Abstract
This study reports the synthesis and characterization of four novel rare earth-gallic acid complexes, Sm(Gal)3·4H2O, Eu(Gal)3·4H2O, Tb(Gal)3·4H2O, and Dy(Gal)3·4H2O. These complexes were synthesized under optimized conditions (60 °C, pH 4–5) and characterized using the Ln3+ elemental content method, infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), mass spectrometry (MS), and fluorescence spectroscopy. IR spectra confirmed the coordination of rare earth ions (Ln3+) with gallic acid through carboxylate oxygen atoms. TGA revealed the thermal decomposition pathways, while MS identified the molecular ion peaks and fragmentation patterns. All complexes exhibited strong luminescence under UV excitation, with emission peaks corresponding to characteristic transitions of Sm3+, Eu3+, Tb3+, and Dy3+. Biological assays demonstrated significant antimicrobial activity against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, with Dy(Gal)3·4H2O showing the highest efficacy. Additionally, the complexes displayed inhibitory effects on MCF7 breast cancer cells, with Tb(Gal)3·4H2O exhibiting the lowest IC50 value (11.3 µM). These findings suggest that rare earth metal complexes with gallic acid have potential applications in biomedical fields, particularly as antimicrobial and anticancer agents.
1. Introduction
Rare earth metals (REMs) have garnered significant attention in recent years due to their unique optical, magnetic, and catalytic properties [1,2]. Their ability to form stable complexes with organic ligands, such as carboxylic acids, has opened new avenues for applications in materials science, catalysis, and biomedicine [3,4,5,6,7,8,9,10,11,12,13]. The combination of REMs with organic compounds has the potential to enhance these biological activities due to the synergistic effects of the metal ions and the organic ligand [4,5,6,7,8,9,10,11,12,13,14]. Metal complexes of gallic acid, particularly with aluminum and iron, have demonstrated superior antibacterial properties against Escherichia coli ATCC 25922. While gallic acid exhibited bacteriostatic effects at 4.0 μmol/mL, its aluminum and iron complexes showed bactericidal activity at the same concentration [15]. This enhanced activity is attributed to increased pro-oxidative and genotoxic effects, leading to elevated malondialdehyde and hydrogen peroxide levels and higher SOS induction factors in DNA-repair-deficient E. coli strains [15]. The cytotoxic activity of the platinum (II) complex was evaluated against chronic myelogenous leukemia cells (K562) and a non-cancerous cell line (LLCMK2). The complex exhibited significantly higher activity compared to gallic acid, cisplatin, and carboplatin against the K562 cell line, with an impressive IC50 value of 0.75 μM. In antimicrobial assays, the complex also demonstrated notable efficacy against Mycobacterium tuberculosis, with a minimum inhibitory concentration (MIC) of 3.75 μg/mL. These findings further support the antitumor and antimycobacterial potential of metal complexes incorporating gallic acid and its derivatives [14]. Despite these advancements, there are still gaps in the literature regarding the systematic study of REM complexes with gallic acid, particularly their antimicrobial and anticancer properties. Moreover, the thermal stability and structural characterization of these complexes remain underexplored. This study aims to address these gaps by synthesizing and characterizing Sm, Eu, Tb, and Dy complexes with gallic acid and evaluating their biological activities.
2. Results and Discussion
2.1. Character of Structure of Complexes
The synthesis of the rare earth metal complexes with gallic acid was carried out under optimized conditions, yielding complexes with the general formula Ln(Gal)3·4H2O (Ln = Sm, Eu, Tb, and Dy). The lanthanide content (Ln3+) in the synthesized rare earth gallate complexes Ln(Gal)3·4H2O (Ln = Sm, Eu, Tb, and Dy) was quantitatively determined via complexometric titration using EDTA as the titrant and arsenazo III as a colorimetric indicator. The method provided reliable results, as evidenced by the close agreement between the theoretical and experimentally determined lanthanide content (Table 1). The deviation in all cases was within ±0.2%, indicating both the purity of the isolated complexes and the accuracy of the titrimetric protocol. The experimental data aligned well with the theoretical Ln3+ content values and matched with the lanthanide contents reported by Lis et al. [13] in analogous gallic acid complexes. Thus, it can be concluded that the proposed formula of the complex is consistent with the experimental percentage content of the lanthanide ions.

Table 1.
Ln3+ elemental content in the Ln(Gal)3·4H3O complexes.
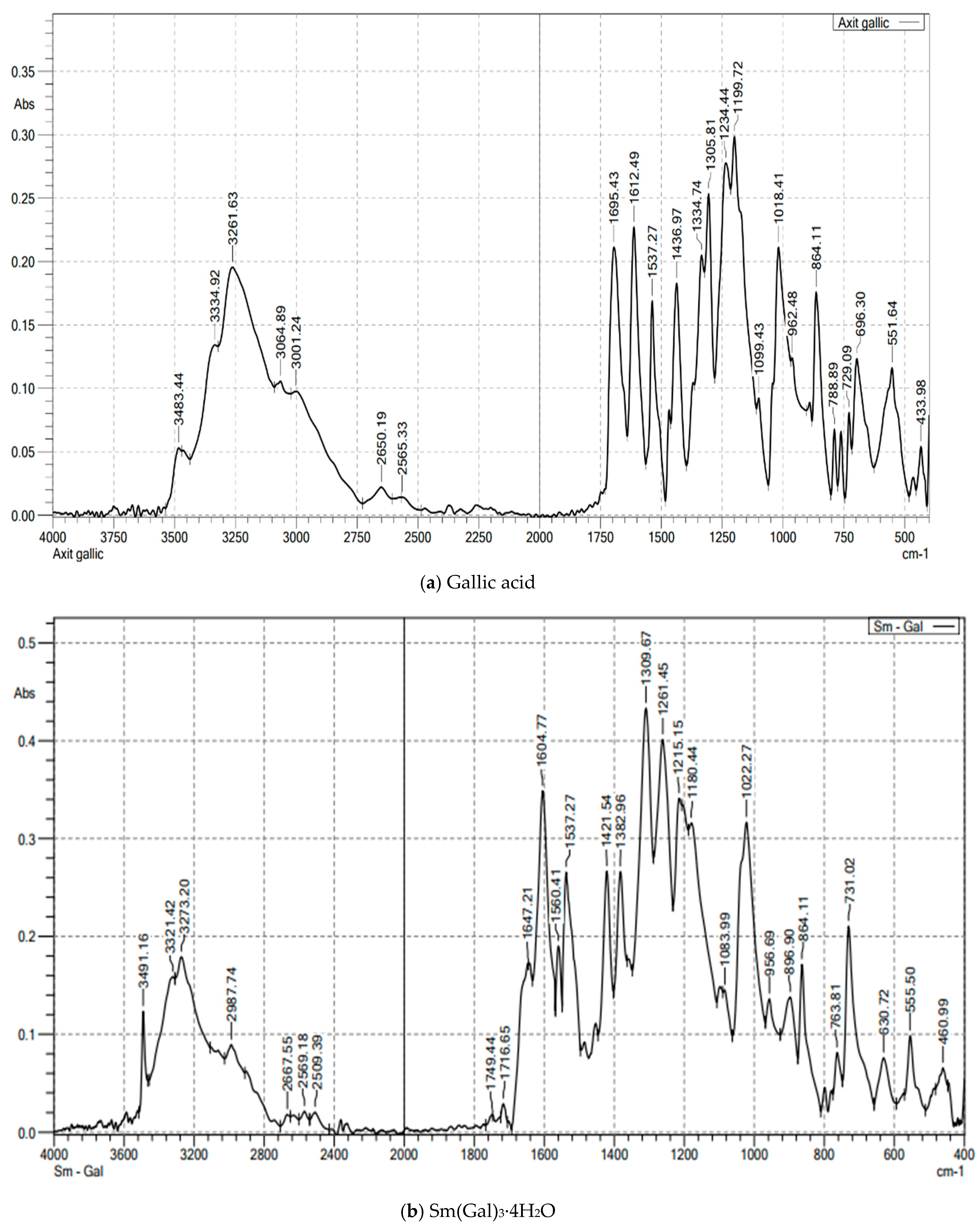
The FT-IR spectral data of gallic acid (Figure 1a; Table 2) showed peaks at 1695 cm−1 (νas(COOH)) and 1436 cm−1 (νs(COOH)). In the complexes (Figure 1b and Figure S1), νas(COO−) shifted to 1604 cm−1, confirming deprotonation and coordination via carboxylate oxygen. Peaks at 555–557 cm−1 were assigned to Ln–O vibrations. Broad bands at 3273–3277 cm−1 indicated hydrated water [10,11,12,13,14]. The IR spectra of the complexes revealed characteristic peaks corresponding to the coordination of Ln3+ ions with the carboxylate groups of gallic acid. The disappearance of the -COOH stretching vibration in the IR spectra of the complexes, compared to free gallic acid, confirmed the formation of the metal-ligand bond.
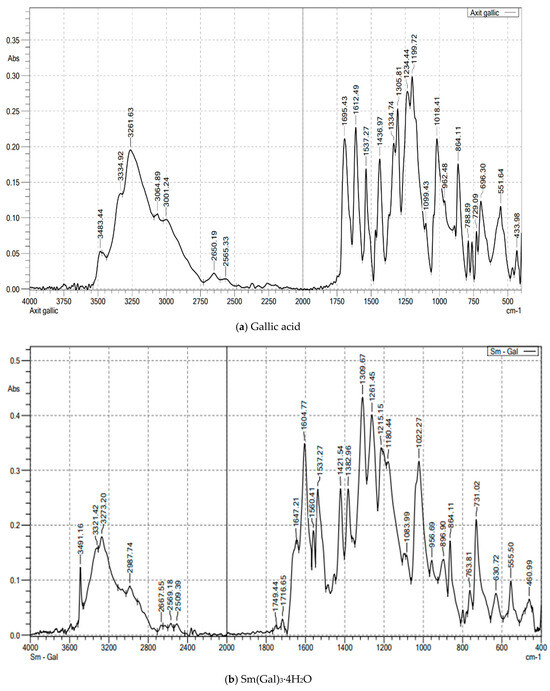
Figure 1.
The FT-IR spectroscopy of gallic acid and Sm(Gal)3·4H2O complex.

Table 2.
Characteristic absorption wavenumbers in the infrared spectra of the ligand and the complexes (cm−1).
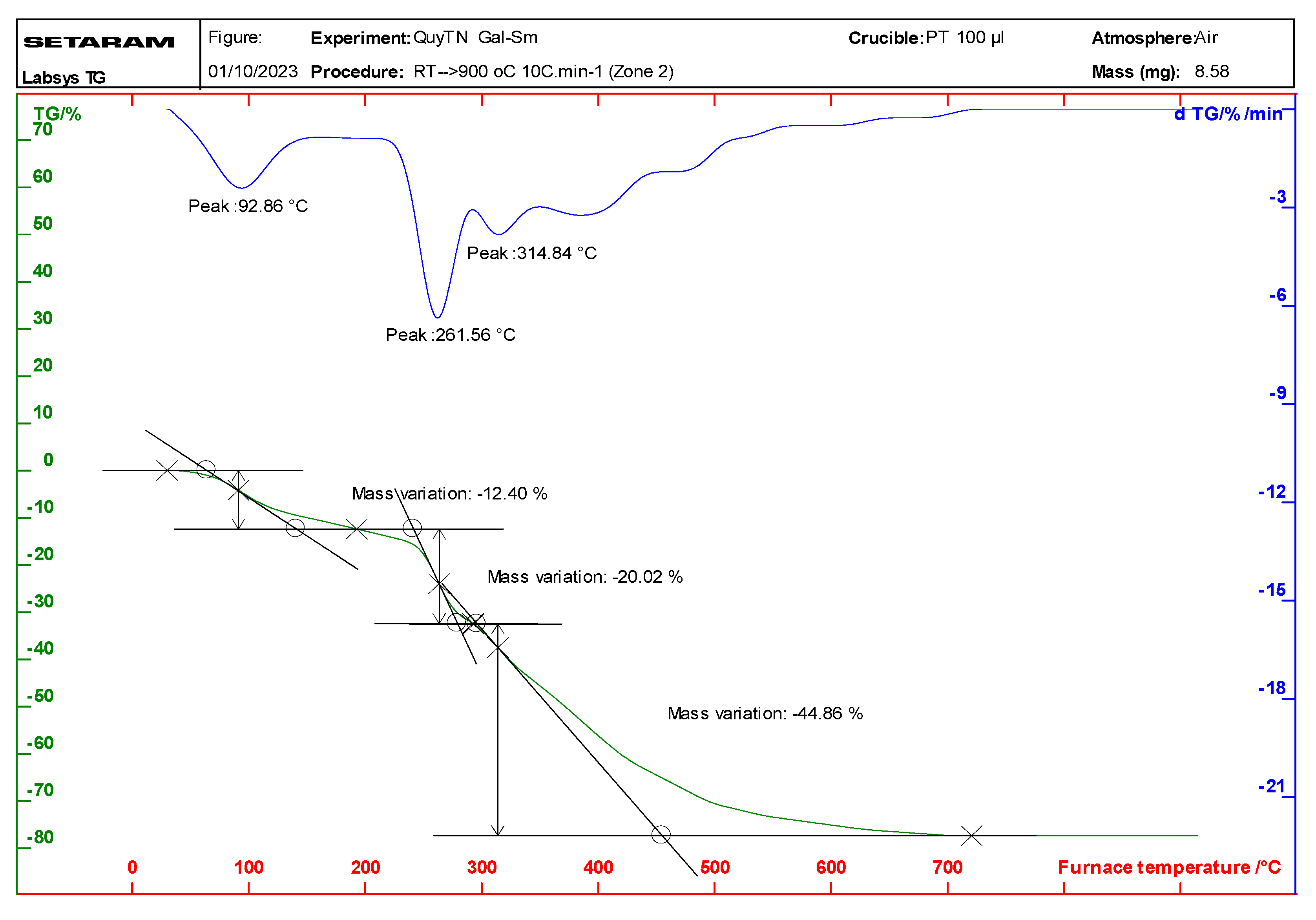
Thermal analysis (TGA/DTA) of the complexes indicated that they undergo a two-step decomposition process (Figure 2 and Figure S2; Table 3). The first step, occurring between 92 and 112 °C, corresponds to the loss of water molecules, while the second step, between 261 and 272 °C, involves the decomposition of the gallate ligands. The final decomposition product was identified as the corresponding rare earth metal oxide Ln2O3 for Sm, Eu, and Dy; otherwise, Tb(Gal)3·4H2O in the decomposition may obtain Tb+4 to leading affordable the oxide Tb4O7 [16,17]. The thermal stability of the complexes was found to be in the following order: Tb(Gal)3·4H2O > Eu(Gal)3·4H2O > Sm(Gal)3·4H2O > Dy(Gal)3·4H2O.
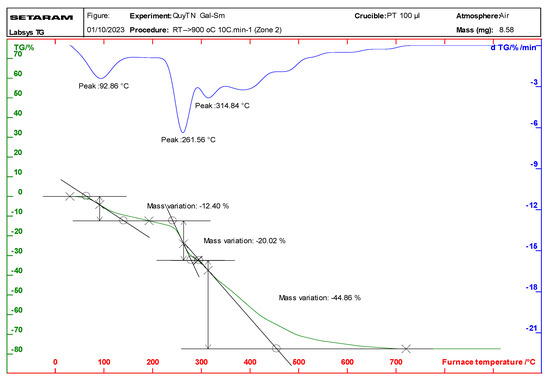
Figure 2.
Thermal analysis diagram of the Sm(Gal)3·4H2O complex.

Table 3.
Thermal analysis results of the complexes.
Mass spectrometry (MS) analysis confirmed the molecular structure of the complexes, with the presence of [Ln(Gal)3]+ ions as the dominant species. ESI-MS spectral data (Table 4) showed dominant peaks corresponding to [Ln(Gal)3]+ (m/z: 657–669), alongside dimeric [Ln2(Gal)3]+ (m/z: 808–832) and fragmented [Ln(Gal)2]+ (m/z: 488–500) ions. The MS spectral data also revealed the formation of dimeric species [Ln2(Gal)3]+, indicating partial polymerization of the complexes under the experimental conditions. In the mass spectra of all four complexes, the most intense peaks appear at m/z values of 657, 659, 666, and 669, corresponding to the Sm(III), Eu(III), Tb(III), and Dy(III) complexes, respectively. These values match the molecular ion masses of [Ln(Gal)3]+ (Ln = Sm, Eu, Tb, and Dy).

Table 4.
Mass spectral data of the complexes.
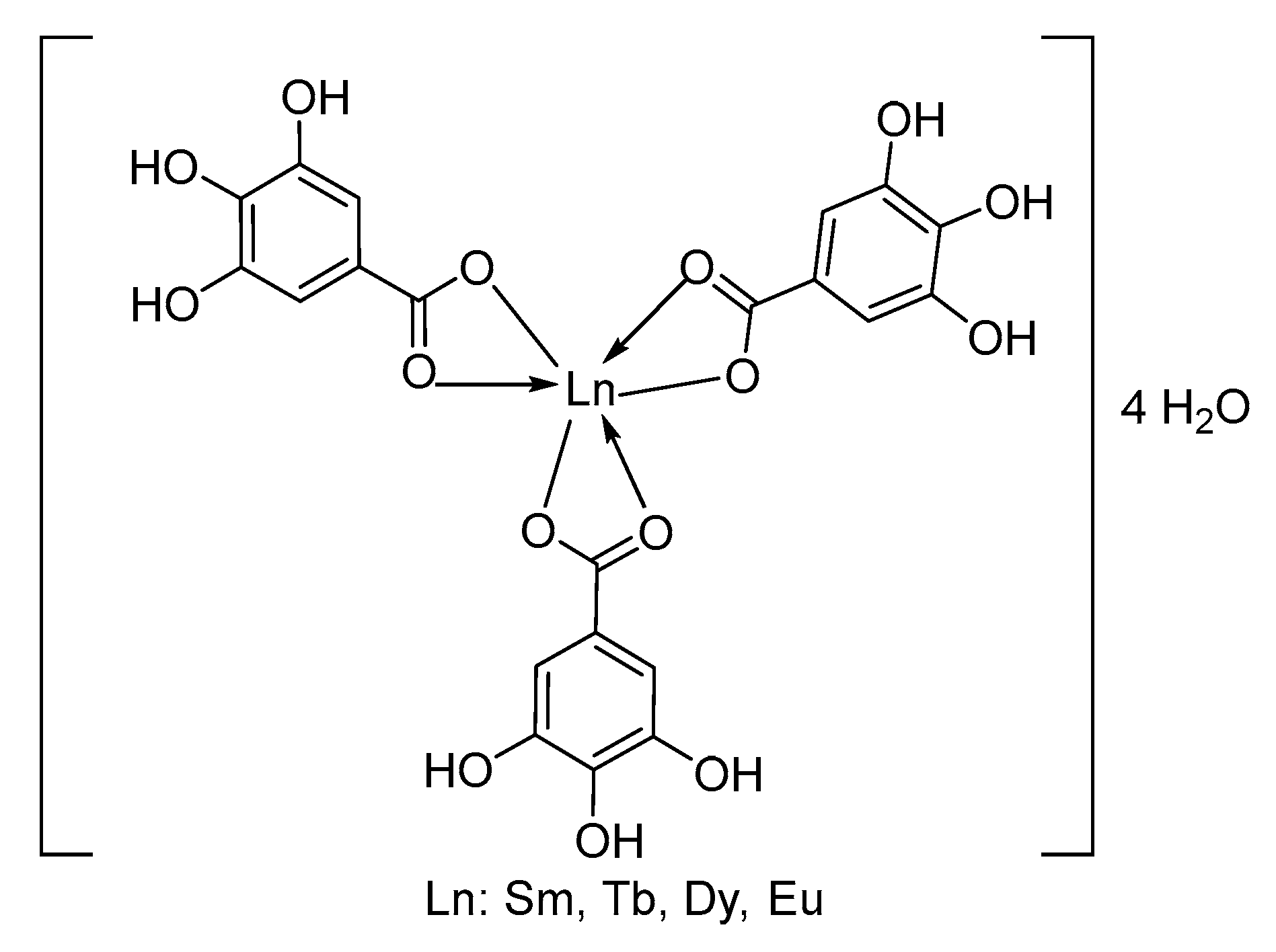
This indicates that, under the ionization conditions, after the removal of four hydration water molecules, the remaining molecular ions have the same composition, [Ln(Gal)3]+, confirming that all the complexes share the same molecular formula: Ln(Gal)3·4H2O (Ln = Sm, Eu, Tb, and Dy). Additionally, in the mass spectra of all four complexes, two weaker peaks are observed. One set of peaks appears at m/z values of 808, 811, 825, and 832, corresponding to the Sm(III), Eu(III), Tb(III), and Dy(III) complexes, respectively. These values match the molecular ion masses of dimeric fragments [Ln2(Gal)3]+, indicating that the complexes undergo partial polymerization under the mass spectrometric conditions. The third set of peaks, at m/z values of 488, 490, 497, and 500, respectively, for Sm(III), Eu(III), Tb(III), and Dy(III), are attributed to the presence of monomeric ions, [Ln(Gal)2]+. Thus, the vapor-phase composition of all four complexes is similar and includes the presence of one dimeric fragment ion, [Ln2(Gal)3]+, and two monomeric fragment ions, [Ln(Gal)3]+ and [Ln(Gal)2]+. Combining the mass spectrometry results with data obtained from infrared spectroscopy, thermal analysis, and elemental analysis, we propose that the complexes have the following structural formula (Figure 3):
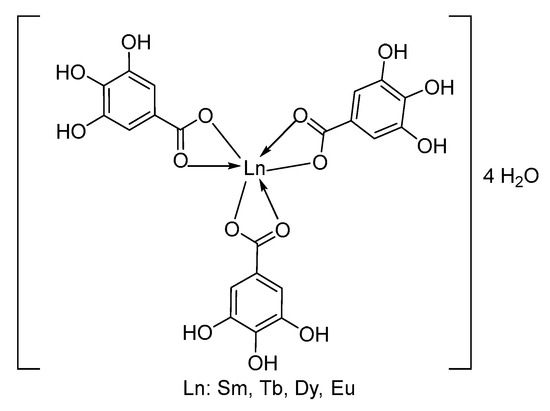
Figure 3.
Structure of complex.
2.2. Fluorescence Character of Synthesized Complexes
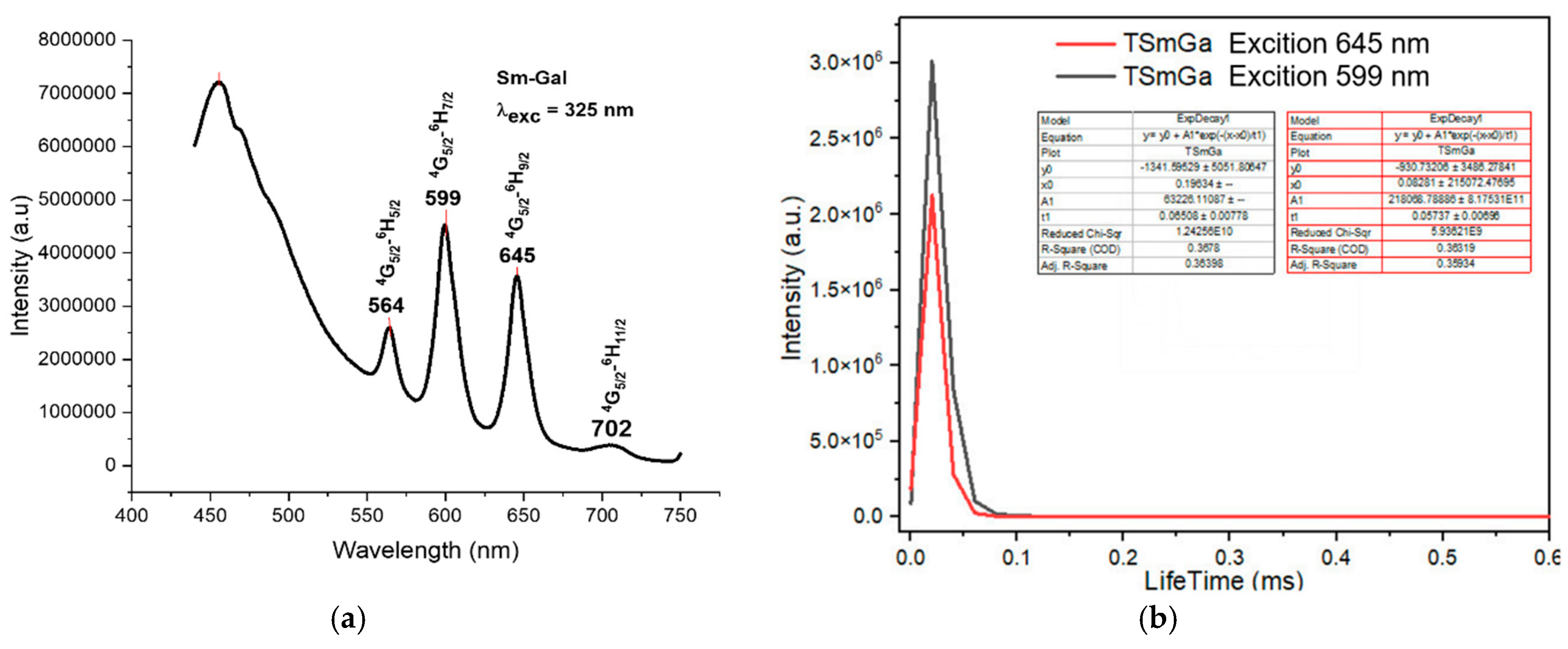
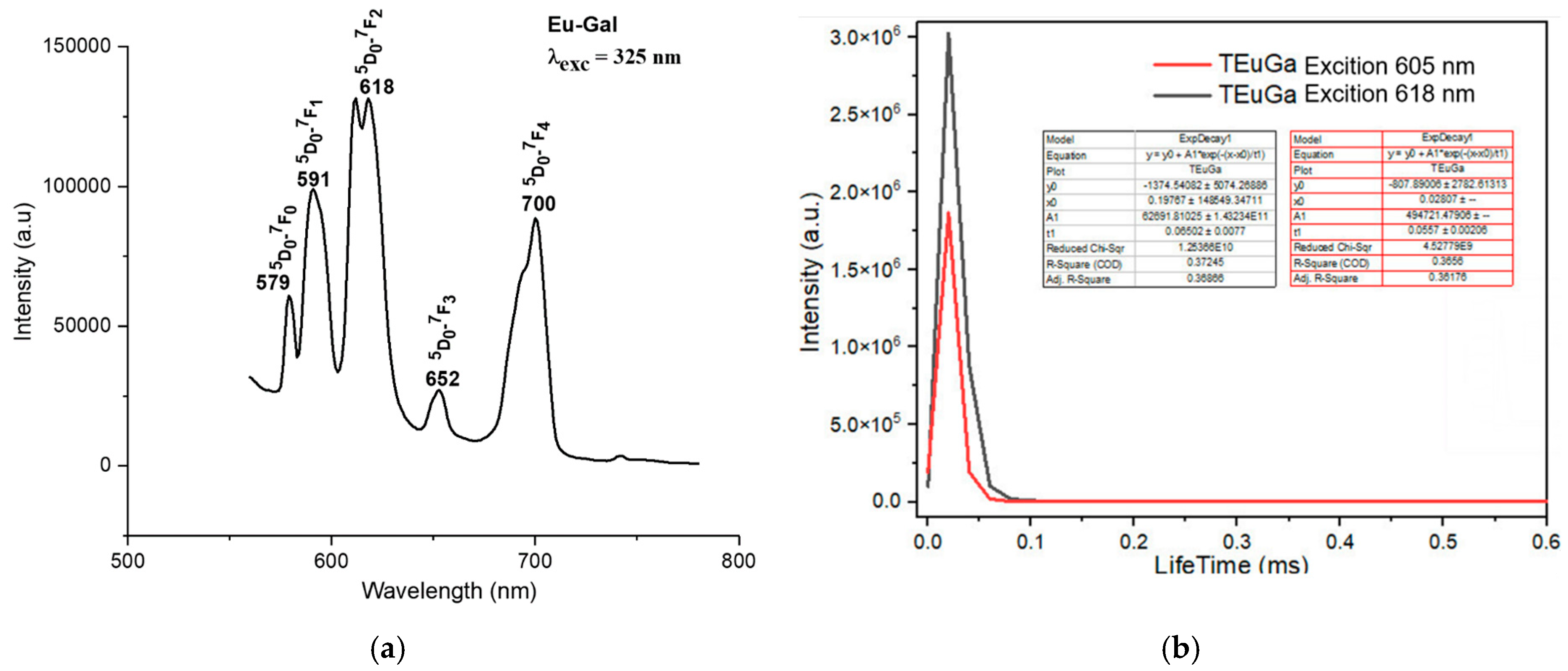
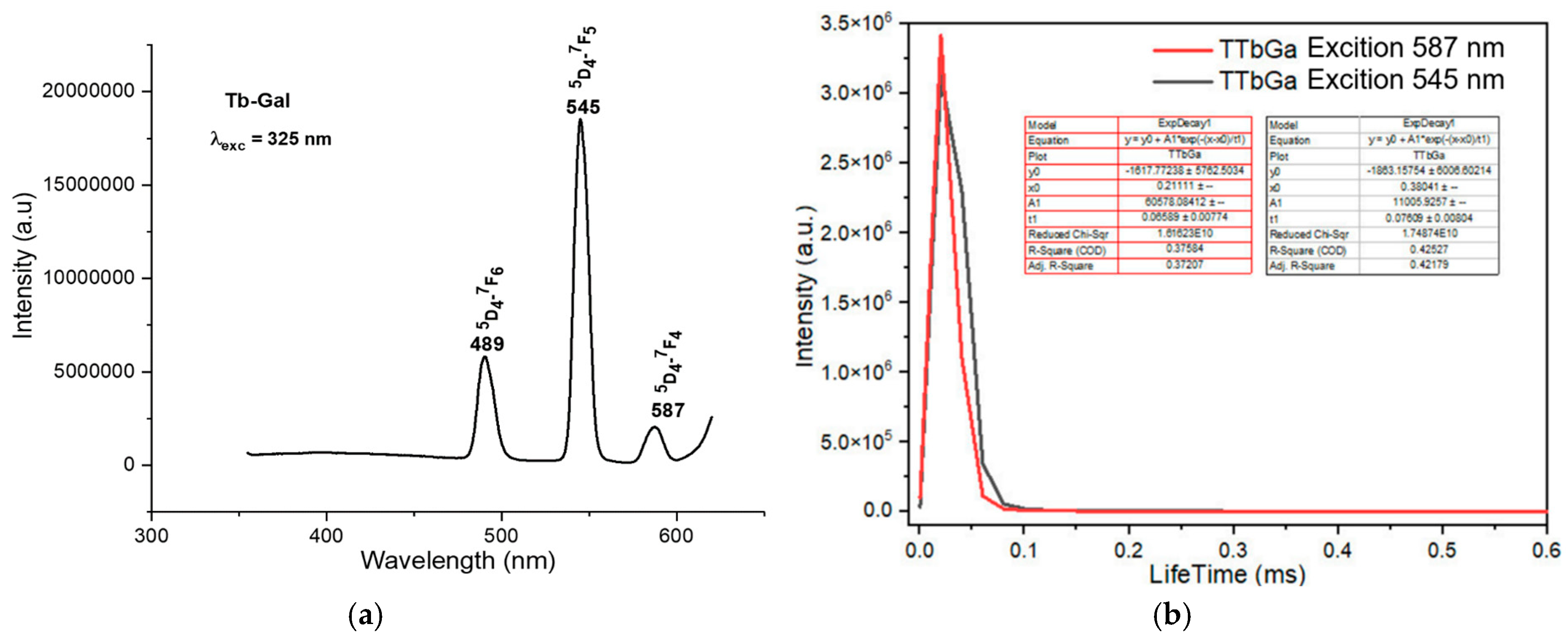
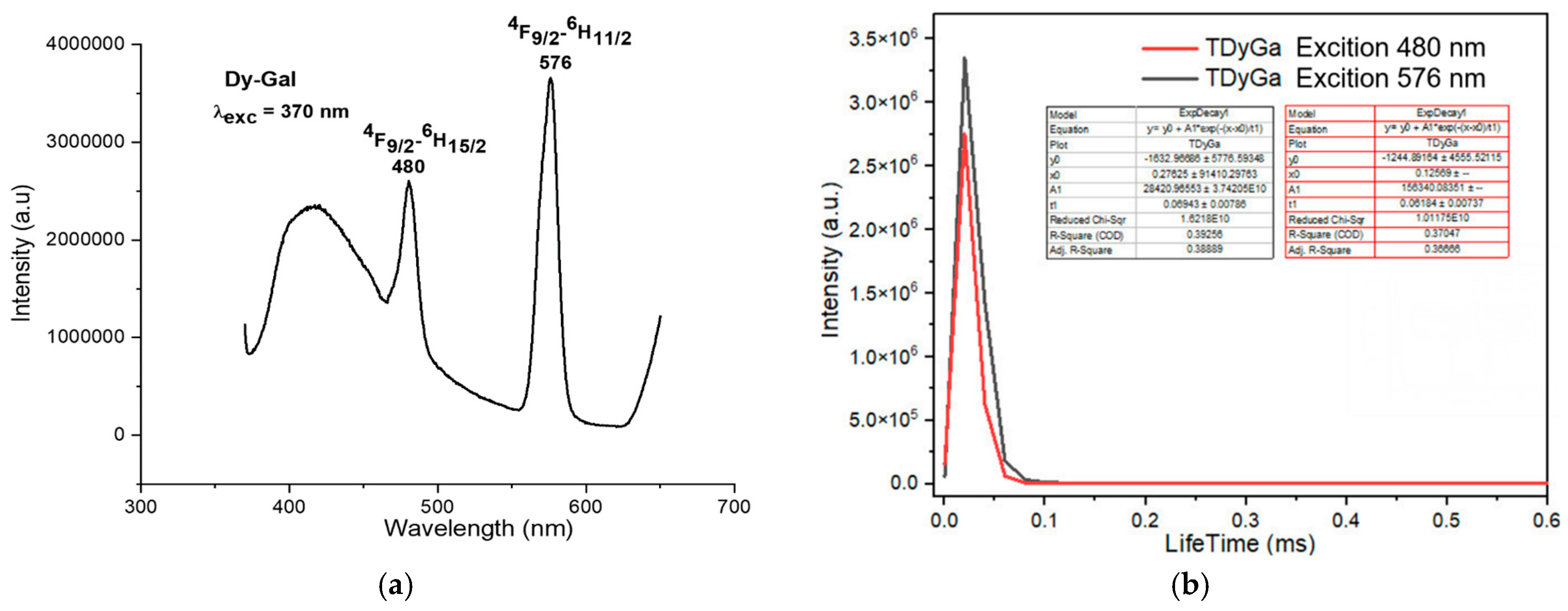
The fluorescence spectra of the complexes exhibited strong emission bands in the visible region, corresponding to the characteristic transitions of the Ln3+ ions. For instance, the Sm(III) complex showed emission bands at 564 nm, 599 nm, 645 nm, and 702 nm, corresponding to the transitions from the 4G5/2 excited state to the 6H5/2, 6H7/2, 6H9/2, and 6H11/2 states, respectively (Figure 4a). The Eu(III) complex exhibited emission bands at 579 nm, 591 nm, 618 nm, 652 nm, and 700 nm, corresponding to the 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 transitions, respectively (Figure 5a). The Tb(III) and Dy(III) complexes also showed strong luminescence, with emission bands corresponding to their respective electronic transitions (Figure 6a and Figure 7a).
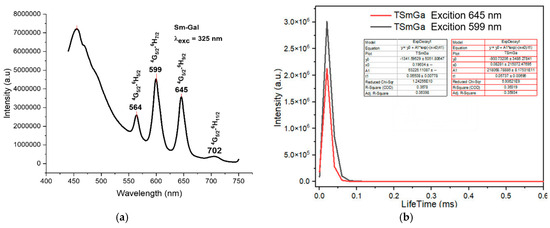
Figure 4.
Fluorescence spectrum of the complex Sm(Gal)3 4H2O. (a) Fluorescence emission spectrum, (b) fluorescence decay spectrum.
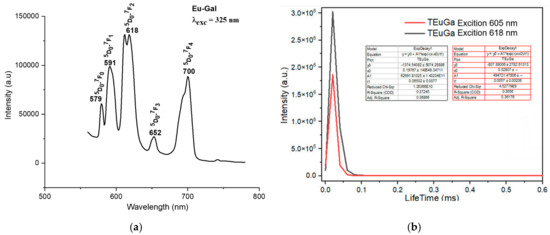
Figure 5.
Fluorescence spectrum of the complex Eu(Gal)3 4H2O. (a) Fluorescence emission spectrum, (b) fluorescence decay spectrum.
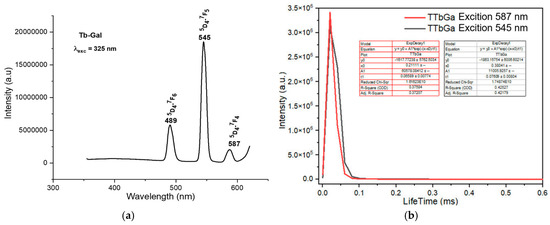
Figure 6.
Fluorescence spectrum of the complex Tb(Gal)3·4H2O. (a) Fluorescence emission spectrum, (b) fluorescence decay spectrum.
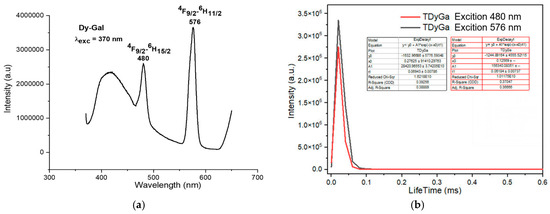
Figure 7.
Fluorescence spectrum of the complex Dy(Gal)3·4H2O. (a) Fluorescence emission spectrum, (b) fluorescence decay spectrum.
Under UV excitation at 325 nm, the Sm(Gal)3·4H2O complex exhibits strong fluorescence emission in the range of 450–750 nm, with four emission bands observed at 564 nm, 599 nm, 645 nm, and 702 nm. These emission bands correspond to light in the green region (564 nm), orange region (599 nm), and red region (645 nm and 702 nm). These emissions are assigned to the following electronic transitions of the Sm3+ ion: 4G5/2 → 6H5/2 (564 nm), 4G5/2 → 6H7/2 (599 nm), 4G5/2 → 6H9/2 (645 nm), and 4G5/2 → 6H11/2 (702 nm) [11]. Among the four emission bands, the orange emission at 599 nm and the red emission at 645 nm exhibit the highest intensities. The green emission at 564 nm has moderate intensity, while the weakest emission is observed at 702 nm. The fluorescence decay spectrum of the Sm(Gal)3·4H2O complex (Figure 4b) shows that under 325 nm excitation, the orange emission at 599 nm has a lifetime of 0.06508 ± 0.00778 ms, and the red emission at 645 nm has a lifetime of 0.05737 ± 0.00698 ms.
The fluorescence emission spectrum of the Eu(Gal)3 4H2O complex appears in the range of 550–720 nm. This complex exhibits fluorescence with five narrow and sharp consecutive emission peaks at 579 nm, 591 nm, 618 nm, 652 nm, and 700 nm (Figure 5a). Among these, the emission peak at 652 nm has the weakest intensity, followed by the peak at 579 nm. The emission peaks at 591 nm and 700 nm show relatively strong intensities, while the emission peak at 618 nm is the strongest (Figure 5a). These emission bands correspond to visible light regions: green (579 nm), orange (591 nm; 618 nm), and red (652 nm, 700 nm). These spectral bands are assigned to the following transitions of the Eu3+ ion: 5D0 → 7F0 (579 nm), 5D0 → 7F1 (591 nm), 5D0 → 7F2 (618 nm), 5D0 → 7F3 (652 nm), and 5D0 → 7F4 (700 nm) [16]. The fluorescence decay spectrum of the Eu(Gal)3·4H2O complex shows that under 325 nm excitation, the orange emission peak at 605 nm has a lifetime of 0.0557 ± 0.0026 ms, and the major emission at 618 nm exhibits a lifetime of 0.06502 ± 0.0077 ms (Figure 5b). Upon excitation at 325 nm, the Tb(Gal)3 4H2O complex emits three consecutive narrow and sharp emission peaks at 489 nm, 545 nm, and 587 nm (Figure 6a). Among them, the green emission peak at 545 nm has the strongest intensity, corresponding to the 5D4 → 7F5 transition. The blue emission peak at 489 nm, with a weaker intensity, corresponds to the 5D4 → 7F6 transition, while the orange emission peak at 587 nm, with the weakest intensity, is assigned to the 5D4 → 7F4 transition (Figure 6a). The fluorescence decay spectrum of the Tb(Gal)3 4H2O complex reveals that under 325 nm excitation, the orange emission peak at 587 nm exhibits a lifetime of 0.06589 ± 0.00774 ms, while the emission peak at 545 nm shows a lifetime of 0.07609 ± 0.00804 ms (Figure 6b) [2,7,18,19,20,21].
The study of the luminescent properties of the Dy(Gal)3·4H2O complex reveals that its fluorescence emission spectrum (Figure 7a) appears in the range of 400–700 nm. When excited by energy at 370 nm, the complex exhibits fluorescence with two narrow and sharp emission peaks at 480 nm and 576 nm. Among these, the emission peak at 480 nm has medium intensity, while the peak at 576 nm shows very strong intensity. These emission bands correspond to visible light in the indigo–blue region at 480 nm and green at 576 nm. The spectral bands are assigned to the following transitions of the Dy3+ ion: 4F9/2 → 6H15/2 (480 nm) and 4F9/2 → 6H11/2 (576 nm) [7,18,19,20,21]. The fluorescence decay spectrum of the Dy(Gal)3·4H2O complex shows that under 325 nm excitation, the orange emission peak at 576 nm has a lifetime of 0.06943 ± 0.00706 ms, while the emission peak at 480 nm exhibits a lifetime of 0.06184 ± 0.00737 ms (Figure 7b).
2.3. Biological Activities of Metal Complexes with Gallic Acid
The antimicrobial efficacy of synthesized Ln(Gal)3·4H2O complexes (Ln = Dy, Tb, Eu, and Sm) was systematically evaluated against three clinically relevant bacterial strains: Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The results were presented in Table 5. Among the four tested complexes, Dy(Gal)3·4H2O and Tb(Gal)3·4H2O demonstrated the highest activity, producing inhibition zones comparable to the standard antibiotic amoxicillin at the maximum tested concentration (100 µg/mL). Specifically, Dy(Gal)3·4H2O showed inhibition diameters of 26 mm, 27 mm, and 25 mm against E. coli, S. aureus, and P. aeruginosa, respectively, whereas Tb(Gal)3·4H2O exhibited notable activity against P. aeruginosa (28 mm). In contrast, Eu(Gal)3·4H2O and Sm(Gal)3·4H2O displayed relatively weaker activities, particularly at lower concentrations, with Sm-complexes losing detectable activity at 20 µg/mL. These differences in antibacterial efficacy can be rationalized based on the electronic configuration, ionic radius, and redox potential of the central lanthanide ions. Dy3+ and Tb3+ possess relatively smaller ionic radii and higher charge densities compared to Eu3+ and Sm3+, which likely enhance electrostatic interactions with the negatively charged bacterial cell membranes, increasing membrane permeability or causing direct membrane disruption [22]. Furthermore, lanthanide ions with favorable redox characteristics, such as Dy3+ and Tb3+, are known to participate in reactive oxygen species (ROS) generation, which contributes to oxidative damage of bacterial DNA, proteins, and lipids, ultimately leading to cell death [23]. Another critical factor contributing to antimicrobial activity is the presence of gallic acid as the coordinating ligand, which possesses antibacterial properties through mechanisms including membrane depolarization, iron chelation, and enzyme inhibition [24]. Complexation of gallic acid with lanthanide ions likely enhances its lipophilicity, facilitating cellular uptake and improving the stability of the active species under physiological conditions [25]. The synergistic action between the lanthanide ion and the gallic acid ligand may therefore play a crucial role in the enhanced bioactivity observed for Dy and Tb complexes. These findings suggest the potential utility of such lanthanide-based complexes as promising candidates for the development of novel antimicrobial agents, particularly in the context of rising antibiotic resistance.

Table 5.
Antimicrobial activity of Ln(Gal)3·4H2O complexes.
The in vitro cytotoxicity of the lanthanide–gallate complexes was assessed against the MCF-7 (human breast adenocarcinoma) cell line, and their half-maximal inhibitory concentration (IC50) values are summarized in Table 6. The Terbium(III) complex Tb(Gal)3·4H2O demonstrated the highest cytotoxic potency, with an IC50 value of 11.3 ± 1.2 µM, followed by Sm(Gal)3·4H2O (IC50 = 13.7 ± 0.6 µM) and Eu(Gal)3·4H2O (IC50 = 15.4 ± 0.5 µM). Notably, Dy(Gal)3·4H2O exhibited the weakest activity (IC50 = 34.4 ± 0.4 µM), suggesting a possible dependence on the nature of the central metal ion. All complexes were significantly less potent than the reference drug ellipticine (IC50 = 0.55 ± 0.03 µM), a known DNA intercalator and topoisomerase II inhibitor [26].

Table 6.
IC50 values against MCF7 cells.
The differences in cytotoxic activity among the lanthanide complexes can be rationalized by considering several physicochemical and biological factors. Firstly, the size and coordination behavior of the lanthanide ions likely influence cellular uptake and biomolecular interactions. Although lanthanide contraction results in minor changes in ionic radii, the electronic configuration and coordination dynamics vary significantly, potentially affecting ligand field strength and complex stability in the biological environment [27]. Secondly, reactive oxygen species (ROS)-mediated mechanisms are implicated in the anticancer activity of many metal-based compounds. The terbium and samarium complexes may generate higher intracellular ROS levels, contributing to oxidative stress, mitochondrial dysfunction, and apoptosis [28]. Gallate, as a polyphenolic ligand, can exhibit both antioxidant and pro-oxidant effects depending on the redox conditions within cancer cells, which could synergize with the metal center to modulate cytotoxic effects [29]. Moreover, the capacity of these complexes to interact with DNA or enzyme targets, such as topoisomerases or kinases, could also account for their bioactivity. Lanthanide ions can coordinate with phosphate backbones and nucleobases, leading to structural perturbations in DNA, inhibition of replication, or activation of apoptotic pathways [30]. The relatively lower activity of Dy(Gal)3·4H2O may be attributed to lower cellular permeability or weaker interaction with biological targets, possibly due to steric or electronic effects associated with Dysprosium(III).
3. Materials and Methods
3.1. Synthesis of Ln(Gal)3 Complexes (Ln = Sm, Eu, Dy, and Yb)
Complexes were synthesized under optimized conditions at a temperature of 60 °C and pH in the range of 4 to 5. Gallic acid (HGal) was completely dissolved in ethanol (C2H5OH). A solution of LnCl3 (Ln = Sm, Eu, Dy, and Yb) was then slowly added to the ligand solution. The molar ratio of LnCl3 to HGal was maintained at 1:3. The reaction mixture was stirred using a magnetic stirrer at 60 °C while maintaining the pH at approximately 4–5. After 5–6 h of stirring, the complex gradually precipitated from the solution. The precipitate was collected by vacuum filtration, washed with distilled water, and dried in a desiccator over silica gel until a constant weight was achieved. The synthesis yields ranged from 80% to 85%.
3.2. Determination of Ln3+ Content (%) in the Complexes (Sm3+, Eu3+, Tb3+, and Dy3+)
The content of Ln3+ ions in the rare earth gallate complexes was determined using complexometric titration with arsenazo III as the indicator. An accurately weighed 0.05 g sample of the complex was transferred into a Kjeldahl flask. A few drops of concentrated H2SO4 were added to wet the sample, and the mixture was heated on an electric hotplate until SO2 gas was released. After cooling, 1–2 mL of H2O2 was added, and the solution was heated again to remove any remaining SO2. This process was repeated until a clear solution with a characteristic rare earth ion color was obtained. The resulting solution was transferred to a 50 mL volumetric flask, diluted to the mark with distilled water, and thoroughly mixed. Complexometric titration was then performed. At the equivalence point, the solution color changed from deep blue to wine red. The method is based on the stable complex formation of Ln3+ with EDTA: Using a pipette, transfer exactly 10 mL of the Ln3+ solution into a 100 mL conical flask. Add approximately 5 mL of acetate buffer (pH ≈ 5), followed by 2–3 drops of arsenazo III indicator. The solution will appear blue. Gently heat the solution, then titrate with 10−3 M EDTA solution until the color changes to wine red. Record the volume of EDTA used. Repeat the titration three times and take the average value. The results are presented in Table 1.
3.3. The Infrared Spectroscopy of Complexes (Sm3+, Eu3+, Tb3+, and Dy3+)
The infrared spectroscopy of complexes (Sm3+, Eu3+, Tb3+, and Dy3+) method was used to confirm the formation of the complexes. The spectra were recorded using an FTIR Affinity–IS spectrometer (Shimadzu, Kyoto, Japan), in the wavenumber range of 400 to 4000 cm−1. The results are presented in Figure 1 and Figure S1 and Table 2.
3.4. Thermal Properties of Complexes (Sm3+, Eu3+, Tb3+, and Dy3+)
Thermal analysis was employed to investigate the thermal stability of the complexes. The thermal analysis diagrams were recorded using a Themys thermal analyzers (SETARAM KEP Technologies, Caluire, France) in an air atmosphere. The temperature was increased from room temperature to 900 °C at a heating rate of 10 °C per minute. The results are presented in Table 3, Figure 2 and Figure S2.
3.5. Mass Spectrometry of Complexes (Sm3+, Eu3+, Tb3+, and Dy3+)
Mass spectrometry was employed to investigate the structural forms, vapor-phase composition, and the stability of fragment ions of the complexes. The mass spectra were recorded using an LC/MS–Xevo TQMS instrument (Waters, Taunton, MA, USA) equipped with an ESI (Electrospray Ionization) source. The complexes were dissolved in ethanol as the solvent. The nebulizer gas pressure was set at 30 psi, the ionization temperature was 325 °C, and nitrogen was used as the ionization assistance gas. The mass spectral data of the complexes are shown in Table 4.
3.6. Antibacterial Activity
The Gram-negative bacterial strains used in this study included Pseudomonas aeruginosa (PA) and Escherichia coli, while the Gram-positive strain was Staphylococcus aureus (SA). All strains were provided by the Faculty of Biology, Thai Nguyen University of Education. The antibacterial activity of the complex was evaluated using the disc agar diffusion method, whereby the diameter of the inhibition zones around the wells was measured to determine the complexes antibacterial efficacy. The complex was diluted in DMSO at concentrations of 25 µg/mL, 50 µg/mL, and 100 µg/mL. Bacterial strains were cultured on nutrient agar at 30 °C for 24 h. Microbial suspensions were prepared in sterile distilled water to reach a concentration of approximately 108 CFU/mL. A volume of 0.1 mL of the microbial suspension was spread evenly on nutrient agar plates, and five wells (6 mm in diameter) were punched into the agar. Each well received 50 µL of the diluted complex and was incubated at 4 °C for 1 h. Subsequently, the plates were incubated at 37 °C for 24 h. The antibacterial activity was determined by measuring the diameters of the inhibition zones in centimeters. DMSO without any complex was used as the negative control, while amoxicillin at 50 µg/mL served as the positive control. All experiments were conducted in triplicate [31,32]. The antibacterial activity data of the complexes are shown in Table 5.
3.7. Cytotoxicity Activity
The cytotoxic effects of the complexes were tested against MCF-7 (human breast carcinoma), and A549 (adenocarcinomic human alveolar basal epithelial) cell lines using the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), conducted at the Vietnam Academy of Science and Technology. This colorimetric assay is based on the reduction of tetrazolium salt to formazan crystals by metabolically active cells and is widely used to evaluate cell viability, proliferation, and cytotoxicity [33]. The culture medium used was Dulbecco’s Modified Eagle Medium (DMEM) supplemented with L-glutamine, sodium pyruvate, NaHCO3, penicillin/streptomycin, 10% fetal bovine serum (FBS), and 0.05% Trypsin-EDTA. The detailed methodology followed the procedure described by Khang et al. [33]. Optical density was measured at 550 nm using a microplate reader. IC50 values (the concentration of complex required to inhibit 50% of cell growth) were calculated using logarithmic regression analysis. The cytotoxic activity data of the complexes are shown in Table 6.
3.8. Statistical Analysis
All experiments were performed in triplicate. Data were analyzed using two-way ANOVA and are presented as mean ± standard deviation. A p-value of <0.05 was considered statistically significant.
4. Conclusions
In conclusion, this study successfully synthesized and characterized rare earth metal complexes with gallic acid, demonstrating their potential as antimicrobial and anticancer agents. The complexes exhibited strong luminescent properties and significant biological activities, with the Dy(Gal)3·4H2O complex showing the highest antimicrobial activity and the Tb(Gal)3·4H2O complex exhibiting the most potent anticancer effects. These findings highlight the potential of rare earth metal complexes with gallic acid in biomedical applications, particularly in the development of new antimicrobial and anticancer therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13060180/s1, Figure S1: The FT-IR spectroscopy of Ln(Gal)3·4H2O complexes (Ln: Eu (a), Dy (b), and Tb (c)); Figure S2: Thermal analysis diagrams of Ln(Gal)3·4H2O complexes (Ln: Eu (a), Dy (b), and Tb (c)).
Author Contributions
Conceptualization, N.T.H.L. and P.V.K.; methodology, H.P.H. and D.C.T.; software, H.P.H.; writing—original draft preparation, N.T.H.L. and P.V.K.; writing—review and editing, N.T.H.L. and P.V.K.; supervision, P.V.K. and N.T.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by MOET, VietNam (Code: B2023-TNA-28).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Thai Nguyen University of Education (Accordance guide under number 1003/QĐ-ĐHSP). The MCF-7 cell line was provided by Sigmaaldrich Ltd, Singapore (Code 41106514).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
Thank you to Chu Manh Nhuong for editing this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, L.; Li, B.; Yue, S.; Li, M.; Hong, Z.; Li, W. A terbium (III) complex with triphenylamine-functionalized ligand for organic electroluminescent device. J. Lumin. 2008, 128, 620–624. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.; El-Sayed, M.M.; Al-Mohammadi, A.R. Some novel rare earth metal ions complexes: Synthesis, characterization, luminescence and biocidal efficiency. Anal. Biochem. 2020, 598, 113645. [Google Scholar] [CrossRef] [PubMed]
- Pichaimani, P.; Lo, K.M.; Elango, K.P. Synthesis, crystal structures, luminescence properties and catalytic application of lanthanide (III) piperidine dithiocarbamate complexes. Polyhedron 2015, 93, 8–16. [Google Scholar] [CrossRef]
- Buldurun, K.; Turan, N.; Savcı, A.; Çolak, N. Synthesis, structural characterization and biological activities of metal (II) complexes with Schiff bases derived from 5-bromosalicylaldehyde: Ru(II) complexes transfer hydrogenation. J. Saudi Chem. Soc. 2019, 23, 205–214. [Google Scholar] [CrossRef]
- Shaygan, S.; Pasdar, H.; Foroughifar, N.; Davallo, M.; Motiee, F. Cobalt (II) complexes with Schiff base ligands derived from terephthalaldehyde and ortho-substituted anilines: Synthesis, characterization and antibacterial activity. Appl. Sci. 2018, 8, 385. [Google Scholar] [CrossRef]
- Taha, Z.A.; Ajlouni, A.M.; Al Momani, W.; Al-Ghzawi, A.A. Syntheses, characterization, biological activities and photophysical properties of lanthanides complexes with a tetradentate Schiff base ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 81, 570–577. [Google Scholar] [CrossRef]
- Ajlouni, A.M.; Al Momani, W.; Al-Ghzawi, A.A.; Taha, Z.A. Synthesis, characterization, biological activities and luminescent properties of lanthanide complexes with [2-thiophenecarboxylic acid, 2-(2-pyridinylmethylene)hydrazide] Schiff bases ligand. J. Rare Earths 2016, 34, 986–993. [Google Scholar] [CrossRef]
- Kathiresan, S.; Annaraj, J.; Bhuvanesh, N.S. Cu (II) and Ni (II) Complexes of Anthracene-Affixed Schiff Base: A Conflict between Covalent and Stacking Interactions with DNA Bases. ChemistrySelect 2017, 2, 5475–5484. [Google Scholar] [CrossRef]
- Song, X.-Q.; Wang, Z.-G.; Wang, Y.; Huang, Y.-Y.; Sun, Y.-X.; Ouyang, Y.; Xie, C.-Z.; Xu, J.-Y. Syntheses, characterization, DNA/HSA binding ability and antitumor activities of a family of isostructural binuclear lanthanide complexes containing hydrazine Schiff base. J. Biomol. Struct. Dyn. 2020, 38, 733–743. [Google Scholar] [CrossRef]
- Kafi-Ahmadi, L.; Marjani, A.P. Mononuclear Schiff base complexes derived from 5-azophenylsalicylaldehyde with Co (ii), Ni (ii) ions: Synthesis, characterization, electrochemical study and antibacterial properties. S. Afr. J. Chem. 2019, 72, 101–107. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Structural characterization and biological activity of a new metal complexes based of Schiff base. J. Mol. Liq. 2021, 330, 115522. [Google Scholar] [CrossRef]
- Lis, T.; Kaczmarek, M.; Szydłowska, J. Synthesis and characterization of rare earth gallic acid complexes. Polyhedron 2000, 19, 1481–1488. [Google Scholar]
- Silva, R.T.C.; Silva-Caldeira, P.P.; Demarqui, F.; Lopes, C.D.; Albuquerque, S.; Pavan, F.R.; Pereira-Maia, E.C.; Diniz, R.; de Oliveira, A.; Rezende, C.; et al. Crystal structure and in vitro biological studies of a Pt(II) complex based on gallic acid and triphenylphosphine. Inorganica Chim. Acta 2024, 569, 122124. [Google Scholar] [CrossRef]
- Barcelo, J.; Guieb, M.; Ventura, A.; Nacino, A.; Pinasen, H.; Viernes, L.B.G.; Yodong, T.; Estrada, B.L.; Valdez, D.; Binwag, T. Antibacterial, Prooxidative and Genotoxic Activities of Gallic Acid and its Copper and Iron Complexes against Escherichia coli. Asia Pac. J. Multidiscip. Res. 2014, 6, 1–14. [Google Scholar]
- Gao, H.; Zhou, Y.; Chen, K.; Li, X. Synthesis of Tb4O7 complexed with reduced graphene oxide for Rhodamine-B absorption. Mater. Res. Bull. 2016, 77, 111–114. [Google Scholar] [CrossRef]
- Esra, Ö.; Nilgun, K. Synthesis and characterization of terbium oxide (III–IV) doped bismuth trioxide polymorphs. J. Chin. Adv. Mater. Soc. 2013, 2, 90–96. [Google Scholar]
- Sagar Babu, S.V.; Krishna Rao, K.; Ill Lee, Y. Synthesis, characterization, luminescence and DNA binding properties of Ln (III)-Schiff base family. J. Chil. Chem. Soc. 2017, 62, 3447–3453. [Google Scholar] [CrossRef]
- Li, H.; Jing, P.; Lu, J.; Xi, L.; Wang, Q.; Ding, L.; Wang, W.-M.; Song, Z. Multifunctional properties of {Cu II2 Ln III2} systems involving nitrogen-rich nitronyl nitroxide: Single-molecule magnet behavior, luminescence, magnetocaloric effects and heat capacity. Dalton Trans. 2021, 50, 2854–2863. [Google Scholar] [CrossRef]
- Li, H.; Jin, C.; Han, J.; Xi, L.; Song, Z. Tuning Nuclearity of Biradical-Ln Functional Compounds with Single-Molecule Magnet Behavior and Near-Infrared Luminescence. Cryst. Growth Des. 2022, 23, 612–619. [Google Scholar] [CrossRef]
- Li, H.; Jin, C.; Han, J.; Tang, J.; Han, X.; Song, Z. Near-infrared-II photothermal conversion and magnetic dynamic regulation in [Ln 3 Rad 2] aggregation by rigidity modification of nitronyl nitroxide. Inorg. Chem. Front. 2024, 11, 8421–8430. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Wang, M.; Guo, F. The Role of Rare Earth Metal Ions in Antibacterial Activity: Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef]
- Bansal, S.; Chhibber, T.; Singh, A.P.; Katare, O.P. Gallic Acid-Based Nanotherapeutics: A New Paradigm for Antimicrobial and Anticancer Applications. Molecules 2022, 27, 1194. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Pathog. 2013, 66, 21–25. [Google Scholar] [CrossRef]
- Sharma, A.; Dube, A.; Singh, A.; Jain, S. Coordination Complexes of Polyphenols with Lanthanides: Emerging Antibacterial and Anticancer Agents. Coord. Chem. Rev. 2021, 429, 213615. [Google Scholar] [CrossRef]
- Auclair, C. Multimodal action of antitumor agents on DNA: The ellipticine series. Arch. Biochem. Biophys. 1987, 259, 1–14. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Lanthanide luminescence for functional materials and bio-sciences. Chem. Sci. 2013, 4, 1939–1949. [Google Scholar] [CrossRef]
- Kostova, I.; Momekov, G. New lanthanide complexes of bioactive hydrazones: Synthesis, characterization, cytotoxicity and apoptosis induction in leukemia cells. Eur. J. Med. Chem. 2007, 42, 577–585. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Sun, R.W.-Y.; Ma, D.-L.; Wong, E.L.-M.; Che, C.-M. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Trans. 2007, 43, 4884–4892. [Google Scholar] [CrossRef]
- Hadacek, F.; Greger, H. Testing of antifungal natural products: Methodologies, comparability of results and assay choice. Phytochem. Anal. 2000, 11, 137–147. [Google Scholar] [CrossRef]
- Trung, T.Q.; Hiep, H.P.; Khang, P. Chemical compositions of Litsea umbellata and inhibition activities. Open Chem. 2023, 21, 20220294. [Google Scholar] [CrossRef]
- Khang, P.; Hiep, H.; Trung, T. Two new compounds from leaves of Capparis dongvanensis (Sy, B.H. Quang & D.V. Hai) and inhibition activities. Open Chem. 2023, 21, 20220317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).