Abstract
Different morphologies of cobalt oxide (Co3O4) electrodes were prepared through the electrochemical deposition technique with various electrodeposition times from 10 min to 50 min. Platinum (Pt) nanoparticles were deposited on the Co3O4 electrodes through sputter coating. The crystallographic, microstructural, surface functional, textural–structural, and electric properties of the Co3O4 electrodes were investigated. X-ray diffraction analysis identified a pure cubic Co3O4 crystal structure in the samples. In the electrodeposition process, the microstructure of the electrodes varied from hierarchical 3D flower-like to 2D hexagonal porous nanoplates due to an increase in oxygen vacancies. The carrier densities of all samples were between 5.77 × 1014 cm−3 and 8.77 × 1014 cm−3. The flat band potentials of all samples were between −5.91 V and −6.21 V vs. an absolute electron potential, and the potential values for electrodes became more positive as the oxygen vacancy concentration in the film structure increased. The 2D hexagonal porous nanoplate Pt/Co3O4 electrodes offered the highest oxygen vacancies and thus the maximum current density of 102.66 mA/cm2, with an external potential set at 1.5 V vs. an Ag/AgCl reference electrode.
1. Introduction
Recently, electrolytic chlorine production from seawater has become a significant field of research due to increasing demands for chlorine in synthetic chemistry, wastewater treatment, metallurgical engineering, and polymer product manufacturing [1]. Dimensionally stable anodes (DSAs) have become the most significant catalyst for the chlorine evolution reaction (CER) because they have the benefits of (i) low power consumption, (ii) long durability, (iii) excellent electrode stability in the electrolysis process, (iv) no use or production of environmentally harmful materials, (v) relatively consistent and stable electrolysis cell operation at room temperature, and (vi) low labor requirements [2]. Industrial chlorine production requires the use of durable electrodes for chloride anticorrosion, and DSAs can fulfil this requirement due to their compact and crack-free microstructure [3].
Among DSA materials, expensive coating materials have been used to form the electrodes, such as ruthenium oxide films [4], iridium oxide films [5], ruthenium–iridium oxides films [6], platinum–iridium films [7], and ternary system films [8]. However, these coatings have a number of drawbacks, including being high-cost, prone to agglutination and coking after sintering, and readily poisoned [9]. When DSAs become poisoned, they lose their catalytic activity and are subject to degradation. Comparatively, alternative transition metal oxide (TMO) catalysts are much cheaper and have sufficient catalytic activity for industrial electrolysis process applications.
One type of TMO catalyst, cobalt oxide (Co3O4), has received considerable attention in various applications, including chlorine production [10], the oxidation of CO and volatile organic compounds [11,12], wastewater treatment [13], and catalytic additives for improving catalytic anticorrosion and electrochemical performance [14,15,16,17,18]. Co3O4 catalysts have the advantages of low cost and excellent catalytic activity and abundant Co reserves compared to platinum group elements (PGEs). Co3O4 catalysts have diverse microstructures, including sheets [19], belts [20], cubes [21], plates [22], and rods [23], due to the presence of different defects [24]. Z. Wang et al. [25] reported observations of two-dimensional flower-like Co3O4 nanosheets which exhibited a stable performance for low Cl ion concentrations during chlorine evolution. X. Zhang et al. [26] synthesized porous three-dimensional nano-sized Co3O4 particles with a high current density (80 mA/cm2 under 1.5 V vs. an Ag/AgCl reference electrode) in 0.6 M NaCl solution.
Co3O4 catalyst films can be produced through various methods, for example, the sol–gel method [27], chemical vapor deposition [28], sputtering deposition [29], and electrochemical deposition [30]. Among these methods, electrochemical deposition is the easiest and fastest process which allows for the greatest control of the microstructures and film thickness when deposited as a coating on different substrates [31]. Precise control of the elemental molar ratios is a significant factor for thin film fabrication, as the crystal orientation is directly affected by the molar ratios during the process of thin film growth [32]. Among all thin film preparation methods, electrochemical deposition is a favorable technology for industrial applications due to the following factors: (i) relatively low cost, (ii) easy process scale-up, (iii) easy control of the molar ratios for thin films, and (iv) high compatibility for different substrates [33].
Due to the advantages of electrochemical deposition, this paper reports on a study of anodes prepared through the electrochemical deposition of Co3O4 catalyst films onto nickel foam substrates. Electrochemically deposited Co3O4 films were fabricated on nickel foam substrates using different Na2SO4 concentrations and deposition times. The prepared electrodes were then applied to chlorine evolution reactions to evaluate their electrocatalytic performance. Various characterization studies were conducted to investigate the factors affecting the chlorine evolution efficiency.
2. Results and Discussion
2.1. Crystallinity Analysis
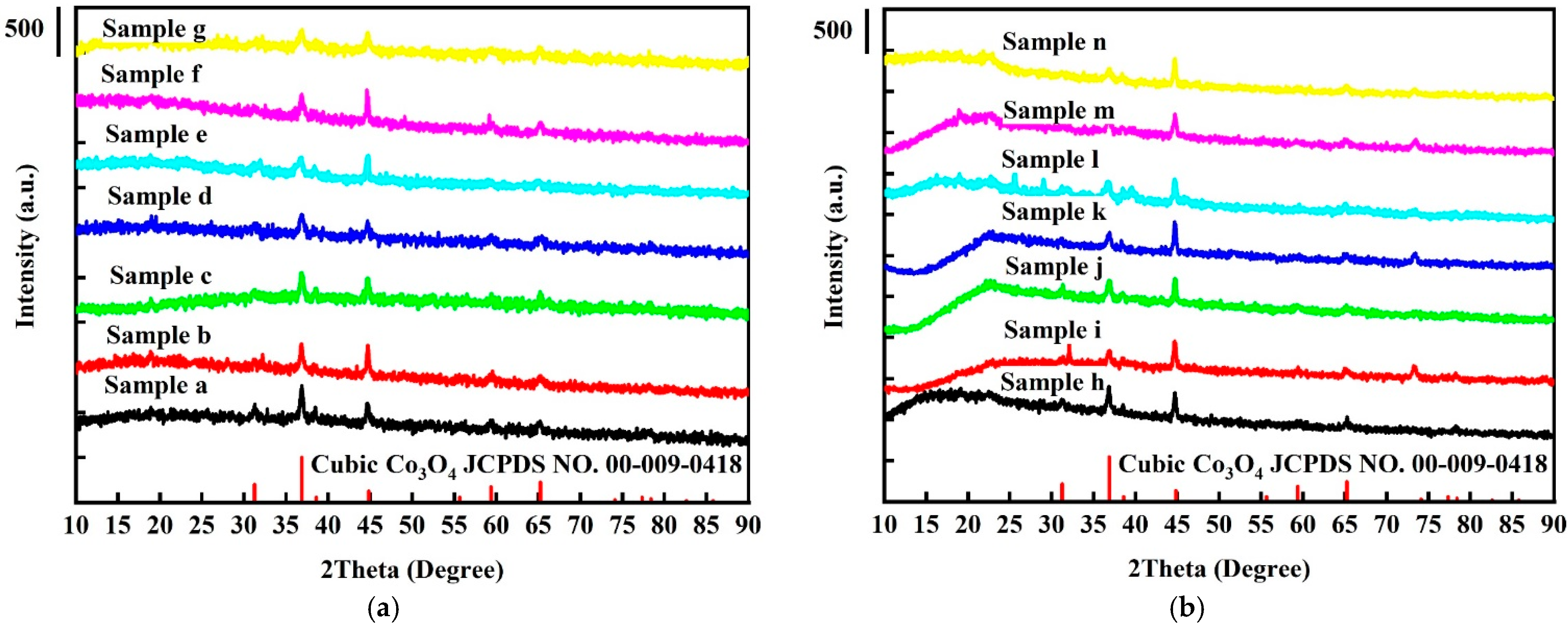
The XRD patterns of samples (a) to (g) (i.e., without platinum nanoparticles) are shown in Figure 1a, along with the standard Co3O4 diffraction peaks from the JCPDS cards. The pure cubic Co3O4 phase is observed in all samples, and no impurity peaks are apparent. Figure 1b shows the XRD patterns of Pt-/Co3O4 electrodes, samples (h) to (n). The XRD patterns are essentially identical to those of the Co3O4 electrodes without platinum nanoparticles, indicating that the platinum nanoparticles were evenly dispersed on the Co3O4 electrodes.
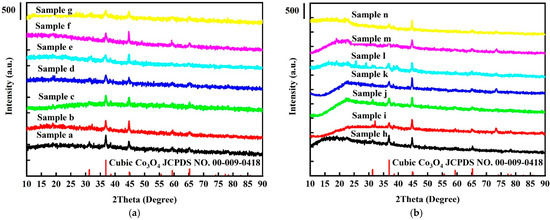
Figure 1.
XRD patterns of (a) pure Co3O4 electrodes and (b) Pt/Co3O4 electrodes, deposited on nickel foam substrates through electrochemical deposition under different conditions.
Table 1 shows the average crystallite size and lattice parameters of all samples. The average crystallite size was estimated from the half-maximum height of the diffraction peaks according to the Scherrer equation. The crystallite size was decreased from 34.91 nm to 19.02 nm as the electrochemical deposition time increased. However, there were no significant changes in the crystallite size when comparing the samples with and without platinum nanoparticles. As the deposition time increased, there was a decrease in the lattice parameters (8.08569 Å to 8.05214 Å) and unit cell volume (528.6288 Å3 to 522.0765 Å3), suggesting that the cobalt atoms moved away from oxygen vacancies in order to bind with other parts of the lattice, thereby decreasing the length of Co-O bonds. This result is consistent with [34].

Table 1.
The average crystallite size and lattice parameters of all samples.
2.2. Raman Spectroscopy Analysis
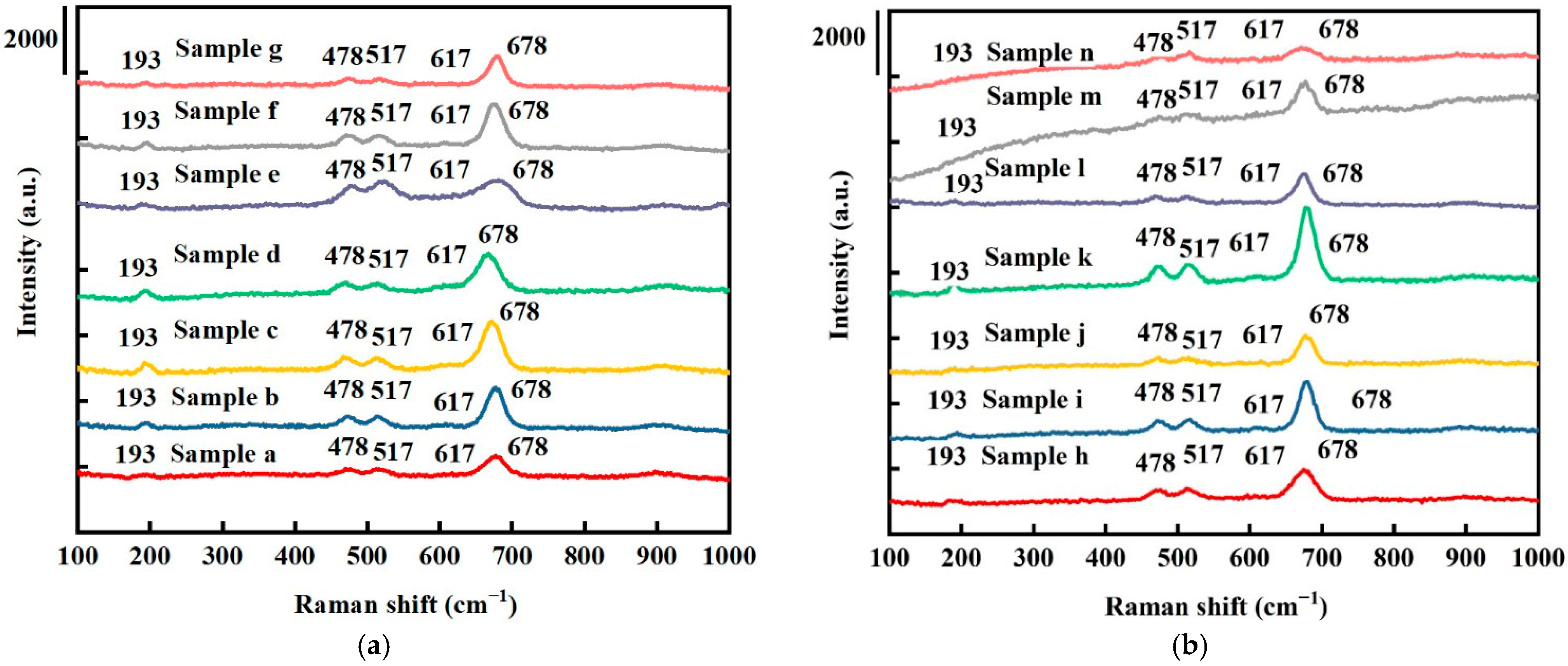
The Raman spectra for all samples are shown in Figure 2. The Raman bands of all samples at 193, 478, 517, 617, and 678 cm−1 correspond to the 3F2g, Eg, and A1g vibrational modes of Co3O4 [35]. The peaks at 193 and 678 cm−1 correspond to the tetrahedral sites (CoO4) and octahedral sites (CoO6) in the Co3O4 phase, respectively. No other phases and impurities were observed, in line with the XRD results. The higher frequencies of the A1g vibrational modes (617 and 678 cm−1) are an indication of oxygen vacancies, where richer oxygen vacancies are indicated by a decrease in the full width at half maximum (FWHM) peaks [9]. As shown in Figure 2a, comparing samples (b), (d), (e), (f), and (g), the peaks of the A1g vibrational modes (617 and 678 cm−1) gradually reduced, indicating increased oxygen vacancies [36]. As shown in Figure 2b, for the Co3O4 electrodes with platinum nanoparticles, no apparent peak position changes were observed.
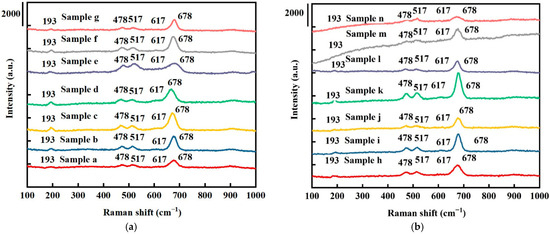
Figure 2.
Raman spectroscopy results for (a) pure Co3O4 electrodes and (b) Pt/Co3O4 electrodes, deposited on nickel foam substrates with varied deposition parameters.
2.3. FTIR Spectrum Studies
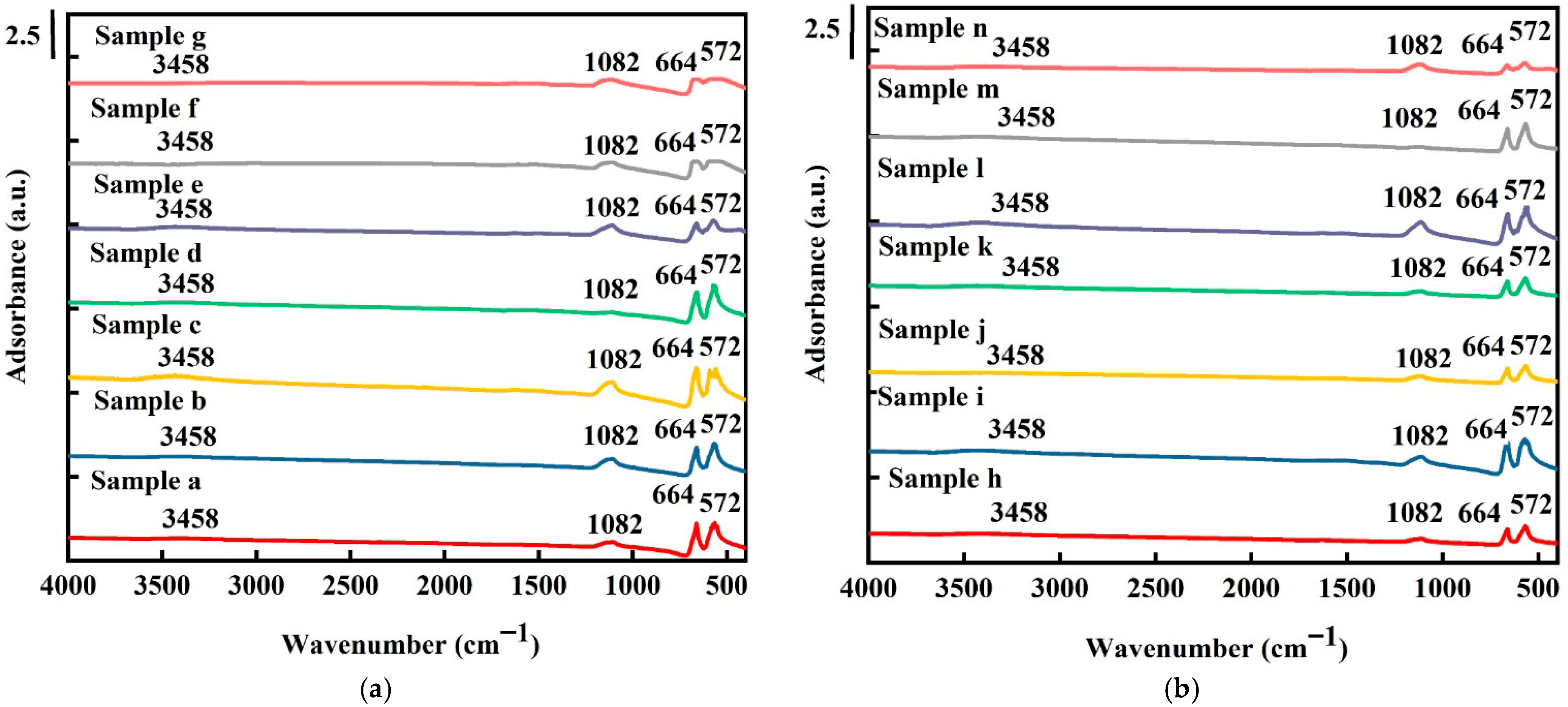
Figure 3 shows the FTIR spectra recorded from 450 to 4000 cm−1 at 25 °C for all samples. Absorption peaks at 572, 664, 1082, and 3458 cm−1 were detected. The two sharp absorption peaks at 572 and 664 cm−1 correspond to the stretching vibration modes of cobalt (II)-O and cobalt (III)-O, respectively, indicating the tetrahedral and octahedral sites in the Co3O4 structure [37]. As shown in Figure 3a, for the pure Co3O4 samples, the vibration frequency of cobalt(II)-O (572 cm−1) and cobalt(III)-O (664 cm−1) decreased with an increase in electrochemical deposition time in the order of sample (d) > sample (e) > sample (f) > sample (g). For both the pure and platinum-coated samples, a slight blue shift in the vibration frequency of both cobalt–O bonds was observed, indicating that oxygen vacancies play the role of donors [38]. A small peak at 1082 cm−1 was detected, which was attributed to C–O from the cobalt oxide precursor. The peaks corresponding to hydroxyl groups at 3458 cm−1 are, however, not evident during the electrochemical deposition reaction due to the occurrence of a dihydroxylation reaction during the deposition process [39]. H. Lin et al. [40] also reported that the hydroxyl group peak at 3458 cm−1 corresponds to two coordinate O2- protonation sites, indicating that the oxygen vacancies of the electrode not only had weakly bridged –OH peaks but were also significantly oxidized.
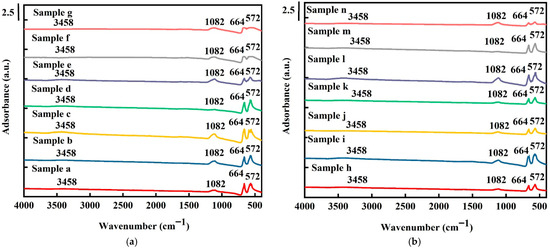
Figure 3.
FTIR spectra of (a) pure Co3O4 electrodes and (b) Pt/Co3O4 electrodes deposited on nickel foam substrates with varied deposition parameters.
2.4. Microstructure and Composition Analysis
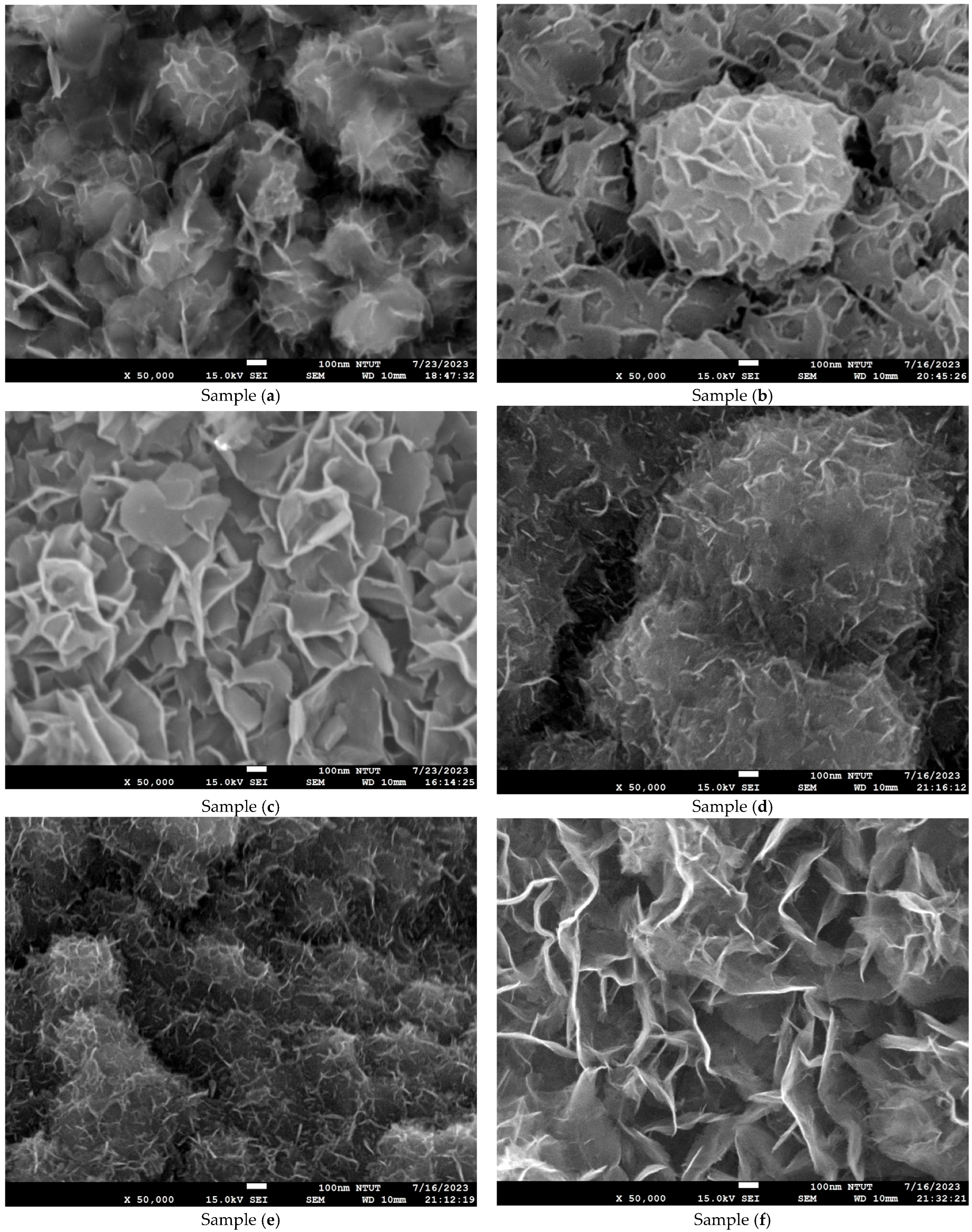
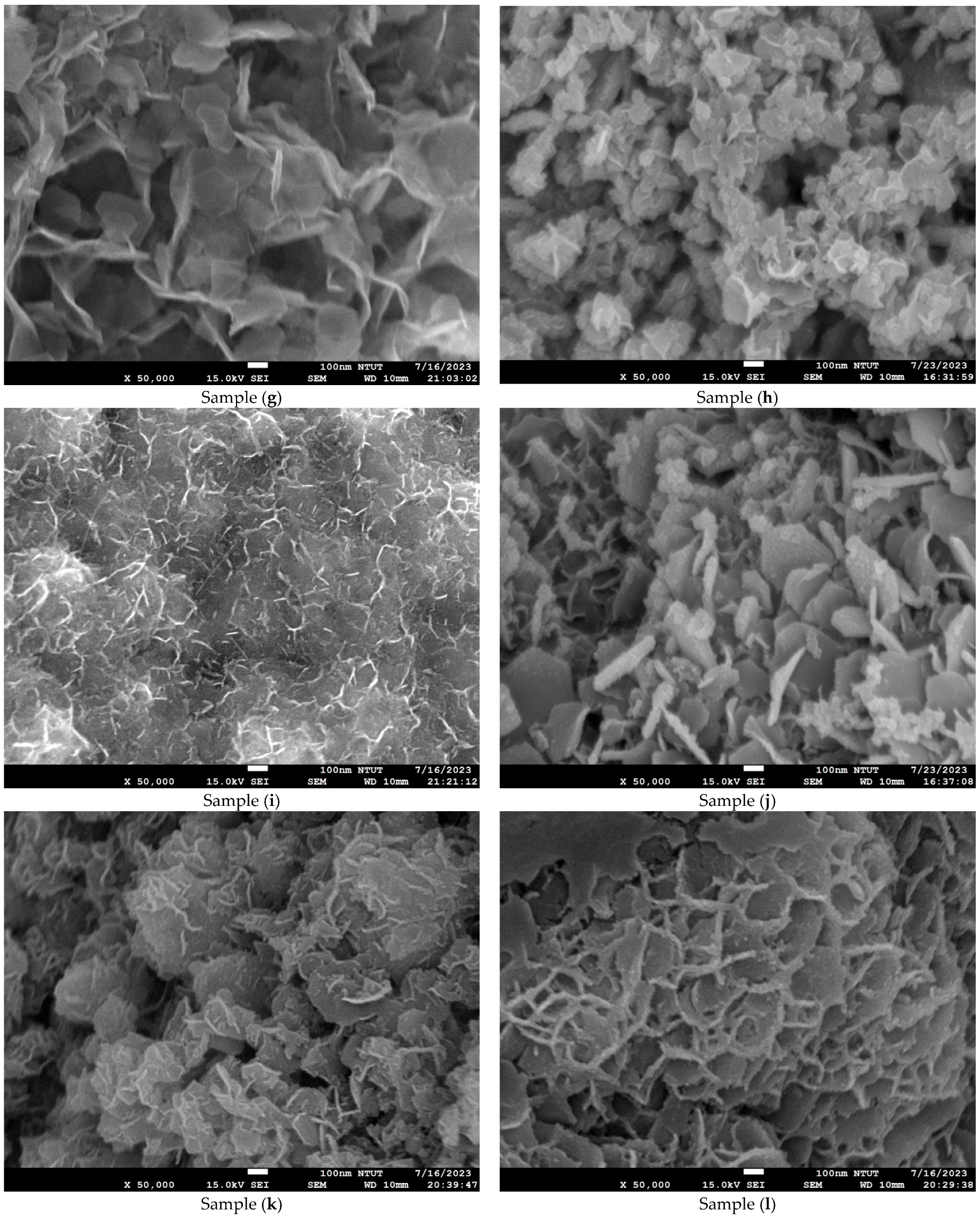
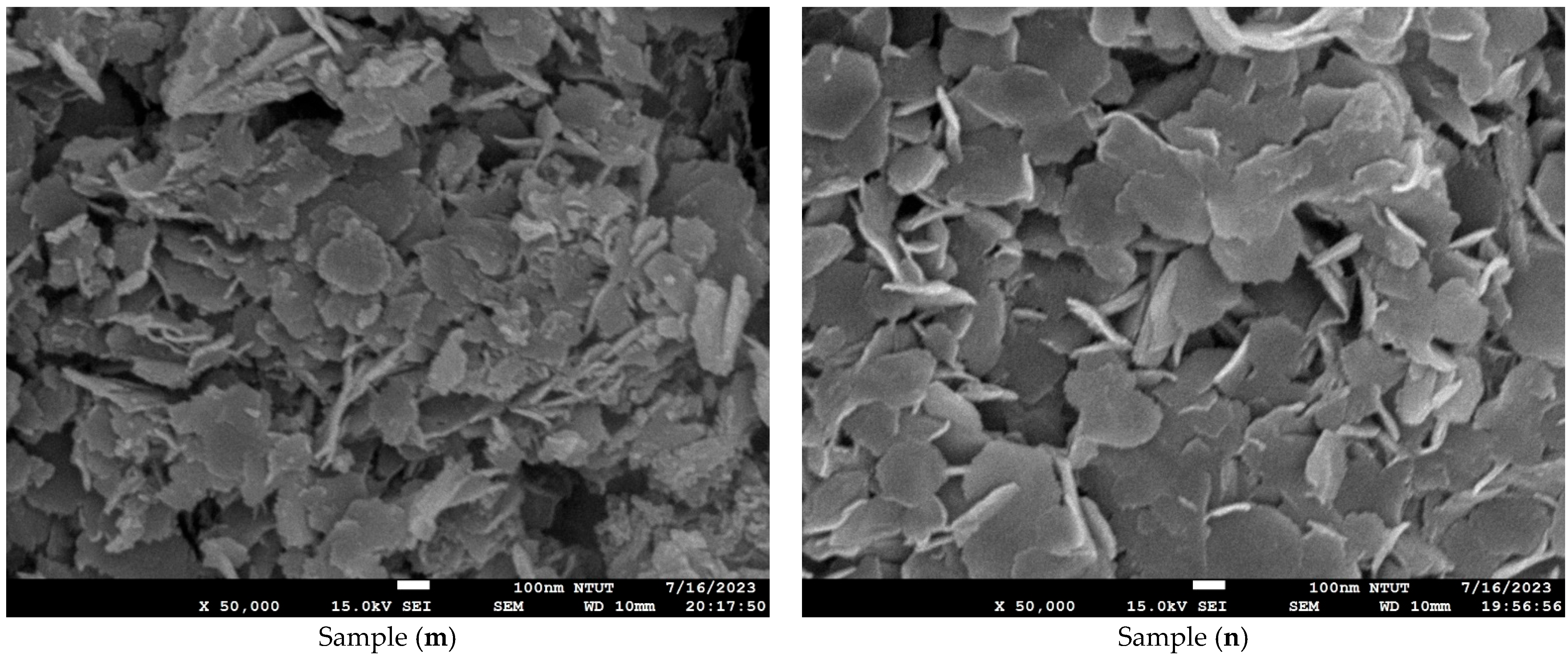
Figure 4 shows microstructure images of all samples obtained using FE-SEM at 50k(x). Curved three-dimensional (3D) hierarchical flower-like microstructures are observed on the surface of samples (a) to (c). Due to dihydroxylation reactions associated with oxygen vacancies on the surface, the microstructure of samples (d) to (e) is agglomerated and cracked [41]. An ultrathin hexagonal pattern can be seen in sample (f) and sample (g). As seen in Figure 4g, the two-dimensional (2D) hierarchical microstructures on the surface of sample (g) are made of hexagonal porous nanoplates. As shown in Figure 4h–n, for samples (h) to (n), the platinum nanoparticles are evenly dispersed on the Co3O4 electrodes. The diameter of the platinum nanoparticles is less than 5 nm. The differences in surface morphologies among the Co3O4 and Pt/Co3O4 electrodes can be attributed to the variations in the deposition parameters, including electrolyte concentration, applied voltage, and deposition time, as summarized in Table 5. At shorter deposition times (samples a–c), incomplete nucleation led to the formation of loose and flower-like structures composed of aggregated nanosheets. With an increasing deposition duration (samples d–g), enhanced hydroxylation and growth kinetics promoted the formation of more compact, interconnected hexagonal nanoplate networks. After platinum sputtering and subsequent electrodeposition (samples h–n), the surface became denser and exhibited fine granular morphologies due to the nucleation of the Pt nanoparticles, which acted as additional active centers and modified the local growth orientation of Co3O4. These morphological transitions indicate that the nucleation-growth behavior of Co3O4 is highly sensitive to the deposition time and electrolyte composition, while the incorporation of Pt nanoparticles refines the surface topology further and improves electron transport pathways.
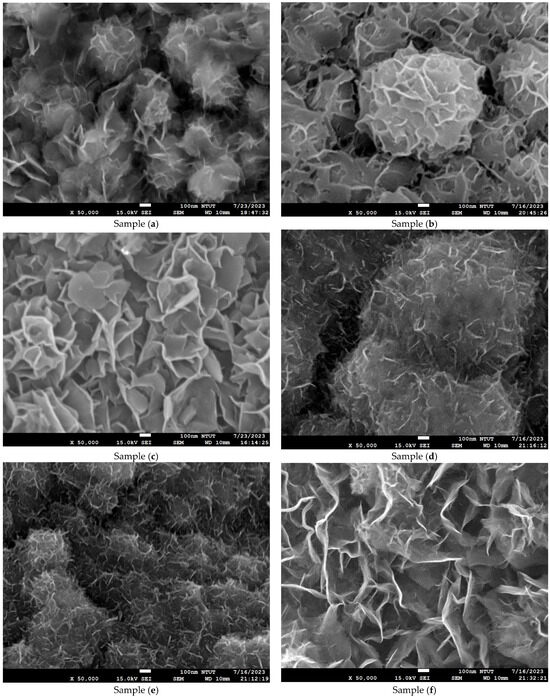
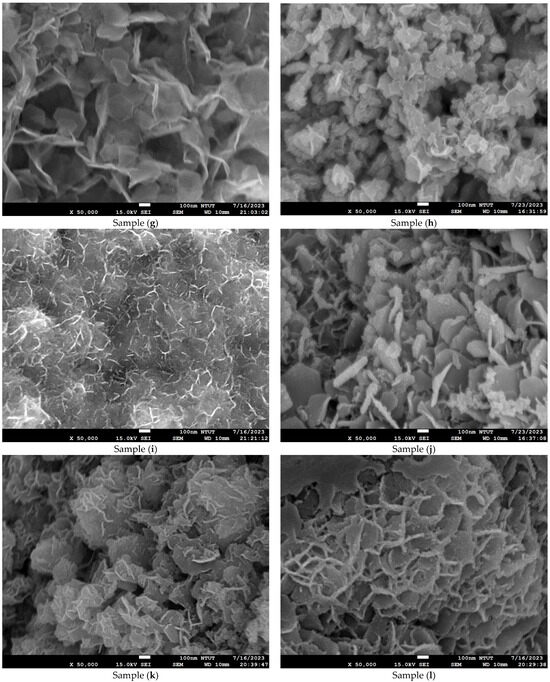
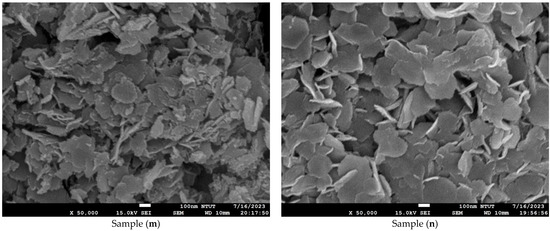
Figure 4.
Microstructure images of all samples with varied deposition parameters, obtained using FE-SEM at a magnification of ×50,000. (a–g) Samples without Pt coating prepared with 0.02 M Co(CH3COO)2·4H2O and varying Na2SO4 concentrations (0.01–0.04 M) under constant-voltage mode (5 V) for 10–50 min; (h–n) Samples coated with Pt by plasma sputtering at 30 kV, 8 mA for 3 min, using the same electrolyte conditions (0.02 M Co(CH3COO)2·4H2O, 0.01–0.04 M Na2SO4, pH 7) and deposition times of 10–50 min.
In particular, the variation in Na2SO4 concentration (0.01–0.04 M) influenced the ionic conductivity of the electrolyte and thus the diffusion rate of Co2+ ions during electrodeposition. A lower Na2SO4 concentration (sample a) resulted in slower ion transport and less uniform nucleation, producing loose, flower-like nanosheet structures. Increasing the Na2SO4 concentration (sample (c)) enhanced ionic conductivity and promoted denser nanosheet stacking with improved surface coverage. However, the XRD results confirmed that the crystal structure remained the same spinel Co3O4 phase, indicating that Na2SO4 concentration primarily affected the morphology rather than the crystallographic structure.
The atomic ratios of each element in the electrode samples were studied using EDX, and the results are presented in Table 2. The ranges of the ratios of cobalt:oxygen:platinum in all samples are 17.4–69.9%:0.0–82.6%:0.0–6.0%, respectively. Comparing samples (b), (d), (e), (f), and (g), it is also apparent that when the deposition time was increased, the atomic ratio of O decreased, indicating an increased oxygen vacancy concentration. A similar trend was observed for the Pt/Co3O4 electrodes. Particle size calculations for the samples were obtained using the ImageJ software (version 1.54f, National Institutes of Health, USA) to analyze the particle size distribution patterns. The order of particle size is as follows—sample (a), 495.081 nm > sample (l), 408.641 nm > sample (n), 238.953 nm—which indicates that the concentration of oxygen vacancies increases as the particle size decreases. The previous results from the XRD patterns, Raman spectroscopy, and FTIR spectra are consistent with this trend.

Table 2.
The atomic ratios of each element in all samples.
2.5. Textural and Structural Characterization
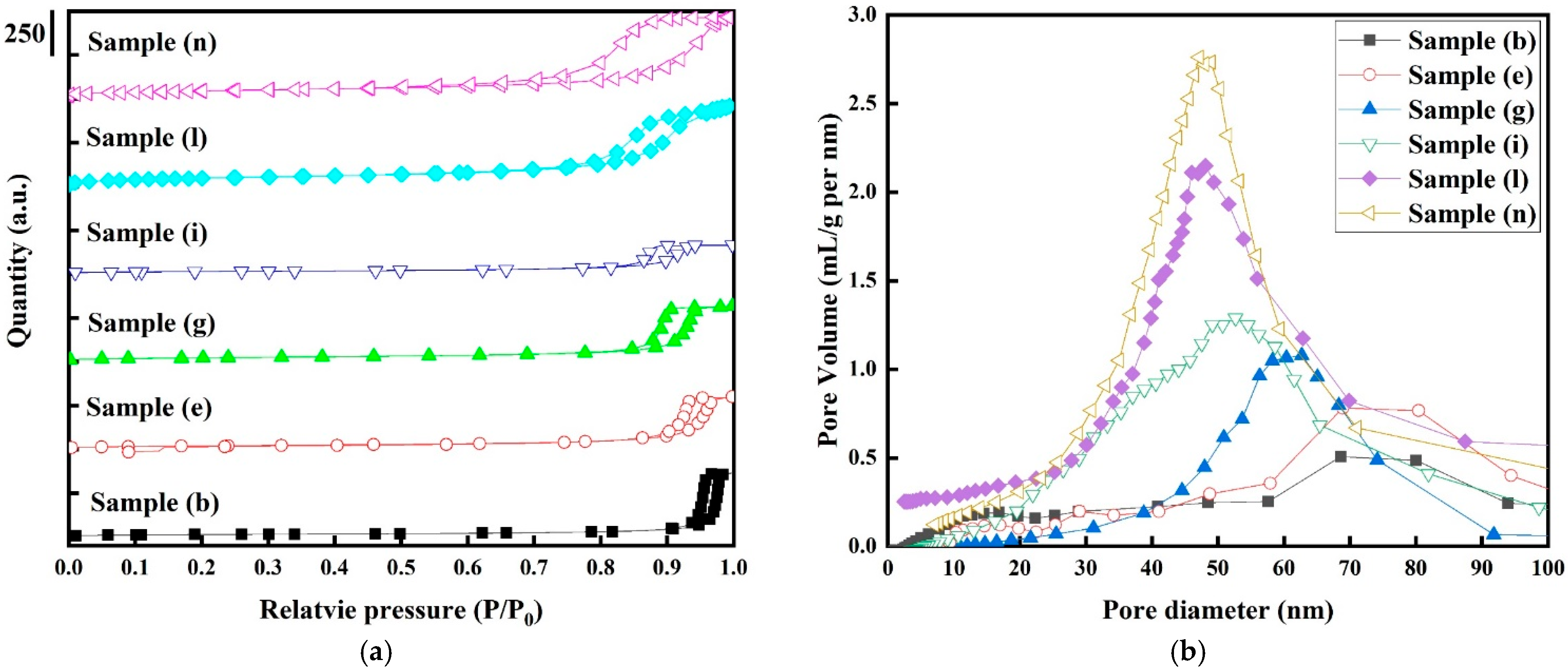
Figure 5a show the hysteresis loop between the nitrogen adsorption and desorption isotherms for the selected samples. According to the BDDT classification of adsorption isotherms, all samples exhibit the classic typical IV sorption isotherms in H2-type hysteresis loops, suggesting that all samples had mesoporous characteristics in their textural structure. Additionally, it is believed that the main reason for mesoporous structure characteristics in the textural structure is due to the interparticle space between Pt and CO3O4 nanoparticles [42]. It is noticeable that the nitrogen adsorption curves steadily rise to a relative pressure (P/P0) of 0.9 for all samples due to the increased volume of adsorbed nitrogen on the electrodes as a result of capillary condensation. The appearance of the H2-type hysteresis loops may be due to ink-bottle-like pores, with highly interconnected, narrow mouths and wider bodies in the samples [43].
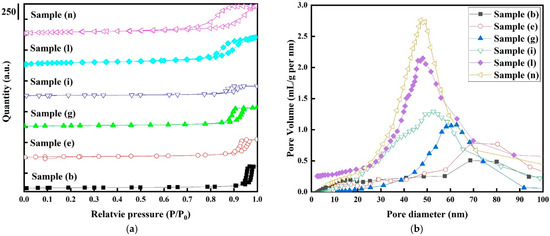
Figure 5.
(a) Nitrogen adsorption–desorption isotherms (hysteresis loops) and (b) pore size distribution curves for the selected electrode samples (b, e, g, i, l, and n). Only representative samples were chosen for BET and BJH analysis.
As seen in Table 3, the pore sizes of sample (b) for the pure Co3O4 electrodes and sample (n) for the Pt/Co3O4 electrodes are 26 nm at a higher relative pressure and 13 nm at a lower relative pressure. Interestingly, it can be discovered that the pore sizes were accompanied with a change in relative pressure [44]. Figure 5b shows the pore size distribution obtained using the Barrett–Joyner–Halenda (BJH) method for the Co3O4 electrodes with and without platinum nanoparticles. The center of the pore size distributions for the pure Co3O4 electrodes without platinum nanoparticles is located at around 75 nm, and the distributions extend to macropore sizes. However, apparent mesoporosity of narrower pores (3 to 87 nm) can be observed for the Pt/Co3O4 electrodes, indicating that the platinum particles introduced a hierarchical mesoporous structures into the samples [45].

Table 3.
Textural–structural characteristics of the selected samples.
The textural and structural characteristics for the selected samples are listed in Table 3. The specific surface areas from the BET analysis of sample (b), sample (e), sample (g), sample (i), sample (l), and sample (n) were 50, 53, 72, 80, 166, and 175 m2/g, respectively, and the average pore diameters were 26, 20, 25, 15, 14, and 16 nm, respectively. Sample (n) has the highest specific surface area from the BET and pore volume results, but the pore size is the lowest among the samples. These textural–structural characteristics are consistent with the results of the microstructural analysis. These samples are suitable for use as an electrode due to the presence of a hierarchical porous textural structure, which acts as a good ion reservoir, allowing for ion transport and charge storage [46].
2.6. Electrochemical Characterization Analysis
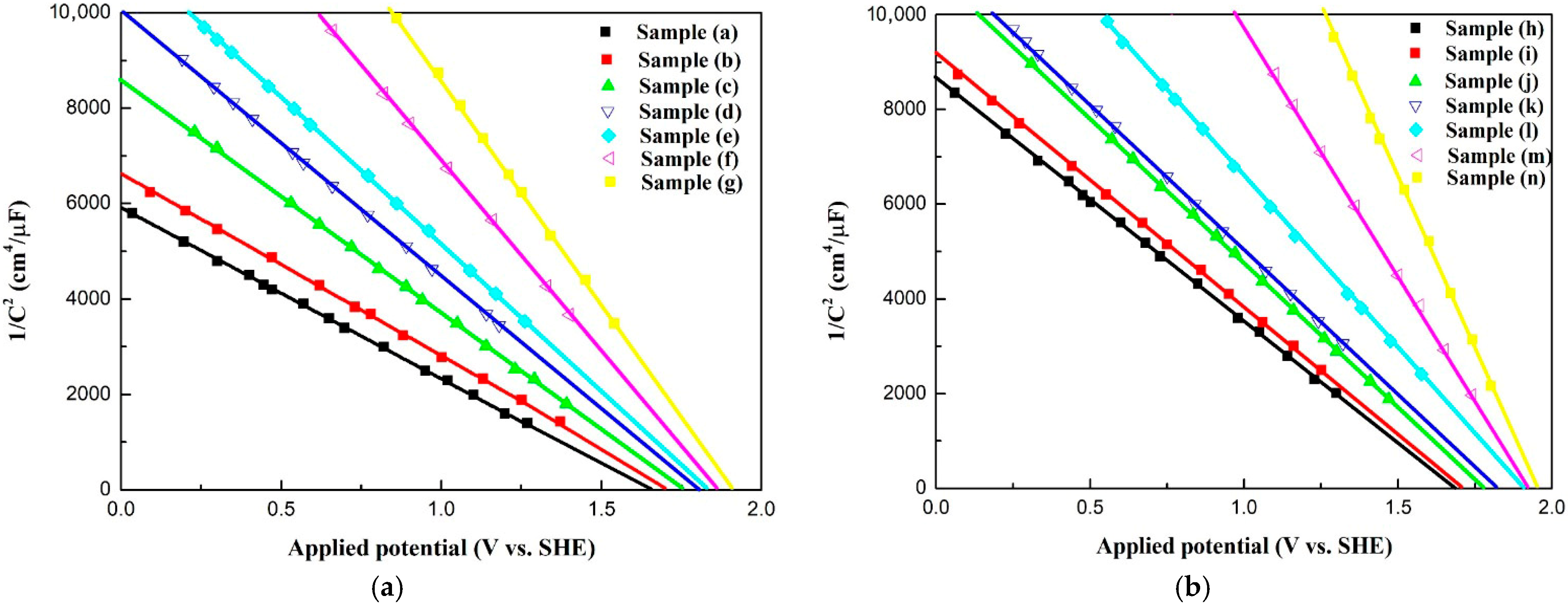
Figure 6 shows the Mott–Schottky curves for all samples. According to Figure 6, both sets of Co3O4 electrodes are p-type semiconductors. The electrochemical properties for all samples are shown in Table 4. The values of the flat band potentials for all samples lie in the range of 1.65 V to 1.95 V vs. the standard hydrogen electrode (SHE) for p-type compounds. The flat band potentials of the Co3O4 electrodes shift toward more positive potentials with an increase in the oxygen vacancy concentration. Table 4 shows the corresponding carrier densities of the Co3O4 electrodes, calculated using the Mott–Schottky curves. The carrier densities of the Co3O4 electrodes are between 5.77 × 1014 cm−3 and 9.60 × 1014 cm−3 and increase with the oxygen vacancy concentration.
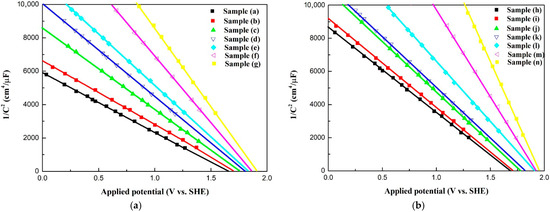
Figure 6.
Mott–Schottky curves for all samples under varied electrodeposition conditions. (a) Samples without Pt coating (a–g); (b) Samples coated with Pt by plasma sputtering (h–n).

Table 4.
Summary of the electric properties of all samples.
The electrocatalytic performance of all samples in chlorine evolution reactions (CERs) was evaluated. CERs were conducted in 0.6 M NaCl electrolyte solution at a pH of 7 based on the following mechanisms [47]:
Reduction at the cathode:
H2O(l) + 2 e− → H2 (g) + 2 OH−
Oxidation at the anode:
2Cl−(aq) + Cl2(g) + 2e−
gfH2O (l) → OH (ads) + H+ + e−
Overall reaction:
NaCl (aq) + H2O(l) → Na+(aq) +OH−(aq) + H2(g) +0.5 Cl2(g)
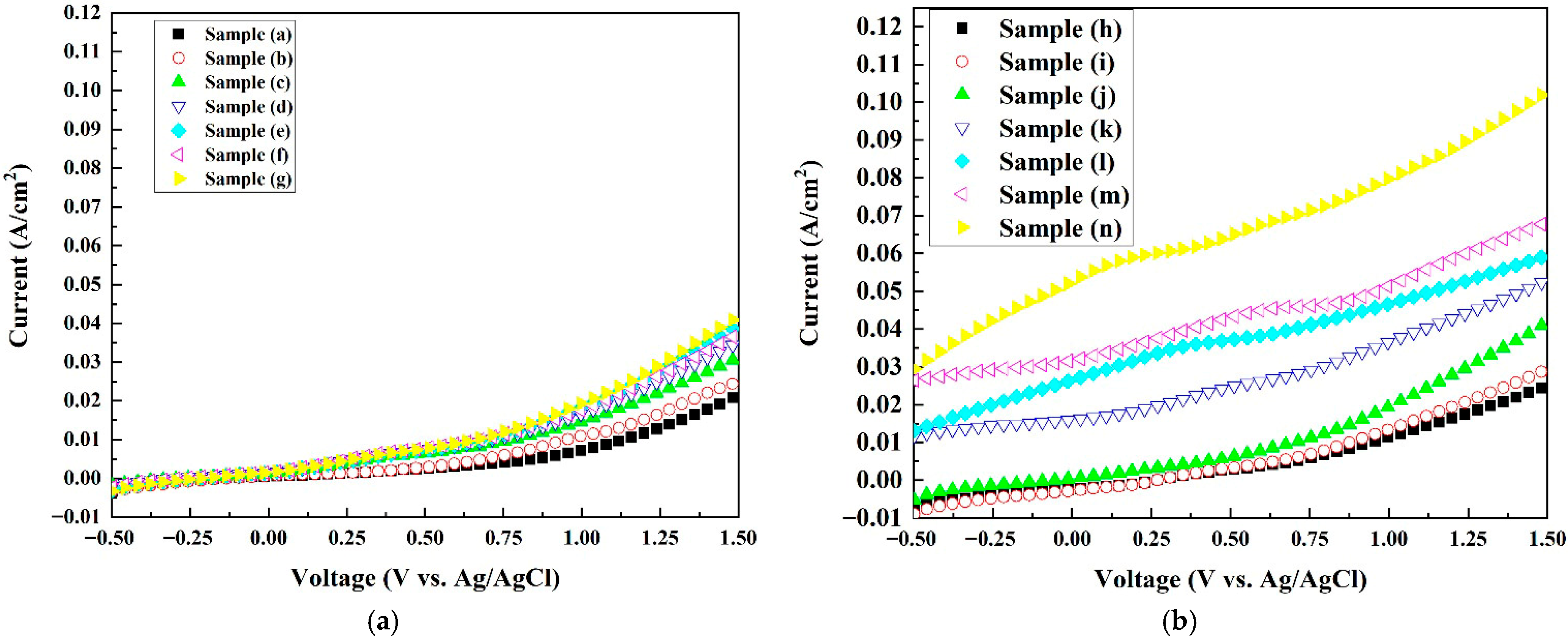
Figure 7 shows the current densities of all samples evaluated via linear scanning voltammetry (LSV) with a sweep rate of 5 mV/s and an applied potential between −0.5 V and +1.50 V versus an Ag/AgCl reference electrode. As shown in Figure 7a, for the pure Co3O4 electrodes, under an external potential of +1.5 V, the current density increased from 0.02 A/cm2 for sample (a) to 0.04 A/m2 for sample (g), signaling the trend of an increasing current density with an increase in the oxygen vacancy concentration. The flat band potentials increased from sample (a) to sample (g), i.e., from 1.65 V to 1.90 V vs. the SHE. This indicates that sample (g) had the highest driving force and thus current density among the pure Co3O4 samples. With the addition of platinum nanoparticles, as shown in Figure 7b, the current density improved from 0.04 A/cm2 for sample (g) to 0.103 A/cm2 for sample (n) at an external potential of +1.5 V.
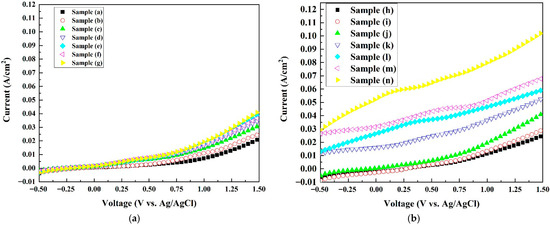
Figure 7.
Current density versus applied voltage curves of the electrode samples measured in 0.6 M NaCl solution at pH = 7. (a) Pure Co3O4 electrodes (samples a–g); (b) Pt/Co3O4 electrodes prepared by plasma sputtering (samples h–n).
Sample (n) shows higher energy potential holes than those in other samples due to its highest positive flat band potential (1.95 V vs. the SHE) among all samples, leading to the largest current density (0.103 A at an external potential of 1.5 V vs. Ag/AgCl). The introduction of transition metals into the catalysts enhanced the catalytic oxidation activity. As deduced in [46,48], due to the interaction between the platinum and the catalyst, the platinum d-band moved away from the flat band potential, thus improving the desorption ability of the intermediate *OH and thus the electrochemical performance.
In comparison, previous studies on Co3O4-based anodes for the chlorine evolution reaction (CER) have reported current densities typically in the range of ~0.01–0.10 A cm−2 at similar potentials. Zhu et al. [20] demonstrated Co3O4 nanobelt arrays achieving a current density of ~0.08 A cm−2 under ~+1.4 V vs. the RHE. Wang et al. [25] reported a Co3O4/graphite-felt electrode achieving 10 mA cm−2 (~0.01 A cm−2) at a ~1.06 V onset potential. These comparisons show that the Pt/Co3O4 electrode in this work (0.103 A cm−2 at +1.5 V) is competitive with and in some cases superior to published Co3O4-based systems for the CER.
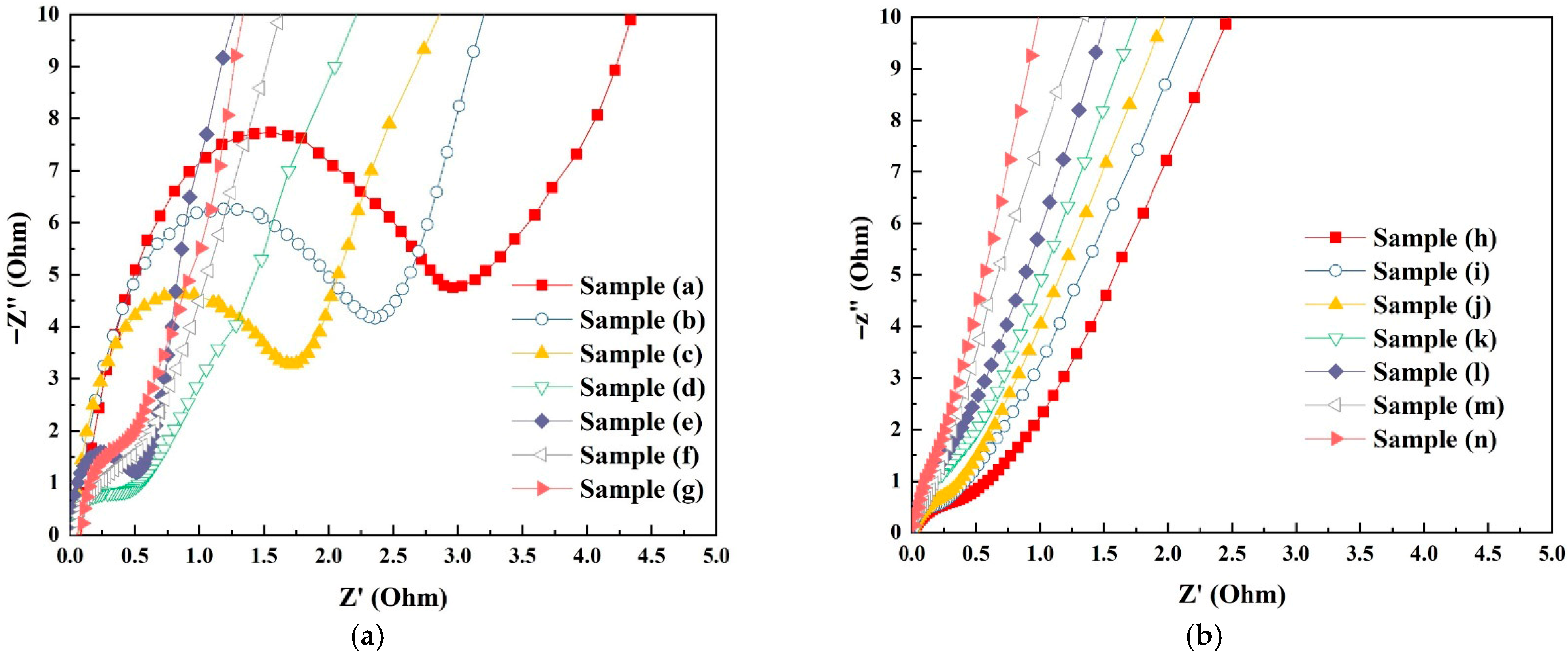
It is well known that electron charge transfer and diffusion play an important role in determining the electrochemical performance. As seen in Figure 8, the Nyquist plots show a straight line in the low-frequency section and semicircle-like shapes in the high-frequency section for all samples. The slope of the straight line and the diameter of the semicircle-like shapes are correlated with the diffusive resistances (the diffusion of the electrolyte and protons in the materials) and the charge transfer resistance (the sum of the electrolyte resistance in the host materials) [49]. The semicircle’s diameter and the linear line’s slope represent the electron transfer resistance and the efficiency of ion diffusion, respectively. As seen in Figure 8a, the AC resistance decreases in the following order, sample (a) (2.97 Ω) > sample (b) (2.37 Ω) > sample (c) (1.72 Ω) > sample (d) (0.53 Ω) > sample (e) (0.50 Ω) > sample (f) (0.45 Ω) > sample (g) (0.34 Ω), following the trend of increasing oxygen vacancies. Lower AC resistance will result in better electrochemical efficiency due to low diffusion resistance between the electrolyte and protons in the electrodes. Figure 8b shows the Nyquist plots for the Pt/Co3O4 electrodes in the frequency range of 0.1 kHz to 10 × 106 Hz at room temperature. It is apparent that there are minimal semicircle-like shapes in these samples, indicating minimal AC resistance and thus a higher charge transfer efficiency [50]. The improvement in AC resistance can be attributed to the increased oxygen vacancies and the interaction between the platinum-active sites and oxygen vacancies [50].
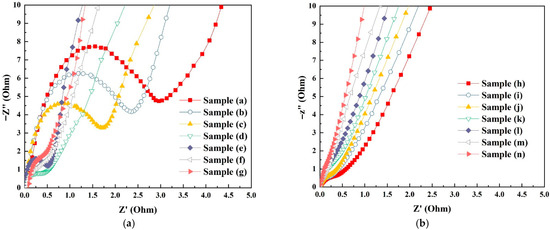
Figure 8.
Nyquist plots showing the real and imaginary impedance components of the electrode samples measured at room temperature in the frequency range of 0.1 kHz to 10 × 106 Hz. (a) Pure Co3O4 electrodes (samples a–g); (b) Pt/Co3O4 electrodes prepared by plasma sputtering (samples h–n).
2.7. Time-Dependent Chlorine Evolution
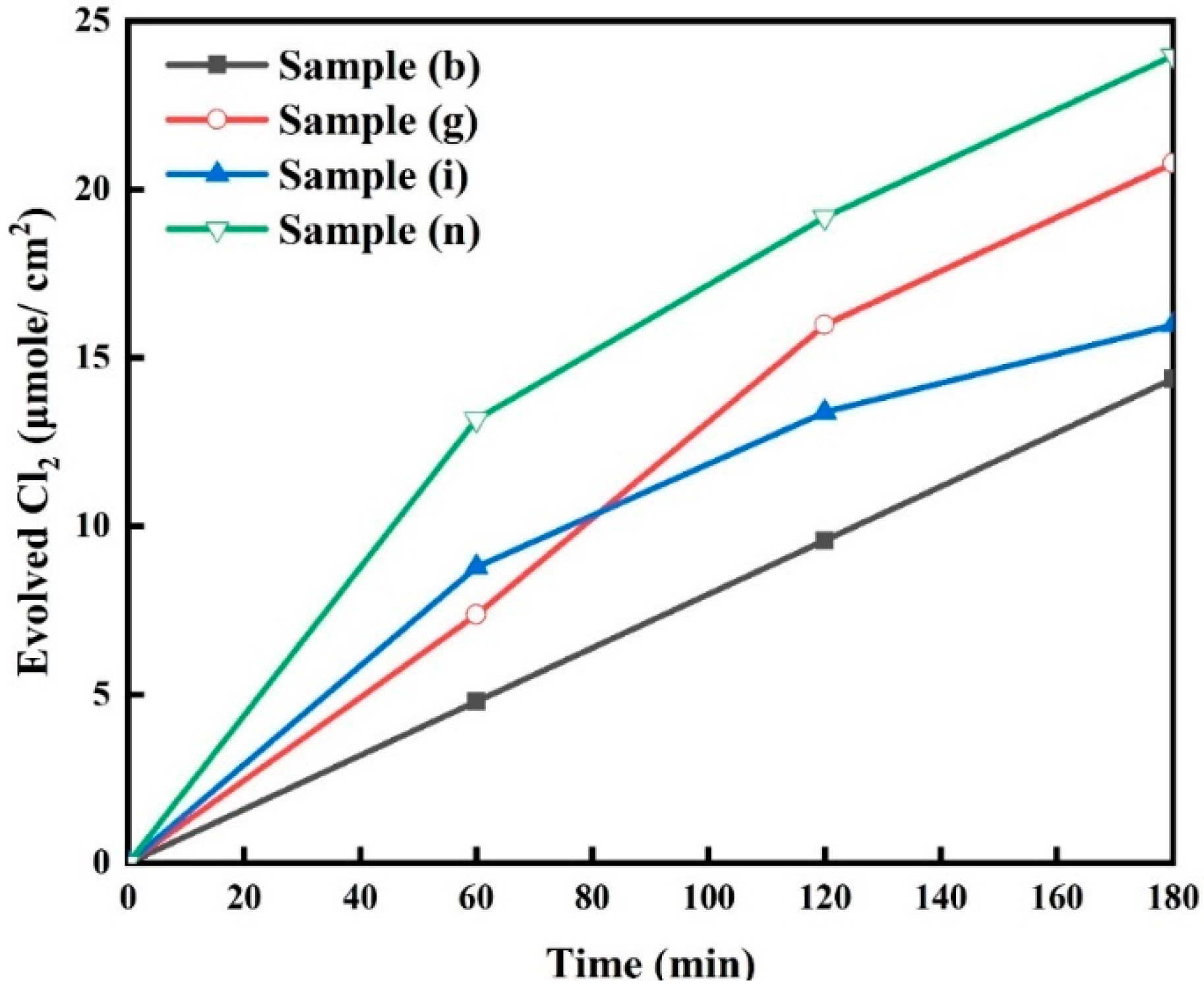
The chlorine evolution results for selected samples, sample (b), sample (g), sample (i), and sample (n), in 0.6 M NaCl solution at a pH of 7 are shown in Figure 9. The amount of evolved chlorine at 180 min was found to be 23.92 µmol/cm2 from sample (n), while the lowest was 14.26 µmol/cm2 from sample (b). The amounts of evolved chlorine from sample (g) and sample (i) were 20.79 µmol/cm2 and 15.91 µmol/cm2, respectively. Sample (n) appears to be the best candidate due to several reasons:
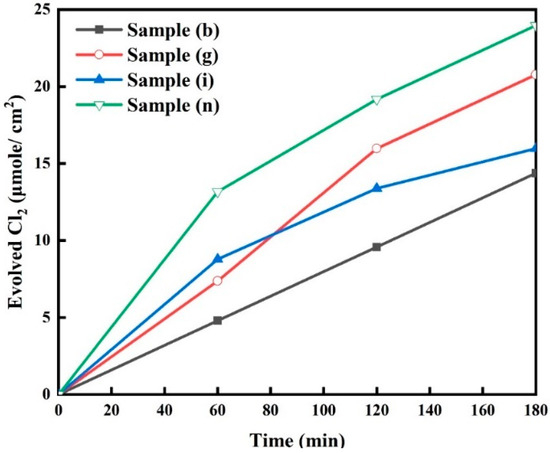
Figure 9.
Catalyzed chlorine evolution results of the electrode samples measured in 0.6 M NaCl solution at pH = 7 for the selected samples (b, g, i and n).
- The sample exhibited the highest charge transfer efficiency with minimal AC resistance and consequently the highest flat band potential (as presented in Figure 8).
These results indicate that increasing the electrodeposition time resulted in richer oxygen vacancies, which altered the textural and microstructural features of the electrode and improved the carrier density. Additionally, introducing Pt into the electrode significantly improved the charge transfer efficiency with minimal AC resistance. These factors (oxygen vacancies and Pt) subsequently increased the electrocatalytic efficiency of the electrode.
X. Li et al. [51] also found that the redox reactivities of MnMoO4 improved when oxygen vacancies were introduced into electronic structures. J. Zeng et al. [52] reported that oxygen vacancies introduced into the structure of a MnO2 catalyst greatly enhanced the catalytic performance and lifetime. When oxygen vacancies were introduced, the potential of the MnO2 catalysts shifted to become more positive, leading to increased oxidative abilities compared with those of pure MnO2 without oxygen vacancies.
3. Experimental
3.1. Materials
Cobalt (II) acetate (Co(CH3COO)2·4H2O), ≥99.5%) and sodium sulfate (Na2SO4, ≥99.5%) were obtained from Sigma Aldrich Co. (Steinheim, Germany). Sodium hydroxide (NaOH, ≥99%) and sulfuric acid (H2SO4, ≥98%) were purchased from Merck Co. (Darmstadt, Germany). These chemicals were used as received with no further purification. Nickel foam (99.8% purity, thickness ≈ 1.5 mm, porosity ≈ 95%) was purchased from Alfa Aesar and used as the conductive substrate for electrodeposition (the SEM image of the pristine nickel foam is shown in Appendix A). Prior to use, the nickel foam was cut into 1 × 1 cm2 pieces. Acetone, deionized water, and ethanol were applied for 30 min to clean the nickel foam substrates prior to deposition. Subsequently, nickel foam substrates were washed using deionized water and blow-dried using nitrogen gas.
3.2. Co3O4 Electrode Fabrication
Co3O4 electrodes were prepared from a solution of Co(CH3COO)2·4H2O (0.8 M, 25 mL) and Na2SO4 (at various concentrations) which was constantly stirred for 30 min before electrodeposition in order to produce a homogeneous solution. All electrodepositions were performed in a three-electrode system, comprising a silver chloride electrode as the reference electrode, a platinum sheet as the counter electrode, and a nickel foam substrate as the working electrode. The electrochemical deposition was carried out at 5 V (vs. Ag/AgCl reference electrode) under various deposition times (10, 20, 30, 40, and 50 min). This produced a black homogeneous layer coating on the nickel foam substrate. The prepared samples were washed with deionized water, blow-dried using nitrogen gas, baked at a constant ramp rate (5 °C per minute) from room temperature to 300 °C, and held for 1 h under ambient air.
The electrodeposition parameters are shown in Table 5. Samples (a), (b), and (c) were used to test the effect of Na2SO4 concentration. Samples (d) to (g) were used to test the effect of electrochemical deposition time. Samples (h) to (n) had platinum nanoparticles deposited via a plasma sputtering process at 30 kV and 8 mA for 3 min.

Table 5.
The electrodeposition parameters of the Co3O4 electrodes.
3.3. Material Characterization
The crystallographic studies of the Co3O4 electrodes were carried out using an X-ray diffractometer (XRD, Model PANalytical Empyrean, Malvern Panalytical B.V., Almelo, The Netherlands) at 45 kV and 40 mA with Cu Kα radiation (λ = 1.5405 Å) in the 2THETA range between 10° and 90°. Raman spectroscopy was conducted using a Micro-Raman (Ramboss 500i, DongWoo Optron Co., Ltd., Gwangju, Republic of Korea) with a charge-coupled device detector and an Argon ion laser (532 nm) in a wavenumber range from 400 to 1000 cm−1. The functional groups on the electrode surface were studied using a Fourier Transform Infrared (FTIR) spectrometer with a Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) technique in a wavenumber range between 400 and 4000 cm−1. The surface microstructure of the Co3O4 electrodes was observed using a Field Emission Scanning Electron Microscope (FE-SEM, Model JEOL JSM-7610F, JEOL Ltd., Tokyo, Japan) with an energy-dispersive X-ray (EDX) analyzer (Oxford Instruments, Abingdon, UK). The accelerating voltage of the FE-SEM was kept at 15 kV. The textural structure of the electrodes was analyzed using measurements of the surface area and porosity with Micromeritics ASAP 2020 (Micromeritics Instrument Corp., Norcross, GA, USA) and using the Brunauer–Emmett–Teller (BET) technique at a temperature of −196.15 °C. Prior to the textural structure analysis, the samples were thermally treated at 150 °C for 24 h using degassing stations to remove impurities.
3.4. Electrical Property Measurements
Current density was measured in a Pyrex cell using a computer-controlled electrochemical workstation (ZIV model SP1) with a three-electrode system consisting of a Co3O4 electrode as the working electrode, a platinum plate as the counter electrode, and an Ag/AgCl electrode as the reference electrode. The applied potential was in the range of −0.5 to 1.5 V. The conductive layer of the working electrode was connected to a copper wire using silver paste, and its back and edge were sealed with epoxy resin. A 0.6 M NaCl solution with a pH of 7 was used as the electrolyte, and this was degassed using ultra-pure nitrogen gas prior to the current density measurement. The electrode resistance was determined through electrochemical impedance spectroscopy (EIS) in a frequency range between 100 kHz and 100 mHz using an open-circuit voltage amplitude of 5 mV. Mott–Schottky measurements of all samples were recorded via a computer-controlled electrochemical workstation (ZIV model SP1) with a frequency response analyzer (FRA modules).
The flat band was determined by measuring the capacities of the interface layer (such as the capacities of the space charge and the Helmholtz layer) between the space charge capacity for catalysts and the Helmholtz layer for the electrolyte via the vital Mott–Schottky curves. The following Mott–Schottky formula relating the space charge capacitance to the applied voltage was used:
where C is the capacitance of the space charge; E is the applied voltage; EFB is the potential of the flat band for electrochemical catalysts; ε is the basic charge for electrochemical catalysts; ε0 is the permittivity in a vacuum; ND is the electron carrier density of the electrochemical catalysts; T is room temperature (25 °C); k is the Boltzmann constant (1.38 × 10−23 J/K); and A is the area of the electrochemical catalyst/electrolyte interface. When electrochemical catalysts are n-type, B is 1. The potential of the flat band can be calculated using the intersection () of and the E plot with the E-axis
3.5. Chlorine Evolution Measurement
Chlorine evolution was conducted using the Co2O4 electrodes with a 1 × 1 cm2 exposure area in 0.6 M NaCl solution at a pH of 7. No bias voltage was applied to the Co3O4 electrodes as the working electrode and the platinum plate as the counter electrode in a two-electrode system. The composition of the evolved chlorine gas was measured using gas chromatography–mass spectrometry (GC–MS) (Agilent, model 7890B/5977A, Santa Clara, CA, USA).
4. Conclusions
Co3O4 electrodes rich in oxygen vacancies were prepared through electrochemical deposition on nickel foam substrates using different deposition parameters. From the XRD patterns, the main crystalline structure for all the electrodes was found to be a cubic Co3O4 phase. Using Mott–Schottky curves, all samples were found to be p-type semiconductors, which was likely due to the presence of oxygen vacancies. Also, from the Mott–Schottky curves, the carrier densities of the samples were found to be between 5.77 × 1014 cm−3 and 8.77 × 1014 cm−3. For an external potential of 1.5 V versus an Ag/AgCl reference electrode, the maximum current density among all of the electrodes was 102.66 mA/cm2. Additionally, the chlorine production rate can be improved from 14.26 µmol/cm2 to 23.92 µmol/cm2, by around 1.67 times, by increasing the oxygen vacancy concentration on the Pt/Co3O4 electrodes.
Author Contributions
Conceptualization, G.-T.P. and A.N.N.; methodology, G.-T.P.; software, G.-T.P.; validation, G.-T.P. and A.N.N.; formal analysis, G.-T.P.; investigation, G.-T.P.; resources, A.N.N.; data curation, G.-T.P.; writing—original draft preparation, G.-T.P.; writing—review and editing, G.-T.P. and A.N.N.; visualization, G.-T.P.; supervision, A.N.N.; project administration, A.N.N.; funding acquisition, A.N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by the authors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Thomas C.-K. Yang and the Centre of Precision Analysis and Material Research Precision Analysis, National Taipei University of Technology, for their assistance with the material analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Figure A1 a SEM image of pristine nickel foam substrate showing its 3D microporous framework and interconnected ligament structure (magnification: ×100). The open-cell structure facilitates electrolyte penetration and provides a large surface area for Co3O4 electrodeposition.

Figure A1.
A SEM image of pristine nickel foam showing the open 3D porous network structure (magnification: ×100).
Figure A1.
A SEM image of pristine nickel foam showing the open 3D porous network structure (magnification: ×100).

References
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Moreno-Hernandez, I.A.; Brunschwig, B.S.; Lewis, N.S. Crystalline nickel, cobalt, and manganese antimonates as electrocatalysts for the chlorine evolution reaction. Energy Environ. Sci. 2019, 12, 1241–1248. [Google Scholar] [CrossRef]
- Nasirpouri, F.; Alipour, K.; Daneshvar, F.; Sanaeian, M.-R. Electrodeposition of anticorrosion nanocoatings. In Corrosion Protection at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2020; pp. 473–497. [Google Scholar]
- Jo, H.-G.; Kim, K.-H.; Ahn, H.-J. Well-dispersed Pt/RuO2-decorated mesoporous N-doped carbon as a hybrid electrocatalyst for Li–O2 batteries. RSC Adv. 2021, 11, 12209–12217. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, H.; Feng, X.; Lei, L.; He, Y.; Zhang, X. Neodymium-Doped IrO2 Electrocatalysts Supported on Titanium Plates for Enhanced Chlorine Evolution Reaction Performance. ChemElectroChem 2021, 8, 1204–1210. [Google Scholar] [CrossRef]
- Clayton, J.A.; Walton, R.I. Development of New Mixed-Metal Ruthenium and Iridium Oxides as Electrocatalysts for Oxygen Evolution: Part II: Mechanistic understanding and practical considerations. Johns. Matthey Technol. Rev. 2022, 66, 406–417. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Y.; Wu, L.; Chen, R.; Wang, J.; Chang, S.; Ma, F.; Li, Y.; Ni, H. RuO2/IrO2 nanoparticles decorated TiO2 nanotube arrays for improved activity towards chlorine evolution reaction. Catal. Today 2022, 400, 26–34. [Google Scholar] [CrossRef]
- Palma-Goyes, R.; Vazquez-Arenas, J.; Ostos, C.; Torres-Palma, R.; González, I. The effects of ZrO2 on the electrocatalysis to yield active chlorine species on Sb2O5-doped Ti/RuO2 anodes. J. Electrochem. Soc. 2016, 163, H818. [Google Scholar] [CrossRef]
- Ren, Q.; Feng, Z.; Mo, S.; Huang, C.; Li, S.; Zhang, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. 1D-Co3O4, 2D-Co3O4, 3D-Co3O4 for catalytic oxidation of toluene. Catal. Today 2019, 332, 160–167. [Google Scholar] [CrossRef]
- Jiang, S.; Suo, H.; Zhang, T.; Liao, C.; Wang, Y.; Zhao, Q.; Lai, W. Recent advances in seawater electrolysis. Catalysts 2022, 12, 123. [Google Scholar] [CrossRef]
- Bae, J.; Shin, D.; Jeong, H.; Kim, B.-S.; Han, J.W.; Lee, H. Highly water-resistant La-doped Co3O4 catalyst for CO oxidation. ACS Catal. 2019, 9, 10093–10100. [Google Scholar] [CrossRef]
- Dissanayake, S.; Wasalathanthri, N.; Amin, A.S.; He, J.; Poges, S.; Rathnayake, D.; Suib, S.L. Mesoporous Co3O4 catalysts for VOC elimination: Oxidation of 2-propanol. Appl. Catal. A Gen. 2020, 590, 117366. [Google Scholar] [CrossRef]
- Anele, A.; Obare, S.; Wei, J. Recent trends and advances of Co3O4 nanoparticles in environmental remediation of bacteria in wastewater. Nanomaterials 2022, 12, 1129. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Barmi, M.J. Novel lead–cobalt composite anodes for copper electrowinning. Hydrometallurgy 2013, 137, 45–52. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Barmi, J.M. Dimensionally Stable Lead-Cobalt Oxide Coated Composite Anodes. Australian Patent 2013901230, 15 April 2013. [Google Scholar]
- D’Aloya, A.; Nikoloski, A.N. An electrochemical investigation of the formation of CoSx and its effect on the anodic dissolution of iron in ammoniacal-carbonate solutions. Hydrometallurgy 2013, 131, 99–106. [Google Scholar] [CrossRef]
- Barmi, M.J.; Nikoloski, A.N. Electrodeposition of lead–cobalt composite coatings electrocatalytic for oxygen evolution and the properties of composite coated anodes for copper electrowinning. Hydrometallurgy 2012, 129, 59–66. [Google Scholar] [CrossRef]
- Nikoloski, A.; Nicol, M. Addition of cobalt to lead anodes used for oxygen evolution—A literature review. Miner. Process. Extr. Metall. Rev. 2009, 31, 30–57. [Google Scholar] [CrossRef]
- Yang, G.; Cheng, Q.; Liao, F.; Mao, L.; Zhao, X.; Chen, L. Two-dimensional porous CeO2@ Co3 O4 sheet-like heterostructures for high-performance aqueous hybrid supercapacitors. Dalton Trans. 2022, 51, 18296–18307. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, P.; Wang, Z.; Liu, Y.; Zheng, Z.; Zhang, Q.; Zhang, X.; Dai, Y.; Whangbo, M.-H.; Huang, B. Co3O4 nanobelt arrays assembled with ultrathin nanosheets as highly efficient and stable electrocatalysts for the chlorine evolution reaction. J. Mater. Chem. A 2018, 6, 12718–12723. [Google Scholar] [CrossRef]
- Ganguli, S.; Koppisetti, H.V.; Ghosh, S.; Biswas, T.; Mahalingam, V. Paradoxical Observance of “Intrinsic” and “Geometric” Oxygen Evolution Electrocatalysis in Phase-Tuned Cobalt Oxide/Hydroxide Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 7957–7968. [Google Scholar] [CrossRef]
- Han, J.-L.; Meng, Q.-F.; Gao, S.-L. Facile synthesis of Co3O4 hexagonal plates by flux method. J. Cryst. Growth 2018, 482, 23–29. [Google Scholar] [CrossRef]
- Li, K.; Chen, C.; Bian, X.; Sun, T.; Jia, J. Electrolytic nitrate reduction using Co3O4 rod-like and sheet-like cathodes with the control of (220) facet exposure and Co2+/Co3+ ratio. Electrochim. Acta 2020, 362, 137121. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, L.; Guo, B.; Huang, Z.-F.; Chen, Z.; Wang, L.; Zhang, X.; Guo, Z.; Xu, W.; Loh, K.P. Tracking the role of defect types in Co3O4 structural evolution and active motifs during oxygen evolution reaction. J. Am. Chem. Soc. 2023, 145, 2271–2281. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, Q.; Li, Y.; Zhou, X.; Liu, X.; Yang, H.; Zhang, Z.; Duan, D.; Liu, S. 3D-Graphite Felt Self-loaded Rich Co3O4 Nanoparticle Electrodes for Chlorine Evolution Reaction at Low Concentration Chloride Ion. Catal. Lett. 2024, 154, 886–898. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Liu, X.; Qiu, Y.; Liu, Z.; Xie, H.; Duan, J.; Hou, B. Efficient electrocatalytic chlorine evolution under neutral seawater conditions enabled by highly dispersed Co3O4 catalysts on porous carbon. Appl. Catal. B Environ. 2023, 330, 122594. [Google Scholar] [CrossRef]
- He, Y.; Tao, X.; Li, Z.; Gao, G.; Zhuang, J.; He, L.; Li, Y.; Wang, Y.; Sun, D.; Xie, A. The Mg-Co3O4 coating on indium tin oxide film with improved electrochromic and energy storage properties by sol-gel spin coating. Ceram. Int. 2023, 49, 32237–32245. [Google Scholar] [CrossRef]
- Roniboss, A.; Sindhu, S.; Kennedy, L.J.; Arockiasamy, S. Synthesis and thermal properties of two novel cobalt (II) Schiff’s base complexes as precursors for coating cobalt oxide (Co3O4) thin film by a Plasma Enhanced Metallo-Organic Chemical Vapour Deposition. J. Mol. Struct. 2023, 1272, 134189. [Google Scholar] [CrossRef]
- Azevedo Neto, N.F.; Angelico, J.C.; da Silva Pelissari, M.R.; Camargo, L.P.; Simões, R.P.; Dall’Antonia, L.H.; Dias da Silva, J.H. Reactive sputtering deposition of Co3O4 films and an evaluation of its use as an electrochemical sensor for ascorbic acid. J. Mater. Sci. Mater. Electron. 2022, 33, 19678–19692. [Google Scholar] [CrossRef]
- Yuan, C.; Li, M.; Wang, M.; Dan, Y.; Lin, T.; Cao, H.; Zhang, M.; Zhao, P.; Yang, H. Electrochemical development and enhancement of latent fingerprints on stainless steel via electrochromic effect of electrodeposited Co3O4 films. Electrochim. Acta 2021, 370, 137771. [Google Scholar] [CrossRef]
- Chen, D.; Kang, Z.; Li, W. One-step electrodeposition to fabricate superhydrophobic coating and its reversible wettability transformation. Mater. Res. Express 2019, 7, 016404. [Google Scholar] [CrossRef]
- Bchiri, Y.; Souissi, R.; Bouricha, B.; Bouguila, N.; Kraini, M.; Vázquez-Vázquez, C.; López-Quintela, M.; Alaya, S. S/In molar ratio effect on the photoconductivity of the sprayed β-In 2 S 3 thin films. J. Mater. Sci. Mater. Electron. 2021, 32, 27995–28006. [Google Scholar] [CrossRef]
- Saha, S.; Johnson, M.; Altayaran, F.; Wang, Y.; Wang, D.; Zhang, Q. Electrodeposition fabrication of chalcogenide thin films for photovoltaic applications. Electrochem 2020, 1, 286–321. [Google Scholar] [CrossRef]
- Janotti, A.; Varley, J.; Rinke, P.; Umezawa, N.; Kresse, G.; Van de Walle, C. Hybrid functional studies of the oxygen vacancy in TiO2. Phys. Rev. B 2010, 81, 085212. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, P.; Zhang, H.; Zhao, J.; Shi, H.; Huang, Y.; Yang, H. Oxygen vacancies in Co3O4 promote CO2 photoreduction. Appl. Catal. B Environ. 2022, 300, 120729. [Google Scholar] [CrossRef]
- Wu, J.; Tao, Y.; Zhang, C.; Zhu, Q.; Zhang, D.; Li, G. Activation of chloride by oxygen vacancies-enriched TiO2 photoanode for efficient photoelectrochemical treatment of persistent organic pollutants and simultaneous H2 generation. J. Hazard. Mater. 2023, 443, 130363. [Google Scholar] [CrossRef]
- Diallo, A.; Beye, A.; Doyle, T.B.; Park, E.; Maaza, M. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: Physical properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, Y.; Zhang, J.; Cai, W. The role of oxygen vacancy in fluorine-doped SnO2 films. Phys. B Condens. Matter 2011, 406, 1822–1826. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Enhanced photocatalytic performance of nanostructured TiO2 thin films through combined effects of polymer conjugation and Mo-doping. J. Mater. Sci. 2019, 54, 5266–5279. [Google Scholar] [CrossRef]
- Lin, H.; Long, J.; Gu, Q.; Zhang, W.; Ruan, R.; Li, Z.; Wang, X. In situ IR study of surface hydroxyl species of dehydrated TiO2: Towards understanding pivotal surface processes of TiO2 photocatalytic oxidation of toluene. Phys. Chem. Chem. Phys. 2012, 14, 9468–9474. [Google Scholar] [CrossRef]
- Ding, Q.; Dou, Y.; Liao, Y.; Huang, S.; Wang, R.; Min, W.; Chen, X.; Wu, C.; Yuan, D.; Liu, H.K. Oxygen Vacancy-Rich Ultrathin Co3O4 Nanosheets as Nanofillers in Solid-Polymer Electrolyte for High-Performance Lithium Metal Batteries. Catalysts 2023, 13, 711. [Google Scholar] [CrossRef]
- Wong, C.P.P.; Lai, C.W.; Lee, K.M.; Pan, G.T.; Huang, C.M.; Juan, J.C.; Yang, T.C.K. Enhancement of discharge capacity and energy density by oxygen vacancies in nickel doped SrTiO3 as cathode for rechargeable alkaline zinc battery. Electrochim. Acta 2022, 404, 139705. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic− inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Ramírez-Campillo, R.; Meylan, C.; Alvarez, C.; Henríquez-Olguín, C.; Martínez, C.; Cañas-Jamett, R.; Andrade, D.C.; Izquierdo, M. Effects of in-season low-volume high-intensity plyometric training on explosive actions and endurance of young soccer players. J. Strength Cond. Res. 2014, 28, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Yang, W.-D.; Lee, K.-C.; Huang, C.-M. An effective electrodeposition mode for porous MnO2/Ni foam composite for asymmetric supercapacitors. Materials 2016, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Mirseyed, S.F.; Jafarzadeh, K.; Rostamian, A.; Semnani, A.; Abbasi, H.M.; Ostadhassan, M. A novel approach to the role of iridium and titanium oxide in deactivation mechanisms of a Ti/(36 RuO2-xIrO2-(64-x) TiO2) coating in sodium chloride solution. Corros. Sci. 2022, 206, 110481. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Han, M.H.; Lim, C.; Yu, S.-H.; Choi, C.H.; Min, B.K.; Choi, J.-Y.; Lee, W.H.; Oh, H.-S. Unveiling the role of Ni in Ru-Ni oxide for oxygen evolution: Lattice oxygen participation enhanced by structural distortion. J. Energy Chem. 2023, 77, 54–61. [Google Scholar] [CrossRef]
- Erusappan, E.; Pan, G.-T.; Chung, H.-Y.; Chong, S.; Thiripuranthagan, S.; Yang, T.C.-K.; Huang, C.-M. Hierarchical nickel–cobalt oxide and glucose-based carbon electrodes for asymmetric supercapacitor with high energy density. J. Taiwan Inst. Chem. Eng. 2020, 112, 330–336. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, X.; Chen, F.; Kuang, X.; Min, J.; Duan, H.; Li, J.; Chen, J. Oxygen vacancy induced interaction between Pt and TiO2 to improve the oxygen reduction performance. J. Colloid Interface Sci. 2023, 650, 901–912. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Wei, F.; Wang, X.; Han, W.; Yue, J. Effect of oxygen vacancies on the electronic structure and electrochemical performance of MnMoO4: Computational simulation and experimental verification. New J. Chem. 2022, 46, 1665–1676. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, H.; Liu, Z.; Liu, X.; Zhou, G.; Jiang, Y. Oxygen vacancy induced MnO2 catalysts for efficient toluene catalytic oxidation. Catal. Sci. Technol. 2021, 11, 6708–6723. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).