Transition Metal (II) Coordination Chemistry Ligated by a New Coplanar Tridentate Ligand, 2,6-Bis(5-isopropyl-1H-pyrazol-3-yl)pyridine
Abstract
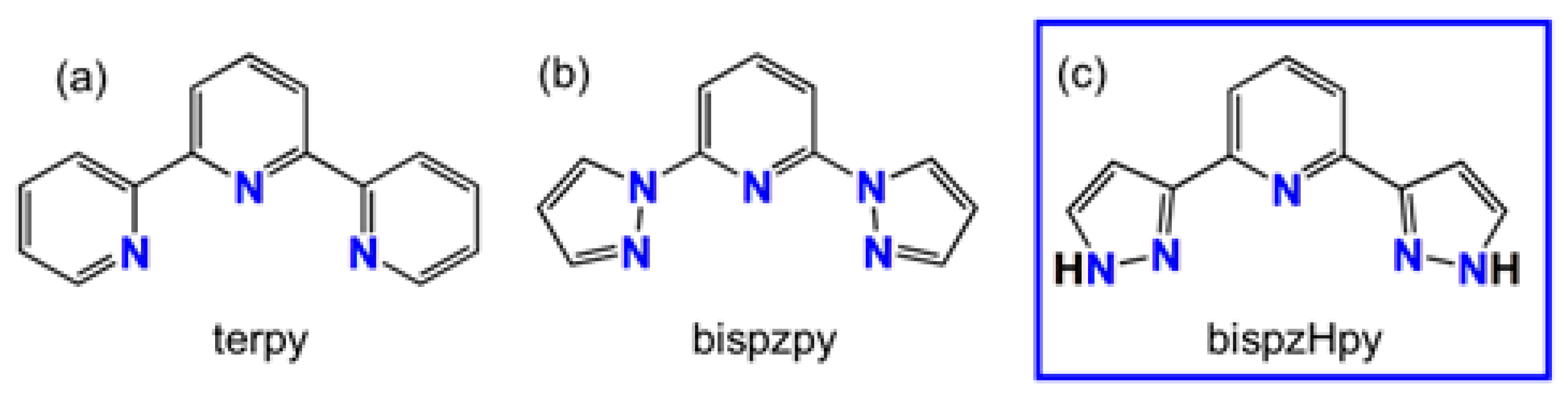
1. Introduction
2. Results and Discussion
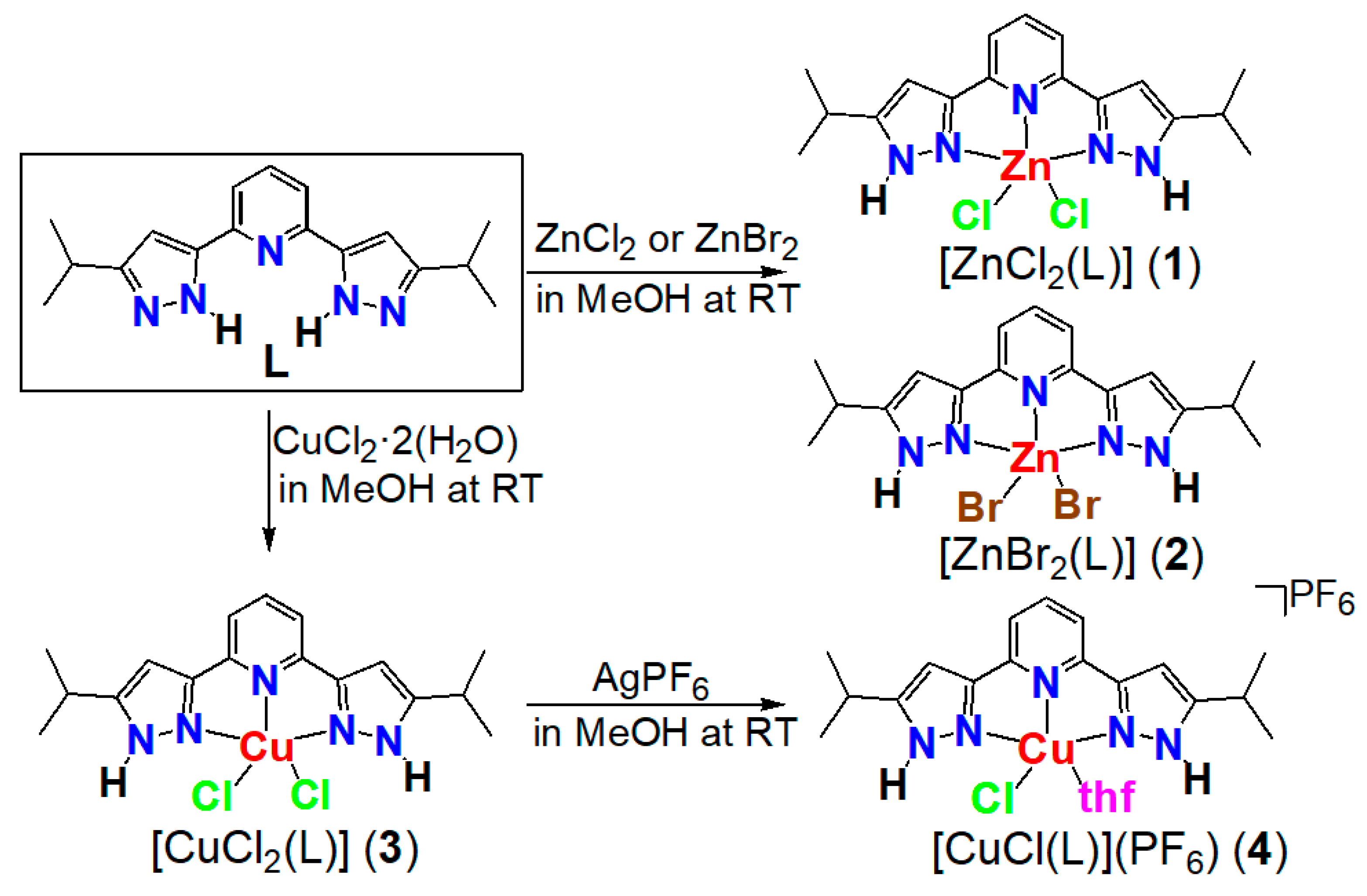
2.1. Synthesis of Ligand
2.2. Synthesis of Complexes
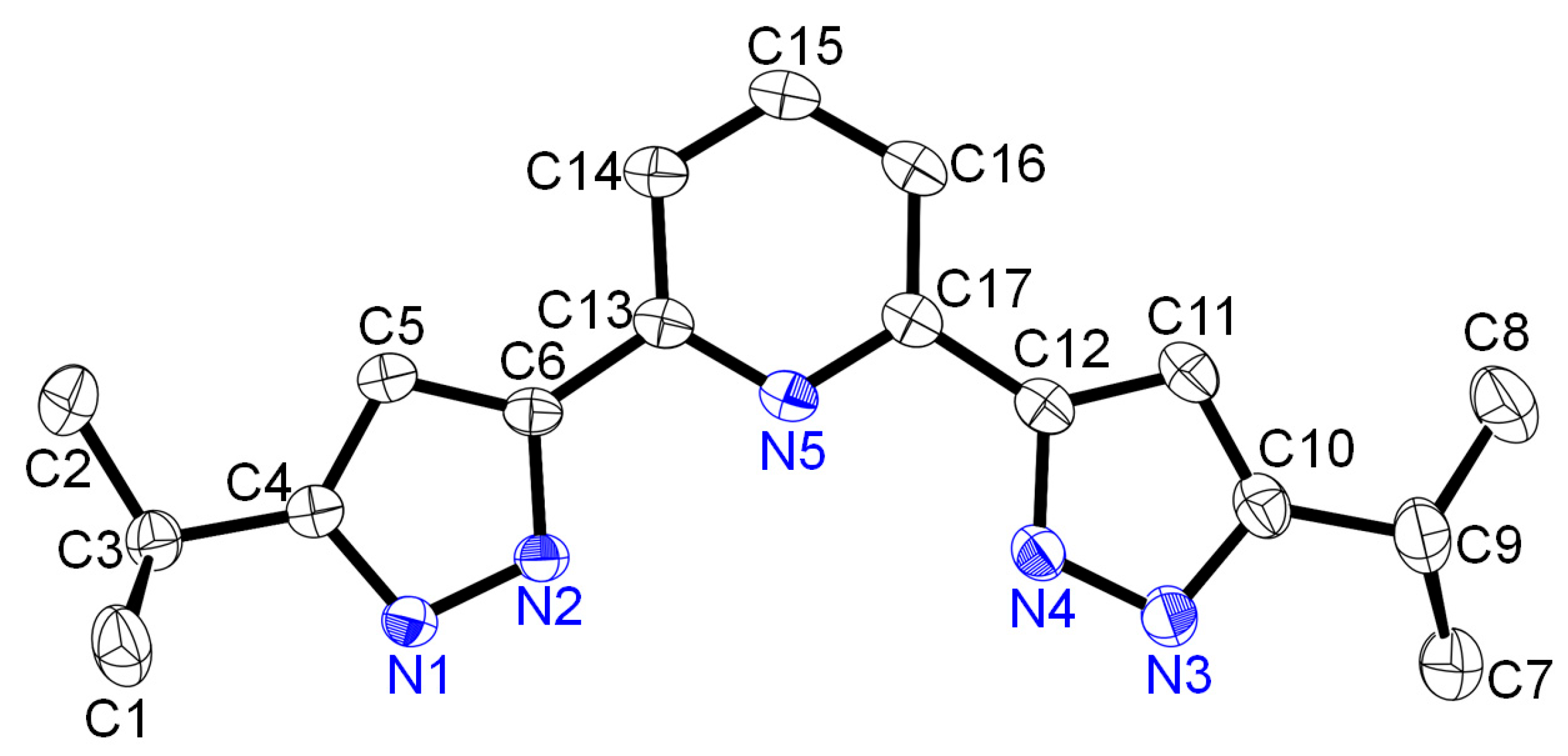
2.3. Structure of Ligand
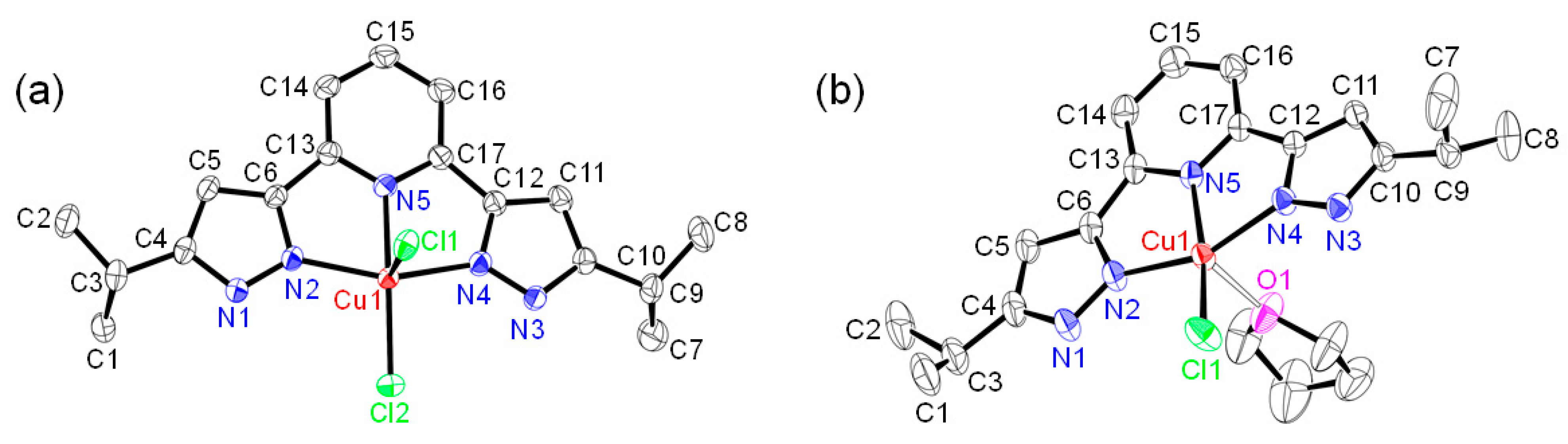
2.4. Structures of Halogenido Metal (II) Complexes
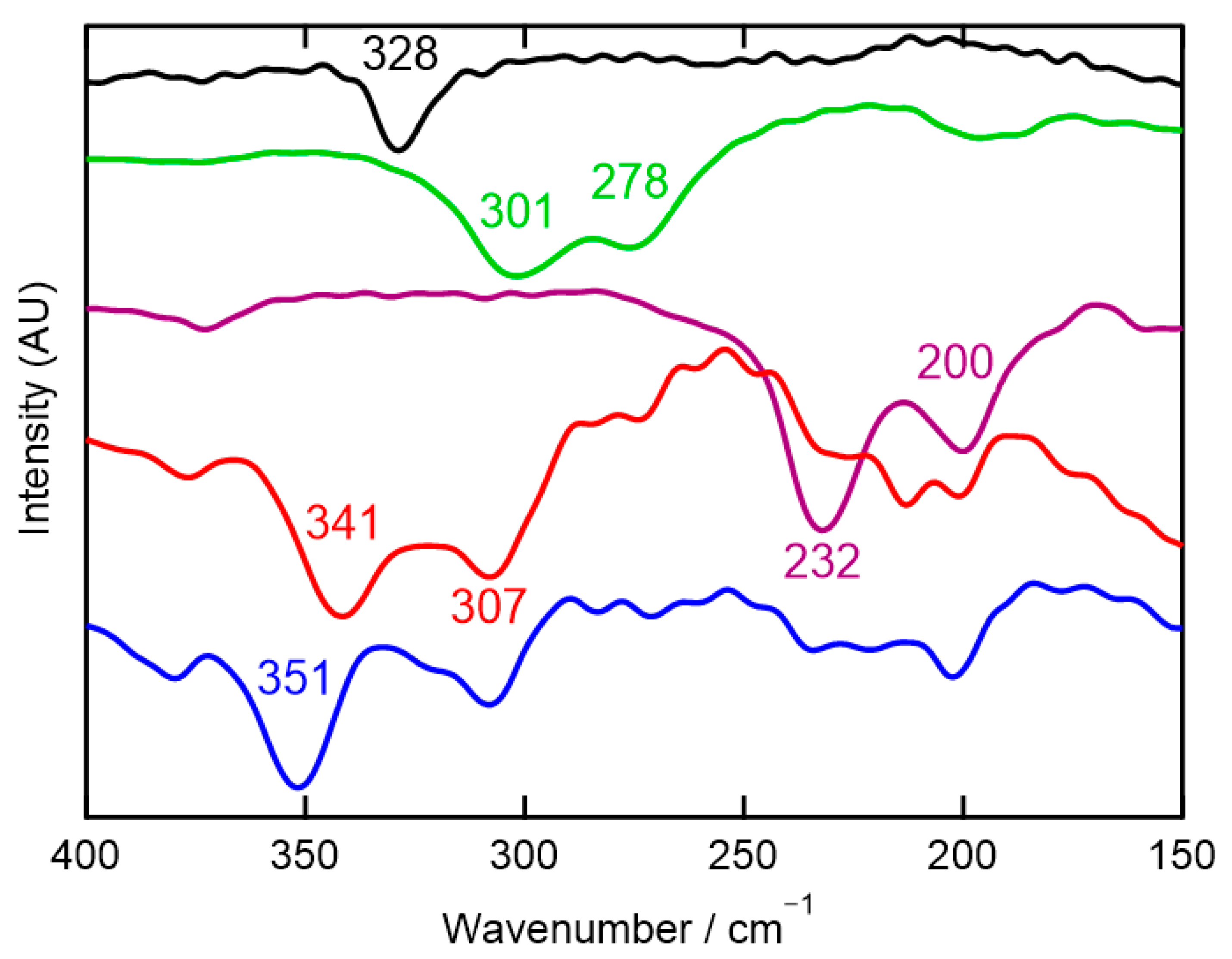
2.5. Infrared and Far-Infrared Spectroscopy
2.6. 1H-NMR Spectroscopy
2.7. EPR Spectroscopy
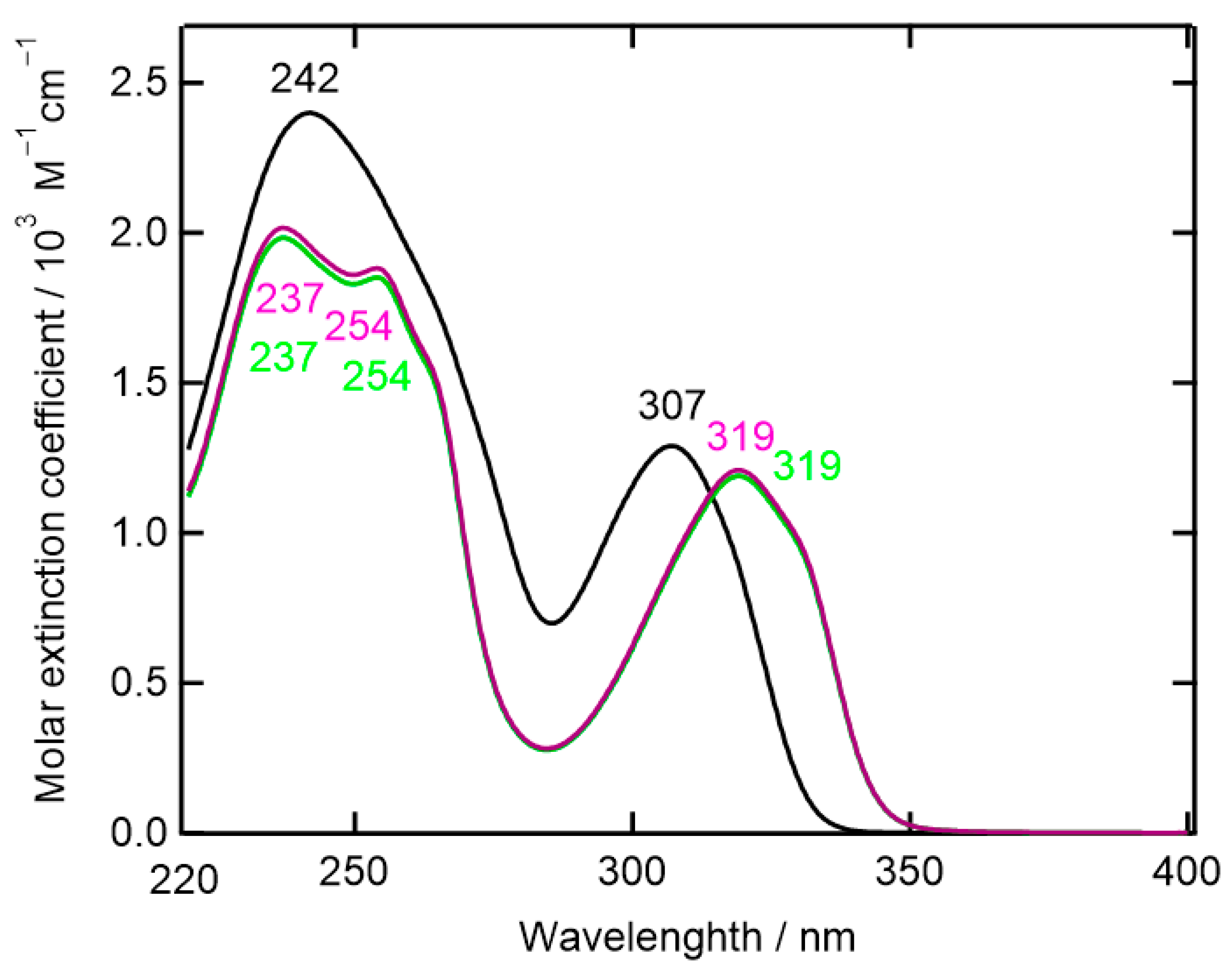
2.8. UV-Vis Absorption Spectroscopy
3. Materials and Methods
3.1. Material and General Techniques
3.2. Instrumentation
3.3. Preparation of Ligand and Complexes
- 2,6-Bis(3-isopropyl-1H-pyrazol-5-yl)pyridine (bispzHpy) (L)
- Reaction of L with NaH
- [ZnCl2(L)] (1)
- [ZnBr2(L)] (2)
- [CuCl2(L)] (3)
- [CuCl(L)(thf)](PF6) (4)
3.4. X-Ray Crystal Structure Determinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPR | Electron Paramagnetic Resonance |
| EtOH | Ethanol |
| EtOAc | Ethyl acetate |
| DR | Diffuse Reflectance |
| IR | Infrared |
| NMR | Nuclear Magnetic Resonance |
| MeOH | Methanol |
| terpy | 2,2’:6’,2’’-Terpyridine |
| thf | Tetrahydrofuran |
| UV-Vis | Ultraviolet-Visible |
References
- Bessel, C.A.; See, R.F.; Jameson, D.L.; Churchill, M.R.; Takeuchi, K.J. Structural considerations of terdentate ligands: Crystal structures of 2,2’: 6′,2″-terpyridine and 2,6-bis(pyrazol-1-yl)pyridine. J. Chem. Soc. Dalton Trans. 1992, 1992, 3223–3228. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2″-terpyridine ligands–versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Mukherjee, R. Coordination chemistry with pyrazole-based chelating ligands: Molecular structural aspects. Coord. Chem. Rev. 2000, 203, 151–218. [Google Scholar] [CrossRef]
- McMillin, D.R.; Moore, J.J. Luminescence that lasts from Pt(trpy)Cl+ derivatives (trpy = 2,2’: 6′,2″-terpyridine). Coord. Chem. Rev. 2002, 229, 113–121. [Google Scholar] [CrossRef]
- Baranoff, E.; Collin, J.; Flamigni, L.; Sauvage, J. From ruthenium(II) to iridium(III): 15 years of triads based on bis-terpyridine complexes. Chem. Soc. Rev. 2004, 33, 147–155. [Google Scholar] [CrossRef]
- Hofmeiera, H.; Schubert, U.S. Recent developments in the supramolecular chemistry of terpyridine–metal complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef]
- Halcrow, M.A. The synthesis and coordination chemistry of 2,6-bis(pyrazolyl)pyridines and related ligands—Versatile terpyridine analogues. Coord. Chem. Rev. 2005, 249, 2880–2908. [Google Scholar] [CrossRef]
- Durand, J.; Milani, B. The role of nitrogen-donor ligands in the palladium-catalyzed polyketones synthesis. Coord. Chem. Rev. 2006, 250, 542–560. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef]
- Olguín, J.; Brooker, S. Spin crossover active iron(II) complexes of selected pyrazole-pyridine/pyrazine ligands. Coord. Chem. Rev. 2011, 255, 203–240. [Google Scholar] [CrossRef]
- Denat, F.; Gros, C.P. Introduction to the nitrogen ligands themed issue. New J. Chem. 2016, 40, 5643. [Google Scholar] [CrossRef]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L. Nitrogen ligands. Dalton Trans. 2019, 48, 13904–13906. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, A.; Bargan, A. Advanced and biomedical applications of schiff-base ligands and their metal complexes: A review. Crystals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Tran, D.P.; Truong, T.N. Computational study on the nature of bonding between silver ions and nitrogen ligands. ACS Omega 2022, 7, 45231–45238. [Google Scholar] [CrossRef]
- Fujisawa, K.; Yamada, A.; Koyama, M.; Young, D.J. The copper(II) coordination chemistry of alkyl substituted bispyrazole pyridine ligands: Structure and spectral properties. Inorg. Chim. Acta 2023, 555, 121567. [Google Scholar] [CrossRef]
- Lin, W.-S.; Kuwata, S. Recent developments in reactions and catalysis of protic pyrazole complexes. Molecules 2023, 28, 3529. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, N.; Bai, F.; Wan, L.; Shan, H.; Hou, Y.; Xing, Y.; Shi, X. Multi-functional d10 metal–organic materials based on bis-pyrazole/pyridine ligands supported by a 2,6-di(3-pyrazolyl)pyridine with different spanning flexible dicarboxylate ligands: Synthesis, structure, photoluminescent and catalytic properties. CrystEngComm 2013, 15, 9135–9147. [Google Scholar] [CrossRef]
- Guan, Q.-L.; Liu, Z.; Wei, W.-J.; Xing, Y.-H.; Liu, J.; Zhang, R.; Hou, Y.-N.; Wang, X.; Bai, F.-Y. Synthesis, structure, spectroscopy of four novel supramolecular complexes and cytotoxicity study by application of multiple parallel perfused microbioreactors. New J. Chem. 2014, 38, 3258–3268. [Google Scholar] [CrossRef]
- Milutinović, M.M.; Bogojeski, J.V.; Klisurić, O.; Scheurer, A.; Elmrothd, S.K.C.; Bugarčić, Ž.D. Synthesis and structures of a pincer-type rhodium(III) complex: Reactivity toward biomolecules. Dalton Trans. 2016, 45, 15481–15491. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, W.; Wang, D. Mononuclear, dinuclear, hexanuclear, and one-dimensional polymeric silver complexes having ligand-supported and unsupported argentophilic interactions stabilized by pincer-like 2,6-bis(5-pyrazolyl)pyridine ligands. Dalton Trans. 2008, 11, 1444–1453. [Google Scholar] [CrossRef]
- Kuei, C.-Y.; Liu, S.-H.; Chou, P.-T.; Lee, G.-H.; Chi, Y. Room temperature blue phosphorescence: A combined experimental and theoretical study on the bis-tridentate Ir(III) metal complexes. Dalton Trans. 2016, 45, 15364–15373. [Google Scholar] [CrossRef]
- Galstyan, A.; Naziruddin, A.R.; Cebrián, C.; Iordache, A.; Daniliuc, C.G.; Cola, L.D.; Strassert, C.A. Correlating the structural and photophysical features of pincer luminophores and monodentate ancillary ligands in PtII phosphors. Eur. J. Inorg. Chem. 2015, 2015, 5822–5831. [Google Scholar] [CrossRef]
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Moro-oka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, T.; Tatsumi, K.; Nakamura, A. A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = i-Pr and Ph). J. Am. Chem. Soc. 1992, 114, 1277–1291. [Google Scholar] [CrossRef]
- Fujisawa, K.; Kobayashi, T.; Fujita, K.; Kitajima, N.; Moro-oka, Y.; Miyashita, Y.; Yamada, Y.; Okamoto, K. Mononuclear copper(II) hydroxo complex: Structural effect of a 3-position of tris(pyrazolyl)borates. Bull. Chem. Soc. Jpn. 2000, 73, 1797–1804. [Google Scholar] [CrossRef]
- Fujisawa, K.; Tada, N.; Ishikawa, Y.; Higashimura, H.; Miyashita, Y.; Okamoto, K. The most hindered hydrotris(pyrazolyl)borate ligand, X-ray structure of chlorocopper(II) complex: [Cu(Cl){HB(3-Ad-5-Pripz)3}] as compared with [Cu(Cl){HB(3-But-5-Pripz)3}]. Inorg. Chem. Commun. 2004, 7, 209–212. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ono, T.; Ishikawa, Y.; Amir, N.; Miyashita, Y.; Okamoto, K.; Lehnert, N. Structural and electronic differences of copper(I) complexes with tris(pyrazolyl)methane and hydrotris(pyrazolyl)borate ligands. Inorg. Chem. 2006, 45, 1698–1713. [Google Scholar] [CrossRef]
- Fujisawa, K. A personal perspective on the discovery of dioxygen adducts of copper and iron by Nobumasa Kitajima. J. Biol. Inorg. Chem. 2017, 22, 237–251. [Google Scholar] [CrossRef]
- Yoshinari, A.; Tazawa, A.; Kuwata, S.; Ikariya, T. Synthesis, structures, and reactivities of pincer-type ruthenium complexes bearing two proton-responsive pyrazole arms. Chem. Asian J. 2012, 7, 1417–1425. [Google Scholar] [CrossRef]
- Minkin, A.I.; Garnovskii, A.D.; Elguero, J.; Katritzky, A.R.; Denisko, G.V. The tautomerism of heterocycles: Five-membered rings with two or more heteroatoms. Adv. Heterocycl. Chem. 2000, 76, 157–323. [Google Scholar] [CrossRef]
- Aguilar-Parrilla, F.; Limbach, H.-H.; Foces-Foces, C.; Cano, F.H.; Jagerovic, N.; Elguero, J. Structure and dynamics of 3,5-di-tert-butylpyrazole probed by combined X-ray crystallography and 15N solid state NMR. J. Org. Chem. 1995, 60, 1965–1970. [Google Scholar] [CrossRef]
- Sugiyarto, K.H.; Scudder, M.L.; Craig, D.C.; Goodwin, H.A. Electronic and structural properties of the spin crossover systems bis(2,6-bis(pyrazol-3-yl)pyridine)iron(II) thiocyanate and selenocyanate. Aust. J. Chem. 2000, 53, 755–765. [Google Scholar] [CrossRef]
- Dong, J.-Y.; You, T.-P. 2,6-Bis(3-phenyl-1H-pyrazol-5-yl)pyridine monohydrate. Acta Cryst. 2008, E64, o820. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.J.; Chen, C.-H.; Pink, M.; Lord, R.L.; Caulton, K.G. Coordination and electronic characteristics of a nitrogen heterocycle pincer ligand. Inorg. Chim. Acta 2016, 451, 82–91. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2’-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Einstein, F.W.D.; Penfol, B.R. The crystal structure of terpyridylzinc chloride: A refinement. Acta Cryst. 1966, 20, 924–926. [Google Scholar] [CrossRef]
- Henke, W.; Kremer, S.; Reinen, D. Cu2+ in five-coordination: A case of a pseudo-Jahn-Teller effect. 1. Structure and spectroscopy of the compounds Cu(terpy)X2·nH2O. Inorg. Chem. 1983, 22, 2858–2863. [Google Scholar] [CrossRef]
- Aghatabay, N.M.; Neshat, A.; Karabiyik, T.; Somer, M.; Haciu, D.; Dülger, B. Synthesis, characterization and antimicrobial activity of Fe(II), Zn(II), Cd(II) and Hg(II) complexes with 2,6-bis(benzimidazol-2-yl)pyridine ligand. Eur. J. Med. Chem. 2007, 42, 205–213. [Google Scholar] [CrossRef]
- Postmus, C.; Ferraro, J.R.; Woznizk, W. Low-frequency infrared spectra of nitrogen-ligand complexes of zinc(II) halides. Inorg. Chem. 1967, 6, 2030–2032. [Google Scholar] [CrossRef]
- Thornton, D.A. Metal complexes of pyridine: Infrared and Raman spectra with particular reference to isotopic labelling studies. Coord. Chem. Rev. 1990, 104, 251–295. [Google Scholar] [CrossRef]
- Fujisawa, K.; Chiba, S.; Miyashita, Y.; Okamoto, K. Copper complexes with neutral N4 tripodal ligands: Influence of the number of nitrogen donors on their structures, properties, and reactivity. Eur. J. Inorg. Chem. 2009, 26, 3921–3934. [Google Scholar] [CrossRef]
- CrystalClear, Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 2001.
- Rigaku Oxford Diffraction. CrysAlis PRO; Oxford Diffraction Ltd.: Oxfordshire, UK, 2015. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Crystal Structure; Version 4.3. Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2003.
- Parsons, S.; Flack, H. Precise absolute-structure determination in light-atom crystals. Acta Cryst. 2004, A60, S61. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Minakawa, Y.; Young, D.J. Transition Metal (II) Coordination Chemistry Ligated by a New Coplanar Tridentate Ligand, 2,6-Bis(5-isopropyl-1H-pyrazol-3-yl)pyridine. Inorganics 2025, 13, 189. https://doi.org/10.3390/inorganics13060189
Fujisawa K, Minakawa Y, Young DJ. Transition Metal (II) Coordination Chemistry Ligated by a New Coplanar Tridentate Ligand, 2,6-Bis(5-isopropyl-1H-pyrazol-3-yl)pyridine. Inorganics. 2025; 13(6):189. https://doi.org/10.3390/inorganics13060189
Chicago/Turabian StyleFujisawa, Kiyoshi, Yurika Minakawa, and David James Young. 2025. "Transition Metal (II) Coordination Chemistry Ligated by a New Coplanar Tridentate Ligand, 2,6-Bis(5-isopropyl-1H-pyrazol-3-yl)pyridine" Inorganics 13, no. 6: 189. https://doi.org/10.3390/inorganics13060189
APA StyleFujisawa, K., Minakawa, Y., & Young, D. J. (2025). Transition Metal (II) Coordination Chemistry Ligated by a New Coplanar Tridentate Ligand, 2,6-Bis(5-isopropyl-1H-pyrazol-3-yl)pyridine. Inorganics, 13(6), 189. https://doi.org/10.3390/inorganics13060189







