Abstract
This article provides a review of modern technologies for processing chromite ores and beneficiation wastes, with a focus on the recovery of magnesium and platinum-group metals (PGMs). It reveals that the traditional use of chromites solely as a source of chromium limits the potential of this raw material, whereas comprehensive processing enables the recovery of associated components, including serpentine minerals, which are widely present in chromite ores and tailings. Pyrometallurgical, hydrometallurgical, plasma-arc, and biotechnological methods are examined, as well as their integration into combined flowsheets. Particular attention is given to sulfation, chloridization, and carbochlorination processes, which ensure a high degree of PGM recovery. Economic and environmental aspects of comprehensive processing are discussed, including carbon footprint reduction, waste minimization, and prospects for the development of “green metallurgy.” It is concluded that the further advancement of resource-efficient and environmentally safe technologies for chromite processing will increase production efficiency, ensure resource independence, and support compliance with global carbon neutrality requirements.
1. Introduction
Chromite ores are the primary source of chromium—an element widely used in metallurgy, the chemical industry, and the production of refractories. Chromium is in high demand primarily as both a component in the manufacture of stainless steels and heat-resistant alloys, and a raw material for producing chromium compounds employed in leather, paint and coatings, and textile industries, among others [1,2,3]. Owing to its ability to form compounds that are resistant to corrosion and oxidation, chromium holds an important position among alloying elements and determines the quality of key industrial materials.
The largest chromite deposits are concentrated in South Africa, Kazakhstan, India, Turkey, and Russia, making these countries strategic players in the global ferrochrome and chromium products market [4,5,6,7]. South Africa alone accounts for over 70% of global chromium reserves, while Kazakhstan and Russia rank among the top five countries in terms of mining and processing volumes. This underscores not only the economic importance of the chromium industry but also its geopolitical role in ensuring resource security (Figure 1) [8].
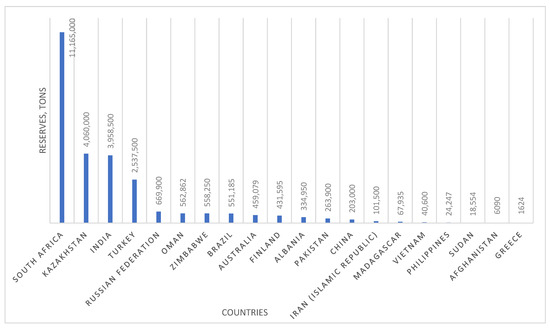
Figure 1.
Major chromite reserves worldwide (2023).
Traditional technologies for processing chromite raw materials are primarily aimed at the production of ferrochrome and chromium oxides. However, significant amounts of associated elements—magnesium and platinum-group metals (PGMs)—remain in dumps and tailings without industrial utilization. This is due both to the complex mineralogical composition of ores (presence of MgO, FeO, Al2O3, and silicates) and the lack of integrated technological flowsheets designed for complete raw material utilization. As a result, a considerable portion of potentially valuable components is lost, while waste volumes continue to grow, creating environmental and socio-economic risks [9,10,11,12,13,14].
In recent decades, there has been a steady increase in interest in developing technologies for the comprehensive processing of chromite raw materials, based on the principles of resource conservation, waste minimization, and maximum utilization of all valuable components. This approach is driven by several factors:
- The gradual depletion of rich ores and the need to process low-grade and refractory deposits;
- Stricter environmental regulations related to tailings storage, waste management, and reducing greenhouse gas emissions;
- Growing global demand for associated elements such as magnesium (used in metallurgy, construction, chemical, and pharmaceutical industries) and PGMs (applied in automotive catalysts, electronics, and jewelry).
Magnesium recovery is of particular importance, as its content in chromite ores and serpentine-bearing beneficiation tailings is relatively high. Magnesium is widely used in the production of alloys, refractories, fertilizers, pharmaceuticals, and chemical reagents. Platinum-group metals, although present in chromites at low concentrations, have high market value and strategic significance for high-tech industries, which justifies the development of technologies for their extraction even from low-grade raw materials [15,16,17].
Research groups in China, Russia, Kazakhstan, South Africa, and several other countries are actively engaged in developing integrated processing schemes for chromite resources. These flowsheets combine pyrometallurgical and hydrometallurgical methods, plasma technologies, and sorption, flotation, and bioleaching processes. As a result, it becomes possible to extract chromium, magnesium, and PGMs simultaneously, thereby improving mineral resource utilization and reducing environmental impacts [18,19,20].
Thus, one of the main priorities of modern metallurgy and beneficiation technology is the development and implementation of integrated approaches to process chromite raw materials. The present review article aims to not only systematize and analyze existing methods and emerging technologies, with particular emphasis on the recovery of magnesium, rare earth elements, and platinum-group metals, but also evaluate their economic and environmental efficiency.
2. Characteristics of Chromite Raw Materials
2.1. Mineralogical and Chemical Composition
Chromite (FeCr2O4) belongs to the spinel group of minerals and is the principal industrial source of chromium. Chromite ores typically contain 46–62% Cr2O3, 15–30% FeO, and 8–18% MgO, along with admixtures of Al2O3, SiO2, CaO, and other oxides.
The chemical composition of chromite is usually highly variable, mainly due to substitution among trivalent elements, ranging from Cr-rich to Al-rich varieties. Various trace elements, such as platinum-group elements (Os, Ir, Ru, Rh, Pt, and Pd) and Ni, Cu, Co, V, Zn, and Ti, may also be present, in some cases, as independent mineral phases [21].
Mineralogical composition varies significantly depending on the deposit: for example, South African ores are characterized by high Cr2O3 content and relatively low MgO impurities, whereas Kazakh and Indian chromites often contain higher levels of MgO and silicate minerals.
The quality of chromite is determined primarily by the mass fraction of chromium oxide (Cr2O3) and the Cr/Fe ratio. In industry, ores are generally processed when they contain at least 46–48% Cr2O3 and have a Cr/Fe ratio greater than 2.0 [9,22,23].
The presence of aluminosilicates, serpentines, and other silicate phases complicates ore processing, as they reduce the efficiency of both pyrometallurgical and hydrometallurgical methods. In addition, ore composition affects its refractoriness, solubility in acids, and the potential for comprehensive recovery of associated elements.
2.2. Magnesium and PGM Content and Occurrence
Magnesium in chromite ores is present as MgO within the spinel crystal lattice, where it partially substitutes for Fe2+ and Cr3+ through isomorphic replacement. In addition, Mg may also occur in silicate minerals such as serpentine, olivine, and pyroxene (Table 1, Figure 1). This complicates its recovery, since it requires either breaking the strong bonds within the spinel lattice or separating it from stable silicate phases [24].

Table 1.
Forms of magnesium occurrence in chromite ores and their processing significance.
The primary industrially significant ores are those composed of magnochromite, (Mg,Fe)Cr2O5 (Figure 2 and Table 1).
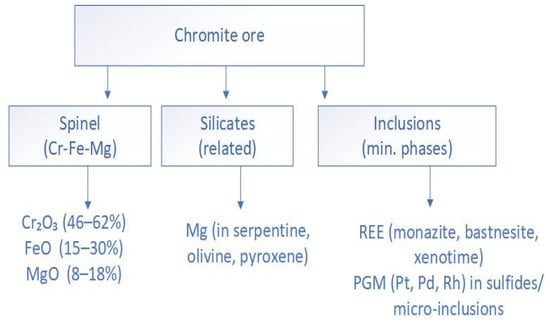
Figure 2.
Mineralogical distribution of components in chromite ore.
Since the earliest geochemical studies of ultramafic rocks [26], chromite has been recognized as a potential concentrator of platinum-group elements (PGEs = Os, Ir, Ru, Rh, Pt, and Pd). These metals occur mainly as minute grains (<20 µm) of specific platinum-group minerals (PGMs), located within chromite crystals, along their grain boundaries, or in the interstitial silicate matrix of chromitite.
Platinum-group metals (PGEs—Pt, Pd, Rh, and others) are usually present in chromites at extremely low concentrations (0.1 to 2 g/t) and occur either as micro-inclusions within the spinel structure or in association with sulfide minerals such as pentlandite and chalcopyrite (Table 2).

Table 2.
Associations of sulfides and PGMs in chromitite layers.
At present, there is a general consensus that primary PGEs represent primary liquidus phases mechanically entrapped within chromites and mafic silicates crystallizing from magma at high temperatures, which appears to be the most plausible explanation. This model readily accounts for the mineralogical variability of composite inclusions containing PGEs, as described in several chromitites, and does not require crystallographic substitution of PGEs into the structure of chromite or mafic silicates [29,30,31,32].
Most authors distinguish PGEs into two genetically different categories based on their textural relationships:
“Primary” PGEs, enclosed in fresh chromite away from fractures and alteration zones;
“Secondary” PGEs, invariably associated with low-temperature mineral assemblages, occurring either within the ferritchromite rims of chromite grains or in the interstitial silicate matrix (serpentine, chlorite, and talc) [33].
Elemental microanalysis confirmed that platinum occurs in trace amounts within the studied chromite material. The detected Pt signal (~0.12 wt%) indicates that chromite ores can contain measurable quantities of platinum-group metals (PGMs), supporting the potential for their recovery as valuable by-products in integrated processing schemes. This observation is consistent with previously reported occurrences of PGM inclusions in chromite ores from layered mafic intrusions and lateritic deposits, where platinum and palladium are commonly hosted as micro-scale inclusions or intergrowths within chromite grains [19].
2.3. Challenges of Comprehensive Recovery
The integrated recovery of valuable components from chromite ores is complicated by several factors:
Mineralogical complexity. Valuable elements are distributed among different mineral phases, often tightly intergrown, which hinders selective beneficiation [34].
- Fine dissemination of minerals. Chromospinelides are associated with olivine, serpentine, and pyroxenes, reducing the efficiency of flotation and gravity separation [35].
- High energy demand for grinding. Ultra-fine grinding is required to liberate minerals, leading to higher costs and decreased recoverability [36].
- Problems of comprehensive utilization. Valuable by-products (e.g., platinum-group metals) are often lost to tailings due to the lack of specialized recovery technologies.
- Environmental risks. Traditional ferrochrome smelting generates toxic Cr(VI) compounds, restricting the implementation of integrated processing technologies [37].
These challenges highlight the need to develop integrated processing schemes that combine pyrometallurgy, hydrometallurgy, and selective beneficiation to enable the recovery of not only chromium but also magnesium and PGEs.
3. Traditional Methods of Chromite Processing
Recent advances in chromite processing emphasize integrated approaches that combine physical beneficiation, pyrometallurgical treatment, and hydrometallurgical recovery to maximize metal extraction while minimizing waste.
Special attention is given to the recovery of magnesium from serpentinite-associated minerals and platinum-group metals (PGMs) from slag and tailings, contributing to the sustainable utilization of resources.
Figure 3 illustrates the integrated processing routes for chromite ores and tailings, highlighting the main technological pathways for magnesium and PGM recovery.
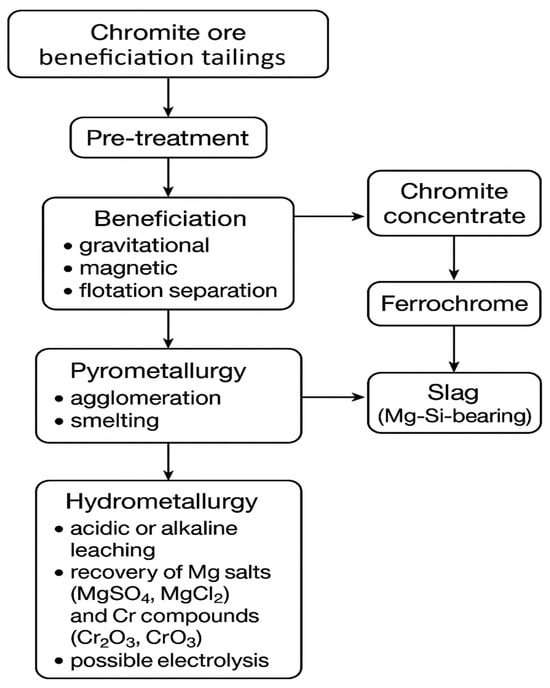
Figure 3.
Integrated processing routes for chromite ores and tailings.
Traditional methods of chromite processing begin with ore beneficiation. As a rule, chromium is extracted from high-grade ores directly, whereas low-grade ores require preliminary beneficiation. The main objective of beneficiation is to increase Cr2O3 content and improve the Cr/Fe ratio, which is achieved by removing iron-bearing and silicate impurities. The applied physico-mechanical and physico-chemical methods include gravity, magnetic, and electrostatic separation; flotation; and classification and grinding (Table 3).

Table 3.
Methods of chromite beneficiation.
Gravity separation is the most common method used in the beneficiation of chromite ores. This technique separates chromite from other minerals based on differences in density, using spiral and shaking methods. It is particularly effective for low-grade ores, enabling higher Cr2O3 content with significant recovery rates [38]. The main equipment includes the following [39]:
Jigging machines: Efficient for coarse fractions (>1 mm).
Spiral separators: Used for fine fractions (0.1–1 mm).
Shaking tables: Applied to finely ground ores.
Dense medium separation: Used for preliminary removal of gangue.
Magnetic separation is used to remove iron impurities from chromite ores. This method is especially valuable for ores with high iron content, as it increases the Cr/Fe ratio by eliminating undesirable iron minerals.
Low-intensity separation (up to 0.2 T) removes strongly magnetic impurities.
High-intensity separation (up to 2 T) concentrates weakly magnetic chromite [40].
In some cases, flotation is applied, which relies on differences in mineral surface wettability. Certain minerals adhere to air bubbles, while others remain in the pulp. This method is used mainly for fine-grained ores or gravity separation tailings. Typical collectors include fatty acids and anionic reagents [41]. The drawback of flotation is its low selectivity, so it is often combined with preliminary magnetic separation.
Crushing and grinding are the preliminary stages of beneficiation, aimed at reducing ore particle size to facilitate further processing. These operations help liberate chromite grains from associated minerals, thereby improving the efficiency of subsequent separation processes [42].
3.1. Pyrometallurgical Technologies
Traditionally, chromite ore, fluxes, and reductants are processed via submerged arc smelting in three-phase furnaces. Prior to charging, chromite pellets must be agglomerated. The main reductant is coke, which results in significant CO2 emissions and makes the process both energy-intensive and environmentally unfavorable [43,44,45].
Recent studies propose replacing part of the traditional operations with plasma technologies, which provide higher temperatures, reduce the consumption of carbonaceous reductants, and allow the use of hydrogen as a reductant. This opens up opportunities to lower the carbon footprint and improve the environmental sustainability of ferrochrome production [46,47].
Another direction for improvement is oxidative sintering of chromite pellets (Outotec FeCr process), which reduces energy consumption during subsequent smelting by partially releasing iron from the spinel lattice. It has been shown that the degree of oxidative treatment can vary significantly depending on the deposit and even within a single batch, thus requiring preliminary characterization of pellets. The use of oxidized feed has demonstrated advantages in its interaction with carbon and increases the efficiency of ferrochrome smelting [48].
3.2. Hydrometallurgical Technologies
An alternative to pyrometallurgy is offered by hydrometallurgical methods, which are based on acid or alkaline leaching. Direct pressure leaching of chromite with sodium hydroxide (NaOH) was investigated by Farrow and Burkin [49] in the temperature range of 190–270 °C. During leaching, chromium is gradually removed from the surface layers and replaced by iron. This results in the formation of an iron-enriched layer, which thickens over time and slows further chromium recovery. Various approaches to chromite decomposition have been tested.
Xu et al. employed NaOH with oxidants (O2, air, O3), producing sodium chromate, which was then converted to dichromate and chromium anhydride by electrolysis [50]. Wang et al. applied an electrochemical field in a KOH medium, achieving up to 99% chromium recovery [51]. However, all such methods are energy-intensive, and, despite reducing toxic waste, none have yet reached industrial-scale application.
Direct acid leaching with nitric acid has demonstrated effectiveness for processing low-grade chromite ores. Optimization of process parameters (acid concentration, temperature, and particle size) enabled recovery of up to 86.7% Cr2O3. Kinetic analysis using the shrinking-core model indicated that the reaction rate is limited by internal diffusion. This method is regarded as a relatively low-cost alternative to pyrometallurgical technologies for chromite beneficiation in the steel industry [52].
Leaching with sulfuric acid has been the subject of numerous studies [53]. Without oxidants, chromium recovery remains low due to the formation of passivating polynuclear compounds. The use of catalysts and oxidants such as perchloric acid or sodium bichromate significantly improves process efficiency: extraction rates of 82–93% have been achieved without generating toxic Cr(VI). The highest recovery (96.4%) was reported under optimized conditions with oxidant addition [54]. Thus, the choice of an appropriate oxidant is a key factor in process intensification, whereas structural features of the spinel lattice, particularly the presence of silicon-bearing phases, limit complete leaching.
A comparative assessment of hydrometallurgical and pyrometallurgical methods is presented in Table 4.

Table 4.
Comparative characteristics of pyrometallurgical and hydrometallurgical processing of chromites.
Both groups of technologies ensure chromium recovery but practically do not address associated elements (Mg and PGEs), highlighting the need to develop comprehensive processing schemes.
Thus, while traditional technologies provide valuable chromium products, they fail to achieve full utilization of raw materials. This underscores the necessity of developing new integrated approaches that enable the simultaneous recovery of chromium, magnesium, rare earth elements, and platinum-group metals.
4. Modern Approaches to Integrated Processing
4.1. Magnesium Recovery
Lizardite, one of the serpentine minerals, is often present in the host rocks of chromite deposits and enters tailings after beneficiation. These tailings can serve as a promising raw material for magnesium extraction and the production of amorphous SiO2. At work [55] reported leaching results for chromite beneficiation tailings dominated by lizardite (containing ~39.3% MgO). Hydrochloric (HCl) and sulfuric (H2SO4) acids were used for magnesium recovery, and optimized conditions achieved yields of up to 98% Mg. The solid residue after leaching was composed mainly of amorphous silica.
In the processing of chromite production wastes, a method of selective magnesium removal has been proposed, involving roasting with (NH4)2SO4 followed by H2SO4 leaching. Under optimal conditions, up to 79.6% Mg was removed, increasing chromium recovery from 84.6% (direct roasting) to 95.4%. This effect was explained by the decomposition of Mg-containing spinels and diopside into soluble phases of Cr2O3 and (Fe,Cr)2O3. The method enhances chromium recovery efficiency and may be applied to other Cr-bearing wastes [56].
The process described in Ref [57] involved leaching high-Mg peridotite (a host rock of chromite ore) to obtain magnesium salts and microsilica. Oxidative leaching with H2O2 prevented gel formation and improved filtration, achieving 92% Mg recovery. Products included Mg(OH)2 and MgSO4 salts, while the solid residue included SiO2 particles (10–30 μm) suitable for high-strength concrete production.
Research on serpentine wastes from the Zhitikara deposit [58] demonstrated the production of valuable magnesium compounds (MgSO4, Mg(OH)2, and MgO). Since serpentines are widespread silicate minerals commonly associated with chromite ores and concentrates, their processing enables the integrated use of ores and beneficiation tailings. The proposed technology is based on acid leaching with subsequent purification, yielding high-purity products. A techno-economic analysis confirmed its resource and energy efficiency, as well as the advantage of producing MgO as the end product.
A patented two-stage sulfuric acid leaching process [59] was also reported for serpentine-bearing feedstocks. The first stage employs filtrate and wash solutions (100–130 g/L H2SO4, L:S (liquid-to-solid ratio) = 3–4:1), while the second stage uses fresh acid (350–600 g/L, L:S (liquid-to-solid ratio) = 1.5–2.5:1). After hydrolytic purification, a magnesium compound is precipitated and thermally decomposed (1100–1500 °C) to yield high-purity MgO. This method achieves up to 83.5% Mg recovery, reduces reagent consumption, and enables processing of low-grade chromite ores and serpentine-rich tailings. The resulting MgO is characterized by >97% purity and high stability in water and acids, making the process both economically and technologically viable for integrated mineral resource utilization.
4.2. Recovery of Platinum-Group Metals (PGMs)
According to [60], the most effective recovery of PGMs from chromite ores is achieved using combined processing methods. These involve preliminary gravity and flotation beneficiation to concentrate sulfide minerals, followed by metallurgical treatment (smelting and acid leaching), and hydrometallurgical processes with prior thermal or mechanochemical activation of the ore. This approach enables more complete utilization of PGMs from refractory chromites and beneficiation tailings.
High efficiency has been demonstrated by methods based on two-stage smelting with gas bubbling of the melt and oxygen injection, combined with solid and gaseous carbonaceous reductants. One such method [61] involves two-stage smelting of PGM-bearing materials. The first stage is performed at high temperatures with intensive bubbling and oxygen-rich injection to ensure complete melting. The second stage is conducted under strongly reducing conditions with intensive bubbling, enabling active reduction in PGMs, formation of an Fe-based alloy that serves as the collector phase, and effective separation of metallic and slag phases.
Similar approaches are described in a patent [62], involving oxidative thermal treatment of the Fe phase (concentrating PGMs) in a converter furnace with air or oxygen injection at 800–1100 °C. Under these conditions, PGMs exhibit a strong chemical affinity for the molten Fe phase, concentrating almost completely while separating from sulfide and silicate phases. Maximum recovery occurs when the feed maintains weight ratios of silicate–iron–sulfide components (as SiO2, Fe oxides, and S) of (2–6):(0.3–3):(0.5–1.5).
A promising alternative is plasma arc smelting, which achieves high PGM concentration in the Fe phase due to localization of reduction and accumulation processes in the cathode zone [63]. The feed is introduced into a DC plasma-arc furnace preheated to 1600–1700 °C, with the cathode at the furnace bottom. Smelting yields a fluid melt concentrated at the cathode zone, while carbonaceous reductants (5–30% of stoichiometric requirement) enable efficient reduction in Fe and PGMs. Plasma arc technologies have emerged as an advanced and sustainable approach in extractive metallurgy, providing ultra-high temperatures (up to 10,000 °C), enhanced reduction kinetics, and efficient energy utilization. According to [64], the use of plasma systems in metallurgical processing enables more efficient energy transfer compared with conventional submerged-arc or induction furnaces, owing to the high energy density and controllable thermal profiles. Plasma systems also allow flexible operation with various gas mixtures and feedstocks, including ores, concentrates, and metallurgical wastes. A promising application of plasma arc technology is the processing of chromite ores for ferrochrome production. As demonstrated by [65], DC plasma smelting reduction can efficiently convert chromite concentrates into high-carbon ferrochrome (HC-FeCr) at 1700–1800 °C. The process achieves nearly complete reduction in Cr2O3, while the carbon content in the alloy can be adjusted by regulating reductant feed and arc parameters. The use of hydrogen–argon plasma significantly reduces CO2 emissions and enhances the selectivity of chromium reduction, aligning with the principles of “green metallurgy.” As summarized by [62], plasma arc furnaces can treat both oxide and sulfide ores, as well as metallurgical slags and dusts. The key technological advantages are as follows:
- Localization of reduction reactions in the cathode zone, improving metal concentration;
- The use of diverse reductants such as carbon, methane, and hydrogen;
- Controlled slag chemistry and higher metal recovery.
For chromite ores, plasma smelting ensures more complete extraction of chromium and iron, while by-products are immobilized in stable glassy slag phases. In experimental studies (Sanjay et al.), chromium recovery rates above 95% were achieved, with a 30% reduction in carbon consumption compared to traditional carbothermic smelting [66].
Among hydrometallurgical routes, a sulfation process has been proposed [67]. Chromite feed is treated with concentrated H2SO4 (85–93 wt.%) in the presence of CrO3 as an oxidant (70–80% of Fe content) at 150–170 °C and L:S (liquid-to-solid ratio) = 4:1–6:1. PGMs are converted into soluble sulfates, with simultaneous Fe oxidation and partial chromite decomposition.
Microwave-assisted extraction has also been tested. A two-step process [68] includes microwave-assisted sulfation roasting with NaHSO4–H2O or KHSO4 and NaClO3, followed by a short (30 min, 105 °C) microwave acid leaching. Reported recoveries were 96% Pd, 85% Pt, and 96% Rh. Sulfation roasting was shown to affect both cordierite materials and rare earths in the wash layer, forming sulfate salts.
Another method involves sulfation roasting with subsequent chlorination, applied to catalyst wastes similar in composition to Cr-rich residues, enabling combined recovery of osmium and other PGMs [69].
Chlorination and carbochlorination methods have proven effective for the selective extraction of PGMs as chlorides, while simultaneously removing Fe and Cr. Advantages of chlorination include low operating temperatures, flexibility to feed composition, and high selectivity. Chlorination at 300–700 °C enables conversion of PGMs to soluble chlorides suitable for hydrometallurgical recovery. For example, FeCl3 vapor chlorination of Cr-rich ores and residues at 573 ± 20 K (300 ± 20 °C) for 35 min, followed by leaching with HCl at 40 °C for 10 min, yielded 92% Pt recovery [70]. Carbochlorination with a CO/Cl2 mixture (4:6) at 550 °C for 1 h achieved recoveries of 95% Pt and 92% Rh [71]. However, emissions of CO and Cl2 pose environmental concerns.
Overall, these technologies demonstrate high potential for the recovery of magnesium and PGMs from chromite ores and residues. However, their industrial implementation requires consideration not only of technical performance but also economic feasibility and environmental sustainability. Consequently, it is essential to evaluate the prospects of integrated processing in terms of both profitability and long-term sustainability.
5. Biotechnological Methods for the Extraction of Platinum-Group Metals and Magnesium from Chromite Ores in the Context of Green Metallurgy
The current trend in metallurgical development is focused on the implementation of green metallurgy principles—technologies that minimize carbon footprint, toxic emissions, and hazardous waste generation while maintaining high recovery efficiency of valuable elements. In this context, chromite ores and their processing residues are of particular interest, as conventional pyrometallurgical processes are energy-intensive and generate hexavalent chromium (Cr(VI)), which is highly toxic to ecosystems.
An alternative approach involves biotechnological methods, which utilize the metabolic activity of microorganisms to dissolve, reduce, and mobilize metals. These processes operate under mild temperature conditions without aggressive reagents, making them environmentally preferable and fully consistent with ESG principles (Environmental, Social, and Governance responsibility) and the circular economy.
Bioprocesses have proven effective not only in detoxifying Cr(VI) but also in mobilizing platinum-group metals (PGMs) and magnesium (Mg) from chromite-bearing materials. As reported in [72], biotechnological pre-treatment of UG-2 concentrate with thermoacidophilic archaea facilitated the removal of iron and nickel, thus enhancing Pt and Pd extraction during the subsequent cyanidation stage.
Similarly, the authors proposed a three-step bioleaching scheme for PGMs: (1) microbial degradation of the silicate matrix, (2) biogenic formation of complexing agents (including CN−), and (3) extraction of soluble Pt, Pd, and Rh species [73]. These approaches highlight the potential of bio-pre-treatment as a “soft” stage within integrated green metallurgy flowsheets for chromite processing.
For high-chromium ores, the authors of [74] described a hybrid process involving roasting with Ca–Mg–Cl salts followed by acid leaching of PGMs. Such thermochemical treatment can be further coupled with bioprocesses to increase PGM liberation and reduce the overall energy demand.
With respect to magnesium, Stanković et al. and Taheri et al. investigated bioleaching of Mg from serpentinites and magnesia–chromite wastes. Their studies showed that acid-producing microorganisms (Aspergillus niger, among others) partially dissolve magnesian silicates, improving Mg accessibility for hydrometallurgical extraction while reducing acid consumption and the overall environmental footprint [75,76].
Therefore, the integration of biotechnological stages into chromite-processing flowsheets aligns closely with the core goals of green metallurgy and sustainable development. Bioprocesses not only lower CO2 emissions and reduce hazardous waste generation but also enable more comprehensive recovery of accompanying elements such as Cr, PGMs, and Mg [77,78,79].
Table 5 presents a concise summary of experimental conditions and key bioleaching outcomes obtained from recent studies on chromite and serpentinite systems, highlighting the diversity of microorganisms, process parameters, and recovery efficiencies achieved under different operational environments.

Table 5.
Summary of experimental conditions and bioleaching outcomes in chromite and serpentinite systems.
For Kazakhstan—one of the world’s leading chromite producers, hosting major deposits such as Donskoye and Khromtau—these environmentally friendly technologies are especially relevant. The application of biotechnological and hybrid approaches could form part of the national strategy for advancing green metallurgy, aimed at the comprehensive utilization of mineral resources, mitigation of environmental risks, and enhancement of value-added production within the domestic metallurgical sector.
To facilitate a clearer comparison between the different chromite ore processing technologies discussed above, Table 6 summarizes their key operational parameters, recovery efficiencies, energy consumption, and environmental impacts. This comparative overview highlights the potential of integrated process schemes that combine pyrometallurgical, hydrometallurgical, plasma, and biotechnological methods for achieving both economic and environmental benefits.

Table 6.
Comparative evaluation of chromite ore processing routes. (Authors’ own compilation based on references [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].)
6. Aspects of Green Chemistry in Chromite Processing
In the context of increasing environmental and regulatory pressures, applying green chemistry principles to chromite processing is becoming crucial. Conventional methods such as high-temperature roasting, the use of aggressive oxidants, and the generation of Cr(VI)-containing waste are associated with large energy consumption, gaseous emissions, and toxic residues. A green chemistry-oriented approach aims to minimize waste and hazardous intermediates, reduce energy consumption, promote reagent recycling and by-product utilization, and ensure the safe stabilization of final residues.
Promising strategies include sub-molten salt or low-temperature oxidative decomposition, which replaces conventional roasting at about 1200 °C with oxidation in a sub-molten alkaline medium (eutectic KOH–NaOH mixtures) at 300–400 °C, thereby reducing energy use and suppressing Cr(VI) formation [80]. Clean hydrometallurgical leaching in controlled redox conditions can decompose chromite while maintaining chromium in the trivalent state, followed by selective iron removal and chromium salt recovery [81]. Chemical and microbial reduction or stabilization of Cr(VI) in residues further decreases its mobility and environmental risk [82].
Another important approach is the valorization and recycling of chromite processing wastes by converting them into secondary raw materials such as chromium-bearing steels, cement additives, or refractory feedstocks [83]. Finally, the use of environmentally benign adsorbents, biochar, and microbial systems for the treatment and detoxification of chromium-containing effluents represents a new direction in sustainable remediation [81].
Implementing these strategies requires careful control of reaction kinetics, prevention of undesired oxidation, economic feasibility assessment, and integration into existing beneficiation flowsheets. Nevertheless, embedding green chemistry principles in chromite metallurgy provides a pathway toward sustainable, low-emission, and environmentally safe chromium production.
7. Conclusions
This analysis of modern approaches for processing chromite raw materials and beneficiation wastes confirms the high relevance of developing comprehensive technologies aimed at maximizing the recovery of all valuable components. Traditionally, chromites have been used primarily as a source of chromium for the production of ferrochrome and stainless steel. However, recent studies convincingly demonstrate the potential for integrated processing, enabling the extraction of magnesium, platinum-group metals (PGMs), and rare earth elements. Such an approach is fully aligned with contemporary priorities of resource conservation and sustainable development.
In this context, serpentine minerals acquire particular importance, being widespread both in chromite ores and beneficiation tailings. The application of acid leaching, plasma-based, and combined methods makes it possible not only to extract magnesium in the form of industrial compounds (MgSO4, Mg(OH)2, and MgO), but also to obtain high-value by-products such as amorphous silica. This highlights the feasibility of practically waste-free processing schemes.
Promising directions for further development include integrated technologies that combine pyrometallurgical, hydrometallurgical, and biotechnological methods, allowing processing flowsheets to be adapted to the mineralogical characteristics of specific deposits. Significant results have also been reported for sulfation, chloridization, and carbochlorination processes, which provide high recovery rates of PGMs from chromite ores and industrial wastes.
From an economic perspective, comprehensive processing enhances profitability by incorporating by-products into production and reducing the cost of final products. From an environmental standpoint, it contributes to reducing the carbon footprint, lowering CO2 emissions and toxic Cr(VI) formation, and minimizing the generation of sludges and tailings. In this way, chromite processing technologies align with the global agenda of “green metallurgy” and the achievement of carbon neutrality goals.
Taken together, the reviewed approaches establish the scientific and technological foundation for a new stage of integrated utilization of chromite resources. Their further development will make it possible to simultaneously improve production efficiency, ensure resource security, and reduce environmental impacts, making this field one of the key directions in modern metallurgy.
To enable transparent comparison between future studies, standardized data reporting is recommended, including ore composition (wt% Cr2O3, Fe2O3, MgO, SiO2, and Al2O3), recovery yield normalized to dry mass (%), specific energy consumption (kWh t−1), and CO2 emissions (kg t−1). Adopting such practices will facilitate reliable benchmarking of process efficiency and environmental performance, aligning with green metallurgy and ESG principles.
Author Contributions
R.A.: project administration, N.A.: writing—original draft preparation, conceptualization; Y.A.: writing—review and editing, visualization; S.G.: methodology, investigation; A.M.: formal analysis, software; A.K.: validation, resource. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan under grant No. AP23489732.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IBM. Mineral Year Book, Part III: Mineral Reviews, 53rd ed.; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 2014; p. 1.
- Raghu Kumar, C.; Rama Murthy, Y.; Bhoja, S.K. Chapter 3–Chromite Ore Beneficiation: Prospects and Challenges. In Mineral Processing; Rajendran, S., Murty, C.V.G.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–116. ISBN 978-0-12-823149-4. [Google Scholar] [CrossRef]
- Yan, J.X.; Chen, J.X.; Hu, L. Metallurgy of Chromium; Metallurgical Industry Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2024.
- Prasad, U. Chapter 4–Metallic Mineral Deposits. In Economic Geology, Economic Mineral Deposits, 2nd ed.; CBS Publication: Delhi, India, 2013; p. 48. [Google Scholar]
- Nafziger, R.H. A Review of the Deposits and Beneficiation of Lower-Grade Chromite. J. S. Afr. Inst. Min. Metall. 1982, 82, 205–226. [Google Scholar]
- Kenzhaliev, B.K.; Kul’deev, E.I.; Luganov, V.A.; Bondarenko, I.V.; Motovilov, I.Y.; Temirova, S.S. Production of Very Fine, Spherical, Particles of Ferriferous Pigments from the Diatomaceous Raw Material of Kazakhstan. Glass Ceram. 2019, 76, 194–198. [Google Scholar] [CrossRef]
- A Deep Dive into the Richest Chromium Producing Countries in 2023: Intro: Chromium Producing Countries. Available online: https://iran-chromite.com/dominating-chromium-production-the-top-chromium-producing-countries/ (accessed on 13 July 2025).
- Cramer, L.A.; Basson, J.; Nelson, L.R. The Impact of Platinum Production from UG2 Ore on Ferrochrome Production in South Africa. J. S. Afr. Inst. Min. Metall. 2004, 104, 517–527. [Google Scholar]
- Kleynhans, E.L.J. Development and Improvement of Pre-Treatment Technologies to Enhance Ferrochrome Production; North-West University: Potchefstroom, South Africa, 2016. [Google Scholar]
- Ospanov, Y.A.; Kvyatkovskiy, S.A.; Kozhakhmetov, S.M.; Sokolovskaya, L.V.; Semenova, A.S.; Dyussebekova, M.; Shakhalov, A.A. Slag heterogeneity of autogenous copper concentrates smelting. Can. Metall. Q. 2023, 62, 594–601. [Google Scholar] [CrossRef]
- Beukes, J.P.; Van Zyl, P.G.; Ras, M. Treatment of Cr(VI)-Containing Wastes in the South African Ferrochrome Industry—A Review of Currently Applied Methods. J. S. Afr. Inst. Min. Metall. 2012, 112, 347–352. [Google Scholar]
- Kenzhaliev, B.K.; Kvyatkovskii, S.A.; Kozhakhmetov, S.M.; Sokolovskaya, L.V.; Kenzhaliev, E.B.; Semenova, A.S. Determination of Optimum Production Parameters for Depletion of Balkhash Copper-Smelting Plant Dump Slags. Metallurgist 2019, 63, 759–765. [Google Scholar] [CrossRef]
- Volodin, V.N.; Abdulvaliyev, R.A.; Trebukhov, S.A.; Nitsenko, A.V.; Linnik, K.A. Recycling of Beryllium, Manganese, and Zirconium from Secondary Alloys by Magnesium Distillation in Vacuum. Kompleks. Ispolz. Miner. Syra = Complex Use Miner. Resour. 2024, 331, 90–100. [Google Scholar] [CrossRef]
- Ibraev, I.K.; Ibraeva, O.T.; Aitkenov, N.B. An Annealing-Free Method for Processing High-Moisture Iron-Containing Sludge of Metallurgical Production. Kompleks. Ispolz. Miner. Syra = Complex Use Miner. Resour. 2025, 333, 59–70. [Google Scholar] [CrossRef]
- Pašava, J.; Knésl, I.; Vymazalová, A.; Vavřín, I.; Gurskaya, L.I.; Kolantsev, L.R. Geochemistry and Mineralogy of Platinum-Group Elements (PGE) in Chromites from Centralnoye I, Polar Urals, Russia. Geosci. Front. 2011, 2, 81–85. [Google Scholar] [CrossRef]
- Legendre, O.; Augé, T. Mineralogy of Platinum-Group Mineral Inclusions in Chromitites from Different Ophiolite Complexes. In Metallogeny of Basic and Ultrabasic Rocks; Gallagher, M.J., Ixer, R.A., Neary, C.R., Prichard, H.M., Eds.; Springer: Amsterdam, The Netherlands, 1986; pp. 361–372. [Google Scholar]
- Economou-Eliopoulos, M. Platinum-Group Element Distribution in Chromite Ores from Ophiolite Complexes: Implications for Their Exploration. Ore Geol. Rev. 1996, 11, 363–381. [Google Scholar] [CrossRef]
- Zaccarini, F.; Garuti, G.; Bakker, R.J.; Pushkarev, E.V. Electron Microprobe and Raman Spectroscopy Investigation of an Oxygen-Bearing Pt–Fe–Pd–Ni–Cu Compound from Nurali Chromitite (Southern Urals, Russia). Microsc. Microanal. 2015, 21, 1070–1079. [Google Scholar] [CrossRef]
- Grieco, G.; Diella, V.; Chaplygina, N.L.; Savelieva, G.N. Platinum Group Elements Zoning and Mineralogy of Chromitites from the Cumulate Sequence of the Nurali Massif (Southern Urals, Russia). Ore Geol. Rev. 2007, 30, 257–276. [Google Scholar] [CrossRef]
- Garuti, G.; Pushkarev, E.; Zaccarini, F. Composition and Paragenesis of Pt-Alloys from Chromitites of the Ural-Alaskan-Type Kitlim and Uktus Complexes, Northern and Central Urals, Russia. Can. Mineral. 2002, 40, 1127–1146. [Google Scholar] [CrossRef]
- Setlhabi, B.; Popoola, A.; Tshabalala, L.; Adeleke, A. Evaluation of Advanced Gravity and Magnetic Concentration of a PGM Tailings Waste for Chromite Recovery. Iran. J. Chem. Chem. Eng. 2019, 38, 61–71. [Google Scholar]
- Latypov, R.; Chistyakova, S.Y.; Letsoele, C. Massive Chromitites of the Bushveld Complex, South Africa: A Critical Review of Existing Hypotheses. Earth Sci. Rev. 2024, 256, 104858. [Google Scholar] [CrossRef]
- Motzer, W.E.; Engineers, T. Chemistry, Geochemistry, and Geology of Chromium and Chromium Compounds. In Chromium(VI) Handbook; CRC Press: Boca Raton, FL, USA, 2004; pp. 23–88. [Google Scholar]
- Nriagu, J.O.; Nieboer, E. Chromium in the Natural and Human Environments; John Wiley & Sons: Hoboken, NJ, USA, 1988; Volume 20. [Google Scholar]
- Melcher, F.; Grum, W.; Thalhammer, T.V.; Thalhammer, O.A.R. The Giant Chromite Deposits at Kempirsai, Urals: Constraints from Trace Elements (PGE, REE) and Isotope Data. Miner. Depos. 1999, 34, 250–270. [Google Scholar] [CrossRef]
- Sorikrat, S.; Laskowski, J. Flotation of Chromite. Trans. Inst. Min. Metall. 1973, 82, 207–213. [Google Scholar]
- Konstantopoulou, G.; Economou-Eliopoulos, M. Distribution of Platinum-Group Elements and Gold within the Vourinos Chromitite Ores, Greece. Econ. Geol. 1991, 86, 1672–1682. [Google Scholar] [CrossRef]
- Kravchenko, G.G. Geological Position and Structure of Chromite Deposits in the Ural Mountains. In Chromitites; UNESCO IGCP-197, Metallogeny of Ophiolites; Petrascheck, W., Ed.; Theophrastus Publications: Athens, Greece, 1986; pp. 3–21. [Google Scholar]
- Stowe, C.W. Compositions and Tectonic Setting of Chromite Deposits through Time. Econ. Geol. 1994, 89, 528–546. [Google Scholar] [CrossRef]
- Zhukova, I.; Stepanov, A.; Jiang, S.-Y.; Zhimulev, F.I.; Gurevich, D.; Polonyankin, A.; Lavrenchuk, A.; Kotlyarov, A. Platinum-Group Elements Geochemistry of Chromite from Kondyor Ultramafic Intrusion, Siberia: Re-Evaluation of Factors Controlling PGE Content of Intrusive Chromite. Chem. Geol. 2025, 691, 122871. [Google Scholar] [CrossRef]
- Constantinides, C.C.; Kingston, G.A.; Fisher, P.C. The occurrence of platinum group minerals in the chromitites of the Kokkinorotsos chrome mine, Cyprus. In Ophiolites, Proceedings of the International Ophiolite Symposium, Cyprus 1979; Panayiotou, A., Ed.; Geological Survey Department: Nicosia, Cyprus, 1980; pp. 93–101. [Google Scholar]
- Stockman, H.W.; Hlava, P.F. Platinum-group minerals in Alpine chromitites from southwestern Oregon. Econ. Geol. 1984, 79, 491–508. [Google Scholar] [CrossRef]
- Augé, T.; Johan, Z. Comparative study of chromite deposits from Troodos, Vourinos, North Oman and New Caledonia ophiolites. In Mineral Deposits within the European Community; Boissonnas, J., Omenetto, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 267–288. [Google Scholar]
- Yaro, S.A. Upgrading of Muro-Toto iron ore gravity pre-concentrate by Reverse Froth Floatation Method. J. Raw Mater. Res. 2004, 1, 54–59. [Google Scholar]
- Burke, T.; Fagliano, J.; Goldoft, M.; Hazen, R.E.; Tglewicz, R.; Mckee, T. Chromite ore processing residue in Hudson County, New Jersey. Environ. Health Perspect. 1991, 92, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.S.; Xie, G. The study of the behavior of chromium ore in roasting process. Min. Metall. Eng. 2006, 26, 57–60. [Google Scholar]
- Gu, F.; Wills, B.A. Chromite—Mineralogy and Processing. Miner. Eng. 1988, 1, 235–240. [Google Scholar] [CrossRef]
- Beukes, J.P.; du Preez, S.P.; van Zyl, P.G.; Paktunc, D.; Fabritius, T.; Päätalo, M.; Cramer, M. Review of Cr(VI) Environmental Practices in the Chromite Mining and Smelting Industry—Relevance to Development of the Ring of Fire, Canada. J. Clean. Prod. 2017, 165, 874–889. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.H.; Wang, Z.K.; Chen, J.Y. Green chemistry and chromium salt industry—A new generation of industrial revolution. Chem. Ind. Eng. Prog. 1986, 10, 172–178. [Google Scholar]
- Murthy, Y.; Tripathy, S. Process optimization of a chrome ore gravity concentration plant for sustainable development. J. S. Afr. Inst. Min. Metall. 2020, 120, 26–268. [Google Scholar] [CrossRef]
- Seifelnasr, A.A.; Tammam, T.; Abouzeid, A.Z.M. Gravity concentration of Sudanese chromite ore using laboratory shaking table. Physicochem. Probl. Miner. Process. 2012, 48, 271–280. [Google Scholar]
- Samata, S.; Misra, R.N.; Bhatnagar, P.P. Thermal Beneficiation of Low-Grade Chrome Ore from Enukonda Deposits, West Godavari District, Andhra Pradesh. NML Tech. J. 1964, 6, 12–16. [Google Scholar]
- Gallios, G.P.; Deliyanni, E.A.; Peleka, E.N.; Matis, K.A. Flotation of chromite and serpentine. Sep. Purif. Technol. 2007, 55, 232–237. [Google Scholar] [CrossRef]
- Maulik, S.; Bhattacharyya, K. Beneficiation of low-grade chromite ores from Sukinda. In Proceedings of the International Seminar on Mineral Processing Technology (MPT-2005), Dhanbad, India, 6–8 January 2005. [Google Scholar]
- Basson, J.; Daavittila, J. High carbon ferrochrome technology. In Handbook of Ferroalloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 317–363. [Google Scholar]
- Baisanov, S.; Shabanov, E.Z.; Grigorovich, K.V.; Toleukadyr, R.T.; Inkarbekova, I.S. Smelting options for carbon ferrochrome based on ore raw materials, middlings and their technological evaluation. Kompleks. Ispolz. Miner. Syra = Complex Use Miner. Resour. 2022, 320, 51–59. [Google Scholar] [CrossRef]
- Akhmadiyeva, N.; Abdulvaliyev, R.; Gladyshev, S.; Sukurov, B.; Abikak, Y.; Manapova, A.; Bakhytuly, N. Optimizing technological parameters for chromium extraction from chromite ore beneficiation tailings. Minerals 2025, 15, 555. [Google Scholar] [CrossRef]
- Farrow, C.J.; Burkin, A.R. Alkali pressure leaching of chromium (III) oxide of chromite mineral. Br. Min. Metall. 1975, 20, 20–27. [Google Scholar]
- Xu, H.B.; Zhang, Y.; Li, Z.H. A Pressure Leaching of Chromite Sodium Chromate Cleaner Production Methods. China Patent 101817561A, 1 September 2010. [Google Scholar]
- Wang, Z.H.; Du, H.; Wang, S.N.; Zheng, S.L.; Zhang, Y.; Hwang, S.; Kim, N.S.; Jeong, T.E. Electrochemical enhanced oxidative decomposition of chromite ore in highly concentrated KOH solution. Miner. Eng. 2014, 57, 16–24. [Google Scholar] [CrossRef]
- Ayinla, K.I.; Baba, A.A.; Oyewumi-Musa, R.T.; Tripathy, B.C.; Anafi, G.A.; Dwari, R.K. Energy saving chemical beneficiation method of improving low grade Nigeria chromite ore for use in steel industries. FUOYE J. Eng. Technol. 2023, 8, 497–502. [Google Scholar] [CrossRef]
- Liu, C.J.; Qi, J.; Jiang, M.F. Experimental study on sulfuric acid leaching behavior of chromite with different temperature. Adv. Mater. Res. 2012, 361, 628–631. [Google Scholar] [CrossRef]
- Shi, P.Y.; Liu, S.L. Experimental study on sulphuric acid leaching of chromite. J. Chin. Rare Earth Soc. 2002, 20, 472–474. [Google Scholar]
- Ciftçi, H.; Arslan, B.; Bilen, A.; Arsoy, Z.; Ersoy, B. Extraction of magnesium from chromite beneficiation tailings. Bull. Miner. Res. Explor. 2021, 164, 251. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, W.; Chu, S.; Liu, Z.; Wu, Z.; Lan, Y.; Galvita, V.V.; Zhang, L.; Su, X. Sufficient extraction of Cr from chromium ore processing residue (COPR) by selective Mg removal. J. Hazard. Mater. 2022, 440, 129754. [Google Scholar] [CrossRef]
- Kallam, V.K.; Vadapalli, S.; Singh, V.; Kapure, G. Processing of peridotite rocks of chromite ore overburden into magnesium salts and micro silica. Min. Metall. Explor. 2020, 37, 1253–1263. [Google Scholar] [CrossRef]
- Auyeshov, A.A.; Eskibayeva, C.Z.; Ibrayeva, A.M.; Arynov, K.T.; Dikanbayeva, A.K. Resource-efficient technology for the utilization of serpentine technogenic waste with the production of magnesium oxide. Int. J. Biol. Chem. 2025, 18, 112–118. [Google Scholar] [CrossRef]
- Kushakova, L.B.; Reznichenko, V.V.; Kospanov, M.M.; Suleymanova, G.A. Method for Processing Magnesium-Containing Materials. RU Patent 23692, 15 February 2011. [Google Scholar]
- Baloyi, N.P.; Nheta, W.; Sibanda, V.; Safari, M. Mineralogical insights into PGM recovery from Middle Group (1–4) chromite tailings. Minerals 2024, 14, 924. [Google Scholar] [CrossRef]
- Ptitsyn, A.M.; Isaev, A.S.; Savin, A.G.; Paretsky, V.M. Method for Processing Materials Containing Platinum Group Metals. RU Patent 2618282, 3 March 2017. [Google Scholar]
- Marakushev, A.A.; Shapovalov, Y.B.; Stolyarova, T.A. Method for the Extraction of Platinum Group Metals. RU Patent 2224034, 20 February 2004. [Google Scholar]
- Gomanov, E.S.; Ortyakov, S.S.; Rublevsky, I.A. Method for the Extraction of Platinum Group Metals. RU Patent 2360984, 10 July 2009. [Google Scholar]
- Bessarabov, D.G.; Van Kaam, T.P.M.; Du Preez, S.P. Hydrogen Plasma Smelting Reduction for Sustainable Ferrochrome Production. Plasma Chem. Plasma Process. 2025, 45, 1045–1062. [Google Scholar]
- Taylor, P.R. Plasma Technology in Extractive and Process Metallurgy. Miner. Eng. 1995, 8, 1081–1097. [Google Scholar] [CrossRef]
- Sanjay, P.; Tangstad, M.; Beukes, J.P.; Ringdalen, E.; Van Zyl, P.G. High-Carbon Ferrochrome Production Using Plasma Arc Technology. J. Sustain. Metall. 2023, 11, 450–465. [Google Scholar]
- Petrov, G.V.; Andreev, Y.V.; Greiver, T.N.; Kovalev, V.N. Method for the Extraction of Platinum Group Metals from Platinum-Containing Raw Materials. RU Patent 2415954, 10 April 2011. [Google Scholar]
- Spooren, J.; Abo Atia, T. Combined microwave assisted roasting and leaching to recover platinum group metals from spent automotive catalysts. Miner. Eng. 2020, 146, 106153. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Joussemet, R.; Mochón, J.; Ruiz-Bustinza, I.; Gaballah, I. An overview study of chlorination reactions applied to the primary extraction and recycling of metals and to the synthesis of new reagents. Thermochim. Acta 2009, 495, 42–50. [Google Scholar] [CrossRef]
- Taninouchi, Y.K.; Sunagawa, K.; Okabe, T.H.; Nakano, H. Extraction of platinum using ferric-chloride-vapor-based chlorination followed by hydrochloric acid leaching. J. Sustain. Metall. 2025, 11, 1835–1847. [Google Scholar] [CrossRef]
- Shemi, A.; Simate, G.; Ndlovu, S. Optimized Bioleaching Pre-treatment of UG-2 PGM Material. J. Sustain. Metall. 2024, 10, 525–541. [Google Scholar] [CrossRef]
- Chipise, L.; Ndlovu, S.; Shemi, A.; Moodley, S.S.; Kumar, A.; Simate, G.S.; Yah, C.S. Towards bioleaching of PGMS. Miner. Eng. 2023, 202, 108291. [Google Scholar] [CrossRef]
- Mpinga, C.N.; Eksteen, J.J.; Aldrich, C.; Dyer, L. Atmospheric Leach Process of High-Chromitite PGM-Bearing Oxidized Mineralized Ore through Single-Stage and Two-Stage Techniques. Miner. Eng. 2018, 125, 216–223. [Google Scholar] [CrossRef]
- Stanković, S.; Atanacković, N.; Štrbački, J.; Kovač, S.; Šarić, K.; Nikšić, Đ.; Schippers, A. Bioleaching and Chemical Leaching of Magnesium from Serpentinites (Zlatibor Mt. Ophiolite Massif, Serbia) with Potential Application in Mineral Carbonation Process for CO2 Sequestration. Front. Microbiol. 2025, 16, 1646341. [Google Scholar] [CrossRef]
- Taheri, B.; Larachi, F. Mineral-Based Magnesium Extraction Technologies: Current and Future Practices. Processes 2025, 13, 2945. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Peng, B.; Chai, L.; Wu, B.; Wu, R. Biotreatment of chromite ore processing residue by Pannonibacter phragmitetus BB. Environ. Sci. Pollut. Res. Int. 2013, 20, 5593–5602. [Google Scholar] [CrossRef]
- Shan, B.; Hao, R.; Zhang, J.; Li, J.; Ye, Y.; Xu, H.; Lu, A. Bioremediation Potential of Cr(VI) by Lysinibacillus cavernae CR-2 Isolated from Chromite-Polluted Soil: A Promising Approach for Cr(VI) Detoxification. Geomicrobiol. J. 2024, 41, 634–647. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, Y.; Li, Z.; Zhang, Y.; Zhao, Z. Green Metallurgical Processing of Chromite. Environ. Prog. Sustain. Energy 2010, 29, 175–182. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, P.; Jiang, M. Advances towards a Clean Hydrometallurgical Process for Chromite. Minerals 2016, 6, 7. [Google Scholar] [CrossRef]
- Pandey, K.; Saharan, B.S.; Kumar, R.; Jabborova, D.; Duhan, J.S. Modern-Day Green Strategies for the Removal of Chromium from Wastewater. J. Xenobiot. 2024, 14, 1670–1696. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Wang, Z.; Zhang, T.; Liu, Y. Effective Cr(VI) reduction and immobilization in chromite ore processing residue (COPR) contaminated soils by ferrous sulfate and digestate: A comparative investigation with typical reducing agents. Ecotoxicol. Environ. Saf. 2023, 265, 115522. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, L.; Li, X.; Liu, W.; Su, X.; Lin, Z. Efficient immobilization and utilization of chromite ore processing residue via hydrothermally constructing spinel phase Fe2+(Cr3+X, Fe3+2-x)O4 and its magnetic separation. Sci. Total Environ. 2022, 813, 152637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).