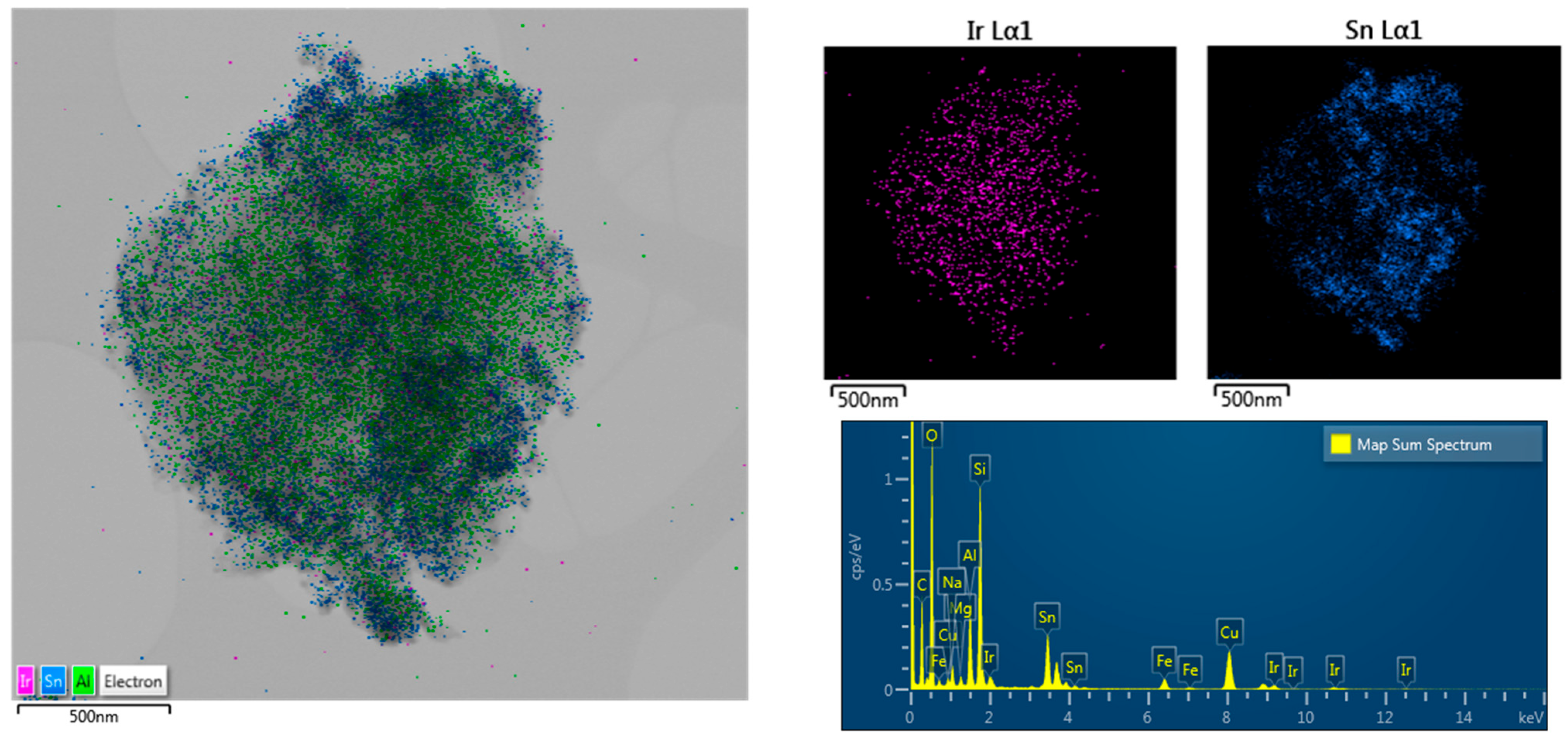
2.1. Structural Characterization
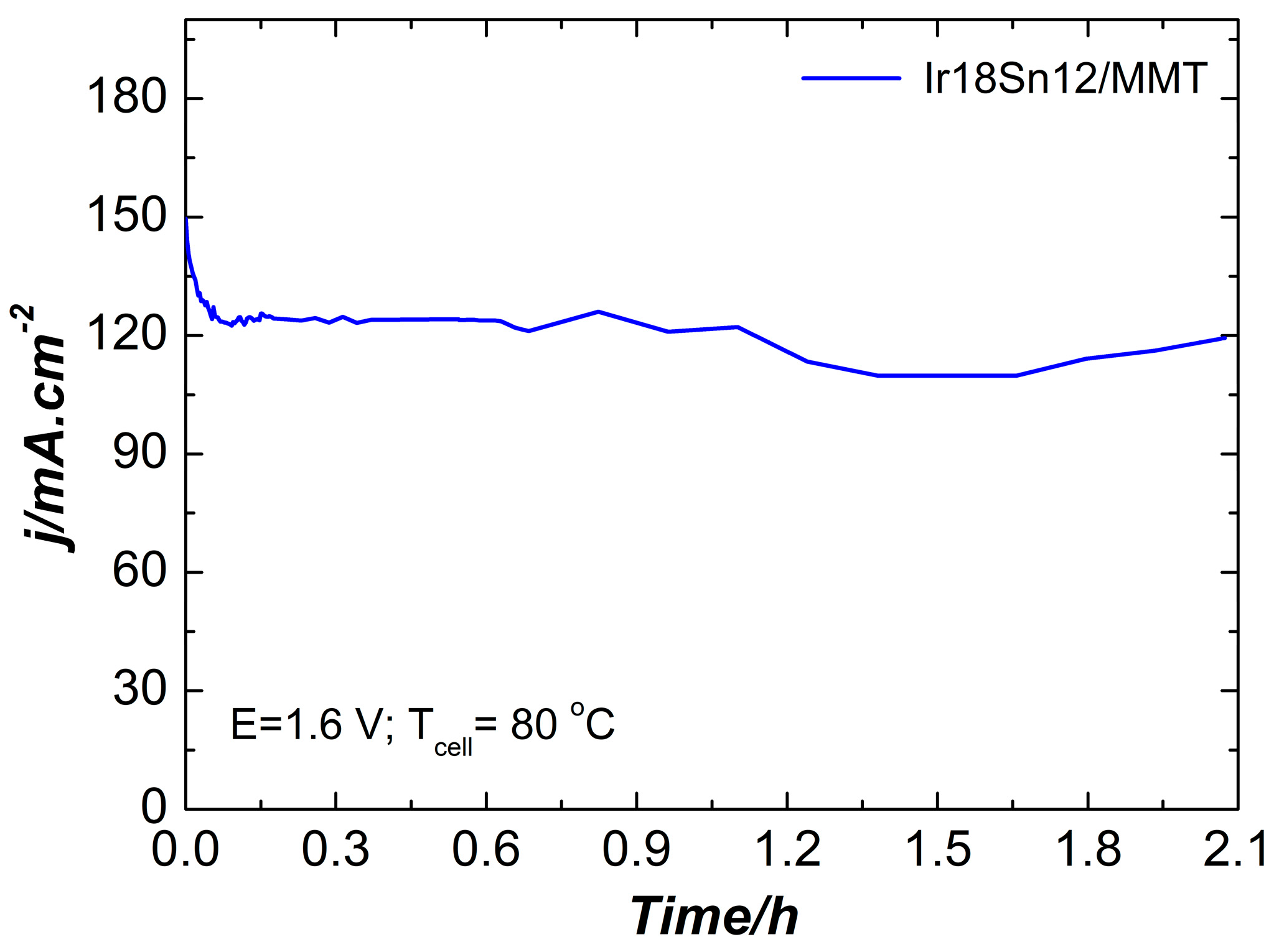
2.2. Electrochemical Characterization
3. Materials and Methods
3.1. Materials
3.2. Synthesis of IrSn/MMT Electrocatalysts
3.3. Physicochemical Characterization of the Electrocatalysts
3.4. Electrochemical Measurements
3.5. Assembly of MEA and PEMEC Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEMEC | Polymer electrolyte membrane electrolysis cell |
| HRTEM | High resolution transmission electron microscopy |
| EDX | Energy-dispersive X-ray spectrometry |
| XRD | X-ray diffraction patterns |
| MEA | Membrane electrode assembly |
| OER | Oxygen evolution reaction |
| RHE | Reference hydrogen electrode |
| PGM | Platinum group metal |
| MMT | Montmorillonite |
| DFT | Density functional theory |
| PAW | Projector augmented wave |
| PBE | Perdew-Burke-Ernzerhof |
| GDE | Gas diffusion electrode |
| OC | Open circuit |
| OCV | Open-circuit voltage |
| LSV | Linear sweep voltammetry |
| CV | Cyclic voltammograms |
References
- Li, G.; Yu, H.; Wang, X.; Sun, S.; Li, Y.; Shao, Z.; Yi, B. Highly effective IrxSn1-xO2 electrocatalysts for oxygen evolution reaction in the solid polymer electrolyte water electrolyser. Phys. Chem. Chem. Phys. 2013, 15, 2858–2866. [Google Scholar] [CrossRef]
- Wang, C.; Lan, F.; He, Z.; Xie, X.; Zhao, Y.; Hou, H.; Guo, L.; Vignesh, M.; Shao, Q.; Liu, H.; et al. Iridium-based Catalysts for Solid Polymer Electrolyte Electrocatalytic Water Splitting. ChemSusChem 2019, 12, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Mao, W.; Jin, L.; Shi, W.; Niu, W.; Wei, C.; He, Y.; Yan, Q.; Wang, R.; Li, Y.; et al. Enhancing heterointerface coupling for durable industrial-level proton exchange membrane water electrolysis. Angew. Chem. 2025, 137, e202502250. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Z.; Tong, Y.; Lin, C.; Zhang, N.; Chen, P. Interfacial bridge bonds induced strong electronic coupling of Co@V-WOx catalyst for enhanced concurrent co-electrolysis performance. Adv. Funct. Mater. 2025, 2502104. [Google Scholar] [CrossRef]
- Lin, H.Y.; Li, W.J.; Lin, M.Y.; Xu, H.G.; Fang, S.R.; Lv, Y.; Li, W.; Guo, J.; Fu, H.Q.; Yuan, H.Y.; et al. Leaching-induced Ti trapping stabilizes amorphous IrOx for proton exchange membrane water electrolysis. Angew. Chem. 2025, 137, e202504212. [Google Scholar]
- Song, S.; Zhang, H.; Ma, X.; Shao, Z.; Baker, R.T.; Yi, B. Electrochemical Investigation of Electrocatalysts for the Oxygen Evolution Reactionin PEM Water Electrolyzers. Int. J. Hydrogen Energy 2018, 33, 4955–4961. [Google Scholar] [CrossRef]
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered Iridium Oxide Films as Electrocatalysts for Water Splitting Via PEM Electrolysis. Electrochim. Acta 2007, 52, 3889–3894. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 4, 483–530. [Google Scholar] [CrossRef]
- Ortel, E.; Reier, T.; Strasser, P.; Kraehnert, R. Mesoporous IrO2 Films Templated by PEO-PB-PEO Block-Copolymers: Self-Assembly, Crystallization Behavior, and Electrocatalytic Performance. Chem. Mater. 2011, 23, 3201–3209. [Google Scholar] [CrossRef]
- Li, G.; Yu, H.; Wang, X.; Yang, D.; Li, Y.; Shao, Z.; Yi, B. Triblock Polymer Mediated Synthesis of Ir-Sn Oxide Electrocatalysts for Oxygen Evolution Reaction. J. Power Sources 2014, 249, 175–184. [Google Scholar] [CrossRef]
- Ardizzone, S.; Trasatti, S. Interfacial Properties of Oxides with Technological Impact in Electrochemistry. Adv. Colloid Interface Sci. 1996, 64, 173–251. [Google Scholar] [CrossRef]
- Mazur, P.; Polonsky, J.; Paidar, M.; Bouzek, K. Non-conductive TiO2 as the Anode Catalyst Support for PEM Water Electrolysis. Int. J. Hydrogen Energy 2012, 37, 12081–12088. [Google Scholar] [CrossRef]
- Wu, X.; Scott, K. RuO2 Supported on Sb-doped SnO2 Nanoparticles for Polymer Electrolyte Membrane Water Electrolysers. Int. J. Hydrogen Energy 2011, 36, 5806–5810. [Google Scholar] [CrossRef]
- Marshall, A.; Børresen, B.; Hagen, H.; Tsypkin, M.; Tunold, R. Electrochemical Characterisation of IrxSn1−xO2 Powders as Oxygen Evolution Electrocatalysts. Electrochim. Acta 2006, 51, 3161–3167. [Google Scholar] [CrossRef]
- Chen, G.H.; Chen, X.M.; Yue, P.L. Electrochemical Behavior of Novel Ti/IrOx−Sb2O5−SnO2 Anodes. J. Phys. Chem. B 2002, 106, 4364–4369. [Google Scholar] [CrossRef]
- Marshall, A.; Haverkamp, R. Electrocatalytic Activity of IrO2–RuO2 Supported on Sb-Doped SnO2 Nanoparticles. Electrochim. Acta 2010, 55, 1978–1984. [Google Scholar] [CrossRef]
- Di Blasi, A.; D’Urso, C.; Baglio, V.; Antonucci, V.; Arico, A.; Ornelas, R.; Matteucci, F.; Orozco, G.; Beltran, D.; Meas, Y.; et al. Preparation and Evaluation of RuO2–IrO2, IrO2–Pt and IrO2–Ta2O5 Catalysts for the Oxygen Evolution Reaction in an SPE Electrolyzer. J. Appl. Electrochem. 2009, 39, 191–196. [Google Scholar] [CrossRef]
- Jiang, G.; Yu, H.; Hao, J.; Chi, J.; Fan, Z.; Yao, D.; Qin, B.; Shao, Z. An Effective Oxygen Electrode Based on Ir0.6Sn0.4O2 for PEM Water Electrolyzers. J. Energy Chem. 2019, 39, 23–28. [Google Scholar] [CrossRef]
- Felix, C.; Maiyalagan, T.; Pasupathi, S.; Bladergroen, B.; Linkov, V. Synthesis. Characterisation and Evaluation of IrO2 Based Binary Metal Oxide Electrocatalysts for Oxygen Evolution Reaction. Int. J. Electrochem. Sci. 2012, 7, 12064–12077. [Google Scholar] [CrossRef]
- Boshnakova, I.; Lefterova, E.; Slavcheva, E. Investigation of Montmorillonite as Carrier for OER. Int. J. Hydrogen Energy 2018, 43, 16897–16904. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Wang, K.; Zhang, C.; Song, Y.; Chen, H.; Xu, Y. Hydrogen Production By Chemical Looping Steam Reforming of Ethanol Using NiO/Montmorillonite Oxygen Carriers in a Fixed-Bed Reactor. Chem. Eng. J. 2016, 298, 96–106. [Google Scholar] [CrossRef]
- Martín, Z.; Jiménez, I.; Gómez, M.A.; Ade, H.W.; Kilcoyne, D.A.; Hernádez-Cruz, D. Spectro microscopy Study of Intercalation and Exfoliation in Polypropylene/Montmorillonite Nanocomposites. J. Phys. Chem. B 2009, 113, 11160–11165. [Google Scholar] [CrossRef] [PubMed]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksata, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Slavcheva, E.; Borisov, G.; Lefterova, E.; Petkucheva, E.; Boshnakova, I. Ebonex Supported Iridiumas Anode Catalystfor PEM Water Electrolysis. Int. J. Hydrogen Energy 2015, 40, 11356–11361. [Google Scholar] [CrossRef]
- Fukushima, Y.; Inagaki, S. Synthesis of an Intercalated Compound of Montmorillonite and 6-Polyamide. J. Incl. Phenom. 1987, 5, 473–482. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, J. Cation Exchange, Interlayer Spacing, and Thermalanalysis of Na/Ca-Montmorillonite Modified with Alkaline and Alkalineearth Metal Ions. J. Therm. Anal. Calorim. 2012, 110, 1199–1206. [Google Scholar] [CrossRef]
- Lanfgord, J.; Wilson, A.A. Survey and some New results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar]
- Burke, L.; Whelan, D.A. Voltammetric Investigation of the Charge Storage Reactions of Hydrous Iridium Oxide Layers. J. Electroanal. Chem. Interfacial Electrochem. 1984, 162, 121–141. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, H. Oxidized Iridium Nanodendrites as Catalysts for Oxygen Evolution Reactions. Catal. Commun. 2011, 12, 408–411. [Google Scholar] [CrossRef]
- Bonanno, M.; Müller, K.; Bensmann, B.; Hanke-Rauschenbach, R.; Aili, D.; Franken, T.; Chromik, A.; Peach, R.; Freiberg, A.T.S.; Thiele, S.; et al. Review and Prospects of PEM Water Electrolysis at Elevated Temperature Operation. Adv. Mater. Technol. 2024, 9, 2300281. [Google Scholar] [CrossRef]

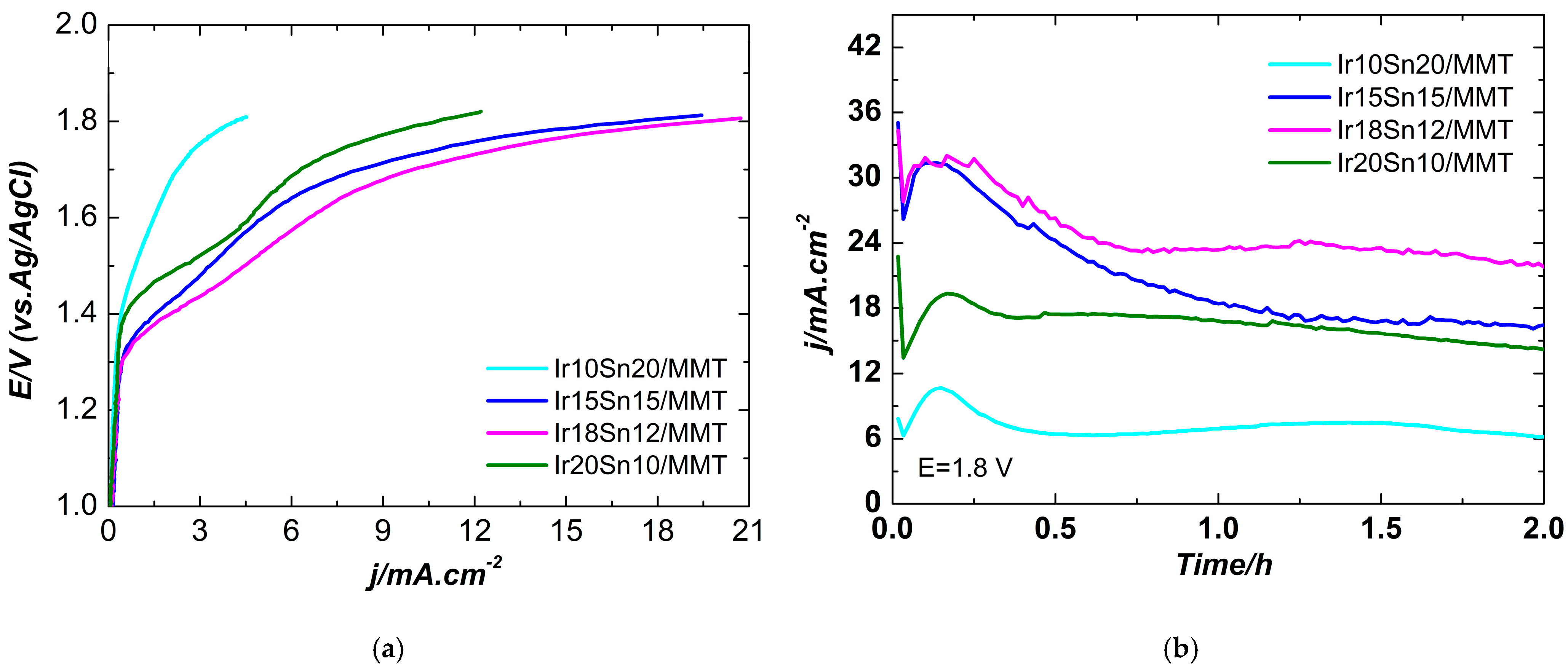
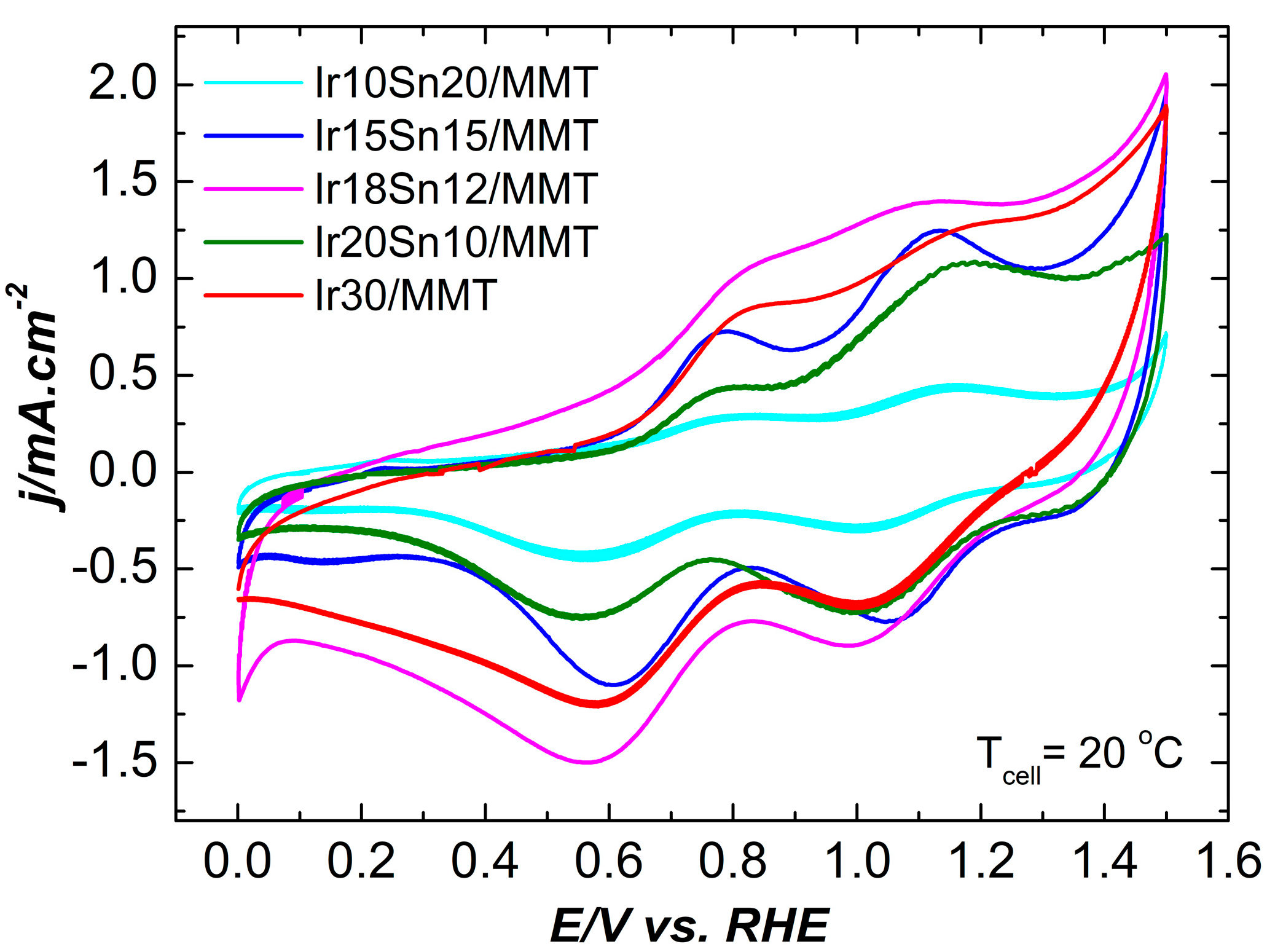
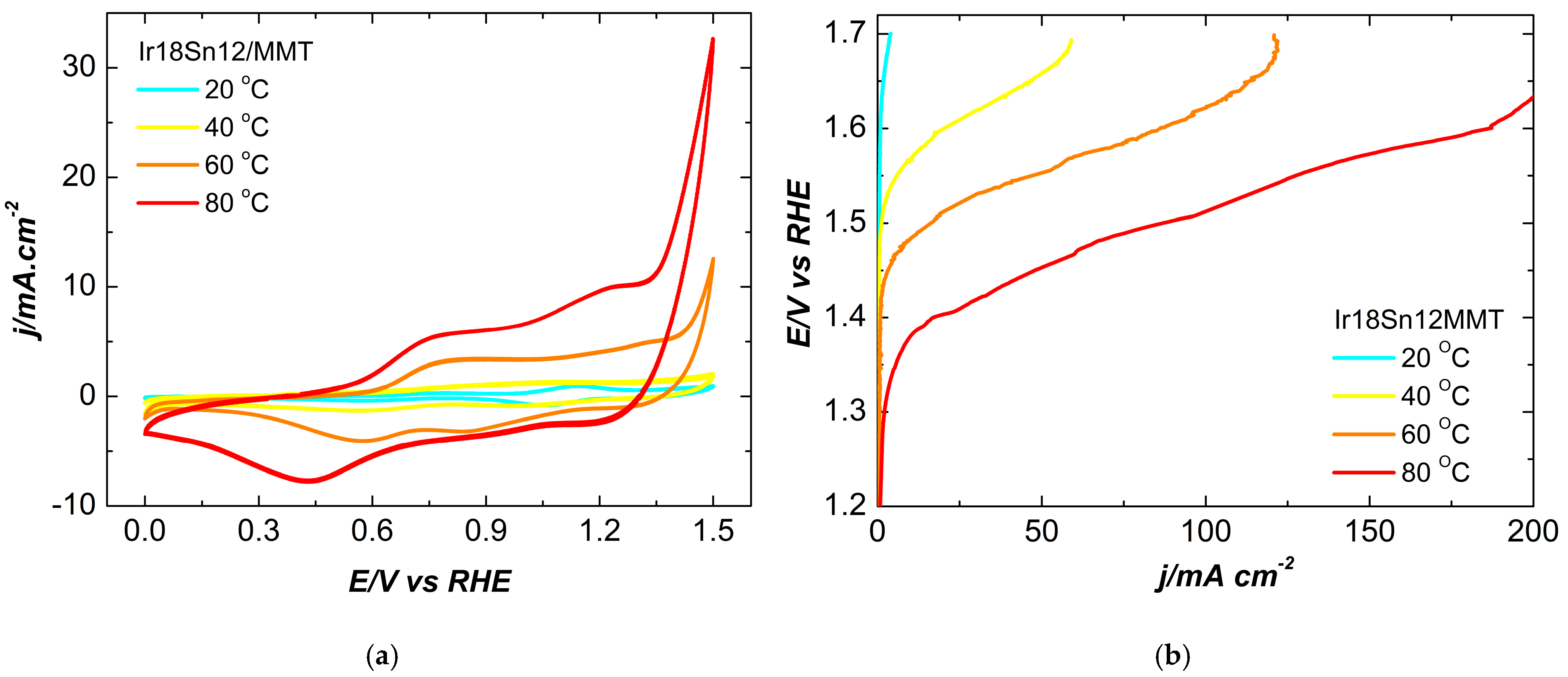
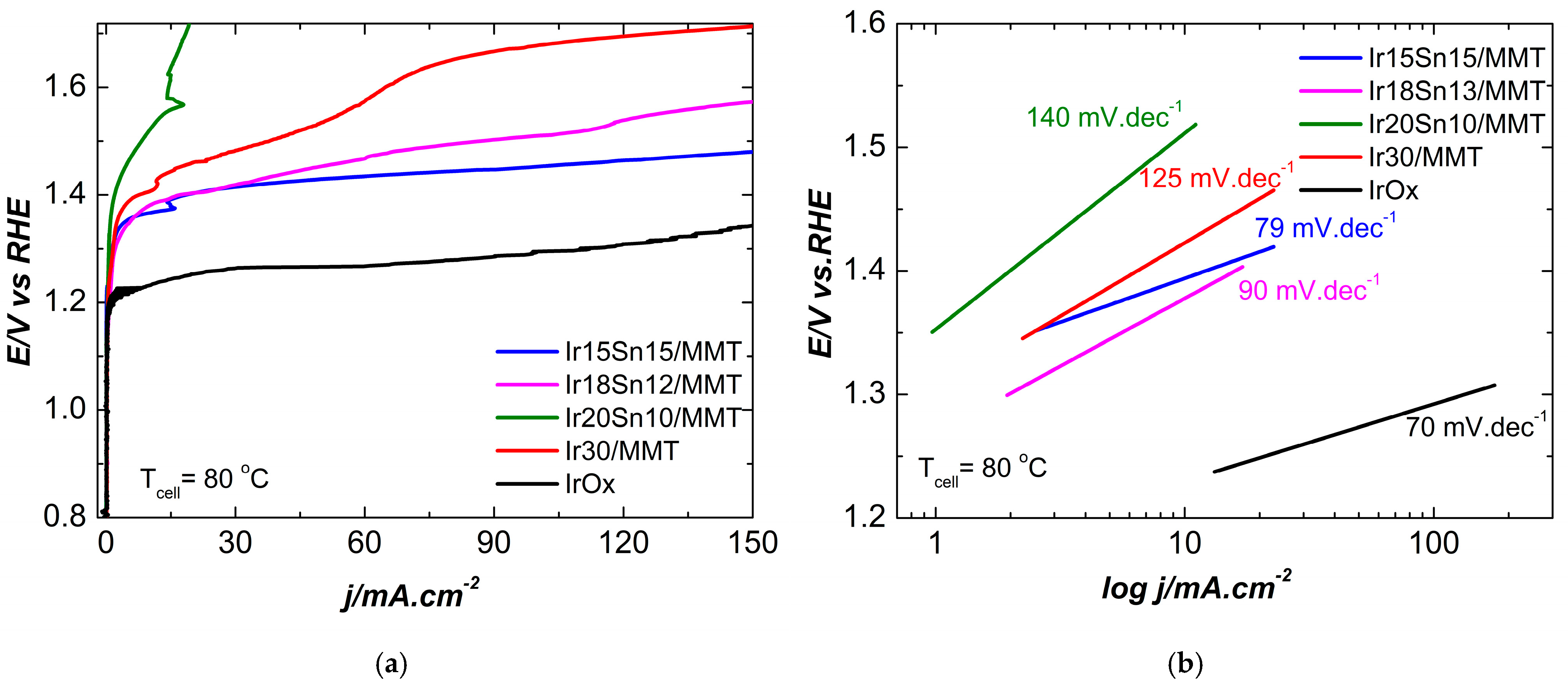
| Temperature | 20 °C | 80 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| Samples | ƞ/mV at j = 1 mA.cm−2 | E(OER) V at j = 1 mA.cm−2 | Tafel Slope mV.dec−1 | j/mA at 1.8 V | ƞ/mV at j = 10 mA.cm−2 | E(OER) V at j = 10 mA.cm−2 | Tafel Slope mV.dec−1 | j/mA at 1.6 V |
| Ir15Sn15/MMT | 407 | 1.63 | 120 | 12.3 | 164 | 1.39 | 79 | 240 |
| Ir18Sn12/MMT | 344 | 1.57 | 180 | 12.8 | 148 | 1.37 | 90 | 220 |
| Ir20Sn10/MMT | 390 | 1.62 | 170 | 8.1 | 290 | 1.52 | 140 | 14 |
| Ir30/MMT | --- | --- | 240 | 6.9 | 194 | 1.42 | 125 | 65 |
| IrOx 250 nm | 0 | 1.23 | 70 | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boshnakova, I.; Lefterova, E.; Borisov, G.; Paskalev, D.; Slavcheva, E. Bimetallic Ir-Sn Non-Carbon Supported Anode Catalysts for PEM Water Electrolysis. Inorganics 2025, 13, 210. https://doi.org/10.3390/inorganics13070210
Boshnakova I, Lefterova E, Borisov G, Paskalev D, Slavcheva E. Bimetallic Ir-Sn Non-Carbon Supported Anode Catalysts for PEM Water Electrolysis. Inorganics. 2025; 13(7):210. https://doi.org/10.3390/inorganics13070210
Chicago/Turabian StyleBoshnakova, Iveta, Elefteria Lefterova, Galin Borisov, Denis Paskalev, and Evelina Slavcheva. 2025. "Bimetallic Ir-Sn Non-Carbon Supported Anode Catalysts for PEM Water Electrolysis" Inorganics 13, no. 7: 210. https://doi.org/10.3390/inorganics13070210
APA StyleBoshnakova, I., Lefterova, E., Borisov, G., Paskalev, D., & Slavcheva, E. (2025). Bimetallic Ir-Sn Non-Carbon Supported Anode Catalysts for PEM Water Electrolysis. Inorganics, 13(7), 210. https://doi.org/10.3390/inorganics13070210







