Prussian Blue Analogues and Their Derivatives for the Oxygen Evolution Reaction: A Review on Active Site Engineering Strategies
Abstract
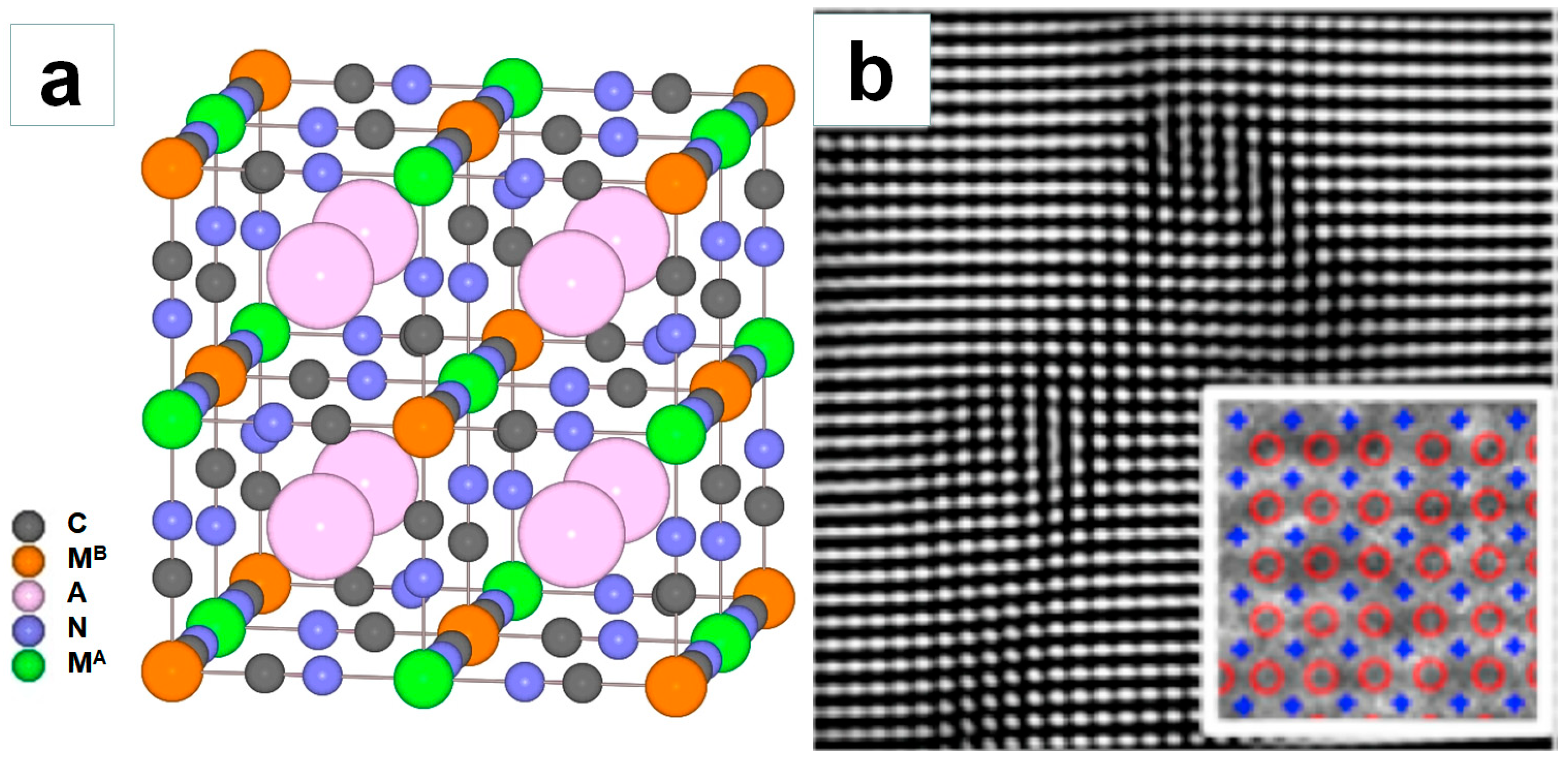
1. Introduction
2. Synthesis Method of PBAs
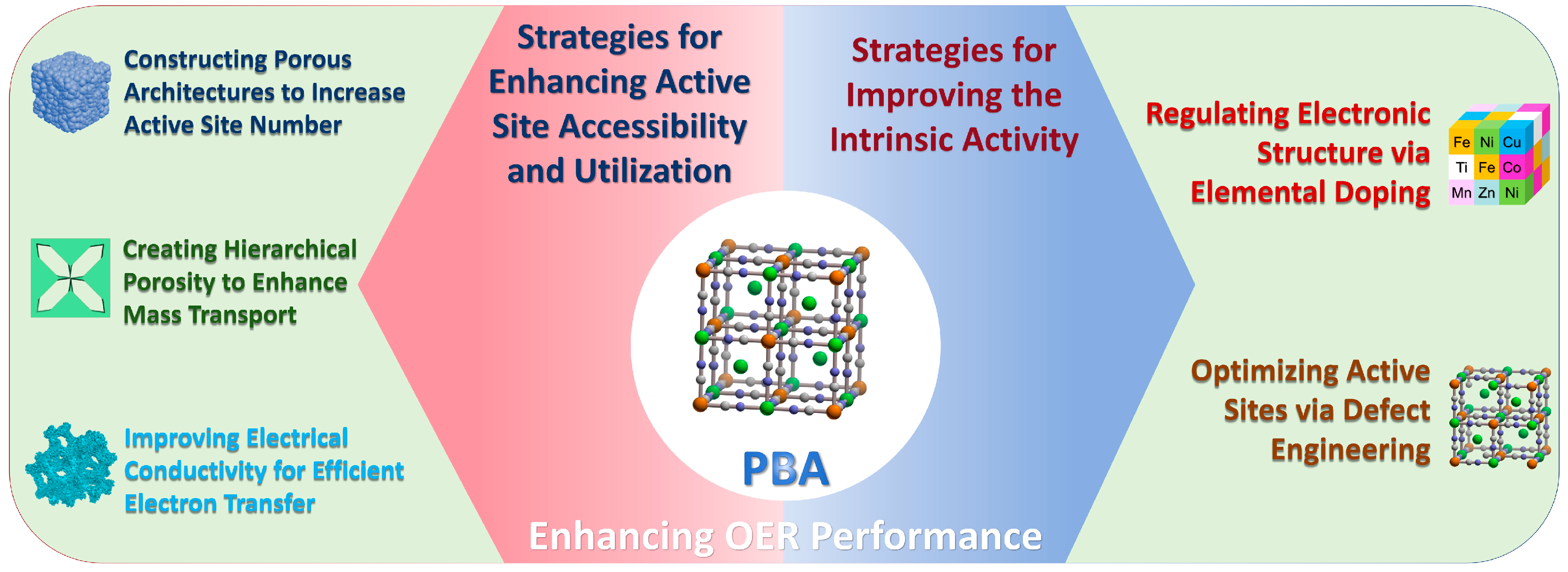
3. Strategies for Enhancing Active Site Accessibility and Utilization
3.1. Constructing Porous Architectures to Increase Active Site Number
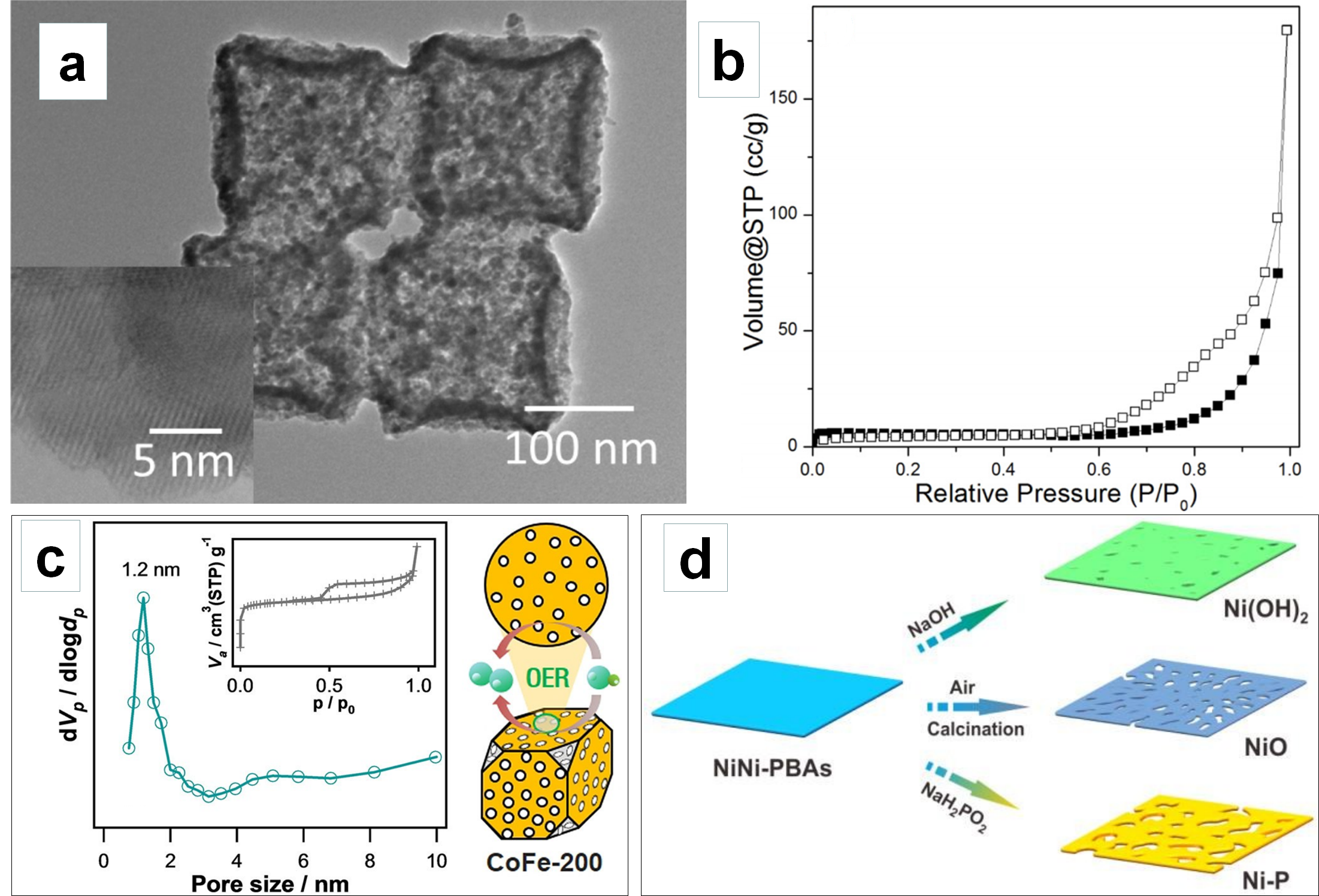
3.2. Creating Hierarchical Porosity to Enhance Mass Transport
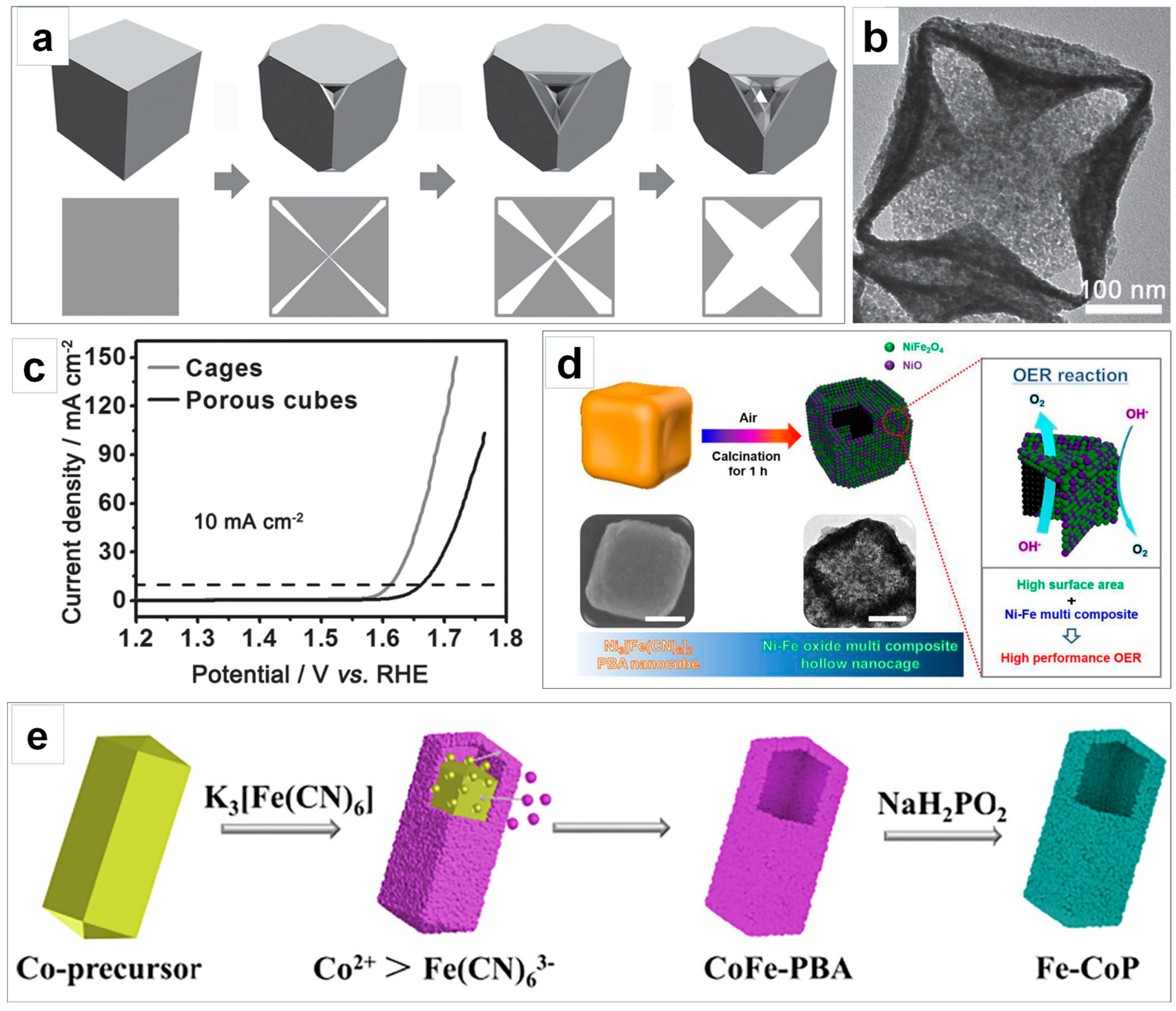
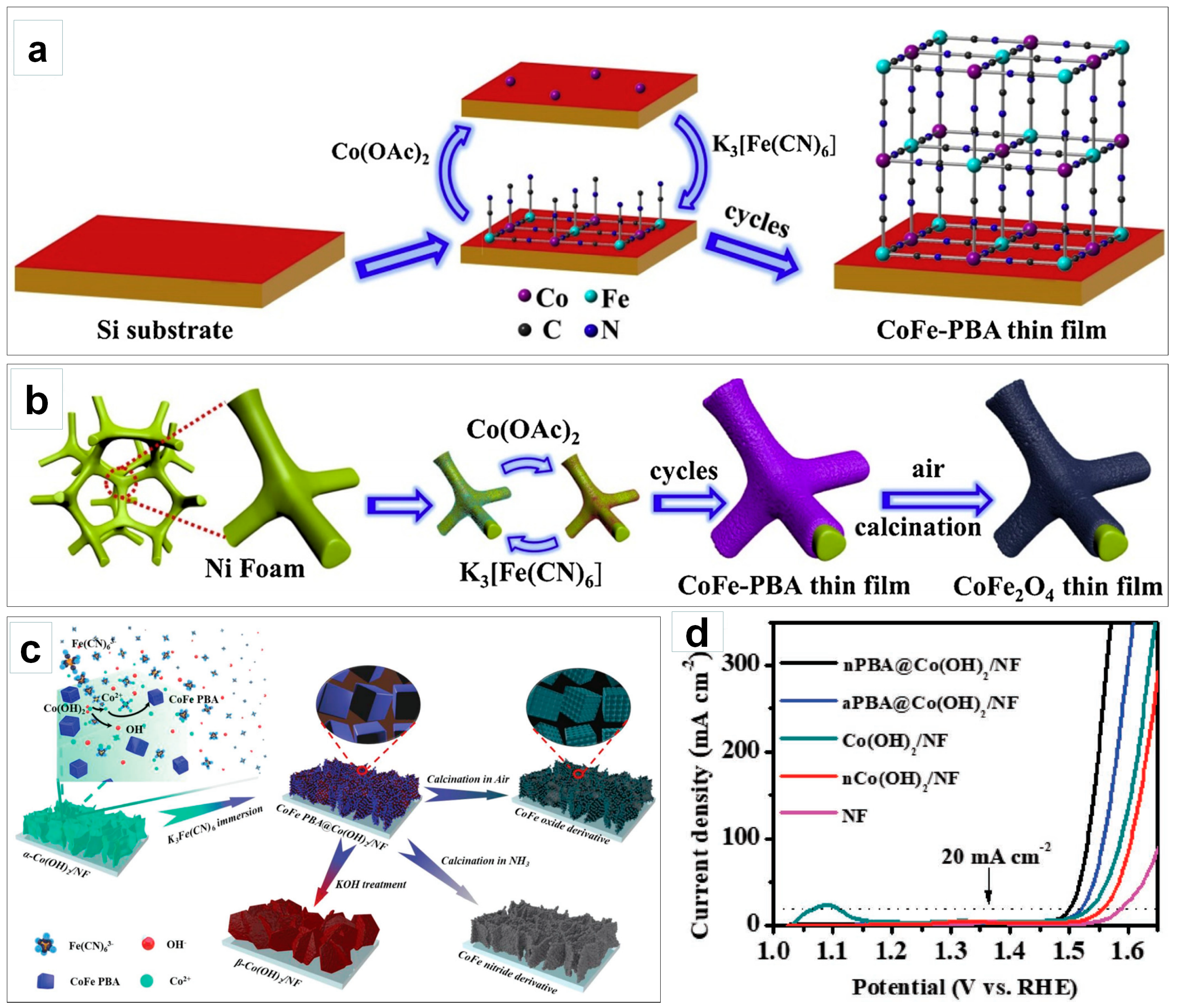
3.3. Improving Electrical Conductivity for Efficient Electron Transfer

4. Strategies for Improving the Intrinsic Activity
4.1. Regulating Electronic Structure via Elemental Doping
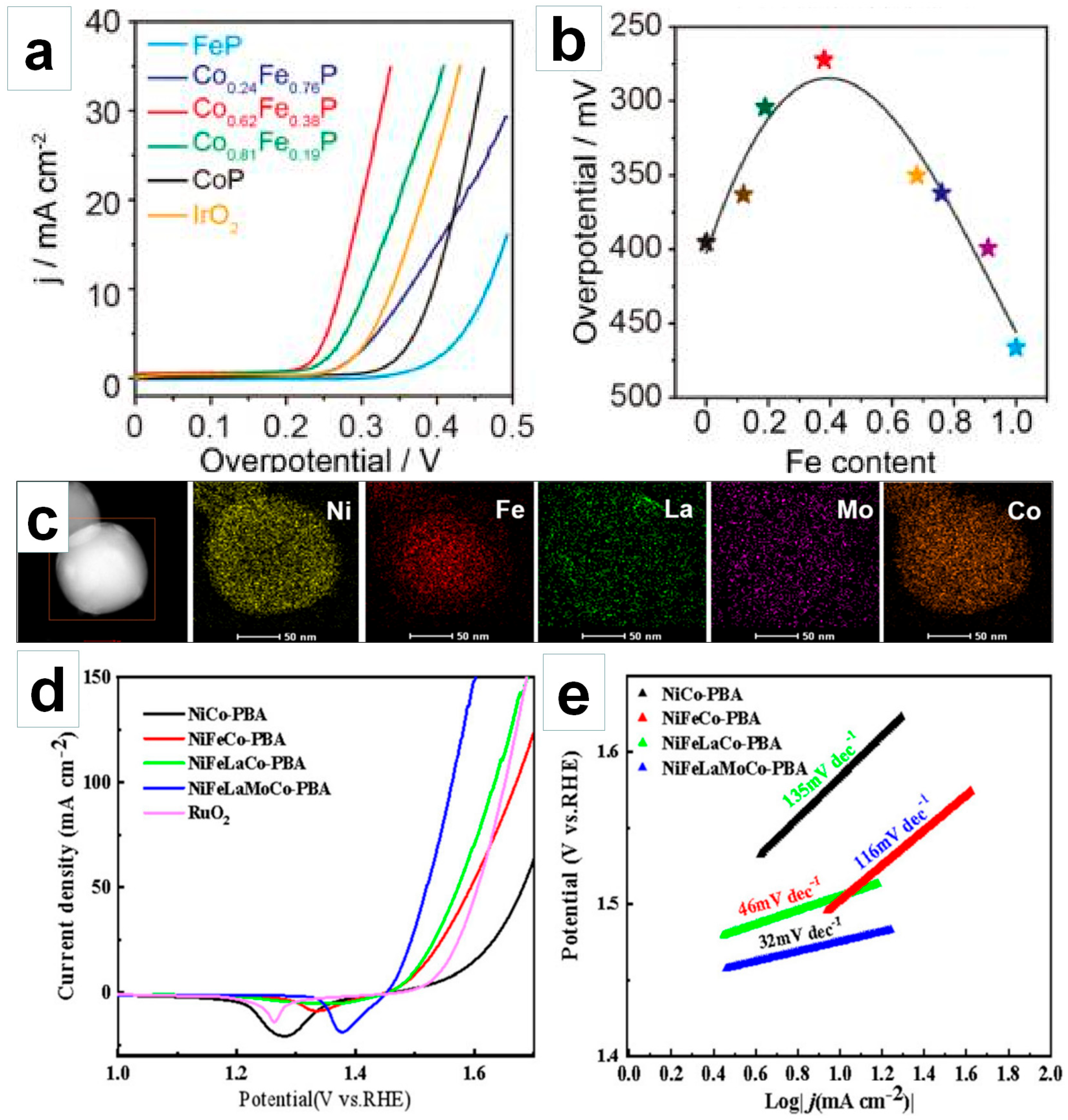
4.2. Optimizing Active Sites via Defect Engineering
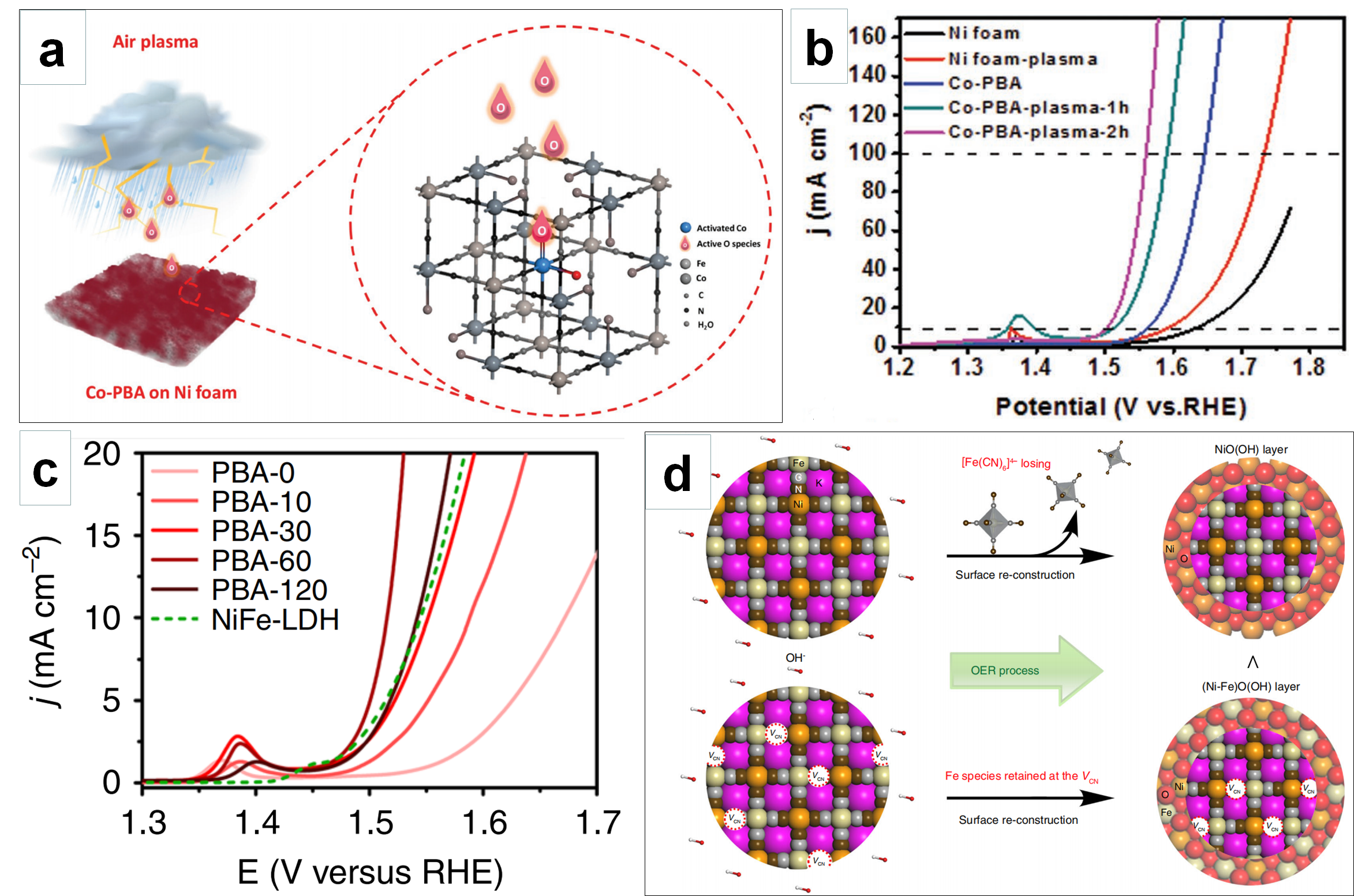

5. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Qin, Y.; Li, Z.-G.; Guo, T.-M.; An, L.-C.; Li, W.; Li, N.; Bu, X.-H. Elastic properties related energy conversions of coordination polymers and metal–organic frameworks. Coord. Chem. Rev. 2022, 470, 214692. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal–Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef]
- Itoi, M.; Jike, T.; Nishio-Hamane, D.; Udagawa, S.; Tsuda, T.; Kuwabata, S.; Boukheddaden, K.; Andrus, M.J.; Talham, D.R. Direct Observation of Short-Range Structural Coherence During a Charge Transfer Induced Spin Transition in a CoFe Prussian Blue Analogue by Transmission Electron Microscopy. J. Am. Chem. Soc. 2015, 137, 14686–14693. [Google Scholar] [CrossRef]
- Chen, J.; Wei, L.; Mahmood, A.; Pei, Z.; Zhou, Z.; Chen, X.; Chen, Y. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion. Energy Storage Mater. 2020, 25, 585–612. [Google Scholar] [CrossRef]
- Xie, J.-Y.; Dong, B. Hollow and substrate-supported Prussian blue, its analogs, and their derivatives for green water splitting. Chin. J. Catal. 2021, 42, 1843–1864. [Google Scholar] [CrossRef]
- Singh, B.; Indra, A. Prussian blue- and Prussian blue analogue-derived materials: Progress and prospects for electrochemical energy conversion. Mater. Today Energy 2020, 16, 100404. [Google Scholar] [CrossRef]
- Cao, M.; Wu, X.; He, X.; Hu, C. Shape-controlled synthesis of Prussian blue analogue Co3[Co(CN)6]2 nanocrystals. Chem. Commun. 2005, 2241–2243. [Google Scholar] [CrossRef]
- Catala, L.; Mallah, T. Nanoparticles of Prussian blue analogs and related coordination polymers: From information storage to biomedical applications. Coord. Chem. Rev. 2017, 346, 32–61. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments. Coord. Chem. Rev. 2017, 352, 328–345. [Google Scholar] [CrossRef]
- Moulik, S.P.; De, G.C.; Panda, A.K.; Bhowmik, B.B.; Das, A.R. Dispersed Molecular Aggregates. 1. Synthesis and Characterization of Nanoparticles of Cu2[Fe(CN)6] in H2O/AOT/n-Heptane Water-in-Oil Microemulsion Media. Langmuir 1999, 15, 8361–8367. [Google Scholar] [CrossRef]
- Qiu, J.-D.; Peng, H.-Z.; Liang, R.-P.; Li, J.; Xia, X.-H. Synthesis, characterization, and immobilization of Prussian blue-modified Au nanoparticles: Application to electrocatalytic reduction of H2O2. Langmuir 2007, 23, 2133–2137. [Google Scholar] [CrossRef]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of Prussian Blue Nanoparticles with a Hollow Interior by Controlled Chemical Etching. Angew. Chem. Int. Ed. 2012, 51, 984–988. [Google Scholar] [CrossRef]
- Han, L.; Tang, P.; Reyes-Carmona, Á.; Rodríguez-García, B.; Torréns, M.; Morante, J.R.; Arbiol, J.; Galan-Mascaros, J.R. Enhanced Activity and Acid pH Stability of Prussian Blue-type Oxygen Evolution Electrocatalysts Processed by Chemical Etching. J. Am. Chem. Soc. 2016, 138, 16037–16045. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Chen, J.; Zheng, J.; Li, X.; Ostrikov, K. Air Plasma Activation of Catalytic Sites in a Metal-Cyanide Framework for Efficient Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800085. [Google Scholar] [CrossRef]
- Song, N.; Ren, S.; Zhang, Y.; Wang, C.; Lu, X. Confinement of Prussian Blue Analogs Boxes Inside Conducting Polymer Nanotubes Enables Significantly Enhanced Catalytic Performance for Water Treatment. Adv. Funct. Mater. 2022, 32, 2204751. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Cheng, T.; Wang, Y.; Lai, W.-Y.; Pang, H.; Huang, W. A Simple Approach to Boost Capacitance: Flexible Supercapacitors Based on Manganese Oxides@MOFs via Chemically Induced In Situ Self-Transformation. Adv. Mater. 2016, 28, 5242–5248. [Google Scholar] [CrossRef]
- Xie, B.; Sun, B.; Gao, T.; Ma, Y.; Yin, G.; Zuo, P. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord. Chem. Rev. 2022, 460, 214478. [Google Scholar] [CrossRef]
- Gupta, A.; Patel, D.K.; Lee, S.Y.; Rigosi, A.F.; Elmquist, R.E.; Adusumalli, V.N.K.B.; Liang, C.-T.; Park, Y.I. Record-High Responsivity and High Detectivity Broadband Photodetectors Based on Upconversion/Gold/Prussian-Blue Nanocomposite. Adv. Funct. Mater. 2022, 32, 2206496. [Google Scholar] [CrossRef]
- Guari, Y.; Cahu, M.; Félix, G.; Sene, S.; Long, J.; Chopineau, J.; Devoisselle, J.-M.; Larionova, J. Nanoheterostructures based on nanosized Prussian blue and its Analogues: Design, properties and applications. Coord. Chem. Rev. 2022, 461, 214497. [Google Scholar] [CrossRef]
- Zhu, X.; Zong, H.; Pérez, C.J.V.; Miao, H.; Sun, W.; Yuan, Z.; Wang, S.; Zeng, G.; Xu, H.; Jiang, Z.; et al. Supercharged CO2 Photothermal Catalytic Methanation: High Conversion, Rate, and Selectivity. Angew. Chem. Int. Ed. 2023, 62, e202218694. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Gao, X.; An, C.; Wang, Z.; Zuo, C.; Dionysiou, D.D.; He, H.; Jiang, Z. Enhancing photocatalytic CO2 reduction with TiO2-based materials: Strategies, mechanisms, challenges, and perspectives. Environ. Sci. Ecotechnol. 2024, 20, 100368. [Google Scholar] [CrossRef]
- Yuan, Z.; Sun, X.; Wang, H.; Zhao, X.; Jiang, Z. Applications of Ni-Based Catalysts in Photothermal CO2 Hydrogenation Reaction. Molecules 2024, 29, 3882. [Google Scholar] [CrossRef]
- Quan, L.; Jiang, H.; Mei, G.; Sun, Y.; You, B. Bifunctional Electrocatalysts for Overall and Hybrid Water Splitting. Chem. Rev. 2024, 124, 3694–3812. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, W.; Zhou, T.; Yan, W.; Wang, K. Design and Optimization of Nanoporous Materials as Catalysts for Oxygen Evolution Reaction—A Review. Molecules 2024, 29, 4562. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Wang, X.; Qi, Y.; Shen, L.; Tai, X.; Yang, F.; He, X.; Wang, Y.; Yao, Y.; et al. Boosting electrocatalytic performance via electronic structure regulation for acidic oxygen evolution. iScience 2024, 27, 108738. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, T.; Dong, Y.; Zhan, Y.; Wei, F.; Zhang, D.; Long, X. Solid–Solid Interface Design for Hydrogen Production by Direct Seawater Electrolysis: Progress and Challenges. Inorganics 2025, 13, 183. [Google Scholar] [CrossRef]
- Hunter, B.M.; Gray, H.B.; Müller, A.M. Earth-Abundant Heterogeneous Water Oxidation Catalysts. Chem. Rev. 2016, 116, 14120–14136. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Cui, M.; Wu, H.; Liu, Y.; Ou, Q.; Tian, X.; Zhang, S. NiFe-Based Electrocatalysts for Alkaline Oxygen Evolution: Challenges, Strategies, and Advances Toward Industrial-Scale Deployment. Adv. Funct. Mater. 2024, 34, 2410618. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Z.; Yang, B.; Cao, Z.; Jiang, Z.; Cui, W.; Wang, K.; Yu, L.; Lu, J.; Zhang, L. Dealloying fabrication of hierarchical porous Nickel–Iron foams for efficient oxygen evolution reaction. Front. Chem. 2022, 10, 1047398. [Google Scholar] [CrossRef]
- Mom, R.V.; Cheng, J.; Koper, M.T.M.; Sprik, M. Modeling the Oxygen Evolution Reaction on Metal Oxides: The Infuence of Unrestricted DFT Calculations. J. Phys. Chem. C 2014, 118, 4095–4102. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.-J.; Sokaras, D.; Weng, T.-C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Z.; Wang, X. Understanding the Mechanism of the Oxygen Evolution Reaction with Consideration of Spin. Electrochem. Energy Rev. 2021, 4, 136–145. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Sun, L.; Guo, H.; Tai, X.; Li, D.; Liu, L.; Ling, C.; Sun, X. Facilitating active species by decorating CeO2 on Ni3S2 nanosheets for efficient water oxidation electrocatalysis. Chin. J. Catal. 2021, 42, 482–489. [Google Scholar] [CrossRef]
- Yue, K.; Lu, R.; Gao, M.; Song, F.; Dai, Y.; Xia, C.; Mei, B.; Dong, H.; Qi, R.; Zhang, D.; et al. Polyoxometalated metal-organic framework superstructure for stable water oxidation. Science 2025, 388, 430–436. [Google Scholar] [CrossRef]
- Liu, X.; Xi, W.; Li, C.; Li, X.; Shi, J.; Shen, Y.; He, J.; Zhang, L.; Xie, L.; Sun, X.; et al. Nanoporous Zn-doped Co3O4 sheets with single-unit-cell-wide lateral surfaces for efficient oxygen evolution and water splitting. Nano Energy 2018, 44, 371–377. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, T.; Ma, X.; Shen, Y.; Deng, Q.; Zhang, W.; Zhao, Y. Hydrogen Production from Urea Sewage on NiFe-Based Porous Electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 11007–11015. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Wu, T.; Ji, L.; Zhang, R.; Jiang, P.; Chen, H.; Zhao, R.; Asiri, A.M.; Sun, X. One-Step Preparation of Cobalt-Nanoparticle-Embedded Carbon for Effective Water Oxidation Electrocatalysis. ChemElectroChem 2019, 6, 1996–1999. [Google Scholar] [CrossRef]
- Mefford, J.T.; Rong, X.; Abakumov, A.M.; Hardin, W.G.; Dai, S.; Kolpak, A.M.; Johnston, K.P.; Stevenson, K.J. Water electrolysis on La1−xSrxCoO3−δ perovskite electrocatalysts. Nat. Commun. 2016, 7, 11053. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhong, Y.; Ge, L.; Chen, Y.; Veder, J.-P.M.; Guan, D.; O’Hayre, R.; Li, M.; Wang, G.; et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 2020, 11, 2002. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; Zhou, W.; Li, J. Catalyst-Support Interactions Promoted Acidic Electrochemical Oxygen Evolution Catalysis: A Mini Review. Molecules 2023, 28, 2262. [Google Scholar] [CrossRef]
- Cherevko, S.; Zeradjanin, A.R.; Topalov, A.A.; Kulyk, N.; Katsounaros, I.; Mayrhofer, K.J.J. Dissolution of Noble Metals during Oxygen Evolution in Acidic Media. ChemCatChem 2014, 6, 2219–2223. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, L. MOF-derived nanoarrays as advanced electrocatalysts for water splitting. Nanoscale 2022, 14, 12196–12218. [Google Scholar] [CrossRef]
- Kawashima, K.; Márquez, R.A.; Smith, L.A.; Vaidyula, R.R.; Carrasco-Jaim, O.A.; Wang, Z.; Son, Y.J.; Cao, C.L.; Mullins, C.B. A Review of Transition Metal Boride, Carbide, Pnictide, and Chalcogenide Water Oxidation Electrocatalysts. Chem. Rev. 2023, 123, 12795–13208. [Google Scholar] [CrossRef]
- Ji, Y.-R.; Guo, Y.-F.; Liu, X.; Wang, P.-F.; Yi, T.-F. Insights on rational design and regulation strategies of Prussian blue analogues and their derivatives towards high-performance electrocatalysts. Chem. Eng. J. 2023, 471, 144743. [Google Scholar] [CrossRef]
- Xuan, C.; Zhang, J.; Wang, J.; Wang, D. Rational Design and Engineering of Nanomaterials Derived from Prussian Blue and Its Analogs for Electrochemical Water Splitting. Chem. Asian J. 2020, 15, 958–972. [Google Scholar] [CrossRef]
- Cao, L.-M.; Lu, D.; Zhong, D.-C.; Lu, T.-B. Prussian blue analogues and their derived nanomaterials for electrocatalytic water splitting. Coord. Chem. Rev. 2020, 407, 213156. [Google Scholar] [CrossRef]
- Lu, M.; Zheng, Y.; Hu, Y.; Huang, B.; Ji, D.; Sun, M.; Li, J.; Peng, Y.; Si, R.; Xi, P.; et al. Artificially steering electrocatalytic oxygen evolution reaction mechanism by regulating oxygen defect contents in perovskites. Sci. Adv. 2022, 8, eabq3563. [Google Scholar] [CrossRef]
- Ding, J.; Guo, D.; Wang, N.; Wang, H.-F.; Yang, X.; Shen, K.; Chen, L.; Li, Y. Defect Engineered Metal–Organic Framework with Accelerated Structural Transformation for Efficient Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202311909. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Li, L.; Shi, J.; Xie, M.-Y.; Nie, J.; Huang, G.-F.; Li, B.; Hu, W.; Pan, A.; Huang, W.-Q. Oxygen Defect Engineering Promotes Synergy Between Adsorbate Evolution and Single Lattice Oxygen Mechanisms of OER in Transition Metal-Based (oxy)Hydroxide. Adv. Sci. 2023, 10, 2303321. [Google Scholar] [CrossRef]
- Meena, A.; Bathula, C.; Hatshan, M.R.; Palem, R.R.; Jana, A. Microstructure and Oxygen Evolution Property of Prussian Blue Analogs Prepared by Mechanical Grinding. Nanomaterials 2023, 13, 2459. [Google Scholar] [CrossRef]
- Ruan, Q.; Li, D.; Wu, C.; Huang, C.; Chu, P.K. Enhancing oxygen evolution reaction via hydrogen plasma treatment: Unveiling the functionality of CN defects and the role of Fe in NiFe Prussian blue analogs. EcoEnergy 2024, 2, 268–277. [Google Scholar] [CrossRef]
- Wang, W.-B.; Cao, H.-J.; Li, G.-L. In Situ Charge Modification within Prussian Blue Analogue Nanocubes by Plasma for Oxygen Evolution Catalysis. Inorg. Chem. 2023, 62, 10241–10248. [Google Scholar] [CrossRef]
- Xu, H.G.; Zhu, C.; Lin, H.Y.; Liu, J.K.; Wu, Y.X.; Fu, H.Q.; Zhang, X.Y.; Mao, F.; Yuan, H.Y.; Sun, C.; et al. Oxygen Plasma Triggered Co−O−Fe Motif in Prussian Blue Analogue for Efficient and Robust Alkaline Water Oxidation. Angew. Chem. Int. Ed. 2025, 64, e202415423. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Wang, J.; Chen, S.; Wang, H.; Zhang, J. Interfacial Scaffolding Preparation of Hierarchical PBA-Based Derivative Electrocatalysts for Efficient Water Splitting. Adv. Energy Mater. 2019, 9, 1802939. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, T.; Xi, W.; Zhao, Y. Bimetal metal-organic frameworks derived Co0.4Fe0.28P and Co0.37Fe0.26S nanocubes for enhanced oxygen evolution reaction. Electrochim. Acta 2018, 263, 576–584. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharyya, S. Porous NiFe-Oxide Nanocubes as Bifunctional Electrocatalysts for Efficient Water-Splitting. ACS Appl. Mater. Interfaces 2017, 9, 41906–41915. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, Q.; Sun, Y.; Ning, G.; Fan, X.; Wang, H.; Lu, H.; Zhang, Y.; Wang, H. Ni–Fe nanocubes embedded with Pt nanoparticles for hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 20832–20842. [Google Scholar] [CrossRef]
- Jo, S.; Noh, S.; Wee, K.-R.; Shim, J.H. Structural Features of Porous CoFe Nanocubes and Their Performance for Oxygen-involving Energy Electrocatalysis. ChemElectroChem 2020, 7, 3725–3732. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, J.; Yu, J.; Wang, W.; Li, W.; Li, N.; Li, Y. Electrospun prussian blue analogue derived NiCo@N-doped carbon nanofibers as efficient and highly stable electrocatalysts for neutral overall water splitting. Int. J. Hydrogen Energy 2021, 46, 8871–8884. [Google Scholar] [CrossRef]
- Su, J.; Xia, G.; Li, R.; Yang, Y.; Chen, J.; Shi, R.; Jiang, P.; Chen, Q. Co3ZnC/Co nano heterojunctions encapsulated in N-doped graphene layers derived from PBAs as highly efficient bi-functional OER and ORR electrocatalysts. J. Mater. Chem. A 2016, 4, 9204–9212. [Google Scholar] [CrossRef]
- Wang, S.; Huo, W.; Feng, H.; Xie, Z.; Shang, J.K.; Formo, E.V.; Camargo, P.H.C.; Fang, F.; Jiang, J. Enhancing Oxygen Evolution Reaction Performance in Prussian Blue Analogues: Triple-Play of Metal Exsolution, Hollow Interiors, and Anionic Regulation. Adv. Mater. 2023, 35, 2304494. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xu, B.; Zhao, Q.; Xie, S.; Jin, W.; Zhang, X.; Gao, B.; Liu, Z.; Abd-Allah, Z.; Chu, P.K.; et al. C≡N vacancy engineering of Prussian blue analogs for the advanced oxygen evolution reaction. J. Environ. Chem. Eng. 2023, 11, 109407. [Google Scholar] [CrossRef]
- Jo, S.; Kwon, J.; Choi, S.; Lu, T.; Byeun, Y.; Han, H.; Song, T. Engineering [Fe(CN)6]3− vacancy via free-chelating agents in Prussian blue analogues on reduced graphene oxide for efficient oxygen evolution reaction. Appl. Surf. Sci. 2022, 574, 151620. [Google Scholar] [CrossRef]
- Quan, L.; Li, S.; Zhao, Z.; Liu, J.; Ran, Y.; Cui, J.; Lin, W.; Yu, X.; Wang, L.; Zhang, Y.; et al. Hierarchically Assembling CoFe Prussian Blue Analogue Nanocubes on CoP Nanosheets as Highly Efficient Electrocatalysts for Overall Water Splitting. Small Methods 2021, 5, 2100125. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Wang, X.; Dong, A.; Zhu, Z.; Chai, L.; Ding, J.; Zhong, L.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Surfactant-Mediated Morphological Evolution of MnCo Prussian Blue Structures. Small 2020, 16, 2004614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lee, T.H.; Noh, H.; Farha, O.K.; Jang, H.W.; Choi, J.-W.; Shokouhimehr, M. Tailorable Topologies for Selectively Controlling Crystals of Expanded Prussian Blue Analogues. Cryst. Growth Des. 2019, 19, 7385–7395. [Google Scholar] [CrossRef]
- Du, D.; Cao, M.; He, X.; Liu, Y.; Hu, C. Morphology-Controllable Synthesis of Microporous Prussian Blue Analogue Zn3[Co(CN)6]2·xH2O Microstructures. Langmuir 2009, 25, 7057–7062. [Google Scholar] [CrossRef]
- Cui, X.; Ma, F.; Lei, G.; Jiang, W.; Yang, X.; Liu, Z.; Wan, J.; Liu, Y. Trisodium Citrate as a Double-Edged Sword: Selective Etching Prussian Blue Analog Nanocubes into Orthogonal Frustums and Their Derivatives for Supercapacitors. Small 2024, 20, 2403732. [Google Scholar] [CrossRef]
- Ge, Y.; Dong, P.; Craig, S.R.; Ajayan, P.M.; Ye, M.; Shen, J. Transforming Nickel Hydroxide into 3D Prussian Blue Analogue Array to Obtain Ni2P/Fe2P for Efficient Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800484. [Google Scholar] [CrossRef]
- Wang, X.; Yu, L.; Guan, B.Y.; Song, S.; Lou, X.W. Metal–Organic Framework Hybrid-Assisted Formation of Co3O4/Co-Fe Oxide Double-Shelled Nanoboxes for Enhanced Oxygen Evolution. Adv. Mater. 2018, 30, 1801211. [Google Scholar] [CrossRef]
- Ding, X.; Huang, H.; Wan, Q.; Guan, X.; Fang, Y.; Lin, S.; Chen, D.; Xie, Z. Self-template synthesis of hollow Fe-doped CoP prisms with enhanced oxygen evolution reaction activity. J. Energy Chem. 2021, 62, 415–422. [Google Scholar] [CrossRef]
- Peixoto, D.A.; Silva, S.C.; Borges, P.H.S.; Lima, R.C.; Nossol, E. Hydrothermal synthesis as a versatile tool for the preparation of metal hexacyanoferrates: A review. J. Mater. Sci. 2023, 58, 2993–3024. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Kuang, Q.; Xu, T.; Jiang, Z.-Y.; Zhang, S.-H.; Xie, Z.-X.; Huang, R.-B.; Zheng, L.-S. Growth of Prussian blue microcubes under a hydrothermal condition: Possible nonclassical crystallization by a mesoscale self-assembly. J. Phys. Chem. C 2007, 111, 4499–4502. [Google Scholar] [CrossRef]
- Xu, S.; Qian, X.; Li, G. Size and morphology-controlled Ni2[Fe(CN)6]·xH2O Prussian Blue analogue fabricated via a hydrothermal route. Mater. Res. Bull. 2008, 43, 135–140. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Z.; Li, X.; Wang, Y.; Niu, C.; Lv, Y. Controlled hydrothermal synthesis of Prussian Blue films with multicolor electrochromic behaviors. J. Solid State Chem. 2023, 325, 124160. [Google Scholar] [CrossRef]
- Ivanov, V.D. Four decades of electrochemical investigation of Prussian blue. Ionics 2020, 26, 531–547. [Google Scholar] [CrossRef]
- Baggio, B.F.; Vicente, C.; Pelegrini, S.; Plá Cid, C.C.; Brandt, I.S.; Tumelero, M.A.; Pasa, A.A. Morphology and Structure of Electrodeposited Prussian Blue and Prussian White Thin Films. Materials 2019, 12, 1103. [Google Scholar] [CrossRef]
- Yang, R.; Qian, Z.; Deng, J. Electrochemical Deposition of Prussian Blue from a Single Ferricyanide Solution. J. Electrochem. Soc. 1998, 145, 2231–2236. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, T.; Chen, H.; Suo, X.; Liang, J.; Zhu, W.; Li, H.; Dai, S. Room temperature synthesis of high-entropy Prussian blue analogues. Nano Energy 2021, 79, 105464. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Niu, Q.; Chang, H.; Ma, G.; Zhu, Y.; Xia, Y. One-step construction of porous Ni/Co metal/oxide nanocubes for highly efficient oxygen evolution. Electrochem. Commun. 2018, 93, 191–196. [Google Scholar] [CrossRef]
- Fang, H.; Huang, T.; Liang, D.; Qiu, M.; Sun, Y.; Yao, S.; Yu, J.; Dinesh, M.M.; Guo, Z.; Xia, Y.; et al. Prussian blue analog-derived 2D ultrathin CoFe2O4 nanosheets as high-activity electrocatalysts for the oxygen evolution reaction in alkaline and neutral media. J. Mater. Chem. A 2019, 7, 7328–7332. [Google Scholar] [CrossRef]
- Guo, B.-Y.; Zhang, X.-Y.; Ma, X.; Chen, T.-S.; Chen, Y.; Wen, M.-L.; Qin, J.-F.; Nan, J.; Chai, Y.-M.; Dong, B. RuO2/Co3O4 Nanocubes based on Ru ions impregnation into prussian blue precursor for oxygen evolution. Int. J. Hydrogen Energy 2020, 45, 9575–9582. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Feng, Y.; Guan, B.; Lou, X.W.; Paik, U. Carbon coated porous nickel phosphides nanoplates for highly efficient oxygen evolution reaction. Energy Environ. Sci. 2016, 9, 1246–1250. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Wang, Z.; Sugahara, Y.; Yamauchi, Y. Hollow Porous Heterometallic Phosphide Nanocubes for Enhanced Electrochemical Water Splitting. Small 2018, 14, 1802442. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, Z.; Chen, S.; Yang, H.; Tang, K. Prussian Blue Analogue-Assisted Formation of Iron–Nickel Selenide Porous Nanosheets for Enhanced Oxygen Evolution. ACS Appl. Energy Mater. 2023, 6, 2178–2186. [Google Scholar] [CrossRef]
- Lu, M.; An, L.; Yin, J.; Jin, J.; Yang, R.; Huang, B.; Hu, Y.; Zhao, Y.-Q.; Xi, P. Electronic engineering of amorphous Fe–Co–S sites in hetero-nanoframes for oxygen evolution and flexible Al–air batteries. J. Mater. Chem. A 2022, 10, 19757–19768. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, T.; Chen, Y.; Liu, J.; Wang, D.; Zhang, W.; Pang, S.; Zhao, Y. A Trimodal Porous Cobalt-Based Electrocatalyst for Enhanced Oxygen Evolution. Adv. Mater. Interfaces 2019, 6, 1900381. [Google Scholar] [CrossRef]
- Han, L.; Yu, X.-Y.; Lou, X.W. Formation of Prussian-Blue-Analog Nanocages via a Direct Etching Method and their Conversion into Ni–Co-Mixed Oxide for Enhanced Oxygen Evolution. Adv. Mater. 2016, 28, 4601–4605. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.-Y.; Paik, U. Formation of Co3O4 microframes from MOFs with enhanced electrochemical performance for lithium storage and water oxidation. Chem. Commun. 2016, 52, 6269–6272. [Google Scholar] [CrossRef]
- Nai, J.; Lu, Y.; Yu, L.; Wang, X.; Lou, X.W. Formation of Ni–Fe Mixed Diselenide Nanocages as a Superior Oxygen Evolution Electrocatalyst. Adv. Mater. 2017, 29, 1703870. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Zhang, H.; Zuo, S.; Wang, Y.; Zhang, S.L.; Zhang, J.; Zang, S.-Q.; Lou, X.W. Manipulating the Local Coordination and Electronic Structures for Efficient Electrocatalytic Oxygen Evolution. Adv. Mater. 2021, 33, 2103004. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Dong, P.; Li, J.; Feng, L.; Huang, J.; Cao, L.; Feng, L.; Kajiyoshi, K.; Wang, C. Boosting the activity of Prussian-blue analogue as efficient electrocatalyst for water and urea oxidation. Sci. Rep. 2019, 9, 15965. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Sathiyan, K.; Vishwanath, R.S.; Zidki, T. Anchoring MoS2 on an ethanol-etched Prussian blue analog for enhanced electrocatalytic efficiency for the oxygen evolution reaction. Mater. Chem. Front. 2022, 6, 1770–1778. [Google Scholar] [CrossRef]
- Lian, Y.; Sun, H.; Wang, X.; Qi, P.; Mu, Q.; Chen, Y.; Ye, J.; Zhao, X.; Deng, Z.; Peng, Y. Carved nanoframes of cobalt–iron bimetal phosphide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2019, 10, 464–474. [Google Scholar] [CrossRef]
- Cai, P.; Huang, J.; Chen, J.; Wen, Z. Oxygen-Containing Amorphous Cobalt Sulfide Porous Nanocubes as High-Activity Electrocatalysts for the Oxygen Evolution Reaction in an Alkaline/Neutral Medium. Angew. Chem. Int. Ed. 2017, 56, 4858–4861. [Google Scholar] [CrossRef]
- He, L.; Cui, B.; Hu, B.; Liu, J.; Tian, K.; Wang, M.; Song, Y.; Fang, S.; Zhang, Z.; Jia, Q. Mesoporous Nanostructured CoFe–Se–P Composite Derived from a Prussian Blue Analogue as a Superior Electrocatalyst for Efficient Overall Water Splitting. ACS Appl. Energy Mater. 2018, 1, 3915–3928. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yu, L.; Wu, H.B.; Lou, X.W. Formation of Nickel Sulfide Nanoframes from Metal–Organic Frameworks with Enhanced Pseudocapacitive and Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 54, 5331–5335. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Woo, M.H.; Lee, J.; Song, Y.H.; Wang, Z.; Guo, Y.; Yamauchi, Y.; Kim, J.H.; Lim, B.; Yoon, D.H. Mesoporous Ni–Fe oxide multi-composite hollow nanocages for efficient electrocatalytic water oxidation reactions. J. Mater. Chem. A 2017, 5, 4320–4324. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, J.; Lou, X.W. Construction of Single-Crystalline Prussian Blue Analog Hollow Nanostructures with Tailorable Topologies. Chem 2018, 4, 1967–1982. [Google Scholar] [CrossRef]
- Nai, J.; Guan, B.Y.; Yu, L.; Lou, X.W. Oriented assembly of anisotropic nanoparticles into frame-like superstructures. Sci. Adv. 2017, 3, e1700732. [Google Scholar] [CrossRef]
- Song, W.; Teng, X.; Niu, Y.; Gong, S.; He, X.; Chen, Z. Self-templating construction of hollow Fe-CoxP nanospheres for oxygen evolution reaction. Chem. Eng. J. 2021, 409, 128227. [Google Scholar] [CrossRef]
- Indra, A.; Paik, U.; Song, T. Boosting Electrochemical Water Oxidation with Metal Hydroxide Carbonate Templated Prussian Blue Analogues. Angew. Chem. Int. Ed. 2018, 57, 1241–1245. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Yu, B.; Hu, Y.; Wang, X.; Yang, D. 3D hollow Co–Fe–P nanoframes immobilized on N,P-doped CNT as an efficient electrocatalyst for overall water splitting. Nanoscale 2019, 11, 17031–17040. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chuang, C.-H.; Hsiao, L.-Y.; Yeh, M.-H.; Ho, K.-C. Oxygen Plasma Activation of Carbon Nanotubes-Interconnected Prussian Blue Analogue for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 42634–42643. [Google Scholar] [CrossRef] [PubMed]
- Aulia, S.; Lin, Y.-C.; Chang, L.-Y.; Wang, Y.-X.; Lin, M.-H.; Ho, K.-C.; Yeh, M.-H. Oxygen Plasma-Activated NiFe Prussian Blue Analogues Interconnected N-Doped Carbon Nanotubes as a Bifunctional Electrocatalyst for a Rechargeable Zinc–Air Battery. ACS Appl. Energy Mater. 2022, 5, 9801–9810. [Google Scholar] [CrossRef]
- Borges, P.H.S.; Gonçalves, J.M.; Breslin, C.B.; Nossol, E. Enhancing Oxygen Evolution Reaction Performance with rGO/CoNi-Prussian Blue-Derived Oxyhydroxide Nanocomposite Electrocatalyst: A Strategic Synthetic Approach. ACS Appl. Mater. Interfaces 2024, 16, 53705–53717. [Google Scholar] [CrossRef]
- Hou, M.; Gong, S.; Ji, L.; Huang, J.; Xu, M.; Chen, Z. Three-dimensional porous ultrathin carbon networks reinforced PBAs-derived electrocatalysts for efficient oxygen evolution. Chem. Eng. J. 2021, 419, 129575. [Google Scholar] [CrossRef]
- Ma, X.; Chang, C.; Zhang, Y.; Niu, P.; Liu, X.; Wang, S.; Li, L. Synthesis of Co-based Prussian Blue Analogues/Dual-Doped Hollow Carbon Microsphere Hybrids as High-Performance Bifunctional Electrocatalysts for Oxygen Evolution and Overall Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 8318–8326. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Q.; Peng, S.; Ramakrishna, S.; Zhang, D.; Zhou, K. Electrospun Inorganic Nanofibers for Oxygen Electrocatalysis: Design, Fabrication, and Progress. Adv. Energy Mater. 2020, 10, 1902115. [Google Scholar] [CrossRef]
- Yang, L.; Feng, S.; Xu, G.; Wei, B.; Zhang, L. Electrospun MOF-Based FeCo Nanoparticles Embedded in Nitrogen-Doped Mesoporous Carbon Nanofibers as an Efficient Bifunctional Catalyst for Oxygen Reduction and Oxygen Evolution Reactions in Zinc-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 5462–5475. [Google Scholar] [CrossRef]
- Ren, A.; Yu, B.; Huang, M.; Liu, Z. Encapsulation of cobalt prussian blue analogue-derived ultra-small CoP nanoparticles in electrospun N-doped porous carbon nanofibers as an efficient bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2024, 51, 490–502. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Wezendonk, T.; Sun, X.; Kapteijn, F.; Gascon, J. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709–1745. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, Y.; Zhao, F.; Nie, K.; Han, N.; Wang, X.; Huang, W.; Song, X.; Zhong, J.; Li, Y. Metallic Cobalt Nanoparticles Encapsulated in Nitrogen-Enriched Graphene Shells: Its Bifunctional Electrocatalysis and Application in Zinc–Air Batteries. Adv. Funct. Mater. 2016, 26, 4397–4404. [Google Scholar] [CrossRef]
- Aparicio, C.; Machala, L.; Marusak, Z. Thermal decomposition of Prussian blue under inert atmosphere. J. Therm. Anal. Calorim. 2012, 110, 661–669. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.-Y.; Paik, U. N-doped graphene layers encapsulated NiFe alloy nanoparticles derived from MOFs with superior electrochemical performance for oxygen evolution reaction. Sci. Rep. 2016, 6, 34004. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, J.; Lv, C.; Hu, C.; Guan, H.; Wang, J.; Shi, Y.; Song, J.; Watanabe, A.; Ge, X. General and scalable preparation of Prussian blue analogues on arbitrary conductive substrates and their derived metal phosphides as highly efficient and ultra-long-life bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 420, 129972. [Google Scholar] [CrossRef]
- Lei, S.; Li, Q.-H.; Kang, Y.; Gu, Z.-G.; Zhang, J. Epitaxial growth of oriented prussian blue analogue derived well-aligned CoFe2O4 thin film for efficient oxygen evolution reaction. Appl. Catal. B Environ. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Zhang, J. Hierarchical Assembly of Prussian Blue Derivatives for Superior Oxygen Evolution Reaction. Adv. Funct. Mater. 2019, 29, 1904955. [Google Scholar] [CrossRef]
- Cao, L.-M.; Hu, Y.-W.; Zhong, D.-C.; Lu, T.-B. Template-Directed Growth of Bimetallic Prussian Blue-Analogue Nanosheet Arrays and Their Derived Porous Metal Oxides for Oxygen Evolution Reaction. ChemSusChem 2018, 11, 3708–3713. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Shan, B.; Wang, W.; Qi, Y.; Tai, X.; Wang, X.; Zheng, D.; Yan, H.; Ying, B.; et al. Recent Advances in Self-Supported Transition-Metal-Based Electrocatalysts for Seawater Oxidation. Acta Phys. -Chim. Sin. 2023, 39, 2303012. [Google Scholar] [CrossRef]
- Jain, P.; Ingole, P.P. Unravelling the potential of prussian blue analogues in oxygen electrocatalysis: A perspective on surface reconstruction. Chem. Phys. Impact 2024, 9, 100693. [Google Scholar] [CrossRef]
- Ede, S.R.; Luo, Z. Tuning the intrinsic catalytic activities of oxygen-evolution catalysts by doping: A comprehensive review. J. Mater. Chem. A 2021, 9, 20131–20163. [Google Scholar] [CrossRef]
- Hu, S.; Ge, S.; Liu, H.; Kang, X.; Yu, Q.; Liu, B. Low-Dimensional Electrocatalysts for Acidic Oxygen Evolution: Intrinsic Activity, High Current Density Operation, and Long-Term Stability. Adv. Funct. Mater. 2022, 32, 2201726. [Google Scholar] [CrossRef]
- Kim, B.; Park, I.; Yoon, G.; Kim, J.S.; Kim, H.; Kang, K. Atomistic Investigation of Doping Effects on Electrocatalytic Properties of Cobalt Oxides for Water Oxidation. Adv. Sci. 2018, 5, 1801632. [Google Scholar] [CrossRef] [PubMed]
- Gerken, J.B.; Shaner, S.E.; Massé, R.C.; Porubsky, N.J.; Stahl, S.S. A survey of diverse earth abundant oxygen evolution electrocatalysts showing enhanced activity from Ni–Fe oxides containing a third metal. Energy Environ. Sci. 2014, 7, 2376–2382. [Google Scholar] [CrossRef]
- Nai, J.; Lu, Y.; Yu, X.-Y. Formation of Ti–Fe mixed sulfide nanoboxes for enhanced electrocatalytic oxygen evolution. J. Mater. Chem. A 2018, 6, 21891–21895. [Google Scholar] [CrossRef]
- Ge, P.; Li, S.; Shuai, H.; Xu, W.; Tian, Y.; Yang, L.; Zou, G.; Hou, H.; Ji, X. Ultrafast Sodium Full Batteries Derived from X-Fe (X = Co, Ni, Mn) Prussian Blue Analogs. Adv. Mater. 2019, 31, 1806092. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, B.-J.; Chen, C.-K. Evaluating Prussian blue analogues MII3[MIII(CN)6]2 (MII = Co, Cu, Fe, Mn, Ni; MIII = Co, Fe) as activators for peroxymonosulfate in water. RSC Adv. 2016, 6, 92923–92933. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.-T.; Jiang, W.-J.; Niu, S.; Wu, M.; Hu, J.-S. Bimetal Prussian Blue as a Continuously Variable Platform for Investigating the Composition–Activity Relationship of Phosphides-Based Electrocatalysts for Water Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 35904–35910. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Li, J.; Zhang, H.; Sharma, S.; Titheridge, L.; Tiffin, C.; Fan, Y.; Zhao, L.; Yang, W.; et al. Achieving High OER Performance by Tuning the Co/Mn Content in Prussian Blue Analogues. ACS Appl. Mater. Interfaces 2024, 16, 58703–58710. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, S.; Vasileff, A.; Qiao, S.Z. Anion and Cation Modulation in Metal Compounds for Bifunctional Overall Water Splitting. ACS Nano 2016, 10, 8738–8745. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.-A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Wygant, B.R.; Kawashima, K.; Mullins, C.B. Catalyst or Precatalyst? The Effect of Oxidation on Transition Metal Carbide, Pnictide, and Chalcogenide Oxygen Evolution Catalysts. ACS Energy Lett. 2018, 3, 2956–2966. [Google Scholar] [CrossRef]
- Ding, S.; Zheng, B.; Wang, X.; Zhou, Y.; Pan, Z.; Chen, Y.; Liu, G.; Lang, L. Intercalated and Surface-Adsorbed Phosphate Anions in NiFe Layered Double-Hydroxide Catalysts Synergistically Enhancing Oxygen Evolution Reaction Activity. Langmuir 2024, 40, 10384–10392. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, Y.; Sun, J.; Qiu, Y.; Liu, C.; Zhou, G.; Cui, Y. In Situ Electrochemically Derived Nanoporous Oxides from Transition Metal Dichalcogenides for Active Oxygen Evolution Catalysts. Nano Lett. 2016, 16, 7588–7596. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Li, Y.; Liu, Y.; Sun, J.; Lee, S.; Lee, J.-S.; Cui, Y. In Situ Electrochemical Oxidation Tuning of Transition Metal Disulfides to Oxides for Enhanced Water Oxidation. ACS Cent. Sci. 2015, 1, 244–251. [Google Scholar] [CrossRef]
- Shi, Y.; Du, W.; Zhou, W.; Wang, C.; Lu, S.; Lu, S.; Zhang, B. Unveiling the Promotion of Surface-Adsorbed Chalcogenate on the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 22470–22474. [Google Scholar] [CrossRef]
- Ahn, W.; Park, M.G.; Lee, D.U.; Seo, M.H.; Jiang, G.; Cano, Z.P.; Hassan, F.M.; Chen, Z. Hollow Multivoid Nanocuboids Derived from Ternary Ni–Co–Fe Prussian Blue Analog for Dual-Electrocatalysis of Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2018, 28, 1802129. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, K.; Dai, F.; Liu, Y.; Zhang, C.; Su, J.; Wang, K. Hierarchical porous tri-metallic NiCoFe-Se/CFP derived from Ni-Co-Fe Prussian blue analogues as efficient electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2023, 642, 638–647. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, B.; Xiang, H.; Ren, F.; Xu, T.; Liu, J.; Xu, H.; Ding, H.; Zhu, Y.; Liu, F. Efficient Oxygen Evolution Reaction Performance Achieved by Tri-Doping Modification in Prussian Blue Analogs. Inorganics 2025, 13, 258. [Google Scholar] [CrossRef]
- Hao, Y.; Du, G.; Fan, Y.; Jia, L.; Han, D.; Zhao, W.; Su, Q.; Xu, B. Prussian blue analogues-derived Ni-doped CoFe2O4 hollow nanocubes as electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. 2023, 614, 156237. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Liu, Z.; Jiao, F.; Zhao, G.; Zhang, Y.; Deng, X. High entropy rare-earth Prussian blue analogues for boosting oxygen evolution catalysis. Nanoscale 2025, 17, 18623–18632. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, L.; Wang, K.; Jin, L.; Liu, Y.; He, G.; Chen, H. Etched High-Entropy Prussian Blue Analogues as Trifunctional Catalysts for Water, Ethanol, and Urea Electrooxidation. Inorg. Chem. 2023, 62, 11271–11277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, Y.; Wei, G.; Xi, W.; Ma, X.; Zhang, W.; Zhu, P.; An, C. Synthesis of ultrathin Co2AlO4 nanosheets with oxygen vacancies for enhanced electrocatalytic oxygen evolution. Sci. China Mater. 2020, 63, 91–99. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.-C.; Dong, C.-L.; Xie, C.; Liu, Z.; Du, S.; Chen, W.; Yan, D.; Tao, L.; Shu, Z.; et al. Operando Identification of the Dynamic Behavior of Oxygen Vacancy-Rich Co3O4 for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2020, 142, 12087–12095. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Xie, M.; Cheng, H.; Wang, T.; Chen, J.; Lu, Y.; Tian, Z.; Lai, Y.; Yu, G. High-Density Cationic Defects Coupling with Local Alkaline-Enriched Environment for Efficient and Stable Water Oxidation. Angew. Chem. Int. Ed. 2023, 62, e202217815. [Google Scholar] [CrossRef]
- Li, S.-F.; Zheng, J.; Yan, D. Cationic Defect Engineering in Perovskite La2CoMnO6 for Enhanced Electrocatalytic Oxygen Evolution. Inorg. Chem. 2023, 62, 11009–11015. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, Z.; Tai, X.; Yu, L.; Ouyang, J.; Li, Y.; Lu, J. Hierarchical Co(OH)2 Dendrite Enriched with Oxygen Vacancies for Promoted Electrocatalytic Oxygen Evolution Reaction. Polymers 2022, 14, 1510. [Google Scholar] [CrossRef]
- Su, X.; Wang, Y.; Zhou, J.; Gu, S.; Li, J.; Zhang, S. Operando Spectroscopic Identification of Active Sites in NiFe Prussian Blue Analogues as Electrocatalysts: Activation of Oxygen Atoms for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 11286–11292. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Duan, Y.; Liu, J.-D.; Chen, Y.; Liu, X.-K.; Liu, W.; Ma, T.; Li, Y.; Zheng, X.-S.; Yao, T.; et al. Unconventional CN vacancies suppress iron-leaching in Prussian blue analogue pre-catalyst for boosted oxygen evolution catalysis. Nat. Commun. 2019, 10, 2799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fan, X.; Cao, S.; Wang, Z.; Yang, Z.; Zhang, W. Thermally activated carbon–nitrogen vacancies in double-shelled NiFe Prussian blue analogue nanocages for enhanced electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 12734–12745. [Google Scholar] [CrossRef]
- Ma, F.; Wang, C.; An, Y.; Liu, Y.; Xin, X.; Fan, K.; Wang, L.; Chen, G.; Hu, Z.; Zhan, T. N2-Plasma-Induced CN-Vacancies in NiCoFe PBAs Enhance Selective Oxygen Evolution Electrocatalytic Performance in Alkaline Seawater. Inorg. Chem. 2025, 64, 11165–11176. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Lee, J.E.; Yun, S.Y.; Kwon, O.; Park, J.K.; Jeong, Y.K. Plasma-Induced Oxygen Vacancies in N-Doped Hollow NiCoPBA Nanocages Derived from Prussian Blue Analogue for Efficient OER in Alkaline Media. Int. J. Mol. Sci. 2023, 24, 9246. [Google Scholar] [CrossRef]
- Diao, F.; Rykær Kraglund, M.; Cao, H.; Yan, X.; Liu, P.; Engelbrekt, C.; Xiao, X. Moderate heat treatment of CoFe Prussian blue analogues for enhanced oxygen evolution reaction performance. J. Energy Chem. 2023, 78, 476–486. [Google Scholar] [CrossRef]
- Khairy, M.; Mahmoud, K.G.; El-Sagher, H.M. Amorphization of nanostructured prussian blue analogues for boosting oxygen evolution reaction toward design of efficient electrocatalysts. Int. J. Hydrogen Energy 2023, 48, 29887–29897. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, D.; Zhang, W.; Jia, G.; Xie, H.; Ye, W.; Zhu, G.; Gao, P. A boronization-induced amorphous–crystalline interface on a Prussian blue analogue for efficient and stable seawater splitting. Chem. Commun. 2022, 58, 6132–6135. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Aulia, S.; Yeh, M.-H.; Hsiao, L.-Y.; Tarigan, A.M.; Ho, K.-C. Graphene quantum dots induced defect-rich NiFe Prussian blue analogue as an efficient electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2023, 648, 193–202. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Yang, T.; Chen, X.; Cheng, Z.; Hou, Q.; Lin, X.; Liu, S.; Wei, S.; Wang, Z. Co3+-rich CoFe-PBA encapsulated in ultrathin MoS2 sheath as integrated core-shell architectures for highly efficient OER. J. Alloy Compd. 2023, 942, 169004. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, L.; Liu, C.-S.; Pang, H. PBA@POM Hybrids as Efficient Electrocatalysts for the Oxygen Evolution Reaction. Chem. Asian J. 2019, 14, 2790–2795. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Sun, Y.; Tang, L.; Liu, Q.; Zhang, X. Loading Pt Nanoparticles on Metal–Organic Frameworks for Improved Oxygen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 11577–11583. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Chae, K.; Yu, D.; Thu Tran, N.A.; Khoi, T.M.; Kim, J.; Cho, H.-S.; Cho, Y. Carbon-Coated CeO2-CoFe Core-Shell electrocatalysts derived from Prussian blue analogues for high-performance oxygen evolution reactions. Sep. Purif. Technol. 2025, 362, 131699. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Jin, L.; Chen, C.; Wang, C.; Du, Y. Boosting electrocatalytic oxygen evolution over Prussian blue analog/transition metal dichalcogenide nanoboxes by photo-induced electron transfer. J. Mater. Chem. A 2019, 7, 26905–26910. [Google Scholar] [CrossRef]
- Azeem, S.; Soriano-López, J.; Brotons-Alcázar, I.; Allen, C.; Torres-Cavanillas, R.; Sanchis-Gual, R.; Coronado, E. Design of Core@Shell Nanoparticles Based on Gold and Magnetic NiFe Prussian-Blue Analogues Featuring Shape-Dependent Magnetic and Electrochemical Activity. Inorg. Chem. 2025, 64, 6510–6518. [Google Scholar] [CrossRef]
- Sanchis-Gual, R.; Otero, T.F.; Coronado-Puchau, M.; Coronado, E. Enhancing the electrocatalytic activity and stability of Prussian blue analogues by increasing their electroactive sites through the introduction of Au nanoparticles. Nanoscale 2021, 13, 12676–12686. [Google Scholar] [CrossRef] [PubMed]
| Sample | Electrolyte | Overpotential ηmA cm−2 [mV] | Tafel [mV dec−1] | Ref. |
|---|---|---|---|---|
| IrOx | 1M KOH | η10 = 285 | 55.4 | [58] |
| IrO2 | 1M KOH | η10 = 351 | 78 | [59] |
| RuO2 | 1M KOH | η10 = 326 | 64 | [60] |
| Ir/C | 1M KOH | η10 = 325 | 46.6 | [61] |
| IrO2 | 1M PBS | η10 = 418 | 134.6 | [62] |
| Pt/C RuO2 | 1M KOH | η10 = 566 η10 = 377 | 151.2 88.6 | [63] |
| RuO2 | 1M KOH | η10 = 338 | 120.4 | [64] |
| IrO2 | 1M KOH | η50 = 460 | 94 | [65] |
| RuO2 | 1M KOH | η10 = 303 | 120.1 | [66] |
| RuO2 | 1M KOH | η10 = 290 | 135 | [67] |
| Sample | Morphological/Structural Characteristics | Electrolyte | Overpotential ηmA cm−2 [mV] | Tafel [mV dec−1] | Stability | Ref. |
|---|---|---|---|---|---|---|
| Co3O4/Co-Fe oxide DSNBs | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 297 | 61 | 10 | [74] |
| Fe-CoP prisms | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 235 | 32.9 | 45 | [75] |
| Ni2[Fe(CN)6] | Defects | 1 M KOH | η20 = 288 | 86 | 40 | [53] |
| NiCoOx-400 | Porous Structure | 0.1 M KOH | η10 = 280 | 74 | 0.83 | [84] |
| NiFe-NCs | Porous Structure | 1 M KOH | η10 = 271 | 48 | 18 | [59] |
| Ultrathin CoFe2O4 Nanosheets | 2D and Porous Structure | 1 M KOH | η10 = 275 | 42.1 | 10 | [85] |
| RuO2/Co3O4 | Interface and Porous Structure | 1 M KOH | η10 = 302 | 74.37 | 1000C | [86] |
| Ni-Fe-Pt NCs | Interface and Porous Structure | 1 M KOH | η10 = 333 | 64 | 12 1000C | [60] |
| CoFe-200/GCE | Porous Structure | 1 M KOH | η10 = 316 | 49.6 | 10,000C | [61] |
| Ni-P | 2D and Porous Structure | 1 M KOH | η10 = 300 | 64 | -- | [88] |
| NiCoFeP | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 273 | 35 | -- | [89] |
| FeSe-NiSe/NF | Self-supported Electrode | 1 M KOH | η10 = 234 | 22 | 120 | [90] |
| FeCoSx-PBA | Hierarchical Nanoporous Structure and amorphous sites | 1 M KOH | η10 = 266 | 33 | -- | [91] |
| Ni–Co mixed oxide cages | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 380 | 50 | 10 | [93] |
| Co3O4 microframes | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 370 | 53 | 1000C | [94] |
| Ni-Fe-Se cages | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 240 | 22 | 10 500C | [95] |
| Ni-Fe-Se cages | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 249 | 35 | 144 | [96] |
| NiFe hollow cages | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 313 | 20 | 5000C | [97] |
| Etched-PBA-MoS2 | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 260 | 55 | 1000C | [98] |
| Co0.6Fe0.4P | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 298 | 48 | 120 | [99] |
| A-CoS4.6O0.6 PNCs | Hierarchical Nanoporous Structure and Amorphous sites | 1 M KOH 0.1 M PBS | η10 = 290 ηonset = 270 | 67 164 | -- | [100] |
| Mesoporous Nanostructured CoFe−Se−P | Hierarchical Nanoporous Structure | 0.1 M KOH | η10 = 210 | 98 | 40 1000C | [101] |
| NiO/NiFe2O4 multicomposite hollow NCs | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 303 | 58.5 | 12 | [103] |
| Co-Fe mixed oxide NAFSs | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 340 | 57 | 8 | [105] |
| Fe-CoxP hollow nanosphere | Hierarchical Nanoporous Structure | 1 M KOH | η10 = 345 | 49 | 12 | [106] |
| t-CoIICoIII | Hierarchical Structure | 1 M KOH | η10 = 220 | 79 | 50 | [107] |
| CoFeP NFs/NPCNT. | High Electrical Conductivity | 1 M KOH | η10 = 278 | 39.5 | 40 3000C | [108] |
| O-CNT/NiFe 1:18 | High Electrical Conductivity and Defect | 1 M KOH | η10 = 279 | 42.8 | -- | [109] |
| O-PBA/N-CNT 1:2 | High Electrical Conductivity and Defect | 1 M KOH | η10 = 280 | 48 | 24 1000C | [110] |
| rGO/CoNiPBd-OOH | High Electrical Conductivity | 1 M KOH | η10 = 346 | 33 | 15 | [111] |
| CoFe/CoFeOx@3DNC | Porous Structure and High Electrical Conductivity | 1 M KOH | η10 = 335 | 50.5 | 20 2000C | [112] |
| PB-Co/Co−N-PHCS | Interface and High Electrical Conductivity | 0.1 M KOH | η10 = 370 | 92 | 28 | [113] |
| FeCo-NCNFs-800 | High Electrical Conductivity | 0.1 M KOH | η10 = 456 | 105.48 | 1000C | [115] |
| CoP@CNF | High Electrical Conductivity | 1 M KOH | η10 = 300 | 73.8 | 24 2000C | [116] |
| NiCo@NC-900 | Porous Structure and High Electrical Conductivity | 1 M PBS | η10 = 396 | 89.2 | 25 3000C | [62] |
| Co3ZnC/Co nanojunctions@NC | High Electrical Conductivity and Interface | 1 M KOH | η10 = 366 | 81 | 5000C | [63] |
| M-NiFe-700@C | High Electrical Conductivity | 1 M KOH | η10 = 281 | 53 | 2000C | [120] |
| NiFeP/CC | High Electrical Conductivity and Self-supported Electrode | 1 M KOH | η200 = 260 | 39.4 | 300 | [121] |
| CoFe2O4 Thin Film | High Electrical Conductivity and Self-supported Electrode | 1 M KOH | η10 = 266 | 53 | 24 | [122] |
| nPBA@Co(OH)2/NF | Hierarchical Structure and Self-supported Electrode | 1 M KOH | η10 = 256 | 46 | 2000 | [57] |
| CuFe Oxide/CF | Hierarchical Structure and Self-supported Electrode | 1 M KOH | η10 = 294 | 68 | 600 | [123] |
| Fe–NiO/CC | High Electrical Conductivity and Hierarchical porous Structure | 1 M KOH | η10 = 218 | 47 | 50 | [124] |
| c-Ti-Fe-S boxes | Metal element doping and Porous Structure | 1 M KOH | η10 = 350 | 55 | 12 | [131] |
| Co0.62Fe0.38P | Metal element doping | 1 M KOH | η10 = 230 | 51 | 15 | [134] |
| FeCo0.41Mn0.42 | Metal element doping | 1 M KOH | η10 = 261 | 47.7 | 72 | [135] |
| NCF MOF | Metal element doping and Hierarchical nanoporous Structure | 0.1 M KOH | η10 = 190 | 49 | 5.5 | [143] |
| NiCoFe-Se/CFP | Metal element doping and High Electrical Conductivity | 1 M KOH | η10 = 221 | 38.58 | 40 1000C | [144] |
| Fe-Mn-Co/PBA | Metal element doping | 1 M NaOH | η10 = 260 | 48 | 20 | [145] |
| PBA-Se 350 | Metal element doping | 1 M KOH | η10 = 184 | 43.4 | 30 | [64] |
| Ni-doped CoFe2O4 hollow nanocubes | Metal element doping and Hierarchical porous Structure | 1 M KOH | η10 = 330 | 72.6 | 12 | [146] |
| NiFeLaMoCo-PBA | High-Entropy Materials | 1 M KOH | η10 = 244 | 32 | 48 | [147] |
| HE-PBA-e | High-Entropy Materials and Defect | 1 M KOH | η10 = 332 | 86.3 | 100 | [148] |
| Co-PBA-plasma-2h | Defect | 1 M KOH | η10 = 274 | 53 | 16 | [14] |
| Co0.4Fe0.28P | Metal element doping and Porous Structure | 1 M KOH | η10 = 270 | 25.6 | 3 | [58] |
| VCN-mediated Ni–Fe PBA | CN vacancies | 1 M KOH | η10 = 283 | 54 | 25 | [157] |
| Oxygen Plasma-CoFe-PBA | Lattice oxygen activation | 1 M KOH | η10 = 218 η1000 = 276 | 26.7 22.9 | 400 | [56] |
| NiFe PBA-H2 4min | CN vacancies | 1 M KOH | η10 = 400 | 104 | 100 | [54] |
| PBA-plasma 3h-Air | CN vacancies | 1 M KOH | η10 = 251 | 62.1 | 100 | [55] |
| N90-NiCoPBA | Oxygen vacancies | 1 M KOH | η10 = 289 | 70 | 24 | [160] |
| Double-shelled NiFe PBAs with VCN | CN vacancies | 1 M KOH | η20 = 267 | 79 | 80 | [158] |
| N2-NiCoFe-PBA | CN vacancies | 1 M KOH 1 M KOH/0.5M NaCl 1 M KOH/seawater | η100 = 286 η100 = 293 η100 = 323 | 41.5 41.9 54.8 | 120 | [159] |
| Ni Fe PBAs with VCN | CN vacancies | 1 M KOH | η50 = 270 | 53 | 80 | [65] |
| v-NiFe PBA@rGO | [Fe(CN)6]3− vacancies | 1 M KOH | η10 = 251 | 36.2 | 200 | [66] |
| Ar-U-CoFe PBA | Amorphous Structure | 1 M KOH | η10 = 305 | 36.1 | 20 | [161] |
| NiFe PBA-N2 300 °C | Amorphous Structure | 1 M KOH | η10 = 330 | 73 | 10 | [162] |
| Boron-modified PBA/NFs 2h | Amorphous Structure | 1 M KOH | η100 = 311 | 88 | 50 | [163] |
| O-GQD-NiFe PBA | Interface and Defects | 1 M KOH | η10 = 259 | 52.5 | 100 | [164] |
| CoIIIFe-PBA/MoS2−x | Interface | 1 M KOH | η100 = 306 | 36.2 | 30 | [165] |
| PBA@POM | Interface and Hierarchical Porous Structure | 1 M KOH | η10 = 440 | 23.45 | 16 | [166] |
| Co-PB/Pt | Interface and Hierarchical Structure | 1 M KOH | η10 = 300 | 68 | 500C | [167] |
| NC@CeO2-CoFe | Interface | 1 M KOH | η10 = 255 | 47 | 1000 4000C | [168] |
| CoFe PBA/CoS2-12 CNBs | Interface | 1 M KOH | η10 = 301 | 59.2 | 45 | [169] |
| CoFe PBA@CoP/NF | Interface and Hierarchical Structure | 1 M KOH | η10 = 171 | 75.7 | 15 | [67] |
| AuNSt@PBA | Strain | 0.1 M PBS 1 M NaNO3 | η10 = 800 | ~190 | -- | [170] |
| Au@CoFe | High Electrical Conductivity | 1 M KOH | η10 = 300 | 63 | 24 | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Shi, H.; Zhou, T.; Yan, W.; Song, J.; Feng, P.; Wang, K.; Jiang, Z. Prussian Blue Analogues and Their Derivatives for the Oxygen Evolution Reaction: A Review on Active Site Engineering Strategies. Inorganics 2025, 13, 354. https://doi.org/10.3390/inorganics13110354
Cao Z, Shi H, Zhou T, Yan W, Song J, Feng P, Wang K, Jiang Z. Prussian Blue Analogues and Their Derivatives for the Oxygen Evolution Reaction: A Review on Active Site Engineering Strategies. Inorganics. 2025; 13(11):354. https://doi.org/10.3390/inorganics13110354
Chicago/Turabian StyleCao, Zhen, Haozhe Shi, Tingting Zhou, Wenhui Yan, Jiahong Song, Pengqi Feng, Kaili Wang, and Zaiyong Jiang. 2025. "Prussian Blue Analogues and Their Derivatives for the Oxygen Evolution Reaction: A Review on Active Site Engineering Strategies" Inorganics 13, no. 11: 354. https://doi.org/10.3390/inorganics13110354
APA StyleCao, Z., Shi, H., Zhou, T., Yan, W., Song, J., Feng, P., Wang, K., & Jiang, Z. (2025). Prussian Blue Analogues and Their Derivatives for the Oxygen Evolution Reaction: A Review on Active Site Engineering Strategies. Inorganics, 13(11), 354. https://doi.org/10.3390/inorganics13110354






