A Heterobimetallic Au(I)–Ru(II) Complex Bridged by dppb: Synthesis, Structural and Solution Characterization, BSA Interaction and In Vivo Toxicity Evaluation in Wistar Rats †
Abstract
1. Introduction
2. Results and Discussion
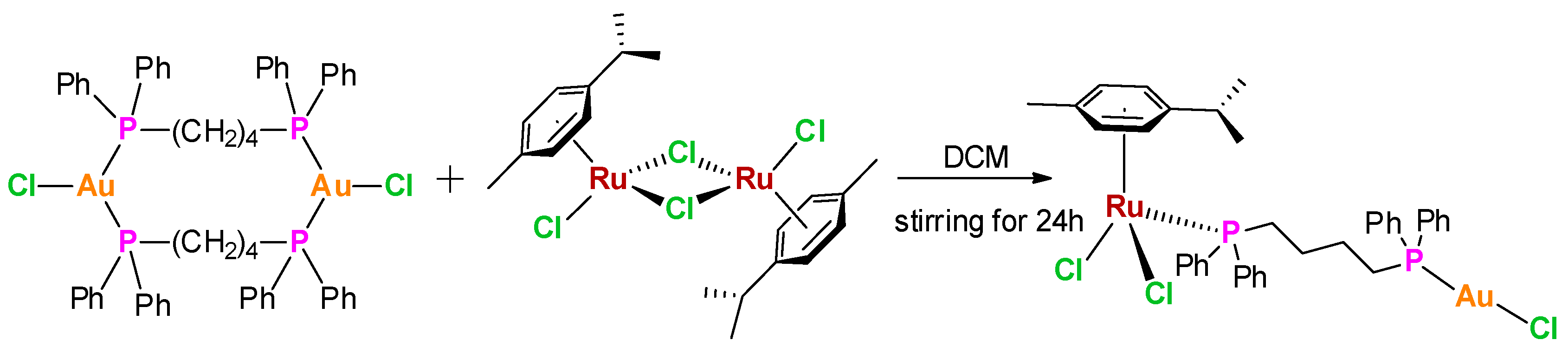
2.1. Design and Syntheses
2.2. Molecular and Crystal Structure
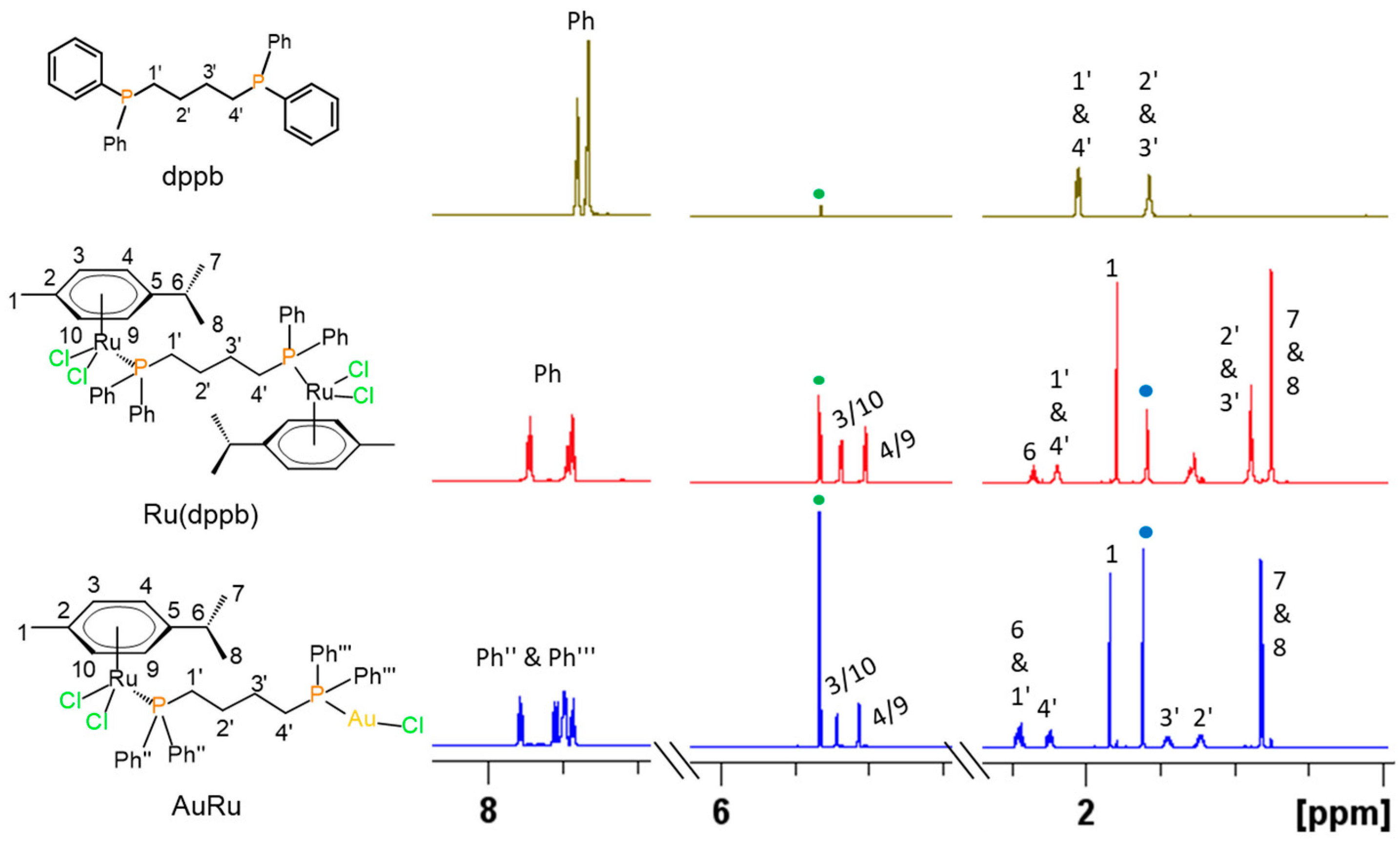
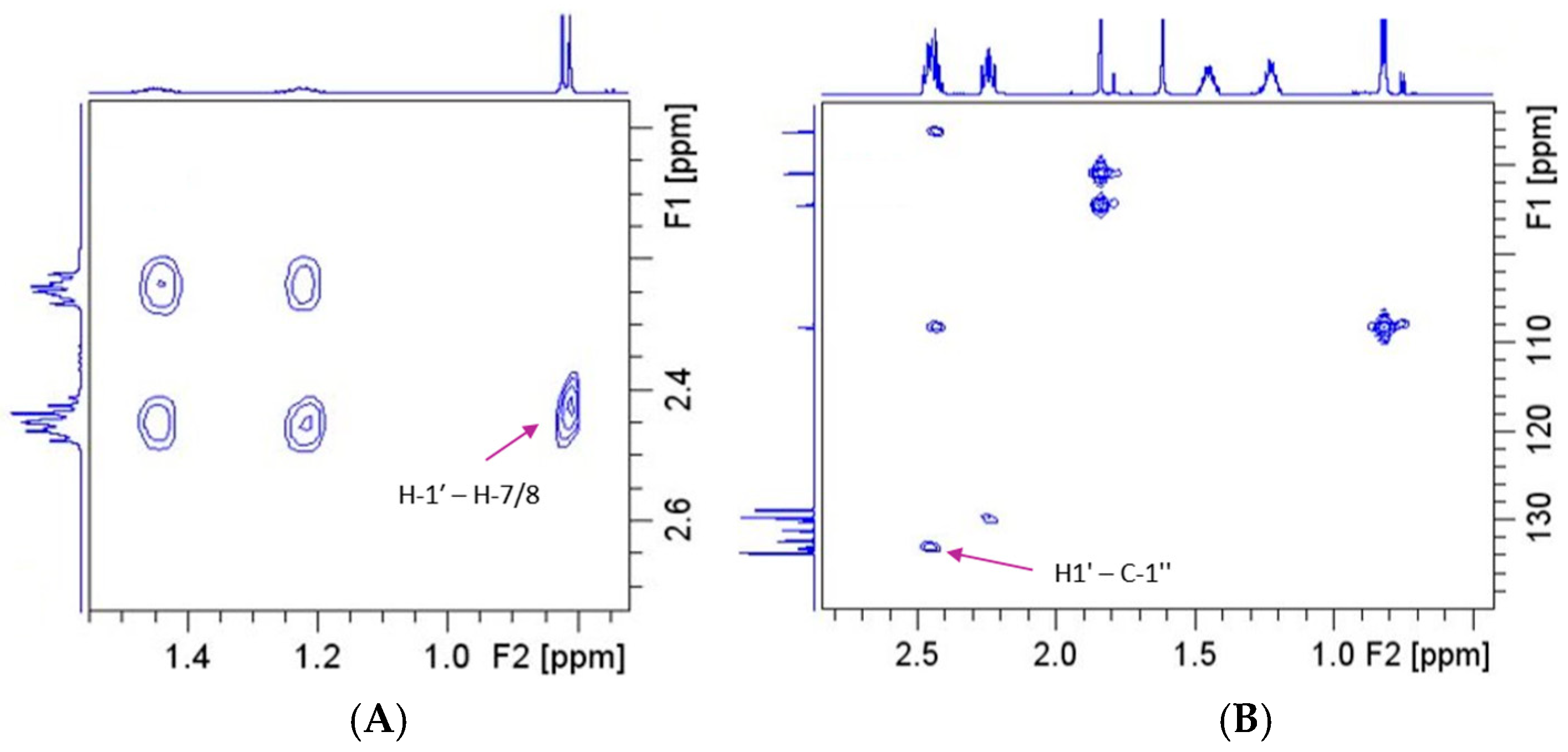
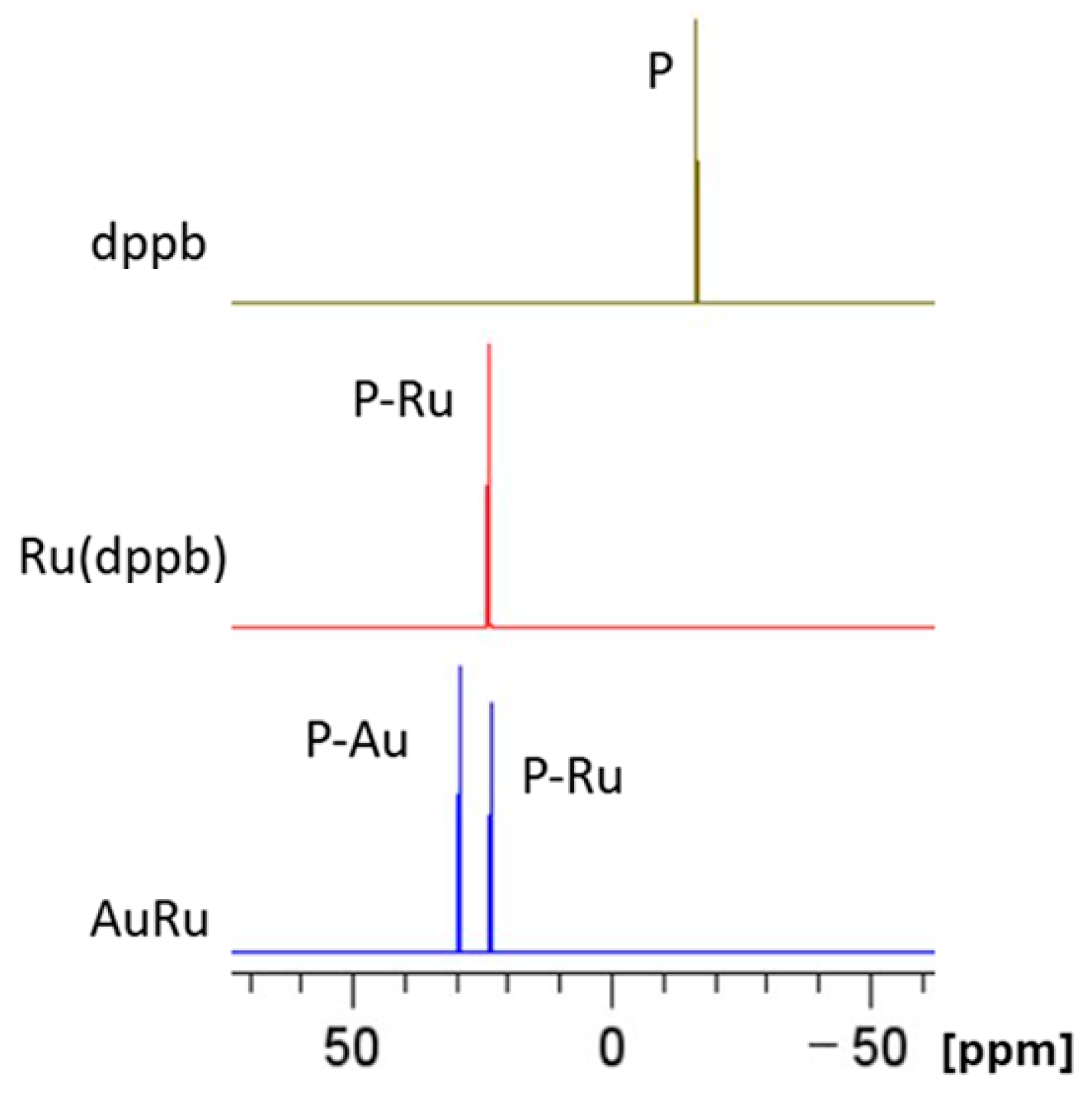
2.3. Chemical and Spectroscopic Characterization
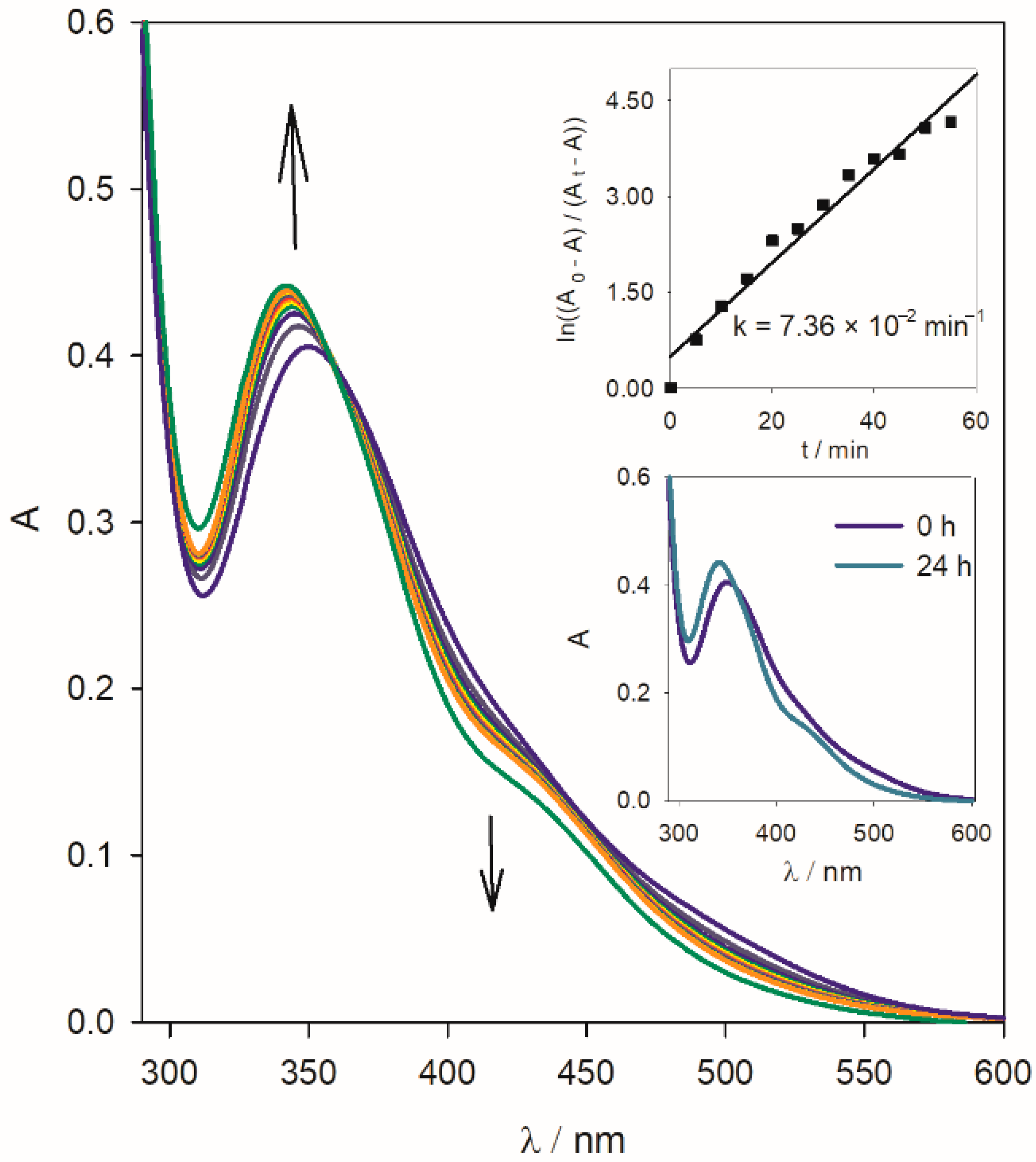
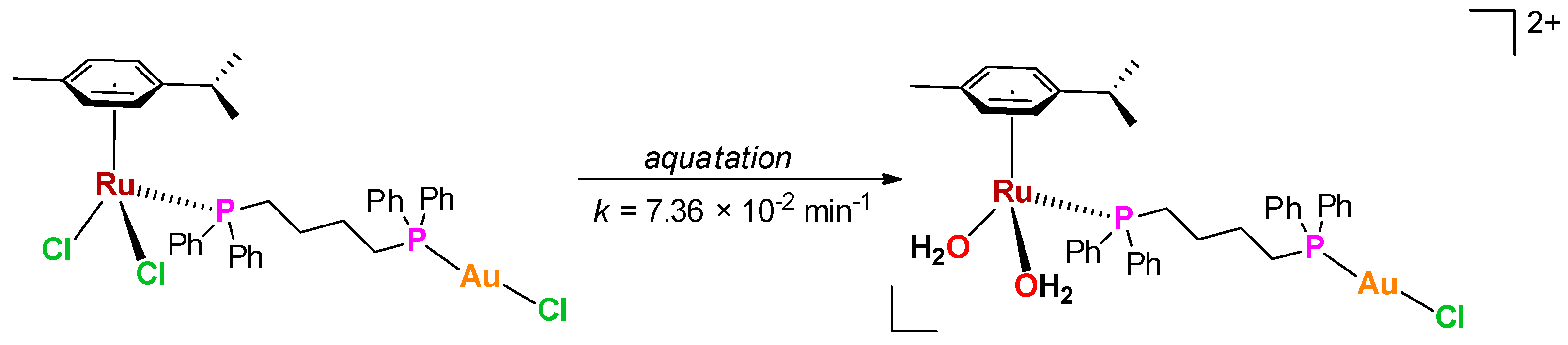
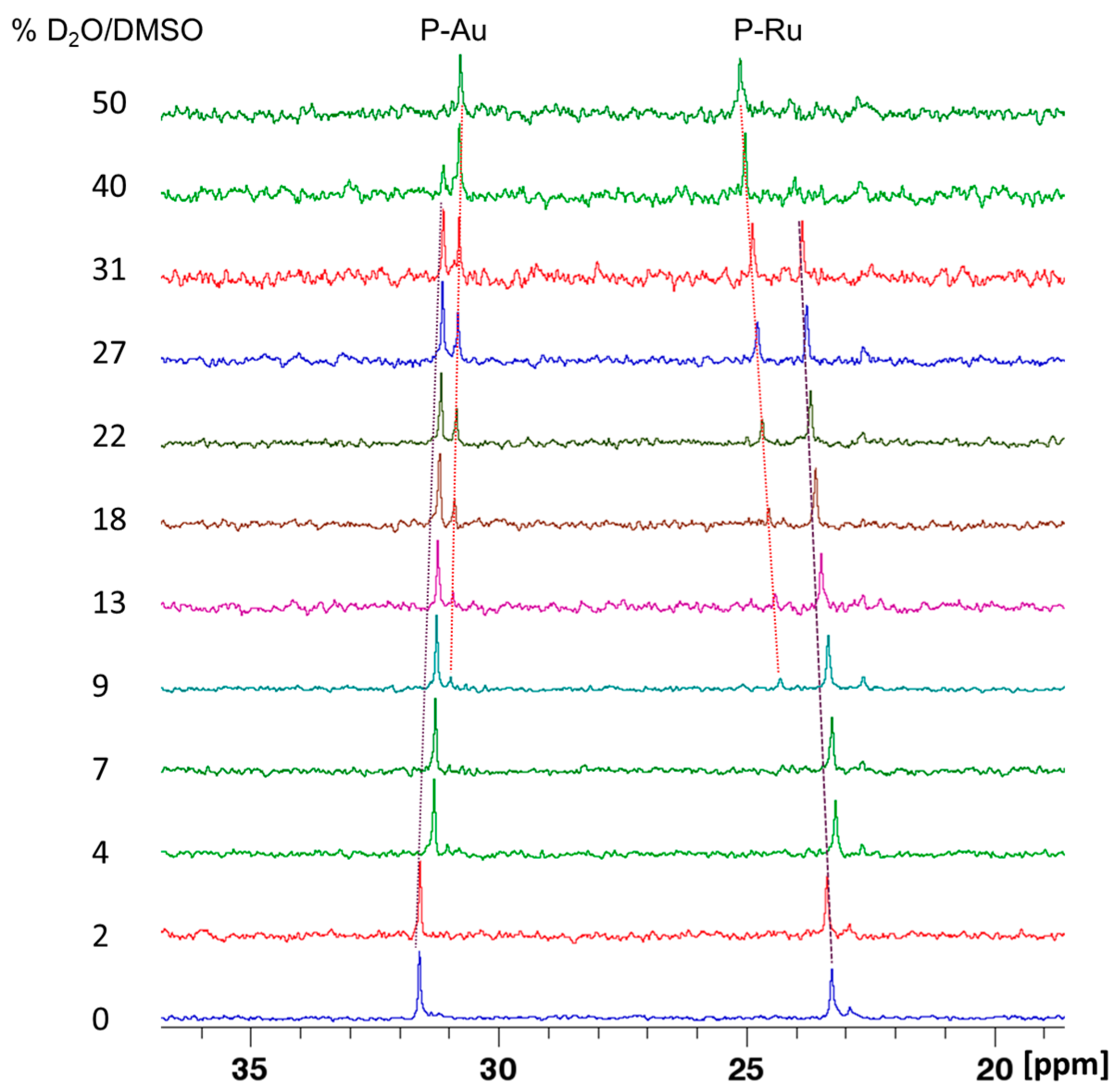
Behavior in Aqueous Solution
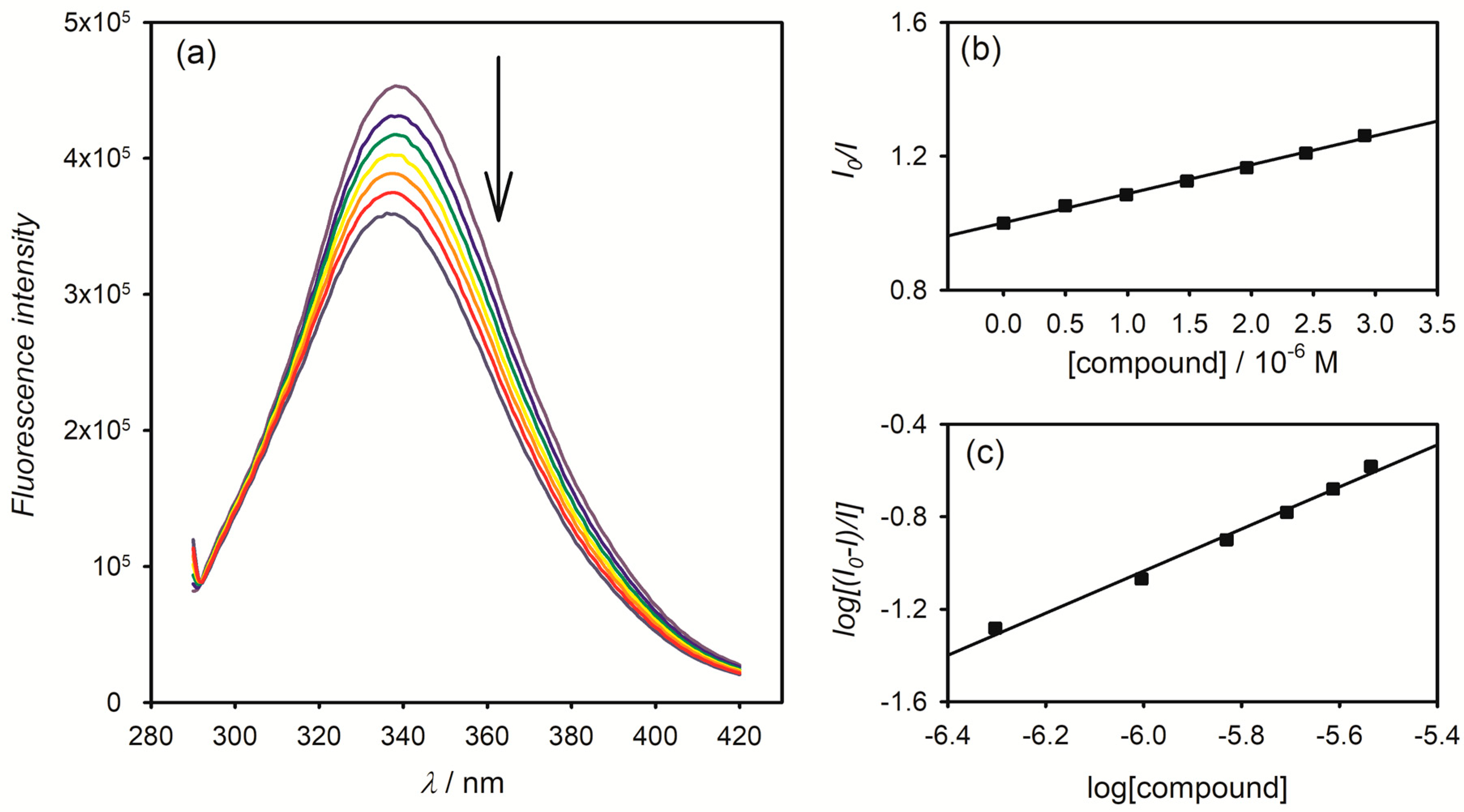
2.4. Interaction with BSA
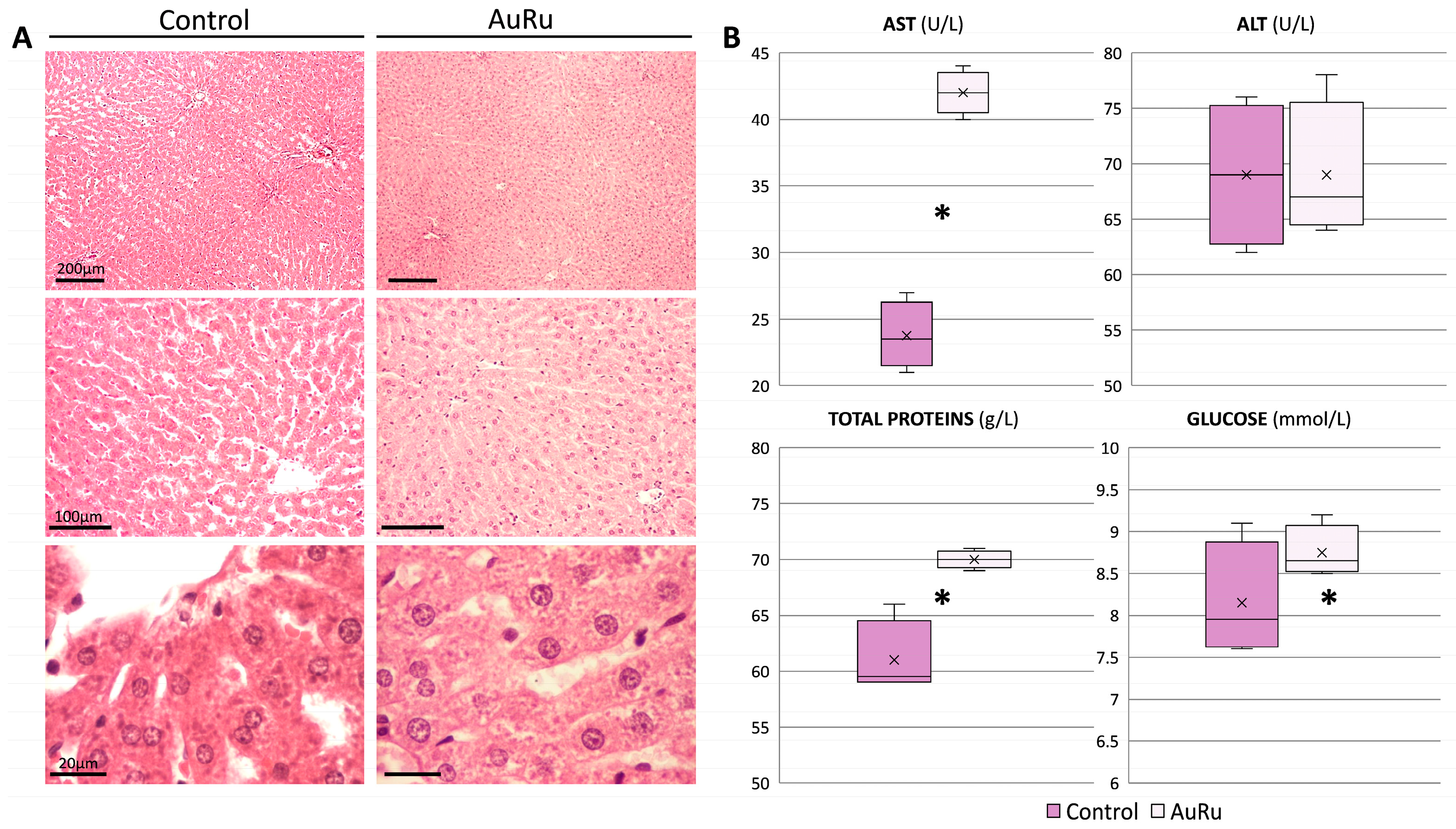
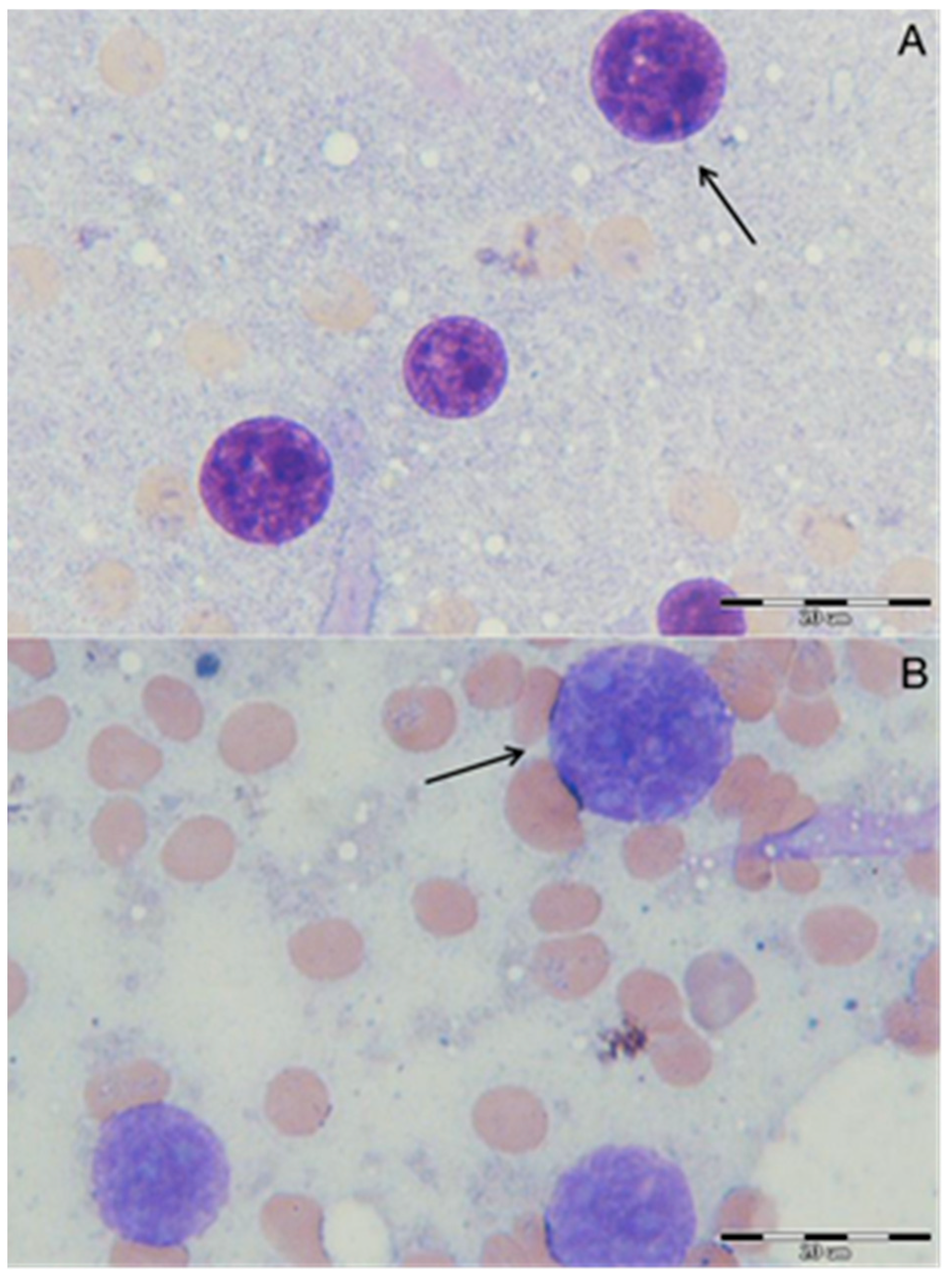
2.5. In Vivo Testing
3. Experimental Section
3.1. Chemicals
3.2. Physical Measurements
3.3. Synthesis of [(cym)Cl2Ru-μ-dppb-AuCl] (AuRu)
3.4. X-Ray Crystallography
3.5. Interaction with BSA
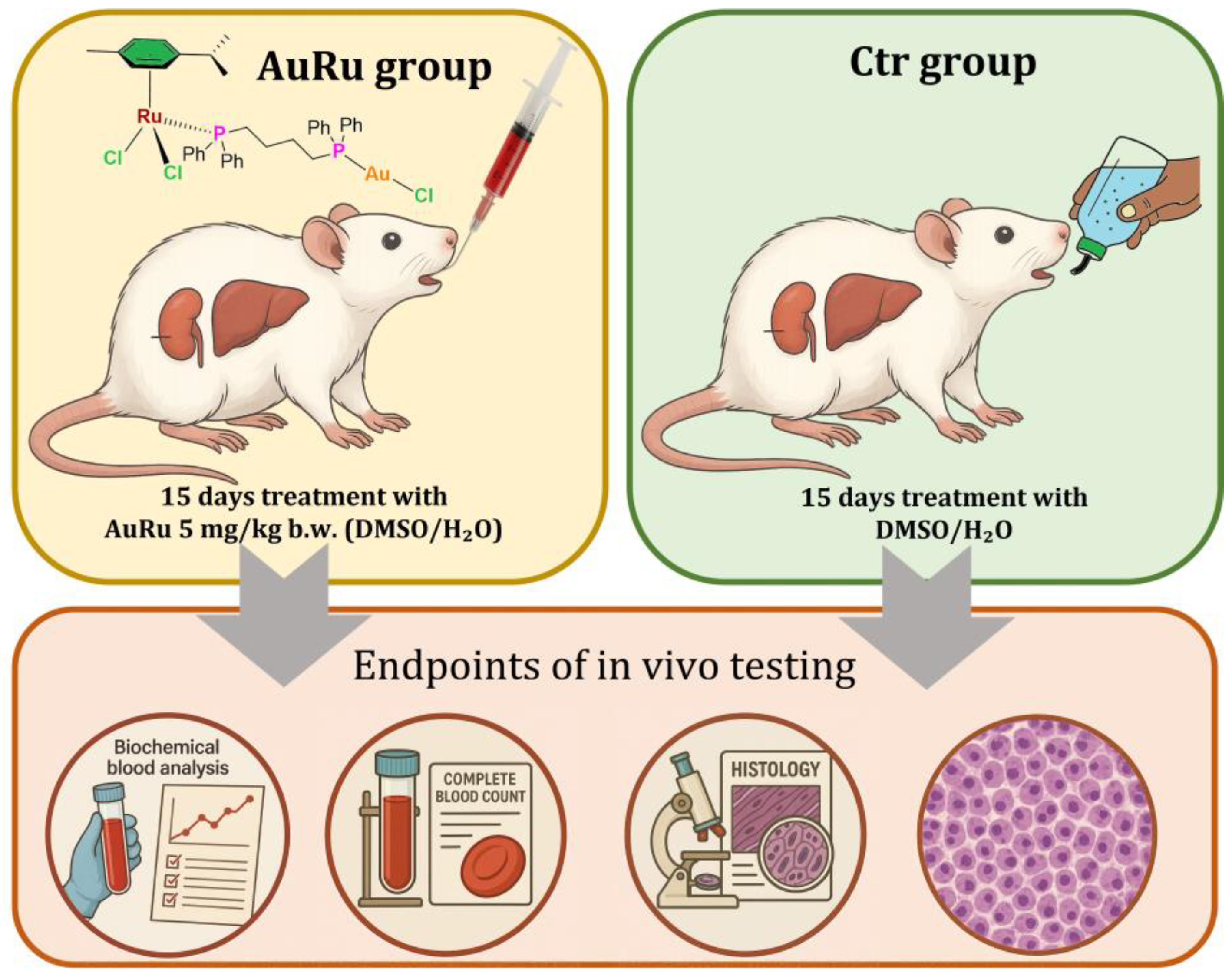
3.6. In Vivo Testing—Experimental Protocol
3.7. Biochemistry and Hematology
3.8. Hepatocytes Nuclei Morphology Observation
3.9. Histology
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, R.A.; Asim, A.; Kakkar, R.; Gupta, D.; Bagchi, V.; Arjmand, F.; Tabassum, S. A chloro-bridged heterobimetallic (η6-Arene) ruthenium–organotin complex as an efficient topoisomerase Iα inhibitor. Organometallics 2013, 32, 2546–2551. [Google Scholar] [CrossRef]
- Massai, L.; Fernández-Gallardo, J.; Guerri, A.; Arcangeli, A.; Pillozzi, S.; Contel, M.; Messori, L. Design, synthesis and characterisation of new chimeric ruthenium (II)–gold (I) complexes as improved cytotoxic agents. Dalton Trans. 2015, 44, 11067–11076. [Google Scholar] [PubMed]
- Wenzel, M.; Bigaeva, E.; Richard, P.; Le Gendre, P.; Picquet, M.; Casini, A.; Bodio, E. New heteronuclear gold (I)–platinum (II) complexes with cytotoxic properties: Are two metals better than one? J. Inorg. Biochem. 2014, 141, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dougan, S.J.; Sadler, P.J. The design of organometallic ruthenium arene anticancer agents. Chimia 2007, 61, 704. [Google Scholar] [CrossRef]
- Dyson, P.J. Systematic design of a targeted organometallic antitumour drug in pre-clinical development. Chimia 2007, 61, 698. [Google Scholar] [CrossRef]
- Ronconi, L.; Sadler, P.J. Using coordination chemistry to design new medicines. Coord. Chem. Rev. 2007, 251, 1633–1648. [Google Scholar] [CrossRef]
- Süss-Fink, G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar]
- Allardyce, C.S.; Dyson, P.J.; Ellis, D.J.; Heath, S.L. [Ru (η6-p-cymene) Cl2 (pta)](pta= 1, 3, 5-triaza-7-phosphatricyclo-[3.3. 1.1] decane): A water soluble compound that exhibits pH dependent DNA binding providing selectivity for diseased cells. Chem. Commun. 2001, 15, 1396–1397. [Google Scholar] [CrossRef]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In vitro and in vivo evaluation of ruthenium (II)− arene PTA complexes. J. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J.; Ellis, D.J.; Salter, P.A.; Scopelliti, R. Synthesis and characterisation of some water soluble ruthenium (II)–arene complexes and an investigation of their antibiotic and antiviral properties. J. Organomet. Chem. 2003, 668, 35–42. [Google Scholar]
- Renfrew, A.K.; Phillips, A.D.; Egger, A.E.; Hartinger, C.G.; Bosquain, S.S.; Nazarov, A.A.; Keppler, B.K.; Gonsalvi, L.; Peruzzini, M.; Dyson, P.J. Influence of Structural Variation on the Anticancer Activity of RAPTA-Type Complexes: Ptn versus pta. Organometallics 2009, 28, 1165–1172. [Google Scholar] [CrossRef]
- Vock, C.A.; Scolaro, C.; Phillips, A.D.; Scopelliti, R.; Sava, G.; Dyson, P.J. Synthesis, characterization, and in vitro evaluation of novel ruthenium (II) η6-arene imidazole complexes. J. Med. Chem. 2006, 49, 5552–5561. [Google Scholar] [CrossRef]
- Morris, R.E.; Aird, R.E.; del Socorro Murdoch, P.; Chen, H.; Cummings, J.; Hughes, N.D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D.I. Inhibition of cancer cell growth by ruthenium (II) arene complexes. J. Med. Chem. 2001, 44, 3616–3621. [Google Scholar] [CrossRef]
- Habtemariam, A.; Melchart, M.; Fernández, R.; Parsons, S.; Oswald, I.D.; Parkin, A.; Fabbiani, F.P.; Davidson, J.E.; Dawson, A.; Aird, R.E. Structure—Activity relationships for cytotoxic ruthenium (II) arene complexes containing N, N-, N, O-, and O, O-chelating ligands. J. Med. Chem. 2006, 49, 6858–6868. [Google Scholar] [CrossRef]
- Schmid, W.F.; John, R.O.; Arion, V.B.; Jakupec, M.A.; Keppler, B.K. Highly antiproliferative ruthenium (II) and osmium (II) arene complexes with paullone-derived ligands. Organometallics 2007, 26, 6643–6652. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Carlos Lima, J.; Rodriguez, L. Phosphine-gold (I) compounds as anticancer agents: General description and mechanisms of action. Anti-Cancer Agents Med. Chem. Anti-Cancer Agents 2011, 11, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-targeted chemotherapeutics: The rational design of gold (I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef] [PubMed]
- Oehninger, L.; Rubbiani, R.; Ott, I. N-Heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans. 2013, 42, 3269–3284. [Google Scholar] [CrossRef]
- Kamei, H.; Koide, T.; Kojima, T.; Hashimoto, Y.; Hasegawa, M. Effect of gold on survival of tumor-bearing mice. Cancer Biother. Radiopharm. 1998, 13, 403–406. [Google Scholar] [CrossRef]
- Stallings-Mann, M.; Jamieson, L.; Regala, R.P.; Weems, C.; Murray, N.R.; Fields, A.P. A novel small-molecule inhibitor of protein kinase Cι blocks transformed growth of non–small-cell lung cancer cells. Cancer Res. 2006, 66, 1767–1774. [Google Scholar] [CrossRef]
- Meyer, A.; Bagowski, C.P.; Kokoschka, M.; Stefanopoulou, M.; Alborzinia, H.; Can, S.; Vlecken, D.H.; Sheldrick, W.S.; Wölfl, S.; Ott, I. On the biological properties of alkynyl phosphine gold (I) complexes. Angew. Chem. Int. Ed. 2012, 51, 8895. [Google Scholar] [CrossRef] [PubMed]
- Chui, C.-H.; Wong, R.S.-M.; Gambari, R.; Cheng, G.Y.-M.; Yuen, M.C.-W.; Chan, K.-W.; Tong, S.-W.; Lau, F.-Y.; Bo-San Lai, P.; Lam, K.-H. Antitumor activity of diethynylfluorene derivatives of gold (I). Bioorganic Med. Chem. 2009, 17, 7872–7877. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lok, C.-N.; Bierla, K.; Che, C.-M. Gold (I) complex of N, N′-disubstituted cyclic thiourea with in vitro and in vivo anticancer properties—Potent tight-binding inhibition of thioredoxin reductase. Chem. Commun. 2010, 46, 7691–7693. [Google Scholar] [CrossRef] [PubMed]
- Köster, S.D.; Alborzinia, H.; Can, S.; Kitanovic, I.; Wölfl, S.; Rubbiani, R.; Ott, I.; Riesterer, P.; Prokop, A.; Merz, K. A spontaneous gold (I)-azide alkyne cycloaddition reaction yields gold-peptide bioconjugates which overcome cisplatin resistance in a p53-mutant cancer cell line. Chem. Sci. 2012, 3, 2062–2072. [Google Scholar] [CrossRef]
- Simon, T.M.; Kunishima, D.H.; Vibert, G.J.; Lorber, A. Screening trial with the coordinated gold compound auranofin using mouse lymphocytic leukemia P388. Cancer Res. 1981, 41, 94–97. [Google Scholar]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Madeira, J.; Gibson, D.; Kean, W.; Klegeris, A. The biological activity of auranofin: Implications for novel treatment of diseases. Inflammopharmacology 2012, 20, 297–306. [Google Scholar] [CrossRef]
- Bertrand, B.; Citta, A.; Franken, I.L.; Picquet, M.; Folda, A.; Scalcon, V.; Rigobello, M.P.; Le Gendre, P.; Casini, A.; Bodio, E. Gold (I) NHC-based homo-and heterobimetallic complexes: Synthesis, characterization and evaluation as potential anticancer agents. JBIC J. Biol. Inorg. Chem. 2015, 20, 1005–1020. [Google Scholar] [CrossRef]
- Elie, B.T.; Pechenyy, Y.; Uddin, F.; Contel, M. A heterometallic ruthenium–gold complex displays antiproliferative, antimigratory, and antiangiogenic properties and inhibits metastasis and angiogenesis-associated proteases in renal cancer. JBIC J. Biol. Inorg. Chem. 2018, 23, 399–411. [Google Scholar] [CrossRef]
- Fernández-Gallardo, J.; Elie, B.T.; Sanaú, M.; Contel, M. Versatile synthesis of cationic N-heterocyclic carbene–gold (I) complexes containing a second ancillary ligand. Design of heterobimetallic ruthenium–gold anticancer agents. Chem. Commun. 2016, 52, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, L.K.; Ortiz, D.; Dyson, P.J. Histidine targeting Heterobimetallic ruthenium (II)–gold (I) complexes. Inorg. Chem. 2019, 58, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Zahirović, A.; Roca, S.; Fočak, M.; Fetahović, S.; Višnjevac, A.; Topčagić, A.; Suljević, D.; Mitrašinović-Brulić, M.; Ostojić, J.; Muzika, V. Heterobinuclearity vs. Homobinuclearity in Ruthenium (II) and Gold (I) Organometallics with Diphosphine Ligands: Synthesis, Characterization, BSA Binding, and In Vivo Toxicity. In Proceedings of the 5th International Congress of Chemists and Chemical Engineers of Bosnia and Herzegovina, Sarajevo, Bosnia and Herzegovina, 27–30 June 2024; Book of Abstracts. p. 128. [Google Scholar]
- Yang, Y.; Abboud, K.A.; McElwee-White, L. Heterobimetallic complexes with dppm-bridged Ru/Pd, Ru/Pt, Ru/Au and Ru/Cu centers. Dalton Trans. 2003, 22, 4288–4296. [Google Scholar] [CrossRef]
- Maidich, L.; Zuri, G.; Stoccoro, S.; Cinellu, M.A.; Zucca, A. Assembly of symmetrical and unsymmetrical platinum (II) rollover complexes with bidentate phosphine ligands. Dalton Trans. 2014, 43, 14806–14815. [Google Scholar] [CrossRef]
- Hancock, R.D. Chelate ring size and metal ion selection. The basis of selectivity for metal ions in open-chain ligands and macrocycles. J. Chem. Educ. 1992, 69, 615. [Google Scholar] [CrossRef]
- Zahirović, A.; Fetahović, S.; Feizi-Dehnayebi, M.; Višnjevac, A.; Bešta-Gajević, R.; Kozarić, A.; Martić, L.; Topčagić, A.; Roca, S. Dual Antimicrobial-Anticancer Potential, Hydrolysis, and DNA/BSA binding affinity of a novel Water-Soluble Ruthenium-Arene ethylenediamine Schiff base (RAES) organometallic. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 318, 124528. [Google Scholar] [CrossRef]
- Zahirović, A.; Roca, S.; Kahrović, E.; Višnjevac, A. Low DNA and high BSA binding affinity of cationic ruthenium (II) organometallic featuring pyridine and 2’-hydroxychalcone ligands. J. Mol. Struct. 2021, 1236, 130326. [Google Scholar] [CrossRef]
- Zahirović, A.; Roca, S.; Višnjevac, A.; Kahrović, E. Ruthenium organometallics of chloro-substituted 2′-hydroxychalcones–A story of catecholase biomimetics beyond copper. J. Organomet. Chem. 2021, 945, 121863. [Google Scholar] [CrossRef]
- Scolaro, C.; Hartinger, C.G.; Allardyce, C.S.; Keppler, B.K.; Dyson, P.J. Hydrolysis study of the bifunctional antitumour compound RAPTA-C, [Ru (η6-p-cymene) Cl2 (pta)]. J. Inorg. Biochem. 2008, 102, 1743–1748. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Parsons, S.; Oswald, I.D.; Davidson, J.E.; Sadler, P.J. Kinetics of Aquation and Anation of Ruthenium (II) Arene Anticancer Complexes, Acidity and X-ray Structures of Aqua Adducts. Chem. A Eur. J. 2003, 9, 5810–5820. [Google Scholar] [CrossRef] [PubMed]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine serum albumin interactions with metal complexes. Clujul Med. 2014, 87, 215. [Google Scholar] [CrossRef]
- Yamasaki, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Albumin–drug interaction and its clinical implication. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 5435–5443. [Google Scholar] [CrossRef]
- Zahirović, A.; Žilić, D.; Pavelić, S.K.; Hukić, M.; Muratović, S.; Harej, A.; Kahrović, E. Type of complex–BSA binding forces affected by different coordination modes of alliin in novel water-soluble ruthenium complexes. New J. Chem. 2019, 43, 5791–5804. [Google Scholar] [CrossRef]
- Coffer, M.T.; Shaw, C.F., III; Eidsness, M.; Watkins, J.; Elder, R. Reactions of auranofin and chloro (triethylphosphine) gold with bovine serum albumin. Inorg. Chem. 1986, 25, 333–339. [Google Scholar] [CrossRef]
- Pratesi, A.; Cirri, D.; Ciofi, L.; Messori, L. Reactions of auranofin and its pseudohalide derivatives with serum albumin investigated through ESI-Q-TOF MS. Inorg. Chem. 2018, 57, 10507–10510. [Google Scholar] [CrossRef]
- Hacker, R.M.; Smith, J.J.; Platt, D.C.; Brennessel, W.W.; Jones, M.A.; Webb, M.I. Ruthenium (II)–Arene Complexes with a 2, 2′-Bipyridine Ligand as Anti-Aβ Agents. Biomolecules 2025, 15, 475. [Google Scholar] [CrossRef]
- Badr, H.E.; Elsayed, S.A.; Kawde, A.-N.; El-Hendawy, A.M. Development of Piano-Stool ruthenium (II) complexes: Synthesis, characterization, DNA/BSA binding affinity, in vitro cytotoxic and wound healing activity. Inorg. Chem. Commun. 2025, 181, 115287. [Google Scholar] [CrossRef]
- Dömötör, O.; Enyedy, É.A. Binding mechanisms of half-sandwich Rh (III) and Ru (II) arene complexes on human serum albumin: A comparative study. JBIC J. Biol. Inorg. Chem. 2019, 24, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Bhuin, S.; Halder, S.; Saha, S.K.; Chakravarty, M. Binding interactions and FRET between bovine serum albumin and various phenothiazine-/anthracene-based dyes: A structure–property relationship. RSC Adv. 2021, 11, 1679–1693. [Google Scholar] [CrossRef]
- Zahirović, A.; Tüzün, B.; Hadžalić, S.; Osmanković, I.; Roca, S.; Begić, S.; Fočak, M. Moderate DNA and high SARS-CoV-2 spike protein affinity of oxidovanadium (IV) complexes of 2-furoic acid hydrazones: In silico and in vitro approach. J. Mol. Struct. 2023, 1294, 136564. [Google Scholar] [CrossRef]
- Yinhua, D.; Foroughi, M.M.; Aramesh-Boroujeni, Z.; Jahani, S.; Peydayesh, M.; Borhani, F.; Khatami, M.; Rohani, M.; Dusek, M.; Eigner, V. The synthesis, characterization, DNA/BSA/HSA interactions, molecular modeling, antibacterial properties, and in vitro cytotoxic activities of novel parent and niosome nano-encapsulated Ho (III) complexes. RSC Adv. 2020, 10, 22891–22908. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Alkhawajah, A.M.M.; Al-Tamimi, D.M.; Shawarby, M.A.; Isab, A.A.; Badar, A. Biological alterations in renal and hepatic tissues by a novel gold (III) anti-cancerous compound. Iran. J. Basic Med. Sci. 2018, 21, 1064. [Google Scholar]
- Mabuza, L.P.; Gamede, M.W.; Maikoo, S.; Booysen, I.N.; Ngubane, P.S.; Khathi, A. Effects of a ruthenium schiff base complex on glucose homeostasis in diet-induced pre-diabetic rats. Molecules 2018, 23, 1721. [Google Scholar] [CrossRef]
- Hou, S.-M.; Hsia, C.-H.; Velusamy, M.; Jayakumar, T.; Hsia, C.-W.; Chang, C.-C.; Lin, K.-H.; Lu, Y.-C. Ruthenium complex, TQ-5, protects against LPS-induced macrophage inflammation and acute liver injury in mice via downregulating NF-κB pathways. Int. J. Mol. Med. 2019, 44, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Manibusan, M.K.; Odin, M.; Eastmond, D.A. Postulated carbon tetrachloride mode of action: A review. J. Environ. Sci. Health Part C 2007, 25, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Beytur, A.; Vardi, N.; Ozdemir, I. Evaluation of reproductive toxicity in male rats treated with novel synthesized ruthenium (II) and gold (I)-NHC complexes. Drug Dev. Ind. Pharm. 2012, 38, 40–46. [Google Scholar] [CrossRef]
- Yue, S.; Luo, M.; Liu, H.; Wei, S. Recent advances of gold compounds in anticancer immunity. Front. Chem. 2020, 8, 543. [Google Scholar] [CrossRef]
- Rinaldi-Neto, F.; Ribeiro, A.B.; Ferreira, N.H.; Squarisi, I.S.; Oliveira, K.M.; Orenha, R.P.; Parreira, R.L.T.; Batista, A.A.; Tavares, D.C. Anti-melanoma effect of ruthenium (II)-diphosphine complexes containing naphthoquinone ligand. J. Inorg. Biochem. 2021, 222, 111497. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef]
- Bertrand, B.; Casini, A. A golden future in medicinal inorganic chemistry: The promise of anticancer gold organometallic compounds. Dalton Trans. 2014, 43, 4209–4219. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Zhou, X.-Q.; Bretin, L.; Zeng, X.; Husiev, Y.; Polanco, E.A.; Zhao, G.; Wijaya, L.S.; Biver, T. Cyclic ruthenium-peptide conjugates as integrin-targeting phototherapeutic prodrugs for the treatment of brain tumors. J. Am. Chem. Soc. 2023, 145, 14963–14980. [Google Scholar] [CrossRef]
- Bennett, M.; Huang, T.N.; Matheson, T.; Smith, A.; Ittel, S.; Nickerson, W. 16.(η6-Hexamethylbenzene) ruthenium complexes. Inorg. Synth. 1982, 21, 74–78. [Google Scholar]
- Doherty, S.; Knight, J.G.; Rath, R.K.; Clegg, W.; Harrington, R.W.; Newman, C.R.; Campbell, R.; Amin, H. Ruthenium complexes of six-electron-donor NUPHOS-type diphosphines: Highly selective catalysts for the hydrocarboxylation of terminal alkynes. Organometallics 2005, 24, 2633–2644. [Google Scholar] [CrossRef]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.; Murray, H.; Fackler, J., Jr. (Tetrahydrothiophene) gold (I) or gold (III) complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar]
- Berners-Price, S.J.; Sadler, P.J. Gold (I) complexes with bidentate tertiary phosphine ligands: Formation of annular vs. tetrahedral chelated complexes. Inorg. Chem. 1986, 25, 3822–3827. [Google Scholar] [CrossRef]
- Zahirović, A.; Osmanković, I.; Turkušić, E.; Kahrović, E. Improved method for spectrophotometric determination of ruthenium using 1, 10-phenanthroline: Application for analysis of complex compounds. Anal. Methods 2018, 10, 5078–5083. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- da Silva, M.M.; Ribeiro, G.H.; de Camargo, M.S.; Ferreira, A.G.; Ribeiro, L.; Barbosa, M.I.; Deflon, V.M.; Castelli, S.; Desideri, A.; Correa, R.S. Ruthenium (II) diphosphine complexes with mercapto ligands that inhibit topoisomerase IB and suppress tumor growth in vivo. Inorg. Chem. 2021, 60, 14174–14189. [Google Scholar] [CrossRef]
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium complexes: An alternative to platinum drugs in colorectal cancer treatment. Pharmaceutics 2021, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Mello-Andrade, F.; Cardoso, C.G.; e Silva, C.R.; Chen-Chen, L.; de Melo-Reis, P.R.; de Lima, A.P.; Oliveira, R.; Ferraz, I.B.M.; Grisolia, C.K.; Almeida, M.A.P. Acute toxic effects of ruthenium (II)/amino acid/diphosphine complexes on Swiss mice and zebrafish embryos. Biomed. Pharmacother. 2018, 107, 1082–1092. [Google Scholar] [CrossRef]
- Mihajlovic, K.; Milosavljevic, I.; Jeremic, J.; Savic, M.; Sretenovic, J.; Srejovic, I.; Zivkovic, V.; Jovicic, N.; Paunovic, M.; Bolevich, S. Redox and apoptotic potential of novel ruthenium complexes in rat blood and heart. Can. J. Physiol. Pharmacol. 2021, 99, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Suljevic, D.; Focak, M.; Filipic, F.; Hamzic, A.; Zubcevic, N.; Alijagic, A. Haematopoiesis in the European common toad Bufo bufo (Linnaeus, 1758): New methodological insights to study general, seasonal, and sexual haematopoietic distribution and maturation pattern. Turk. J. Zool. 2018, 42, 198–206. [Google Scholar] [CrossRef]



| Structure | AuRu |
|---|---|
| Brutto form. | C40H45AuCl7P2Ru |
| Mr (gmol−1) | 1133.88 |
| Crystal color and habit | translucent light orange prism |
| Cryst. (mm) | 0.15 × 0.09 × 0.07 |
| F (000) | 1114 |
| μ (mm−1) | 1.688 |
| Space group | P-1 |
| a (Å) | 10.90240(10) |
| b (Å) | 14.7132(2) |
| c (Å) | 15.2437(2) |
| α (°) | 94.9940(10) |
| β (°) | 99.2710(10) |
| γ (°) | 110.6020(10) |
| V (Å3) | 2231.44(5) |
| Z | 2 |
| Rint | 0.0524 |
| Rσ | 0.0474 |
| θ max (°) | 79.703 |
| Unique | 9465 |
| Obs. [I > 2σ(I)] | 8483 |
| Parameters | 460 |
| R1 [I > 2σ(I)] | 0.1185 |
| wR2, all | 0.1156 |
| S | 0.969 |
| ρmax, ρmin(eÅ−3) | 1.717; −1.367 |
| AuRu | |||
|---|---|---|---|
| Distances | Å | Angles | ° |
| C29–Ru1 | 2.184(6) | C34–Ru1–C29 | 37.9(3) |
| C30–Ru1 | 2.224(6) | C34–Ru1–C33 | 36.8(3) |
| C31–Ru1 | 2.240(6) | C29–Ru1–C33 | 67.7(3) |
| C32–Ru1 | 2.239(6) | C34–Ru1–C30 | 67.8(3) |
| C33–Ru1 | 2.221(6) | C29–Ru1–C30 | 37.4(2) |
| C34–Ru1 | 2.176(6) | C33–Ru1–C30 | 80.5(3) |
| P–Ru1 | 2.3592(14) | C34–Ru1–C32 | 66.2(3) |
| Cl1–Ru1 | 2.4213(14) | C29–Ru1–C32 | 78.6(3) |
| Cl2–Ru1 | 2.4104(16) | C33–Ru1–C32 | 37.5(3) |
| Cl3–Au1 | 2.2798(19) | C30–Ru1–C32 | 66.7(3) |
| P1–Au1 | 2.2311(14) | C34–Ru1–C31 | 78.1(3) |
| C29–Ru1–C31 | 66.7(2) | ||
| C33–Ru1–C31 | 66.3(3) | ||
| C30–Ru1–C31 | 37.8(2) | ||
| C32–Ru1–C31 | 35.4(3) | ||
| C34 Ru1–P | 95.55(18) | ||
| C29–Ru1–P | 94.65(17) | ||
| C33–Ru1–P | 120.5(2) | ||
| C30–Ru1–P | 119.42(16) | ||
| C32–Ru1–P | 158.0(2) | ||
| C31–Ru1–P | 157.08(18) | ||
| C34–Ru1–Cl2 | 154.9(2) | ||
| C29–Ru1–Cl2 | 117.08(19) | ||
| C33–Ru1–Cl2 | 152.4(2) | ||
| C30–Ru1–Cl2 | 89.20(19) | ||
| C32–Ru1–Cl2 | 114.9(2) | ||
| C31–Ru1–Cl2 | 89.98(19) | ||
| P–Ru1–Cl2 | 86.90(5) | ||
| C34–Ru1–Cl1 | 116.9(2) | ||
| C29–Ru1–Cl1 | 154.71(19) | ||
| C33–Ru1–Cl1 | 90.58(19) | ||
| C30–Ru1–Cl1 | 155.14(16) | ||
| C32–Ru1–Cl1 | 92.15(18) | ||
| C31–Ru1–Cl1 | 117.50(18) | ||
| P–Ru1–Cl1 | 85.12(5) | ||
| Cl2–Ru1–Cl1 | 88.18(6) | ||
| P1–Au1–Cl3 | 179.39(8) | ||
| Compound | KSV/105 M−1 | kq/1013 M−1 s−1 | Kb/104 M−1 | n |
|---|---|---|---|---|
| Au(dppb) | 1.49 | 1.49 | 14.1 | 1.00 |
| Ru(dppb) | 1.07 | 1.07 | 0.05 | 0.58 |
| AuRu | 0.87 | 0.87 | 2.66 | 0.91 |
| Compound | J/cm3/M | E | R0/nm | r/nm |
|---|---|---|---|---|
| Au(dppb) | 3.17 × 10−16 | 0.30 | 1.38 | 1.59 |
| Ru(dppb) | 2.82 × 10−15 | 0.25 | 1.99 | 2.38 |
| AuRu | 1.62 × 10−15 | 0.21 | 1.81 | 2.26 |
| AuRu | Ctr | Sig. | |
|---|---|---|---|
| SODIUM (mmol/L) | 149.75 ± 2.63 | 155.66 ± 9.99 | 0.03 |
| CHLORIDE (mmol/L) | 101.25 ± 0.96 | 100.16 ± 5.84 | 0.12 |
| LDH (U/L) | 451.5 ± 112.80 | 599.83 ± 182.3 | 0.01 |
| CK (U/L) | 342.25 ± 29.01 | 297.83 ± 116.97 | 0.03 |
| AuRu | Ctr | Sig. | |
|---|---|---|---|
| WBC (×103/µL) | 3.23 ± 0.22 | 7.11 ± 0.58 | 0.00 |
| LYMPH (×103/µL) | 1.41 ± 0.55 | 5.23 ± 0.51 | 0.00 |
| GRAN (×103/µL) | 0.41 ± 0.15 | 1.83 ± 0.83 | 0.00 |
| RBC (×106/µL) | 6.73 ± 1.43 | 7.14 ± 0.45 | 0.30 |
| HGB (g/dL) | 14.4 ± 1.09 | 14.42 ± 0.51 | 0.93 |
| HCT (%) | 39.00 ± 4.96 | 37.92 ± 1.17 | 0.00 |
| MCV (fL) | 57.43 ± 9.03 | 52.58 ± 4.25 | 0.25 |
| MCH (pg) | 22.33 ± 7.31 | 19.21 ± 0.58 | 0.28 |
| MCHC (g/dL) | 38.05 ± 5.68 | 37.24 ± 2.29 | 0.11 |
| PLT (×103/µL) | 404.8 ± 110.84 | 412.16 ± 22.28 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahirović, A.; Roca, S.; Fočak, M.; Fetahović, S.; Muzika, V.; Suljević, D.; Topčagić, A.; Mitrašinović-Brulić, M.; Osmanković, I.; Crans, D.C.; et al. A Heterobimetallic Au(I)–Ru(II) Complex Bridged by dppb: Synthesis, Structural and Solution Characterization, BSA Interaction and In Vivo Toxicity Evaluation in Wistar Rats. Inorganics 2025, 13, 323. https://doi.org/10.3390/inorganics13100323
Zahirović A, Roca S, Fočak M, Fetahović S, Muzika V, Suljević D, Topčagić A, Mitrašinović-Brulić M, Osmanković I, Crans DC, et al. A Heterobimetallic Au(I)–Ru(II) Complex Bridged by dppb: Synthesis, Structural and Solution Characterization, BSA Interaction and In Vivo Toxicity Evaluation in Wistar Rats. Inorganics. 2025; 13(10):323. https://doi.org/10.3390/inorganics13100323
Chicago/Turabian StyleZahirović, Adnan, Sunčica Roca, Muhamed Fočak, Selma Fetahović, Višnja Muzika, Damir Suljević, Anela Topčagić, Maja Mitrašinović-Brulić, Irnesa Osmanković, Debbie C. Crans, and et al. 2025. "A Heterobimetallic Au(I)–Ru(II) Complex Bridged by dppb: Synthesis, Structural and Solution Characterization, BSA Interaction and In Vivo Toxicity Evaluation in Wistar Rats" Inorganics 13, no. 10: 323. https://doi.org/10.3390/inorganics13100323
APA StyleZahirović, A., Roca, S., Fočak, M., Fetahović, S., Muzika, V., Suljević, D., Topčagić, A., Mitrašinović-Brulić, M., Osmanković, I., Crans, D. C., & Višnjevac, A. (2025). A Heterobimetallic Au(I)–Ru(II) Complex Bridged by dppb: Synthesis, Structural and Solution Characterization, BSA Interaction and In Vivo Toxicity Evaluation in Wistar Rats. Inorganics, 13(10), 323. https://doi.org/10.3390/inorganics13100323











