Bimetallic 2,4-Dichlorophenoxyacetates EU(III) and GD(III): Composition, Structure, and Luminescent Properties
Abstract
1. Introduction
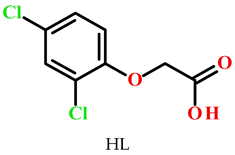
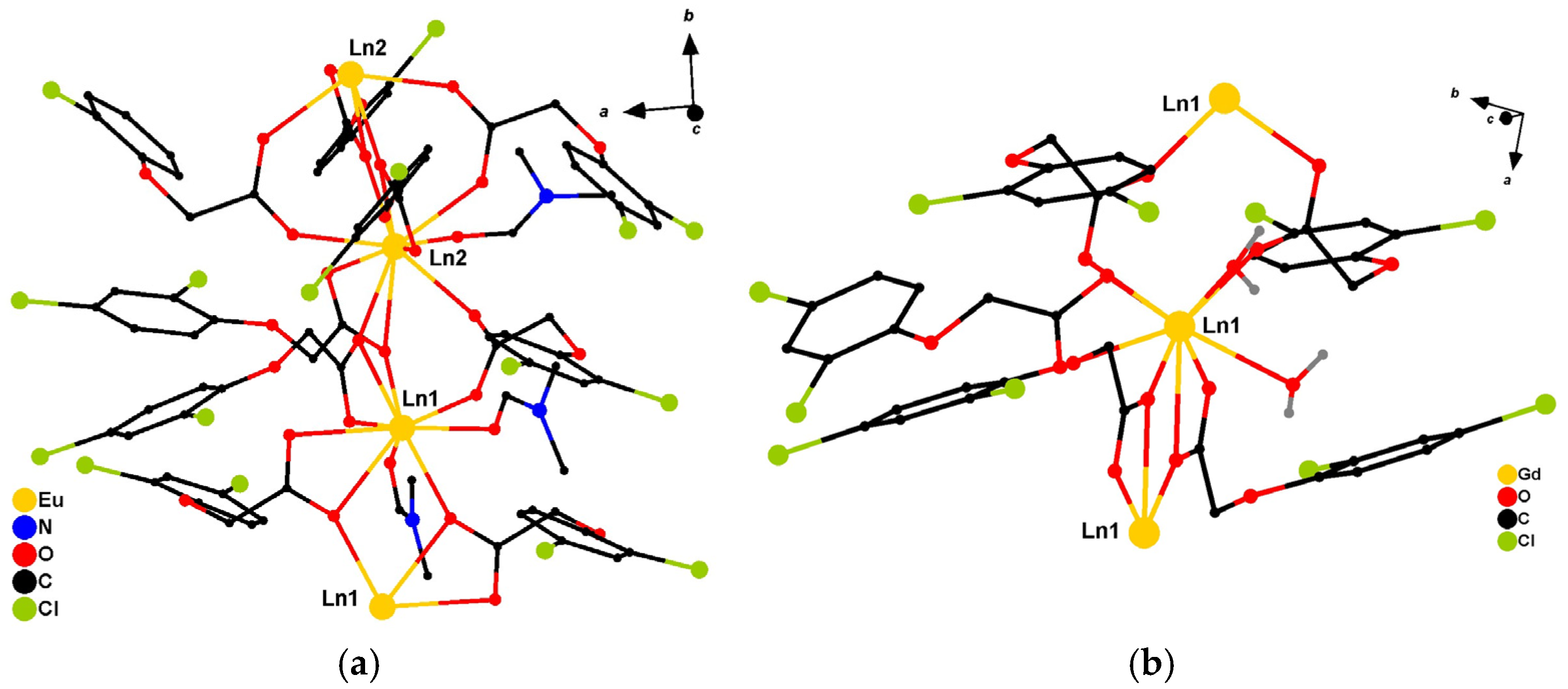
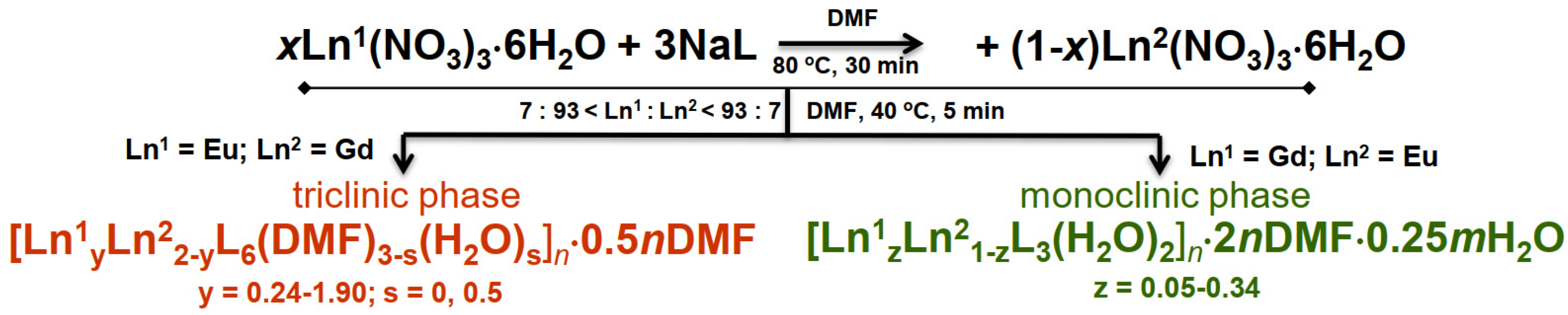
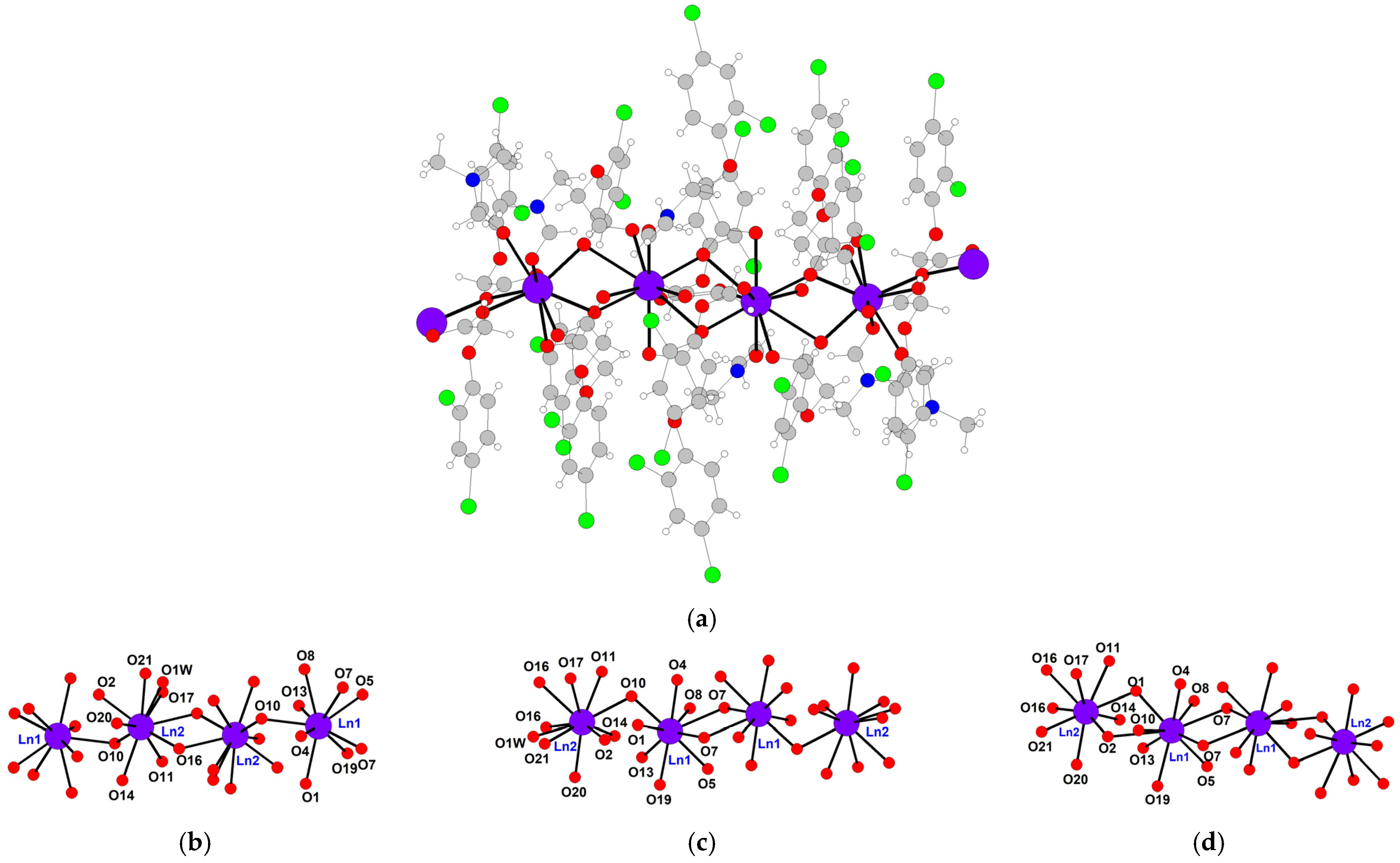
2. Results
General Characterization of Complexes
3. Materials and Methods
Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pode, R. Organic light emitting diode devices: An energy efficient solid state lighting for applications. Renew. Sustain. Energy Rev. 2020, 133, 110043. [Google Scholar] [CrossRef]
- Lim, G.; Lee, K.; Choi, S.; Yoon, H.J. Organometallic and coordinative photoresist materials for EUV lithography and related photolytic mechanisms. Coord. Chem. Rev. 2023, 493, 215307. [Google Scholar] [CrossRef]
- Popy, D.A.; Saparov, B. “This or that”—Light emission from hybrid organic–inorganic vs. coordination Cu(I) halides. J. Mater. Chem. C 2025, 13, 521–560. [Google Scholar] [CrossRef]
- Atzori, M.; Sessoli, R. The Second Quantum Revolution: Role and Challenges of Molecular Chemistry. J. Am. Chem. Soc. 2019, 141, 11339–11352. [Google Scholar] [CrossRef]
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of rare earth elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- de Souza, V.P.; Brandão, P.; Malvestiti, I.; Longo, R.L. Green syntheses of novel luminescent lanthanide compounds based on pentafluorobenzoate. Opt. Mater. 2024, 157, 116158. [Google Scholar] [CrossRef]
- Bolot’ko, A.E.; Shmelev, M.A.; Chistyakov, A.S.; Voronina, J.K.; Varaksina, E.A.; Gogoleva, N.V.; Taydakov, I.V.; Sidorov, A.A.; Eremenko, I.L. Luminescence enhancement by mixing carboxylate benzoate–pentafluorobenzoate ligands in polynuclear {Eu2Zn2} and {Tb2Zn2} complexes. Dalton Trans. 2025, 54, 5708–5720. [Google Scholar] [CrossRef]
- Vialtsev, M.B.; Dalinger, A.I.; Latipov, E.V.; Lepnev, L.S.; Kushnir, S.E.; Vatsadze, S.Z.; Utochnikova, V.V. New approach to increase the sensitivity of Tb-Eu-based luminescent thermometer. Phys. Chem. Chem. Phys. 2020, 22, 25450–25454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-M.; Cui, J.; Zeng, Y.-L.; Ren, N.; Zhang, J.-J. Two novel Sm(III) complexes with different aromatic carboxylic acid ligands: Synthesis, crystal structures, luminescence and thermal properties. Polyhedron 2019, 158, 485–493. [Google Scholar] [CrossRef]
- Thi, D.N.; Thi, N.N.; Vu, A.-T.; Tran, T.Q.; Ngoc, T.N.; Xuan, D.L.; Thi, T.T.; Xuan, T.N. Pyridinedicarboxylate-Tb(III) Complex-Based Luminescent Probes for ATP Monitoring. J. Anal. Methods Chem. 2021, 7, 7030158. [Google Scholar]
- Utochnikova, V.V.; Kuzmina, N.P. Photoluminescence of Lanthanide Aromatic Carboxylates. Russ. J. Coord. Chem. 2016, 42, 679–694. [Google Scholar] [CrossRef]
- Levina, A.A.; Chistyakov, A.S.; Shmelev, M.A.; Varaksina, E.A.; Voronina, J.K.; Gogoleva, N.V.; Taydakov, I.V.; Sidorov, A.A.; Eremenko, I.L. Effect of combining fluorinated non-fluorinated monocarboxylate anions in lanthanide complexes on structure photoluminescent properties. New J. Chem. 2025, 49, 12959–12970. [Google Scholar] [CrossRef]
- Kanzariya, D.B.; Chaudhary, M.Y.; Pal, T.K. Engineering of Metal–Organic Frameworks (MOFs) for Thermometry. Dalton Trans. 2023, 52, 7383–7404. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Liu, B.; Li, H.; Zheng, S.; Jiao, H.; Xu, L. Fluorescent Eu3+/Tb3+ Metal–Organic Frameworks for Ratiometric Temperature Sensing Regulated by Ligand Energy. Inorg. Chem. 2022, 61, 14322–14332. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Cheng, S.; Zhang, Z.; He, M.; Qian, J.; Li, L. Tailoring Energy Transfer in Mixed Eu/Tb Metal–Organic Frameworks for Ratiometric Temperature Sensing. Molecules 2024, 29, 3914. [Google Scholar] [CrossRef]
- Ilmi, R.; Li, X.; Rasbi, N.K.; Zhou, L.; Wong, W.-Y.; Raithby, P.R.; Khan, M.S. Two new red-emitting ternary europium(III) complexes with high photoluminescence quantum yields and exceptional performance in OLED devices. Dalton Trans. 2023, 52, 12885–12891. [Google Scholar] [CrossRef]
- Ilmi, R.; Xia, X.; Oliveira, W.F.; Dutra, J.D.L.; Zhou, L.; Wong, W.-Y.; Raithby, P.R.; Khan, M.S. Photo- and electro-luminescence studies of a new nine-coordinate ternary Eu(III) complex. J. Photochem. Photobiol. A Chem. 2026, 472, 116740. [Google Scholar] [CrossRef]
- Le Natur, F.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Bernot, K.; Ledoux, J.; Le Pollès Roiland, L.C. Coordination polymers based on heterohexanuclear rare earthcomplexes: Toward independent luminescence brightness and color tuning. Inorg. Chem. 2013, 52, 6720–6730. [Google Scholar] [CrossRef]
- Harbuzaru, B.V.; Corma, A.; Rey, F.; Atienzar, P.; Jordá, J.L.; García, H.; Ananias, D.; Carlos, L.D.; Rocha, J. Metal–organic nanoporous structures with anisotropic photoluminescence and magnetic properties and their use as sensors. Angew. Chem. Int. Ed. 2008, 47, 1080–1083. [Google Scholar] [CrossRef]
- Trannoy, V.; Carneiro Neto, A.N.; Brites, C.D.S.; Carlos, L.D.; Serier-Brault, H. Engineering of Mixed Eu3+/Tb3+ Metal-Organic Frameworks Luminescent Thermometers with Tunable Sensitivity. Adv. Opt. Mater. 2021, 9, 2001938. [Google Scholar] [CrossRef]
- Kourtellaris, A.; Lafargue-Dit-Hauret, W.; Massuyeau, F.; Latouche, C.; Tasiopoulos, A.J.; Serier-Brault, H. Tuning of Thermometric Performances of Mixed Eu–Tb Metal–Organic Frameworks through Single-Crystal Coordinating Solvent Exchange Reactions. Adv. Opt. Mater. 2022, 10, 2200484. [Google Scholar] [CrossRef]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chemistry 2016, 22, 14782–14795. [Google Scholar] [CrossRef]
- Golikova, M.V.; Yapryntsev, A.D.; Jia, Z.; Fatyushina, E.V.; Baranchikov, A.E.; Ivanov, V.K. Synthesis and Physicochemical Properties of Yttrium Subgroup REE Lactates Ln(C3H5O3)3·2H2O (Ln = Y, Tb–Lu). Russ. J. Inorg. Chem. 2023, 68, 1414–1424. [Google Scholar] [CrossRef]
- Kendin, M.; Tsymbarenko, D. 2D-Coordination Polymers Based on Rare-Earth Propionates of Layered Topology Demonstrate Polytypism and Controllable Single-Crystal-to-Single-Crystal Phase Transitions. Cryst. Growth Des. 2020, 20, 3316–3324. [Google Scholar] [CrossRef]
- Smith, J.A.; Singh-Wilmot, M.A.; Hossack, C.H.; Cahill, C.L.; Ford, R.A.C. Expanding the Library: Twenty New Ln(III) Metal–Organic Frameworks, Including Two Isoreticular Pairs, from Dihaloterephthalic Acid. Cryst. Growth Des. 2024, 24, 7822–7836. [Google Scholar] [CrossRef]
- Gusev, A.N.; Konnik, O.V.; Shul’gin, V.F.; Pevzner, N.S.; Kiskin, M.A.; Linert, W. Lanthanum and some lanthanides 2,4-dichlorophenoxyactetates: Structure and luminescent properties. Polyhedron 2024, 249, 116749. [Google Scholar] [CrossRef]
- Kiskin, M.A.; Konnik, O.V.; Shul’gin, V.F.; Gusev, A.N. Crystal Structure of Lanthanide Salts with 2,4-Dichlorophenoxyacetic Acid. Russ. J. Coord. Chem. 2024, 50, 476–484. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.1; SHAPE: Barcelona, Span, 2013. [Google Scholar]
- Einkauf, J.D.; Rue, K.L.; Hoeve, H.A.T.; de Lill, D.T. Enhancing luminescence in lanthanide coordination polymers through dilution of emissive centers. J. Lumin. 2018, 197, 412–417. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2016, 295, 1–45. [Google Scholar] [CrossRef]
- Bünzli, J.C. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- Bettinelli, M.; Speghini, A.; Piccinelli, F.; Neto, A.N.C.; Malta, O.L. Luminescence spectroscopy of Eu3+ in Ca3Sc2Si3O12. J. Lumin. 2011, 131, 1026–1028. [Google Scholar] [CrossRef]
- Ferreira, R.A.S.; Nobre, S.S.; Granadeiro, C.M.; Nogueira, H.I.S.; Carlos, L.D.; Malta, O.L. A theoretical interpretation of the abnormal 5D0→7F4 intensity based on the Eu3+ local coordination in the Na9[EuW10O36]∙14H2O polyoxometalate. J. Lumin. 2006, 121, 561–567. [Google Scholar] [CrossRef]
- Savchenko, V.D.; Zhuravlev, K.P.; Tsaryuk, V.I. Judd-Ofelt analysis of dimeric europium carboxylates with gradually changing distortions of the crystal field around Eu3+ ion. J. Lumin. 2024, 276, 120839. [Google Scholar] [CrossRef]
- Loiko, P.A.; Dashkevich, V.I.; Bagaev, S.N.; Orlovich, V.A.; Yasukevich, A.S.; Yumashev, K.V.; Kuleshov, N.V.; Dunina, E.B.; Kornienko, A.A.; Vatnik, S.M.; et al. Spectroscopic and photoluminescence characterization of Eu3+-doped monoclinic KY(WO4)2 crystal. J. Lumin. 2014, 153, 221–226. [Google Scholar] [CrossRef]
- Pelczarska, A.J.; Stefańska, D.; Watras, A.; Macalik, L.; Szczygieł, I.; Hanuza, J. Structural and Luminescence Behavior of Nanocrystalline Orthophosphate KMeY(PO4)2: Eu3+ (Me = Ca, Sr) Synthesized by Hydrothermal Method. Materials 2022, 15, 1850. [Google Scholar] [CrossRef]
- Judd, R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Ilmi, R.; Wang, J.; Dutra, J.D.L.; Zhou, L.; Wong, W.-Y.; Raithby, P.R.; Khan, M.S. Efficient Red Organic Light Emitting Diodes of Nona Coordinate Europium Tris(β-Diketonato) Complexes Bearing 4′-Phenyl-2,2′:6′,2′′-Terpyridine. Chem. Eur. J. 2023, 29, e202300376. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

| Eu1 | Eu2 | Eu4 |
|---|---|---|
| Eu1-O1 2.538 (3) | Eu1-O1 2.5407 (17) | Eu1-O10 2.475 (4) |
| Eu1-O2 2.886 (4) | Eu2-O10 2.4617 (14) | Eu2-O10 2.492 (4) |
| Eu1-O4 2.408 (2) | Eu1-O2 2.904 (2) | Eu1-O1 2.5929 (12) |
| Eu1-O5 i 2.469 (2) | Eu2-O11 2.5673 (15) | Eu2-O11 2.544 (4) |
| Eu1-O7 i 2.633 (2) | Eu1-O4 2.4079 (14) | Eu1-O2 2.8627 (13) |
| Eu1-O7 2.342 (2) | Eu2-O14 2.4683 (14) | Eu2-O2 2.3835 (14) |
| Eu1-O8 i 2.554 (2) | Eu1-O5 i 2.4713 (13) | Eu1-O4 2.4339 (13) |
| Eu1-O10 2.464 (2) | Eu2-O16 2.4174 (15) | Eu2-O13 3.0300 (14) |
| Eu1-O13 2.376 (2) | Eu1-O7 i 2.6356 (14) | Eu1-O5 i 2.4803 (12) |
| Eu1-O19 2.511 (2) | Eu2-O16 ii 2.5300 (15) | Eu2-O14 2.5216 (13) |
| Eu2-O2 2.331 (3) | Eu1-O7 2.3480 (13) | Eu1-O7 i 2.6551 (12) |
| Eu2-O21 2.507 (5) | Eu2-O17 ii 2.5586 (14) | Eu2-O16 2.4200 (14) |
| Eu2-O1 W 2.427 (5) | Eu1-O8 i 2.5534 (14) | Eu1-O7 2.3668 (14) |
| Eu2-O10 2.462 (2) | Eu2-O20 2.4194 (15) | Eu2-O16 ii 2.5388 (13) |
| Eu2-O11 2.567 (3) | Eu1-O10 2.4694 (13) | Eu1-O8 i 2.5805 (12) |
| Eu2-O14 2.460 (2) | Eu1-O13 2.3802 (14) | Eu2-O17 ii 2.5631 (14) |
| Eu2-O16 2.415 (3) | Eu1-O19 2.5179 (14) | Eu1-O13 2.4349 (13) |
| Eu2-O16 ii 2.527 (3) | Eu2-O2 2.3286 (18) | Eu1-O19 2.5633 (12) |
| Eu2-O17 ii 2.553 (2) | Eu2-O21 2.496 (3) | Eu2-O21 2.428 (6) |
| Eu2-O20 2.416 (3) | Eu2-O1W 2.435 (3) | Eu2-O20 2.4457 (14) |
| Eu2-C25 2.873 (3) |
| № | , % | , µs | , µs | Arad, s−1 | Anr, s−1 | Ω2∙1020 | Ω4∙1020 | Gd/Eu Molar Ratio, % | |
|---|---|---|---|---|---|---|---|---|---|
| Gd1 | 6.3 | 578 | 630 | 6.8 | 108 | 1478 | 2.8 | 1.0 | 5/95 |
| Eu1 | 40.4 | 1390 | 1486 | 41.9 | 261 | 411 | 8.0 | 1.2 | 5/95 |
| Gd2 | 7.5 | 615 | 658 | 8.0 | 121 | 1397 | 2.7 | 1.1 | 26/74 |
| Eu2 | 46.3 | 1444 | 1503 | 48.2 | 320 | 344 | 7.9 | 0.9 | 25/75 |
| Gd3 | 5.0 | 598 | 636 | 5.3 | 83 | 1488 | 3.1 | 1.1 | 34/66 |
| Eu3 | 27.3 | 1468 | 1514 | 28.1 | 185 | 474 | 7.6 | 1.2 | 34/66 |
| Eu4 | 12 | 921 | 1045 | 13.6 | 130 | 826 | 7.8 | 1.1 | 88/12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konnik, O.; Gusev, A.; Braga, E.; Nauhatsky, I.; Shpak, M.; Gogoleva, N.; Kiskin, M.; Linert, W. Bimetallic 2,4-Dichlorophenoxyacetates EU(III) and GD(III): Composition, Structure, and Luminescent Properties. Inorganics 2025, 13, 397. https://doi.org/10.3390/inorganics13120397
Konnik O, Gusev A, Braga E, Nauhatsky I, Shpak M, Gogoleva N, Kiskin M, Linert W. Bimetallic 2,4-Dichlorophenoxyacetates EU(III) and GD(III): Composition, Structure, and Luminescent Properties. Inorganics. 2025; 13(12):397. https://doi.org/10.3390/inorganics13120397
Chicago/Turabian StyleKonnik, Oleg, Alexey Gusev, Elena Braga, Igor Nauhatsky, Maxim Shpak, Natalia Gogoleva, Mikhail Kiskin, and Wolfgang Linert. 2025. "Bimetallic 2,4-Dichlorophenoxyacetates EU(III) and GD(III): Composition, Structure, and Luminescent Properties" Inorganics 13, no. 12: 397. https://doi.org/10.3390/inorganics13120397
APA StyleKonnik, O., Gusev, A., Braga, E., Nauhatsky, I., Shpak, M., Gogoleva, N., Kiskin, M., & Linert, W. (2025). Bimetallic 2,4-Dichlorophenoxyacetates EU(III) and GD(III): Composition, Structure, and Luminescent Properties. Inorganics, 13(12), 397. https://doi.org/10.3390/inorganics13120397









