Photocurrent, Photodegradation, and Proton Conductivity of the Stable Dipyridyl and Thiophene-Functionalized CuII2 Supramolecular Compound
Abstract
1. Introduction
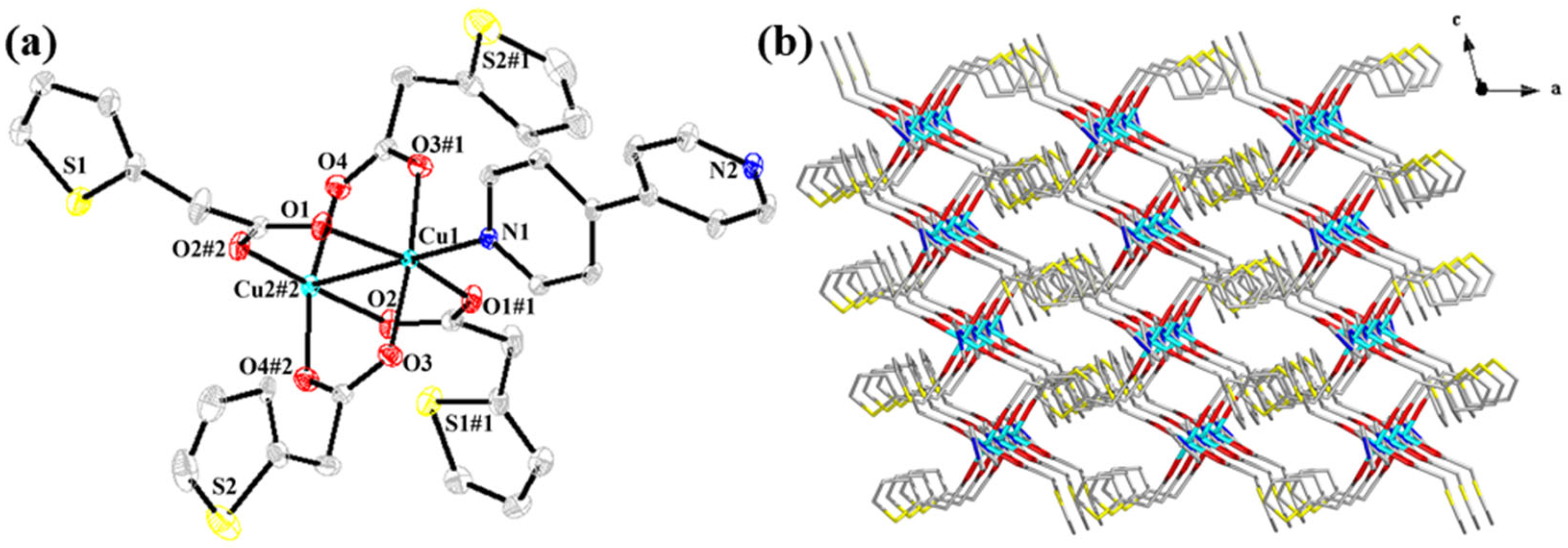
2. Results and Discussion
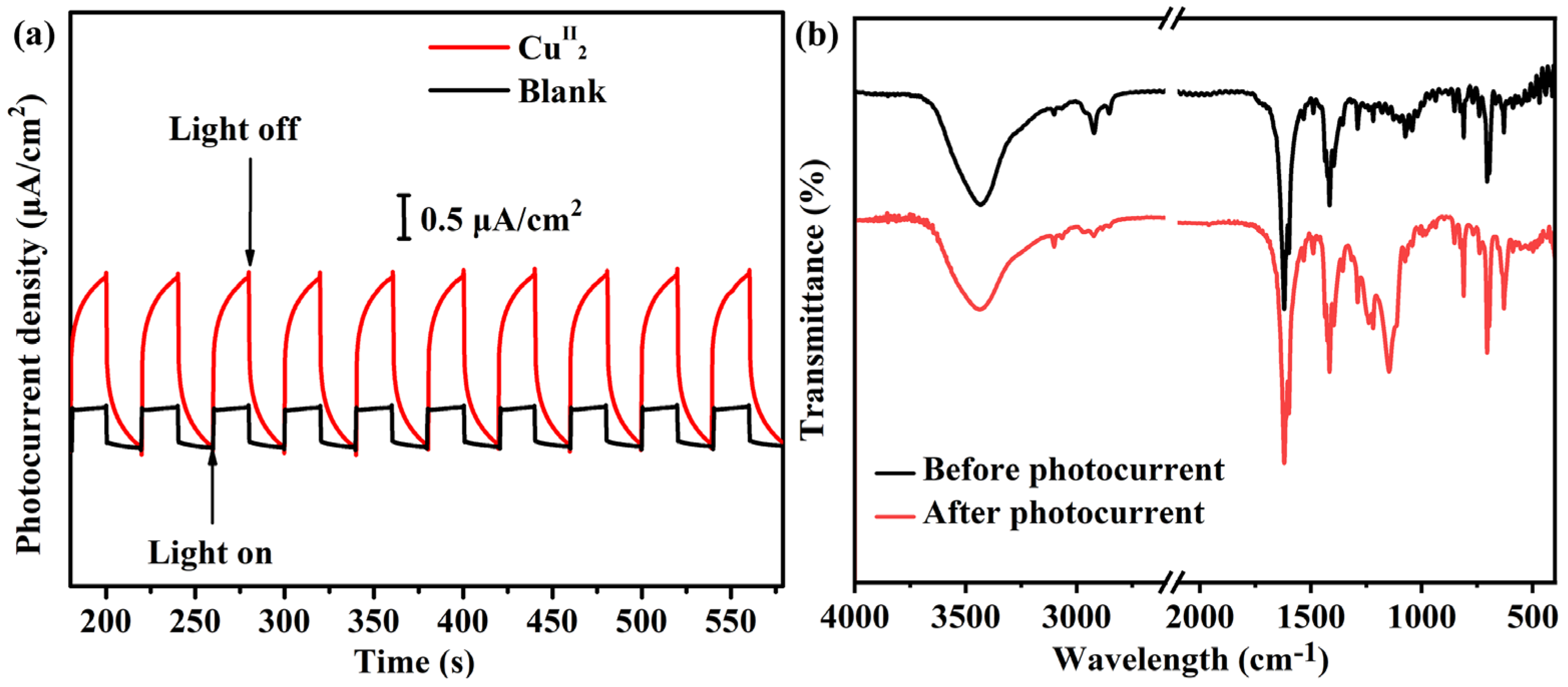
2.1. Photocurrent Response
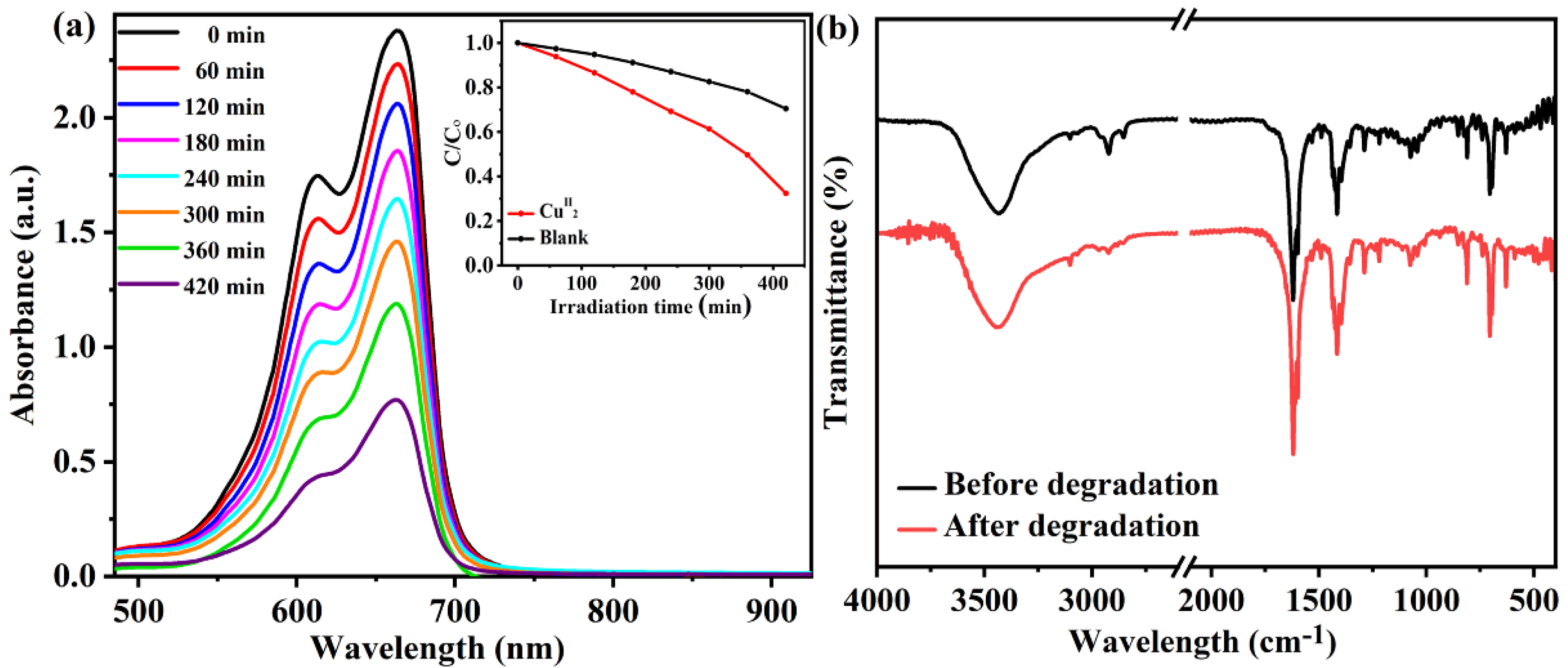
2.2. Photodegradation
2.3. Proton Conductivity
3. Materials and Methods
3.1. Material and Instruments
3.2. Photocurrent Measurement
3.3. Photodegradation Measurement
3.4. Proton Conductivity Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.T.; Li, J.; Qian, C.G.; Luo, X.P.; Wang, K.K.; Zhao, P.X.; Sun, M.G. A multifunctional ternary Cu(II)-carboxylate coordination polymeric nanocomplex for cancer thermochemotherapy. Int. J. Pharmaceut. 2018, 549, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Yang, P.; Wang, R.; Unruangsri, J.; Yang, W.L.; Wang, C.C.; Guo, J. Stable Radical Cation-Containing Covalent Organic Frameworks Exhibiting Remarkable Structure-Enhanced Photothermal Conversion. J. Am. Chem. Soc. 2019, 141, 14433–14442. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Q.; Zhao, M.; Bi, L.-Y.; Hu, Y.-Q.; Gou, G.Y.; Li, J.; Zheng, Y.-Z. Two-Dimensional Silver(I)-Dithiocarboxylate Coordination Polymer Exhibiting Strong Near-Infrared Photothermal Effect. Inorg. Chem. 2019, 58, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Jiang, Z.P.; Li, A.; Chen, X.H.; Ma, Z.K.; Song, H.H. Cu-based MOF-derived porous carbon with highly efficient photothermal conversion performance for solar steam evaporation. J. Mater. Chem. A 2021, 9, 16805–16813. [Google Scholar] [CrossRef]
- Li, W.; Tao, C.-C.; Tang, J.-P.; Zhong, S.-L. Cu-Modified La2Si2O7/TiO2 composite materials: Preparation, characterization and photothermal properties. Dalton Trans. 2022, 51, 12192–12197. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zeng, H.; Zeng, G.; Ru, C.Y.; Li, G.H.; Yan, W.F.; Shi, Z.; Feng, S.H. Multivariate Synergistic Flexible Metal-Organic Frameworks with Superproton Conductivity for Direct Methanol Fuel Cells. Angew. Chem. Int. Ed. 2021, 60, 26577–26581. [Google Scholar] [CrossRef]
- Lan, Q.; Jin, S.-J.; Wang, Z.; Li, X.-Y.; Xiong, Y.; Wang, Z.-C.; Liu, S.-S.; Zhang, Z.-M.; Zhao, Q. Design and synthesis of polyoxovanadate-based framework for efficient dye degradation. Tungsten 2024, 6, 447–453. [Google Scholar] [CrossRef]
- Mohanty, A.; Singh, U.P.; Ghorai, A.; Banerjee, S.; Butcher, R.J. Metal-organic frameworks derived from a semi-rigid anthracene-based ligand and sulfonates: Proton conductivity and dye degradation studies. CrystEngComm 2021, 23, 684–693. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Li, Y.-L.; Zhang, Z.-H.; Li, Z.-F.; Xiao, B.; Li, G. A path to improve proton conductivity: From a 3D hydrogen-bonded organic framework to a 3D copper-organic framework. New J. Chem. 2019, 43, 10637–10644. [Google Scholar] [CrossRef]
- Lian, Y.-X.; Liu, S.-S.; Sun, J.-J.; Luo, P.; Dong, X.-Y.; Liu, X.-F.; Zang, S.-Q. Post-synthesis functionalization of ZIF-90 with sulfonate groups for high proton conduction. Dalton Trans. 2022, 51, 14054–14058. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Z.; Liu, S.-S.; Zhang, H.-J.; Han, Z.; Zhao, G.; Dong, X.-Y.; Zang, S.-Q. Dual-Functional Proton-Conducting and pH-Sensing Polymer Membrane Benefiting from a Eu-MOF. ACS Appl. Mater. Interfaces 2020, 12, 28720–28726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Yuan, J.X.; Fang, Z.Y.; Huang, S.H.; Chen, Z.Y.; Qiu, F.; Lu, C.B.; Zhu, J.H.; Zhuang, X.D. One-dimensional coordination polymers based on metal–nitrogen linkages. Coord. Chem. Rev. 2022, 471, 214735. [Google Scholar] [CrossRef]
- Zhu, L.-G.; Kitagawa, S.; Miyasaka, H.; Chang, H.-C. Syntheses and crystal structures of three one-dimensional copper(II) complexes constructed by salicylate and 4,4′-bipyridine: Ladder, zig-zag, and linear polymeric assembly. Inorg. Chim. Acta 2003, 355, 121–126. [Google Scholar] [CrossRef]
- Si, C.-D.; Hu, D.-C.; Fan, Y.; Dong, X.-Y.; Yao, X.-Q.; Yang, Y.-X.; Liu, J.-C. Three-Dimensional Supramolecular Architectures with CoII Ions Assembled from Hydrogen Bonding and π···π Stacking Interactions: Crystal Structures and Antiferromagnetic Properties. Cryst. Growth Des. 2015, 15, 5781–5793. [Google Scholar] [CrossRef]
- Ferraro, V.; Girotto, M.; Castro, J.; Bortoluzzi, M. Intense millisecond-long red luminescence from heteroleptic Cu(I) 2,1,3-benzothiadiazole complexes. Dye. Pigment. 2023, 217, 111388. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Zhang, M.; Pei, R.-B.; Wang, Q.; Wei, D.-H.; Zang, S.-Q.; Fan, Y.-T.; Mak, T.C.W. A Crystalline Copper(II) Coordination Polymer for the Efficient Visible-Light-Driven Generation of Hydrogen. Angew. Chem. Int. Ed. 2016, 55, 2073–2077. [Google Scholar] [CrossRef]
- Gu, M.-M.; Han, C.-X.; Zhou, K.; Xue, C.-H.; Ji, J.-Y.; Bi, Y.-F. Synthesis, crystal structure and photocurrent response property of a copper(II)-organic supramolecular coordination compound based on [Cu4(phen)4(OH)4(H2O)2]4+ cations and [Cu(EDTA)]2− anions. Inorg. Chim. Acta 2021, 525, 120462. [Google Scholar] [CrossRef]
- Ranjeesh, K.C.; Illathvalappil, R.; Veer, S.D.; Peter, J.; Wakchaure, V.C.; Goudappagouda; Raj, K.V.; Kurungot, S.; Babu, S.S. Imidazole-Linked Crystalline Two-Dimensional Polymer with Ultrahigh Proton-Conductivity. J. Am. Chem. Soc. 2019, 141, 14950–14954. [Google Scholar] [CrossRef]
- Taylor, J.M.; Dawson, K.W.; Shimizu, G.K.H. A water-stable metal-organic framework with highly acidic pores for proton-conducting applications. J. Am. Chem. Soc. 2013, 135, 1193–1196. [Google Scholar] [CrossRef]
- Otsubo, K.; Nagayama, S.; Kawaguchi, S.; Sugimoto, K.; Kitagawa, H. A Preinstalled Protic Cation as a Switch for Superprotonic Conduction in a Metal-Organic Framework. JACS Au 2022, 2, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, H.S.; Aiyappa, H.B.; Bhange, S.N.; Karak, S.; Halder, A.; Kurungot, S.; Banerjee, R. Superprotonic Conductivity in Flexible Porous Covalent Organic Framework Membranes. Angew. Chem. Int. Ed. 2018, 57, 10894–10898. [Google Scholar] [CrossRef] [PubMed]
- Phang, W.J.; Jo, H.; Lee, W.R.; Song, J.H.; Yoo, K.; Kim, B.; Hong, C.S. Superprotonic conductivity of a UiO-66 framework functionalized with sulfonic acid groups by facile postsynthetic oxidation. Angew. Chem. Int. Ed. 2015, 54, 5142–5146. [Google Scholar] [CrossRef] [PubMed]
- Moi, R.; Ghorai, A.; Maity, K.; Banerjee, S.; Biradha, K. Correlation of Structures with Proton Conductivity of 1D Coordination Polymers: Higher Proton Conductivity Due to Synergy of Encapsulated Sulfate Ions and Water Molecules. Cryst. Growth Des. 2022, 22, 7215–7220. [Google Scholar] [CrossRef]
- Yu, H.-G.; Li, B.; Liu, S.; Jiang, C.; Li, Y.-S.; Wu, Y.-P.; Zhao, J.; Li, D.-S. Three new copper(II) coordination polymers constructed from isomeric sulfo-functionalized phthalate tectonics: Synthesis, crystal structure, photocatalytic and proton conduction properties. J. Solid State Chem. 2021, 294, 121860. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Zeng, X.-Y.; Wang, Y.-L.; Liu, Q.-Y. Two coordination polymers constructed from diphenylsulfone-3,3′-disulfo-4,4′-dicarboxylate ligand: Syntheses, structures, and proton conduction. J. Coord. Chem. 2020, 73, 3003–3013. [Google Scholar] [CrossRef]
- Saravanabharathi, D.; Obulichetty, M.; Rameshkumar, S.; Kumaravel, M. Rapid crystallization and proton conductivity of copper (II)-L-tartrate. Synth. Met. 2012, 162, 1519–1523. [Google Scholar] [CrossRef]
- Tayade, S.B.; Lllathvalappil, R.; Lapalikar, V.; Markad, D.; Kurungot, S.; Pujari, B.; Kumbhar, A.S. A copper(ii)-coordination polymer based on a sulfonic–carboxylic ligand exhibits high water-facilitated proton conductivity. Dalton Trans. 2019, 48, 11034–11044. [Google Scholar] [CrossRef]
- Tayade, S.B.; Dhavale, V.M.; Kumbhar, A.S.; Kurungot, S.; Lönnecke, P.; Hey-Hawkins, E.; Pujari, B. Proton conduction in a hydrogen-bonded complex of copper(II)-bipyridine glycoluril nitrate. Dalton Trans. 2017, 46, 6968–6974. [Google Scholar] [CrossRef]
- Liang, G.-M.; Mao, D.-A.; Zhou, Z.-H.; Zhou, K.; Tan, Y.-F.; Ji, J.-Y.; Bi, Y.-F. A stable dipyridyl and thiophene-functionalized copper(II) compound for efficient photo-thermal conversion and boosting photo-thermal synergistic degradation of methyl orange. J. Mol. Struct. 2024, 1316, 139064. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Meng, X.; Wei, M.-J.; Wang, H.-N.; Zang, H.-Y.; Zhou, Z.-Y. Multifunctional luminescent Zn(II)-based metal-organic framework for high proton-conductivity and detection of Cr3+ ions in the presence of mixed metal ions. Dalton Trans. 2018, 47, 1383–1387. [Google Scholar] [CrossRef]
- Liu, L.; Yin, L.Y.; Cheng, D.M.; Zhao, S.; Zang, H.-Y.; Zhang, N.; Zhu, G.S. Surface-Mediated Construction of an Ultrathin Free-Standing Covalent Organic Framework Membrane for Efficient Proton Conduction. Angew. Chem. Int. Ed. 2021, 60, 14875–14880. [Google Scholar] [CrossRef]
- Wang, J.-X.; Wang, Y.-D.; Wei, M.-J.; Tan, H.-Q.; Wang, Y.-H.; Zang, H.-Y.; Li, Y.-G. Inorganic open framework based on lanthanide ions and polyoxometalates with high proton conductivity. Inorg. Chem. Front. 2018, 5, 1213–1217. [Google Scholar] [CrossRef]
- Han, B.-L.; Liu, Z.; Feng, L.; Wang, Z.; Gupta, R.K.; Aikens, C.M.; Tung, C.-H.; Sun, D. Polymorphism in Atomically Precise Cu23 Nanocluster Incorporating Tetrahedral [Cu4]0 Kernel. J. Am. Chem. Soc. 2020, 142, 5834–5841. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, Y.; Wang, H.; Chai, W.X.; Liu, X.F.; Yan, D.G.; Lin, H.; Liu, Y. One-Dimensional Chains in Pentanary Chalcogenides A2Ba3Cu2Sb2S10 (A = K, Rb, Cs) Displaying a Photocurrent Response. Inorg. Chem. 2020, 59, 1577–1581. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Li, L.-J.; Sun, Z.-M. Synthesis, Crystal Structures, and Photochemical Properties of a Family of Heterometallic Titanium Oxo Clusters. Inorg. Chem. 2019, 58, 6312–6319. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.J.; Zhao, W.; Li, C.; Chen, X.D.; Wang, Y.; Zhu, W.H.; Zhong, Q. Influence of metal-porphyrins on the photocatalysis of graphitic carbon nitride. Dye. Pigment. 2018, 153, 241–247. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.W.; Zeng, T.Y.; Shang, Q.G.; Zhou, H.; Pan, Z.Q.; Cheng, Q.G. Two pure MOF-photocatalysts with readily preparation for the degradation of methylene blue dye under visible light. Dalton Trans. 2018, 47, 4251–4258. [Google Scholar] [CrossRef]
- Zhao, D.X.; Cai, C. Cerium-based UiO-66 metal-organic framework for synergistic dye adsorption and photodegradation: A discussion of the mechanism. Dye. Pigment. 2021, 185, 108957. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; Zhou, K.; Liang, G.-M.; Tan, Y.-F.; Ji, J.-Y.; Chen, B.-K.; Bi, Y.-F. Carboxylate Ligand-supported Agn (n=21 and 24) Alkynyl Clusters Induced by Single/Double Carbonate Ions. Chem. Asian J. 2023, 18, e202300041. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.R.; Zhang, Y.Q.; Ma, Y.Y.; Zhao, Q.; Han, Z.G. Hourglass-type polyoxometalate-based crystalline materials as efficient cooperating photocatalysts for the reduction of Cr(VI) and oxidation of dyes. Catal. Sci. Technol. 2020, 10, 2593–2601. [Google Scholar] [CrossRef]
- Kazemi, S.; Teimouri, A.; Salavati, H. Developments of modified magnetic nanoparticle-supported heteropolyacid photocatalytic performances in methylene blue scavenger. J. Chin. Chem. Soc. 2018, 65, 1218–1228. [Google Scholar] [CrossRef]
- Li, H.L.; Huang, L.L.; Lu, Y.; Zhao, S.S.; Shao, X.; Bi, Y.F. A 3D open-framework amino acid templated cerium phosphite-oxalate showing proton conductive property. Solid State Sci. 2024, 157, 107692. [Google Scholar] [CrossRef]
- Wei, M.-J.; Fu, J.-Q.; Wang, Y.-D.; Gu, J.-Y.; Liu, B.-L.; Zang, H.-Y.; Zhou, E.-L.; Shao, K.-Z.; Su, Z.-M. Changes of coordination modes of Cu-based coordination complexes as tuneable proton-conducting solid electrolytes. J. Mater. Chem. A 2017, 5, 1085–1093. [Google Scholar] [CrossRef]
- Ma, H.P.; Liu, B.L.; Li, B.; Zhang, L.M.; Li, Y.-G.; Tan, H.-Q.; Zang, H.-Y.; Zhu, G.S. Cationic Covalent Organic Frameworks: A Simple Platform of Anionic Exchange for Porosity Tuning and Proton Conduction. J. Am. Chem. Soc. 2016, 138, 5897–5903. [Google Scholar] [CrossRef]
- Zhou, E.L.; Qin, C.; Huang, P.; Wang, X.L.; Chen, W.C.; Shao, K.Z.; Su, Z.M. A Stable Polyoxometalate-Pillared Metal-Organic Framework for Proton-Conducting and Colorimetric Biosensing. Chem.-Eur. J. 2015, 21, 11894–11898. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as proton conductors-challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]
- Wang, J.-X.; Liang, S.; Tan, H.Q.; Wang, Y.-H.; Zang, H.-Y.; Li, Y.-G. Construction of Strandberg-Type Polyoxometalate-Based Inorganic-Organic Hybrid Material with Water-Assisted Proton Conductivity. ChemistrySelect 2020, 5, 5883–5888. [Google Scholar] [CrossRef]
- Jiang, Y.S.; Song, J.S.; Xiu, Z.J.; Huang, L.L.; Gao, F.; Jiao, S.F.; Bi, Y.F. Solvent-free syntheses of two pcu topological indium phosphite-oxalates with a novel butterfly motif and proton conductivity. Micropor. Mesopor. Mater. 2019, 289, 109643. [Google Scholar] [CrossRef]

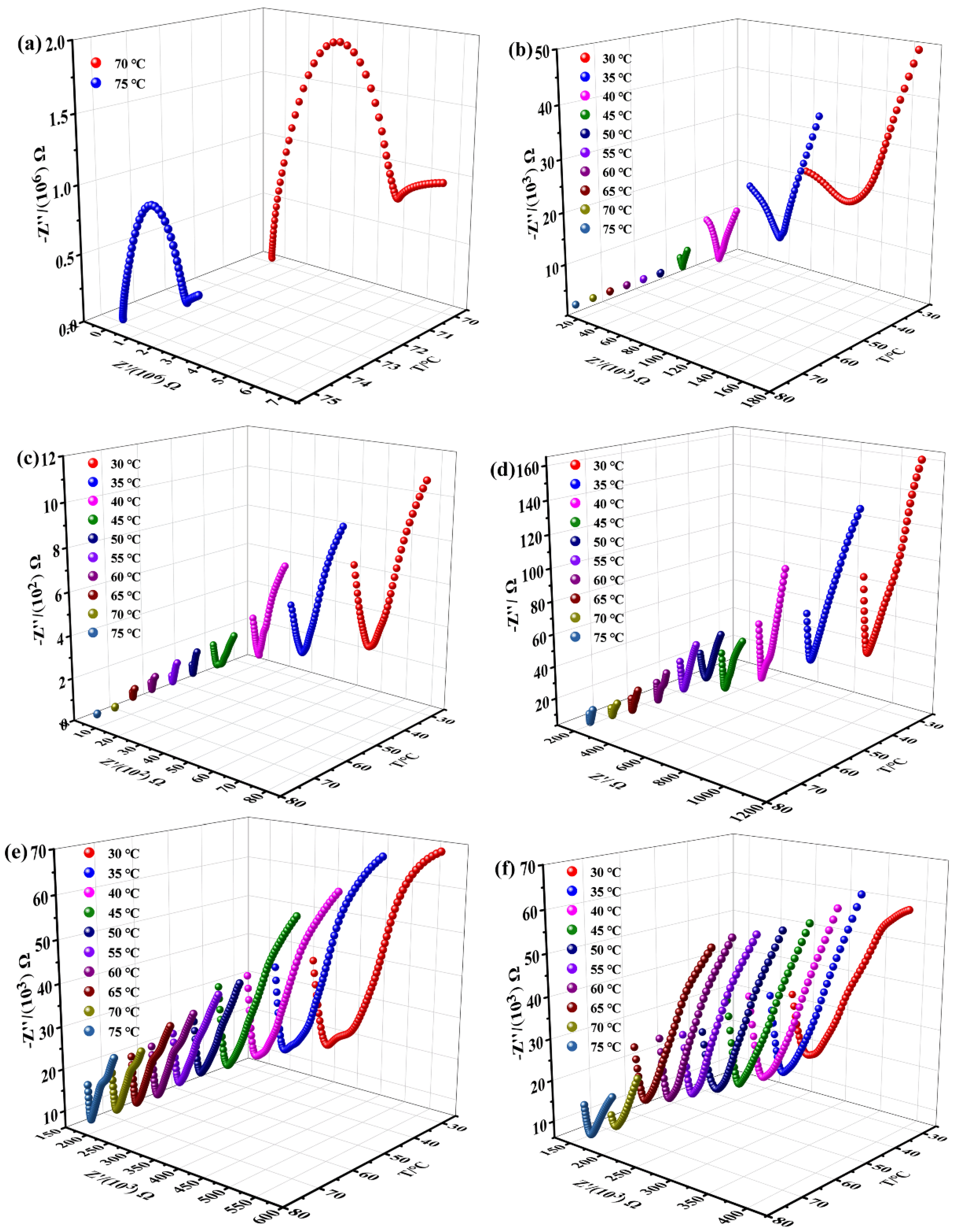
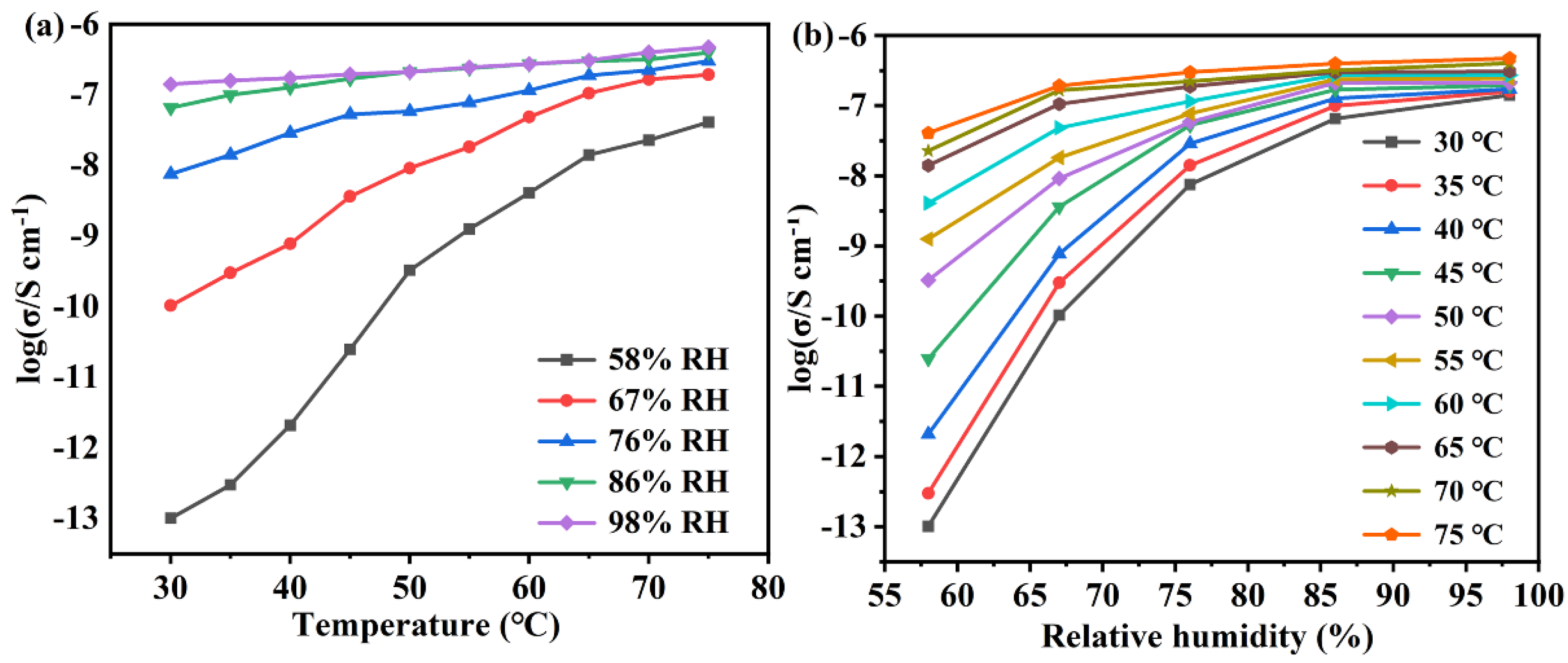
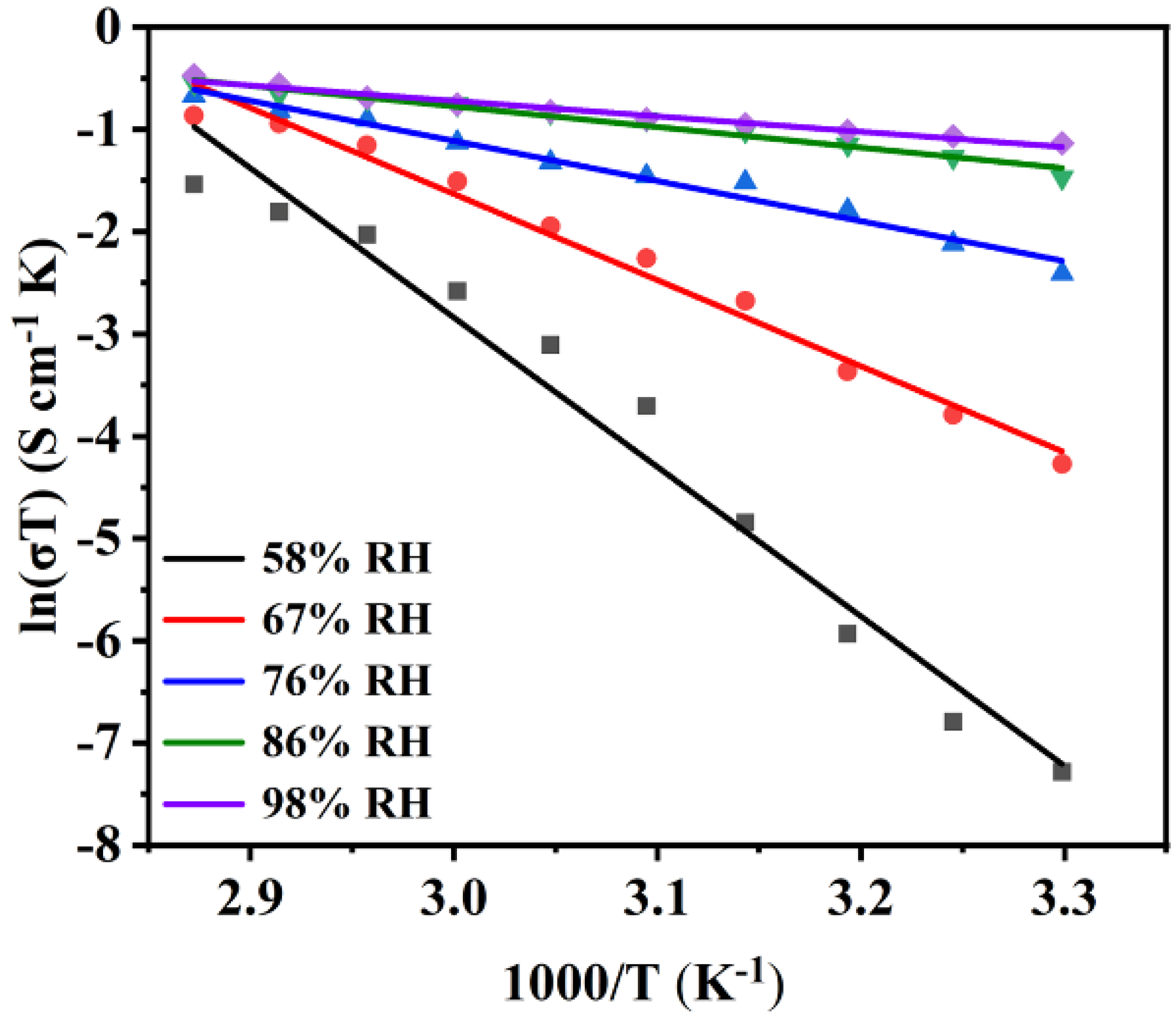
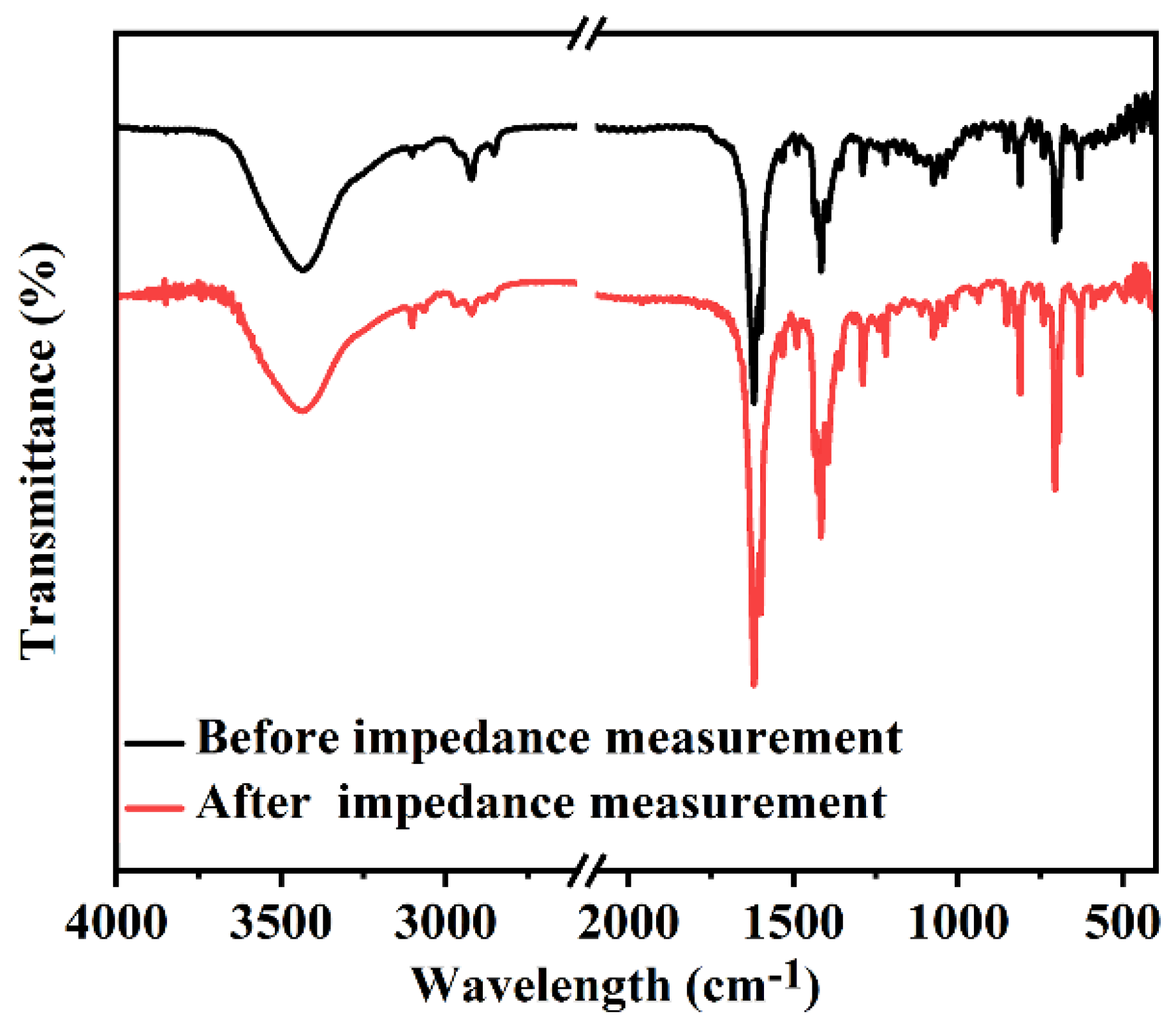
| T/°C | 44% RH | 58% RH | 67% RH | 76% RH | 86% RH | 98% RH |
|---|---|---|---|---|---|---|
| 30 | 2.27 × 10−6 | 4.61 × 10−5 | 2.96 × 10−4 | 7.58 × 10−4 | 1.06 × 10−3 | |
| 35 | 3.64 × 10−6 | 7.31 × 10−5 | 3.90 × 10−4 | 9.11 × 10−4 | 1.11 × 10−3 | |
| 40 | 8.46 × 10−6 | 1.10 × 10−4 | 5.31 × 10−4 | 1.01 × 10−3 | 1.15 × 10−3 | |
| 45 | 2.48 × 10−5 | 2.16 × 10−4 | 6.92 × 10−4 | 1.14 × 10−3 | 1.22 × 10−3 | |
| 50 | 7.61 × 10−5 | 3.23 × 10−4 | 7.20 × 10−4 | 1.26 × 10−3 | 1.26 × 10−3 | |
| 55 | 1.36 × 10−4 | 4.35 × 10−4 | 8.14 × 10−4 | 1.33 × 10−3 | 1.34 × 10−3 | |
| 60 | 2.27 × 10−4 | 6.65 × 10−4 | 9.71 × 10−4 | 1.41 × 10−3 | 1.41 × 10−3 | |
| 65 | 3.88 × 10−4 | 9.31 × 10−4 | 1.20 × 10−3 | 1.47 × 10−3 | 1.48 × 10−3 | |
| 70 | 4.52 × 10−8 | 4.78 × 10−4 | 1.13 × 10−3 | 1.29 × 10−3 | 1.51 × 10−3 | 1.67 × 10−3 |
| 75 | 9.32 × 10−8 | 6.17 × 10−4 | 1.21 × 10−3 | 1.47 × 10−3 | 1.66 × 10−3 | 1.79 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-H.; Liang, G.-M.; Ji, J.-Y.; Gong, X.-J.; Huang, L.-L.; Zhao, L.-P.; Xie, W.-X.; Zhou, K. Photocurrent, Photodegradation, and Proton Conductivity of the Stable Dipyridyl and Thiophene-Functionalized CuII2 Supramolecular Compound. Inorganics 2025, 13, 195. https://doi.org/10.3390/inorganics13060195
Wang J-H, Liang G-M, Ji J-Y, Gong X-J, Huang L-L, Zhao L-P, Xie W-X, Zhou K. Photocurrent, Photodegradation, and Proton Conductivity of the Stable Dipyridyl and Thiophene-Functionalized CuII2 Supramolecular Compound. Inorganics. 2025; 13(6):195. https://doi.org/10.3390/inorganics13060195
Chicago/Turabian StyleWang, Jin-He, Guang-Min Liang, Jiu-Yu Ji, Xiao-Jie Gong, Liang-Liang Huang, Li-Ping Zhao, Wen-Xuan Xie, and Kun Zhou. 2025. "Photocurrent, Photodegradation, and Proton Conductivity of the Stable Dipyridyl and Thiophene-Functionalized CuII2 Supramolecular Compound" Inorganics 13, no. 6: 195. https://doi.org/10.3390/inorganics13060195
APA StyleWang, J.-H., Liang, G.-M., Ji, J.-Y., Gong, X.-J., Huang, L.-L., Zhao, L.-P., Xie, W.-X., & Zhou, K. (2025). Photocurrent, Photodegradation, and Proton Conductivity of the Stable Dipyridyl and Thiophene-Functionalized CuII2 Supramolecular Compound. Inorganics, 13(6), 195. https://doi.org/10.3390/inorganics13060195






