MIL Series in MOFs for the Removal of Emerging Contaminants: Application and Mechanisms
Abstract
1. Introduction
2. Classification of MILs
2.1. MIL-53
2.2. MIL-88
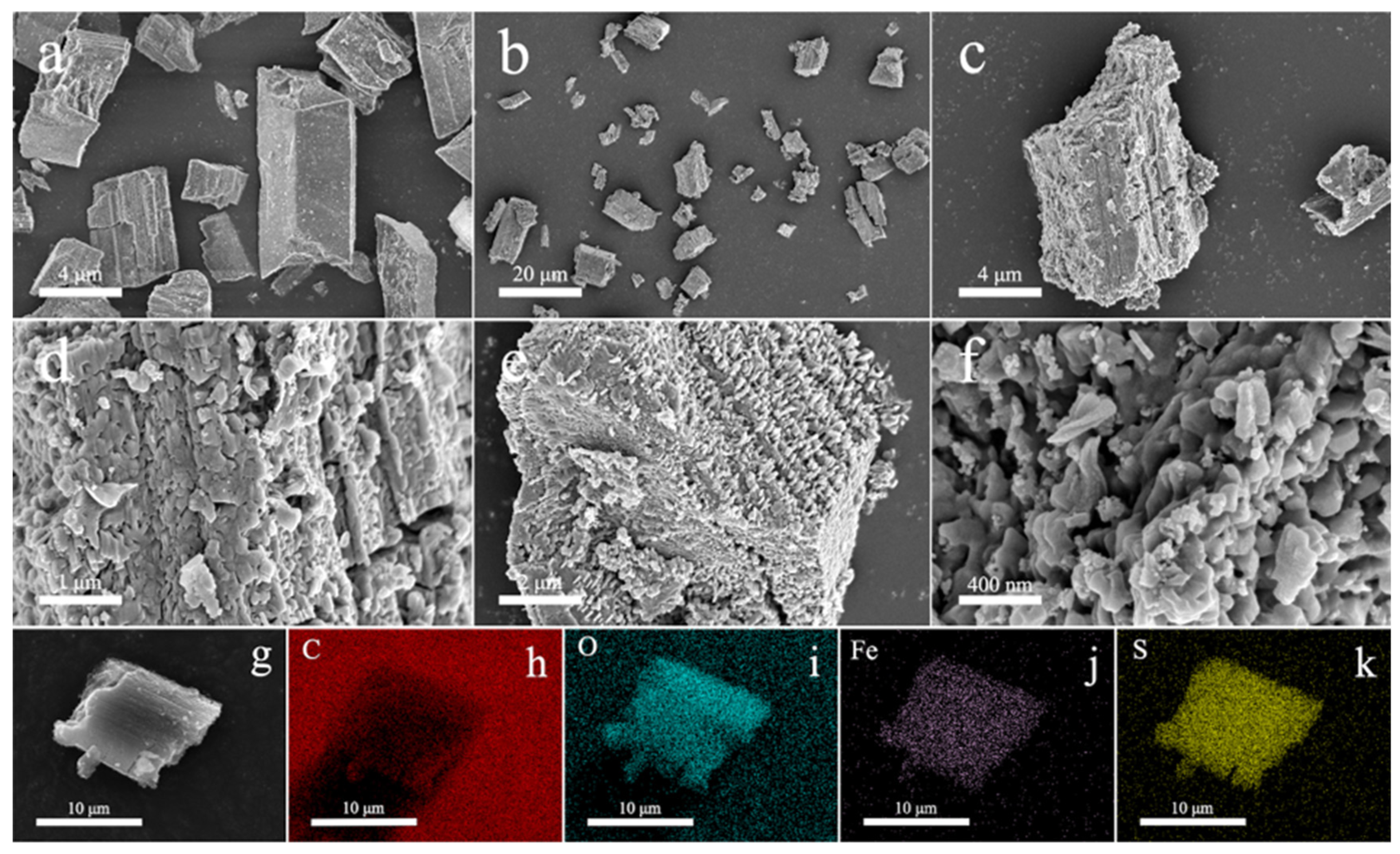
2.3. MIL-100
2.4. MIL-101
2.5. MIL-125
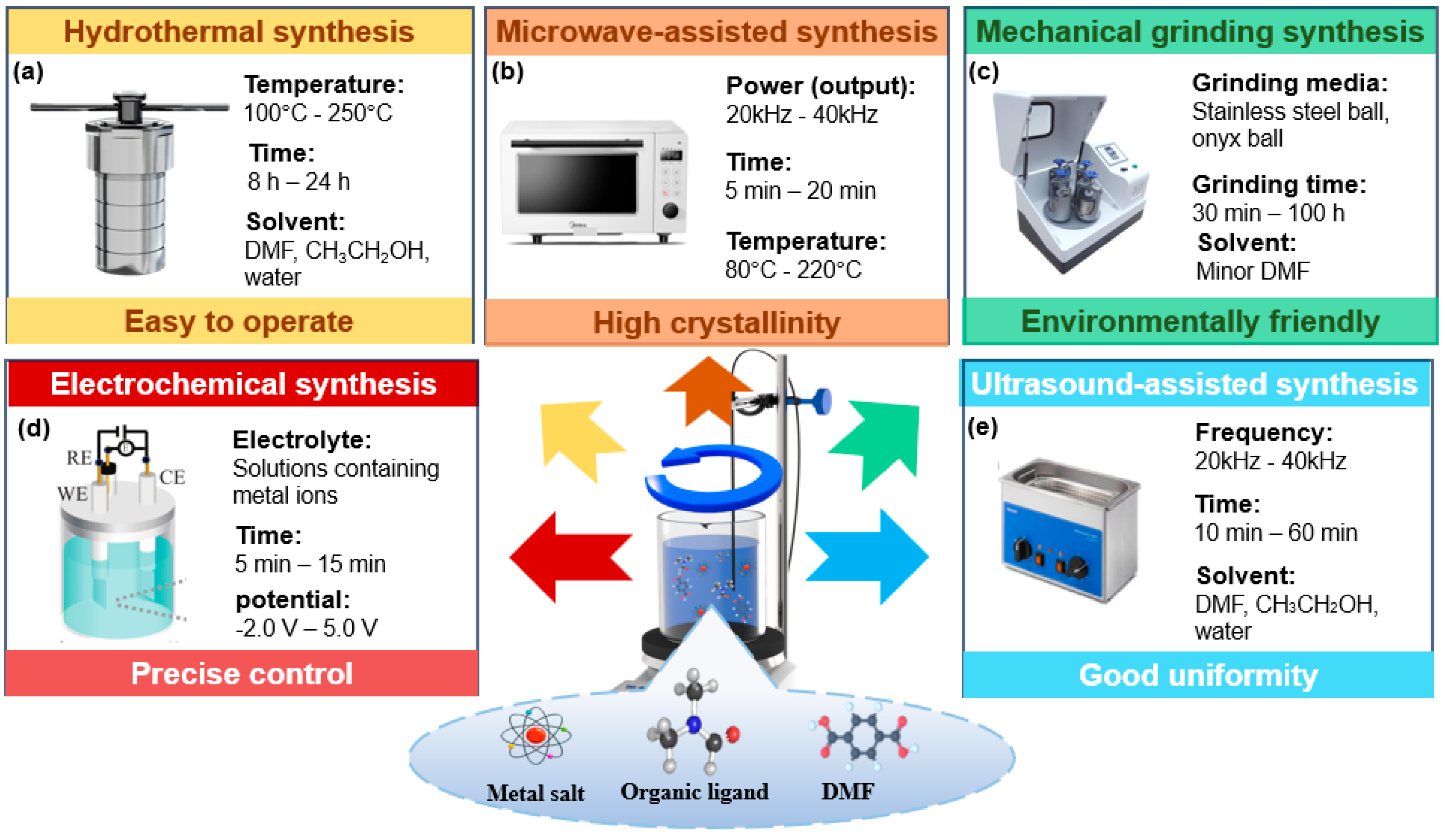
3. Synthesis Method of MILs
3.1. Solvothermal Synthesis/Hydrothermal Synthesis
3.2. Microwave-Assisted Synthesis
3.3. Mechanical Grinding Synthesis
3.4. Electrochemical Synthesis
3.5. Ultrasound-Assisted Synthesis
3.6. Other Synthetic Methods
4. Modification of MILs
4.1. Elemental Doping
4.1.1. Transition Metals
4.1.2. Main Group Elements
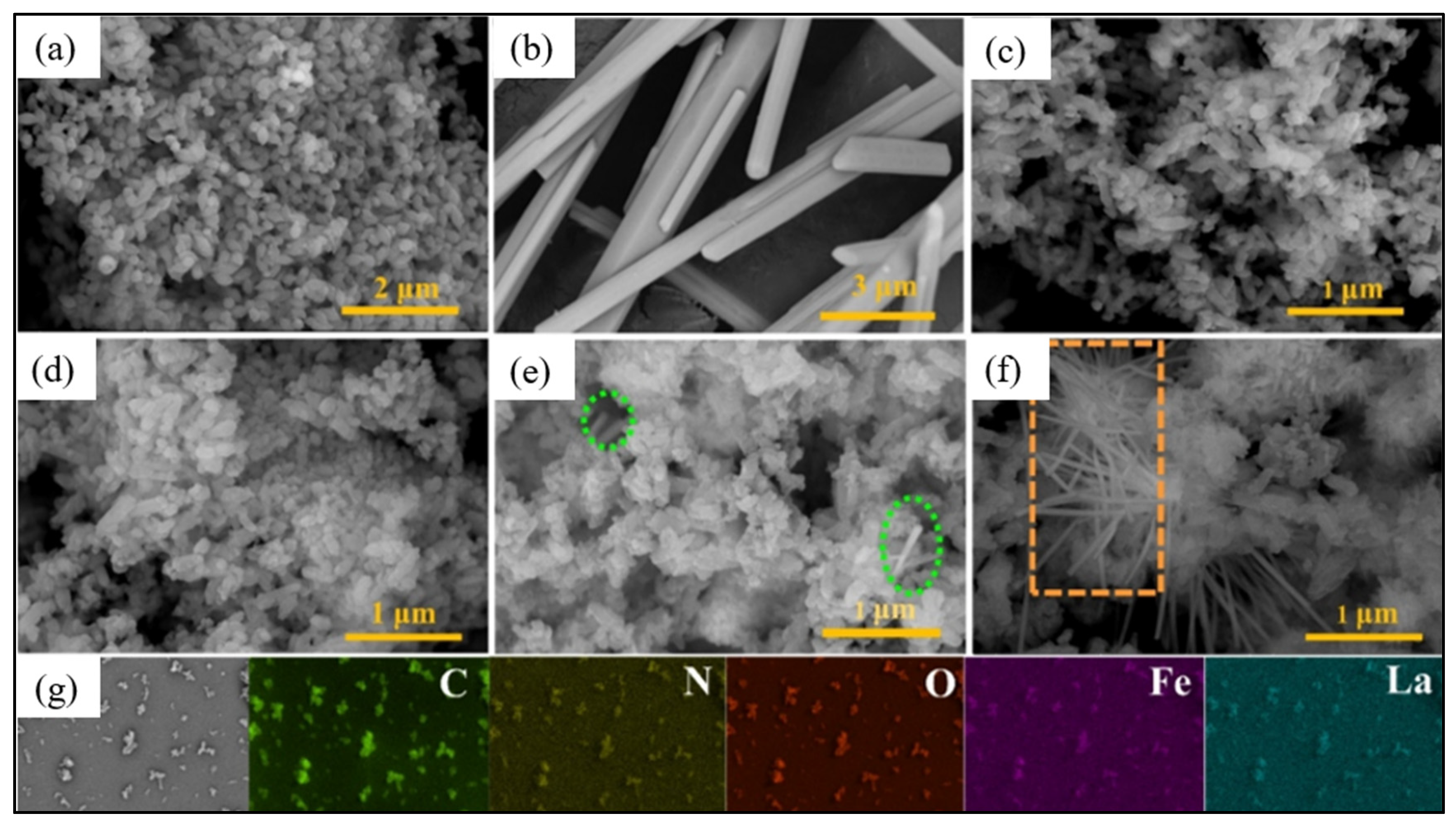
4.1.3. Lanthanides
4.2. Ligand Functionalization
4.2.1. Amino-Functionalized Ligand
4.2.2. Sulfonated-Functionalized Ligands
4.2.3. Other Group-Functionalized Ligands
4.3. Structural Heterogeneity
4.3.1. The Z-Scheme Heterojunctions’ Structure
4.3.2. The S-Scheme Heterojunctions’ Structure
4.3.3. Double S-Scheme Heterojunctions’ Structure
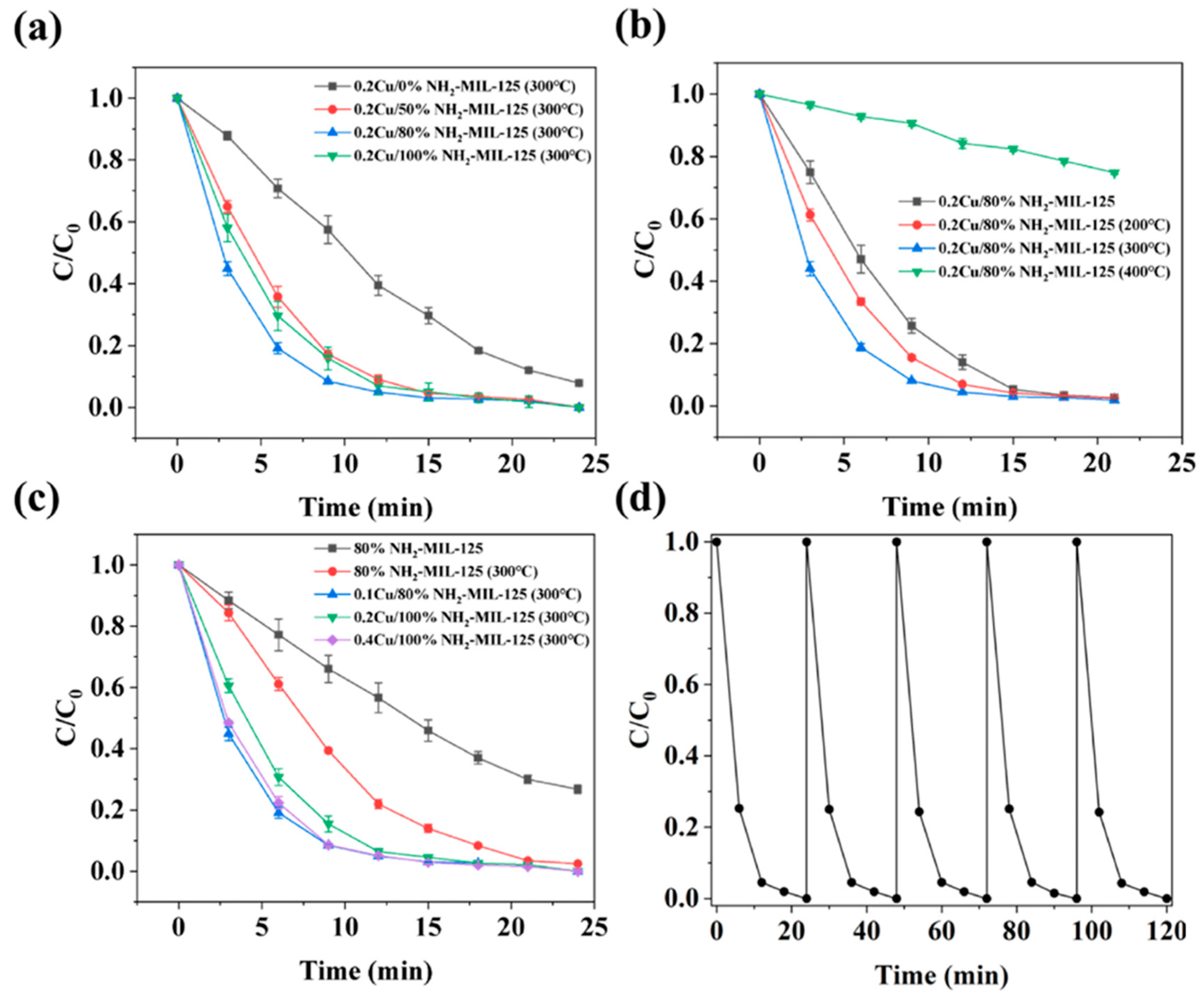
4.3.4. Stability and Recyclability
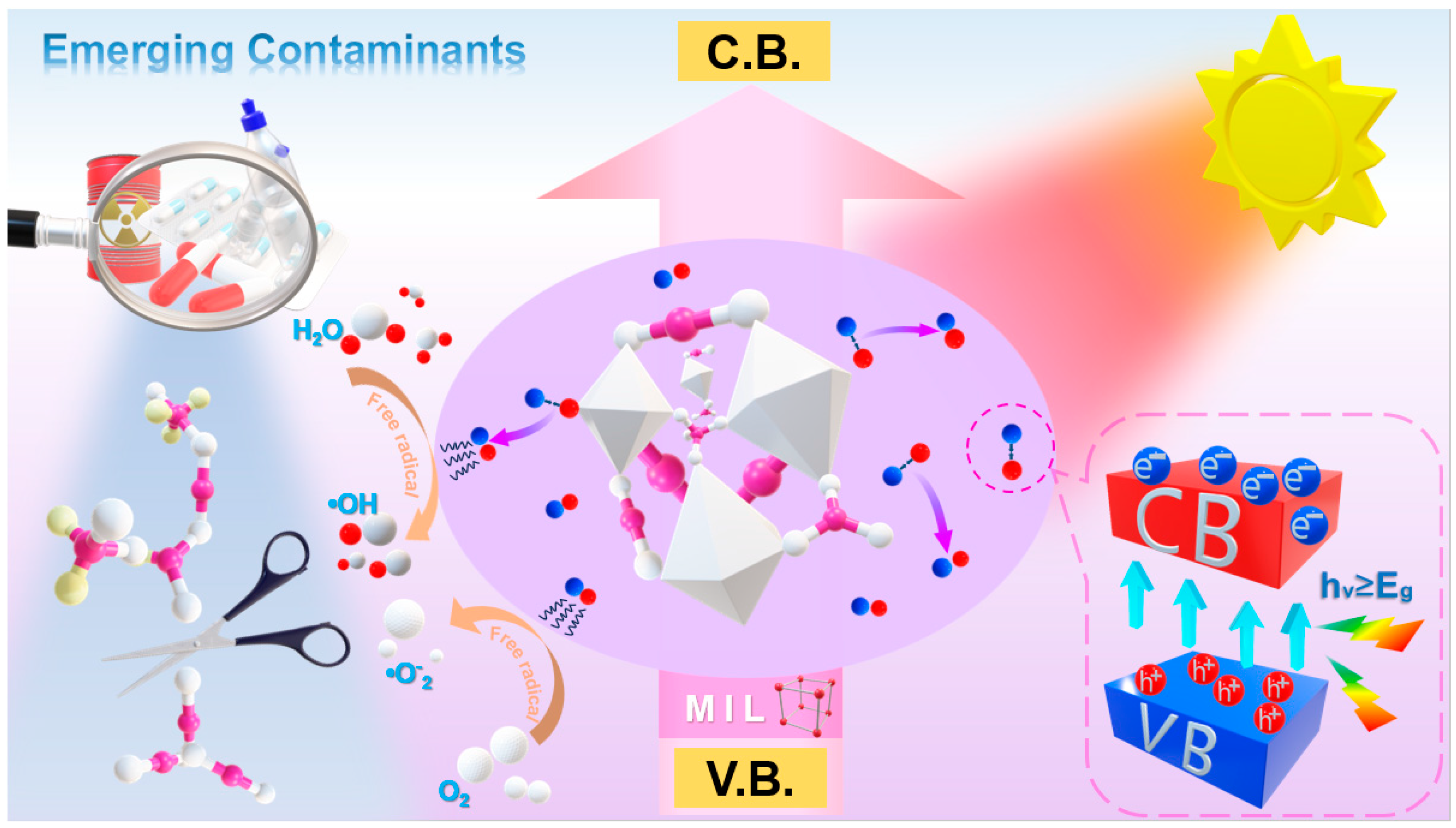
5. The Utilization of MIL Materials in the Removal of Emerging Contaminants
5.1. Persistent Organic Pollutants (POPs)
5.1.1. Pesticides
5.1.2. Industrial Chemicals
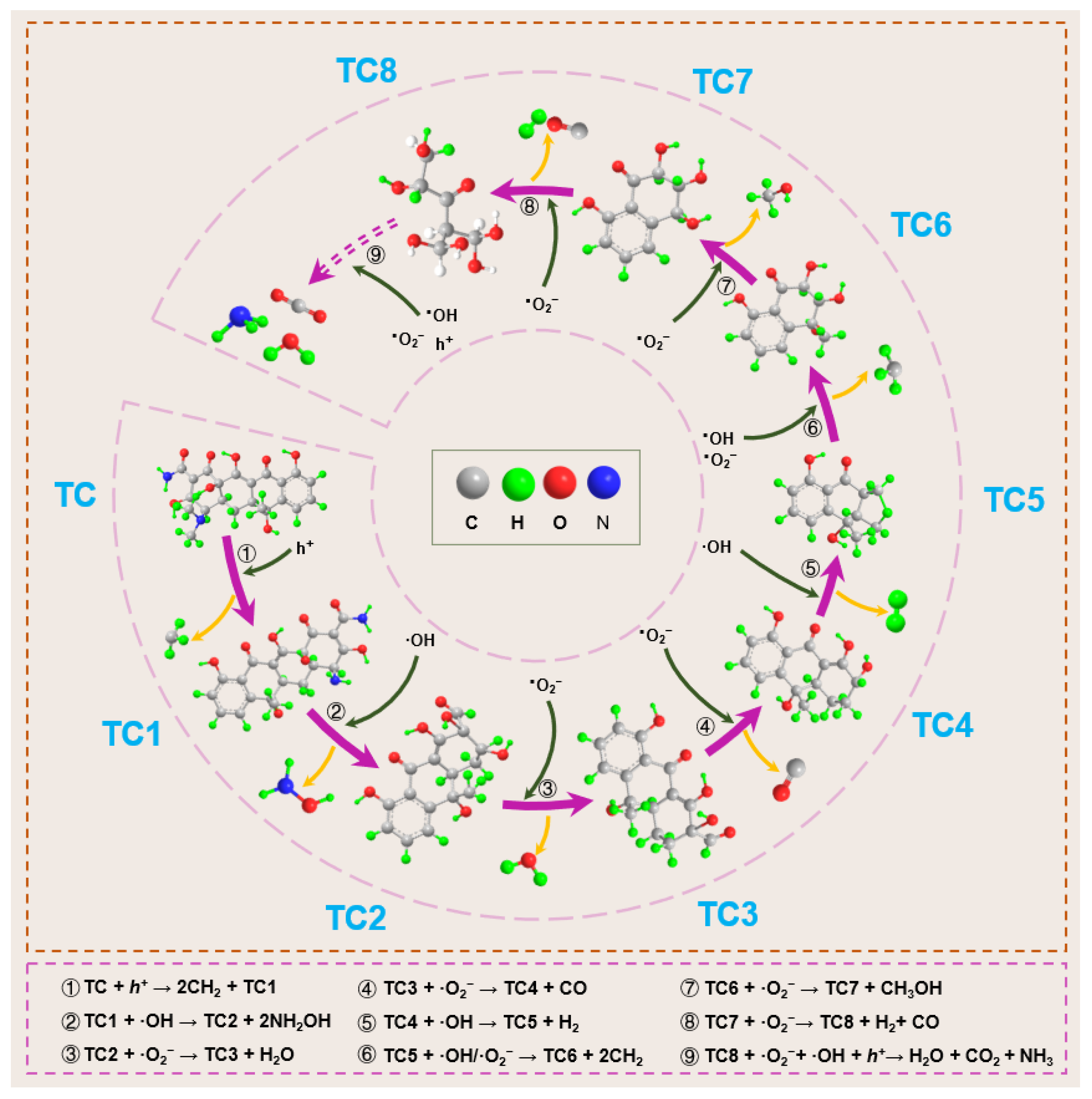
5.2. Pharmaceuticals and Personal Care Products (PPCPs)
5.3. Volatile Organic Compounds (VOCs)
5.3.1. Hydrocarbons
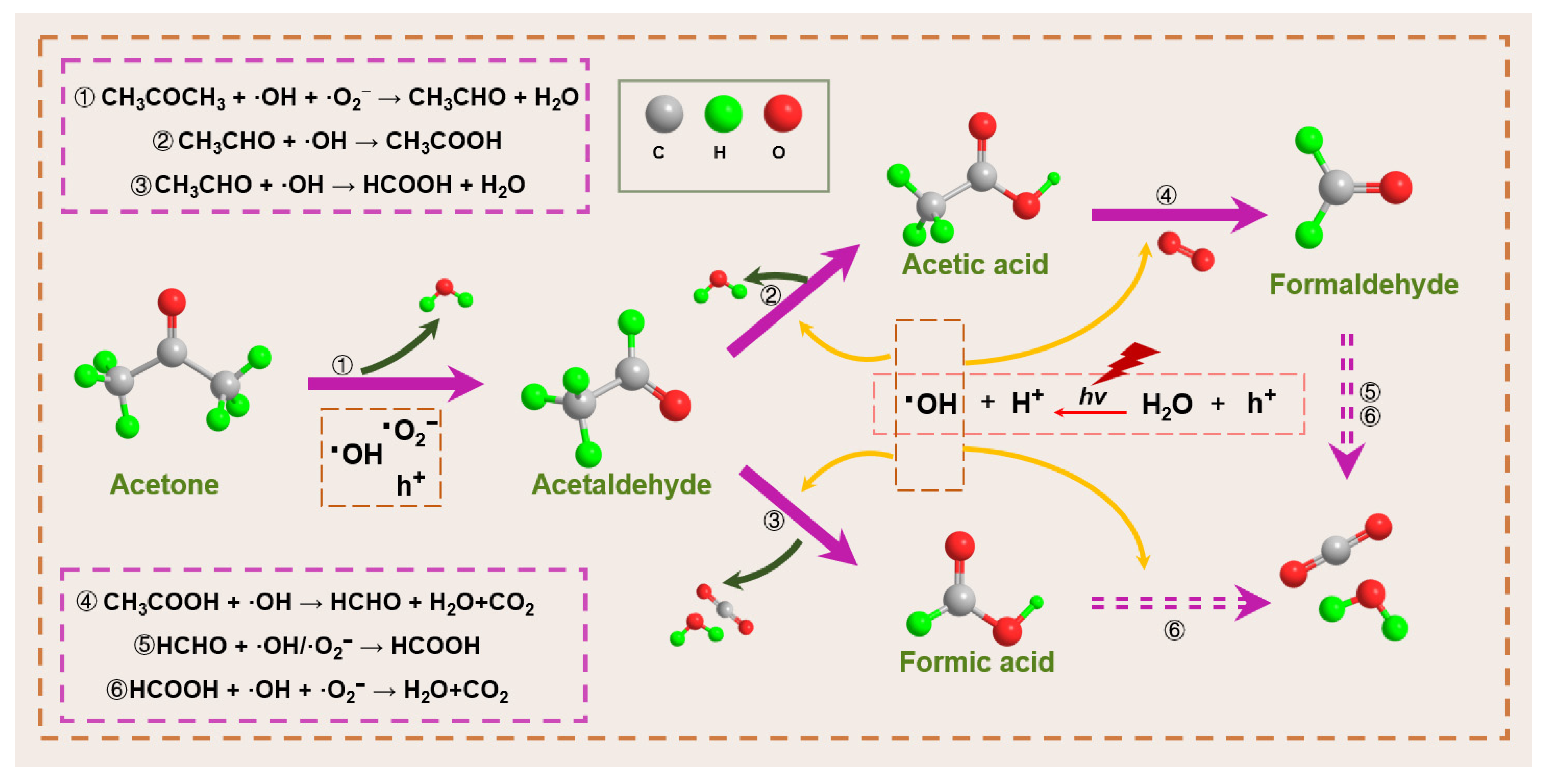
5.3.2. Oxygenated Organic Compounds (OVOCs)
5.3.3. Chlorinated Volatile Organic Compounds (CVOCs)
5.3.4. Sulfur-Containing Volatile Organic Compounds (S-VOCs)
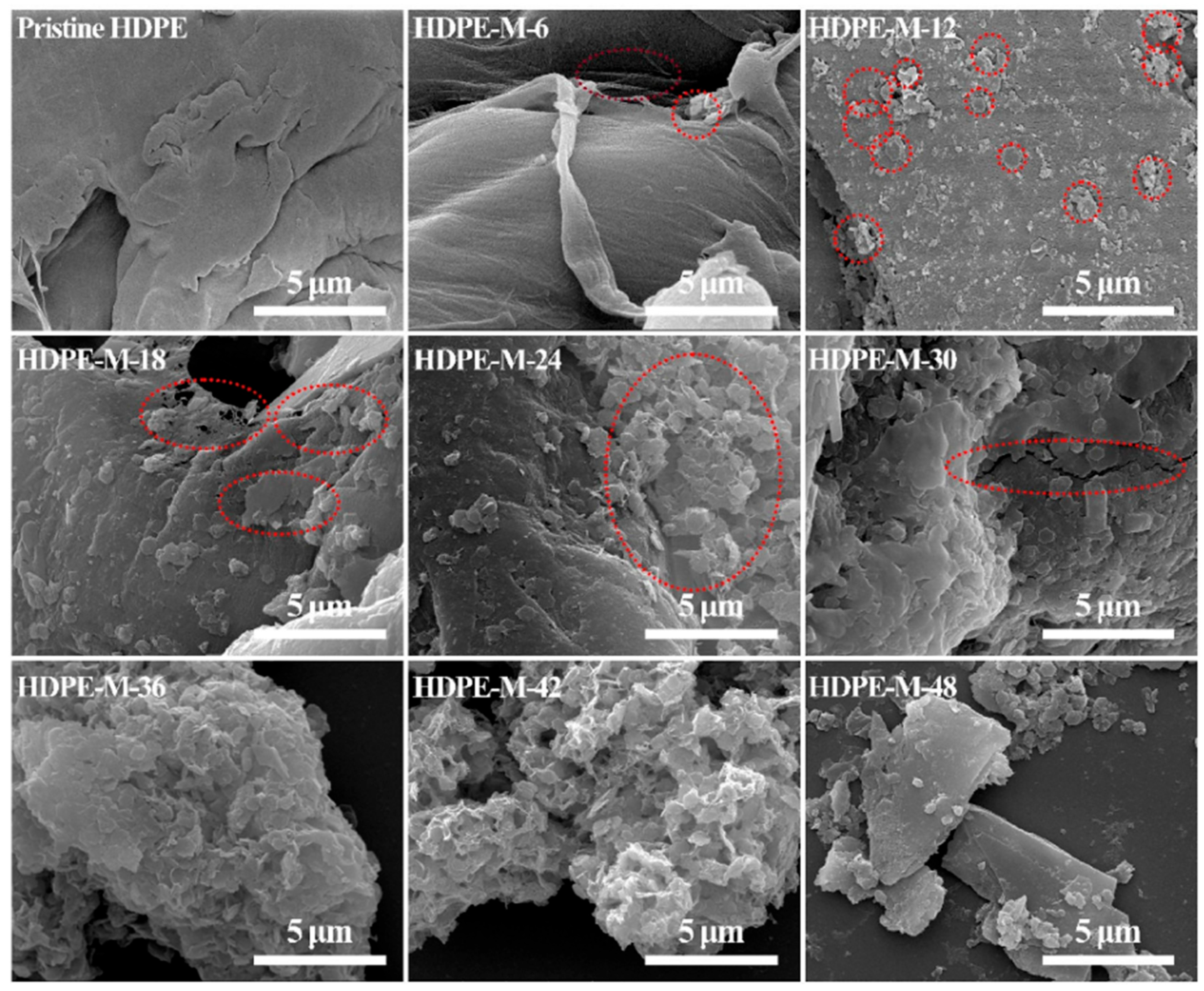
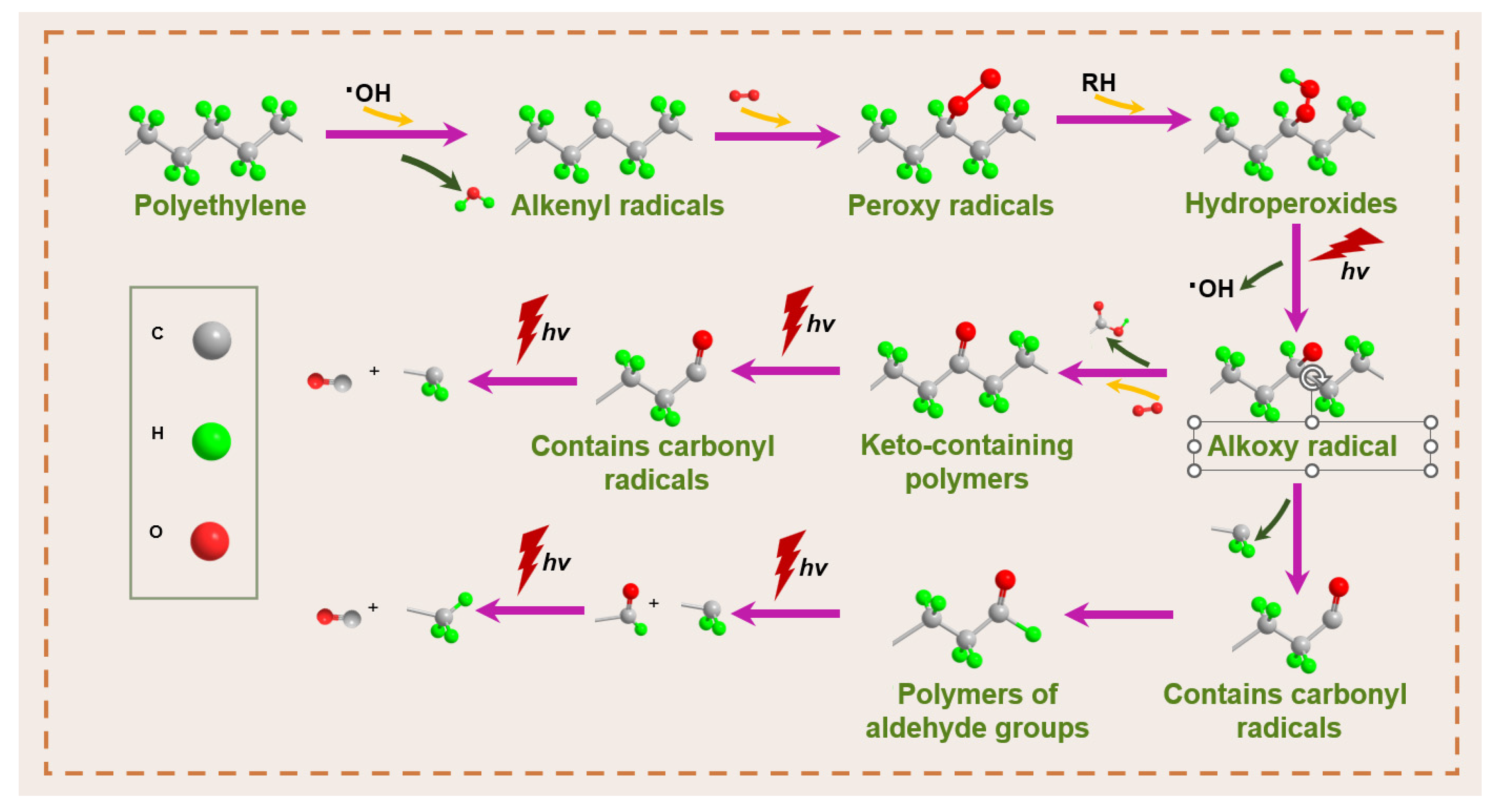
5.4. Microplastics
5.4.1. Micrometer Scale
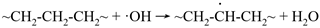
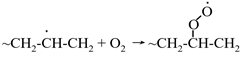
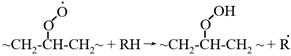
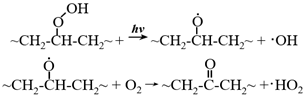
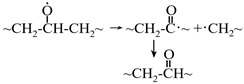
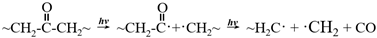
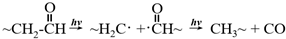
5.4.2. Nanoscale
6. Summary
Funding
Conflicts of Interest
References
- Shah, S.A.R.; Abbas, N.; Serbanescu, L.; Niu, R.; Nassani, A.A. The key challenges and best alternatives to environmental sustainability: A comprehensive study. Sci. Rep. 2025, 15, 7042. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Yang, W.; Zhang, W.; Liu, Y.; Wang, Z.; Li, W. Distribution, sources, partition behavior and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the waters and sediments of Lake Ulansuhai, China. Mar. Pollut. Bull. 2024, 200, 116072. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Shen, X.; Zhu, Z.; Liu, X.; Zhang, H.; Qiu, Y.; Yin, D. Rsearch progress on biochar materials for new pollutants removal in the aquatic environment: A mini-review. Front. Environ. Sci. Eng. 2024, 19, 33. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Zuo, Y. Research and Application of Water Treatment Technologies for Emerging Contaminants (ECs): A Pathway to Solving Water Environment Challenges. Water 2024, 16. [Google Scholar] [CrossRef]
- Guillotin, S.; Delcourt, N. Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14271. [Google Scholar] [CrossRef]
- Rauseo, J.; Spataro, F.; Patrolecco, L. Persistent and Emerging Organic Contaminants in Natural Environments. Water 2025, 17, 436. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Cheng, M.; Li, M.; Zhang, Y.; Gu, X.; Gao, W.; Zhang, S.; Liu, J. Exploring the mechanism of PPCPs on human metabolic diseases based on network toxicology and molecular docking. Environ. Int. 2025, 196, 109324. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Microplastic migration and transformation pathways and exposure health risks. Environ. Pollut. 2025, 368, 125700. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, T.; Ya, H.; Xing, Y.; Wang, X.; Jiang, B. Effects of heavy metals on the adsorption of ciprofloxacin on polyethylene microplastics: Mechanism and toxicity evaluation. Chemosphere 2023, 315, 137745. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zeng, G.; Cheng, M.; Huang, D.; Liu, Y.; Zhou, C.; Xiong, W.; Yang, Y.; Wang, W.; et al. Materials Institute Lavoisier (MIL) based materials for photocatalytic applications. Coord. Chem. Rev. 2021, 438, 213874. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Dutta, S.; Kamal, S.; Gómez-Graña, S.; Pastoriza-Santos, I.; Wuttke, S.; Polavarapu, L. State-of-the-Art, Insights, and Perspectives for MOFs-Nanocomposites and MOF-Derived (Nano) Materials. Adv. Mater. 2025, 2415399. [Google Scholar] [CrossRef]
- Tan, K.L.; Foo, K.Y. Preparation of MIL-100 via a novel water-based heatless synthesis technique for the effective remediation of phenoxyacetic acid-based pesticide. J. Environ. Chem. Eng. 2021, 9, 104923. [Google Scholar] [CrossRef]
- Pouyanfar, N.; Farnam, G.; Ahmadi, M.; Masoudifar, R.; Banan, K.; Asadian, E.; Shahhosseini, S.; Shahbazi, M.-A.; Shirazi, F.H.; Ghorbani-Bidkorpeh, F. Synthesis, modification, characterization, and in vitro evaluation of chitosan-hyaluronic acid coated MIL-100 (Fe) nanoparticles for methotrexate delivery in rheumatoid arthritis. Int. J. Biol. Macromol. 2024, 283, 137715. [Google Scholar] [CrossRef]
- Mo, L.; Chen, G.; Xu, B. Degradation of phenol by peroxymonosulfate catalyzed by cerium-doped amino-functionalized metal-organic frameworks (NH2-MIL-101 (Fe, Ce)). J. Environ. Chem. Eng. 2024, 12, 113256. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL series of metal organic frameworks (MOFs) as novel adsorbents for heavy metals in water: A review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef]
- Khan, S.; Guan, Q.; Liu, Q.; Qin, Z.; Rasheed, B.; Liang, X.; Yang, X. Synthesis, modifications and applications of MILs Metal-organic frameworks for environmental remediation: The cutting-edge review. Sci. Total Environ. 2022, 810, 152279. [Google Scholar] [CrossRef]
- Salvador, F.E.; Tegudeer, Z.; Locke, H.; Gao, W.-Y. Facile mechanochemical synthesis of MIL-53 and its isoreticular analogues with a glance at reaction reversibility. Dalton Trans. 2024, 53, 4406–4411. [Google Scholar] [CrossRef] [PubMed]
- Smoljan, C.S.; Sha, F.; Campitelli, P.; Xie, H.; Eddaoudi, M.A.; Mian, M.R.; Di Nicola, C.; Kirlikovali, K.O.; Snurr, R.Q.; Farha, O.K. Constraining Flexibility in the MIL-88 Topology through Integration of 3-Dimensional Linkers. Cryst. Growth Des. 2024, 24, 3941–3948. [Google Scholar] [CrossRef]
- Quijia, C.R.; Lima, C.; Silva, C.; Alves, R.C.; Frem, R.; Chorilli, M. Application of MIL-100(Fe) in drug delivery and biomedicine. J. Drug Deliv. Sci. Technol. 2021, 61, 102217. [Google Scholar] [CrossRef]
- Pederneira, N.; Aina, P.O.; Rownaghi, A.A.; Rezaei, F. Performance of MIL-101(Cr) and MIL-101(Cr)-Pore Expanded as Drug Carriers for Ibuprofen and 5-Fluorouracil Delivery. ACS Appl. Bio Mater. 2024, 7, 1041–1051. [Google Scholar] [CrossRef]
- Omrani, A.; Deliballi, Z.; Kaya, K.; Kiskan, B.; Akgun, M. Catalytic Role of Nanoconfinement inside MIL-125 (Ti) on the Ring-Opening Polymerization of Simple Benzoxazines. ACS Appl. Polym. Mater. 2023, 6, 253–264. [Google Scholar] [CrossRef]
- Serre, C.; Millange, F.; Thouvenot, C.; Noguès, M.; Marsolier, G.; Louër, D.; Férey, G. Very Large Breathing Effect in the First Nanoporous Chromium(III)-Based Solids: MIL-53 or CrIII(OH)·{O2C−C6H4−CO2}·{HO2C−C6H4−CO2H}x·H2Oy. J. Am. Chem. Soc. 2002, 124, 13519–13526. [Google Scholar] [CrossRef]
- Millange, F.; Walton, R.I. MIL-53 and its Isoreticular Analogues: A Review of the Chemistry and Structure of a Prototypical Flexible Metal-Organic Framework. Isr. J. Chem. 2018, 58, 1019–1035. [Google Scholar] [CrossRef]
- Tomar, S.; Singh, V.K. Review on synthesis and application of MIL-53. Mater. Today Proc. 2021, 43, 3291–3296. [Google Scholar] [CrossRef]
- Xiao, K.; Shu, B.; Lv, K.; Huang, P.; Chang, Q.; Wu, L.; Wang, S.; Cao, L. Recent Progress of MIL MOF Materials in Degradation of Organic Pollutants by Fenton Reaction. Catalysts 2023, 13, 734. [Google Scholar] [CrossRef]
- Serre, C.; Mellot-Draznieks, C.; Surblé, S.; Audebrand, N.; Filinchuk, Y.; Férey, G. Role of Solvent-Host Interactions That Lead to Very Large Swelling of Hybrid Frameworks. Science 2007, 315, 1828–1831. [Google Scholar] [CrossRef]
- Serre, C.; Millange, F.; Surblé, S.; Férey, G. A Route to the Synthesis of Trivalent Transition-Metal Porous Carboxylates with Trimeric Secondary Building Units. Angew. Chem. Int. Ed. 2004, 43, 6285–6289. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. Applications of Porous Metal–Organic Framework MIL-100(M) (M = Cr, Fe, Sc, Al, V). Cryst. Growth Des. 2018, 18, 7730–7744. [Google Scholar] [CrossRef]
- Patricia, H.; Suzy, S.; Christian, S.; Do-Young, H.; You-Kyong, S.; Jong-San, C.; Jean-Marc, G.; Irene, M.; Gérard, F. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem. Commun. 2007, 2820–2822. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Chen, C.; Ahn, W.-S. Chromium terephthalate metal–organic framework MIL-101: Synthesis, functionalization, and applications for adsorption and catalysis. RSC Adv. 2014, 4, 52500–52525. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Reticular Chemistry: Occurrence and Taxonomy of Nets and Grammar for the Design of Frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.; Wu, Y.; Gao, J.; Zhang, Q. From isolated Ti-oxo clusters to infinite Ti-oxo chains and sheets: Recent advances in photoactive Ti-based MOFs. J. Mater. Chem. A 2020, 8, 15245–15270. [Google Scholar] [CrossRef]
- Yang, S.; Yu, J.; Lu, G.; Song, G.; Shi, G.; Wang, Y.; Xie, X.; Yuan, H.; Ren, X.; Sun, J. Effect of NH2-functionalization of MIL-125 on photocatalytic degradation of o-xylene and acetaldehyde. Chem. Eng. J. 2024, 498, 155251. [Google Scholar] [CrossRef]
- Fallah, M.; Sohrabnezhad, S. Study of synthesis of mordenite zeolite/MIL-101 (Cr) metal–organic framework compounds with various methods as bi-functional adsorbent. Adv. Powder Technol. 2019, 30, 336–346. [Google Scholar] [CrossRef]
- Luo, T.; Jeppesen, H.S.; Schoekel, A.; Bönisch, N.; Xu, F.; Zhuang, R.; Huang, Q.; Senkovska, I.; Bon, V.; Heine, T.; et al. Photocatalytic Dehalogenation of Aryl Halides Mediated by the Flexible Metal–Organic Framework MIL-53(Cr). Angew. Chem. Int. Ed. 2025, 64, e202422776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, T.; Sun, Z. Citric acid-induced defective MIL-53(Fe/Ce) for efficient amoxicillin degradation in electro-Fenton system. Sep. Purif. Technol. 2025, 363, 131993. [Google Scholar] [CrossRef]
- Qin, Z.; Wu, R.; Gao, S.; Tai, R.; Li, P.; Sui, X.; Song, X.; Wang, Q.; Chen, S. Photocatalytic degradation of pollutants by self-Fenton reaction on defective NH2-MIL-88B with on-site production of hydrogen peroxide. Appl. Surf. Sci. 2025, 695, 162870. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Li, Z.; Liu, A.; Wang, E.; Wu, Y.; Tang, X.; Du, H.; Jin, L.; Zhu, H.; et al. Efficient photocatalytic degradation of antibiotics using Z-scheme MIL-88(Fe)/Ti3C2/MoO3: Mechanistic insights and toxicity assessment. J. Hazard. Mater. 2025, 486, 137051. [Google Scholar] [CrossRef]
- Mi, X.; Zhou, X.; Zhan, T.; Chen, Y.; Ma, N.; Dai, W. Interfacial engineering-mediated electronic structure regulation of homologous S-scheme LaFeO3@MIL-100(Fe) heterojunction: Enhancement of photocatalytic activity towards levofloxacin. Sep. Purif. Technol. 2025, 363, 132126. [Google Scholar] [CrossRef]
- Li, Y.; Peng, H.; Li, H.; Ma, Q.; Zhang, X.; Chen, Q.; Li, J.R. Elimination of Trace Tetracycline with Alkyl Modified MIL-101 in Water. Small 2024, 20, e2405436. [Google Scholar] [CrossRef]
- Dai, Q.; Gao, G.; Tang, J.; Jiang, R.; Sun, S.; Ye, Y.; Li, S.; Xie, R.; Zhang, J. The MIL-125(Ti)/Co3O4 towards efficiently removing tetracycline by synergistic adsorption-photocatalysis roles. Mater. Des. 2025, 250, 113608. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.k.; Bouhfid, R. Microwave-assisted synthesis of MIL–53(Fe)/biochar composite from date palm for ciprofloxacin and ofloxacin antibiotics removal. Sep. Purif. Technol. 2023, 308, 122850. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Kaliaguine, S.; Gobara, M.; Elbasuney, S.; Boffito, D.C. Microwave-Assisted Synthesis of the Flexible Iron-based MIL-88B Metal–Organic Framework for Advanced Energetic Systems. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2538–2556. [Google Scholar] [CrossRef]
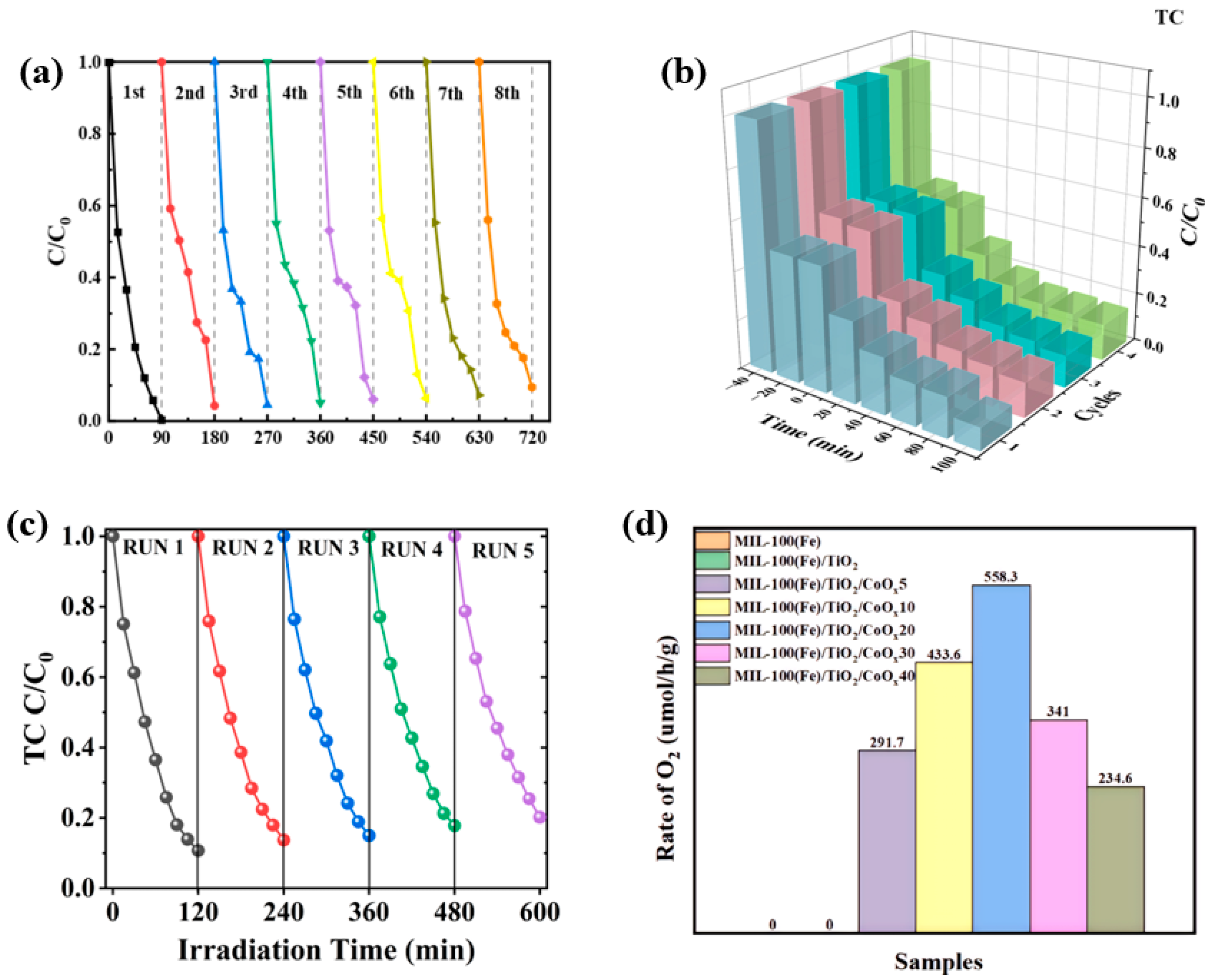
- Hu, W.; Song, Z.; Sun, L.; Zhang, L.; Zhang, Q.; Ren, X.; Li, Y. TiO2/CoOx heterostructure decorated MIL-100(Fe) by atomic layer deposition for enhanced photocatalytic oxygen production. Dalton Trans. 2025, 54, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in situ microwave synthesis of Fe3O4@MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Ramos Corona, A.; Rodríguez López, J.; Rangel Segura, R.; Martínez Garcia, M.M.; Flores, E.; Rodríguez Gattorno, G.; Alvarado Gil, J.J. Microwave-Assisted Synthesis of CdS-MOF MIL-101 (Fe) Composite: Characterization and Photocatalytic Performance. Inorg. Chem. 2024, 63, 19536–19552. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, C.; Li, Y.; Lu, Y.; Huang, N.; Wang, D. Degradation of perfluorooctanoic acid by inductively heated Fenton-like process over the Fe3O4/MIL-101 composite. Chin. Chem. Lett. 2024, 35, 109753. [Google Scholar] [CrossRef]
- Solís, R.R.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Microwave-assisted synthesis of NH2-MIL-125(Ti) for the solar photocatalytic degradation of aqueous emerging pollutants in batch and continuous tests. J. Environ. Chem. Eng. 2021, 9, 106230. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, H.; Feng, D.; Guo, F. Enhanced peroxymonosulfate-activated antibiotic degradation via mechanochemically synthesized cobalt-doped MIL-53(Al). J. Environ. Chem. Eng. 2025, 13, 115356. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Fu, D.; Chang, Y.; Liu, K.; Cui, S.; Li, Y.; Li, B.; Wei, S.; Li, D.; et al. Enhanced nonradical oxidation of thiocyanate by nano γ-Fe2O3 and Fe3C-modified 2D hierarchical porous carbon nanosheets. J. Environ. Chem. Eng. 2025, 13, 115810. [Google Scholar] [CrossRef]
- Hidayat, D.; Lestari, W.W.; Dendy, D.; Khoerunnisa, F.; Handayani, M.; Sanjaya, E.H.; Gunawan, T. Adsorption Studies of Anionic and Cationic Dyes on MIL-100(Cr) Synthesized Using Facile and Green Mechanochemical Method. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1548–1561. [Google Scholar] [CrossRef]
- Souza, B.E.; Tan, J.-C. Mechanochemical approaches towards the in situ confinement of 5-FU anti-cancer drug within MIL-100 (Fe) metal–organic framework. CrystEngComm 2020, 22, 4526–4530. [Google Scholar] [CrossRef]
- Kuzharov, A.A.; Gritsai, M.A.; Butova, V.V.; Soldatov, M.A.; Polyakov, V.A.; Rud, P.A.; Rusalev, Y.V.; Kubrin, S.P.; Roldugin, V.A.; Trigub, A.L.; et al. One-step electrochemical synthesis of γ-Fe2O3@MIL-88a magnetic composite for heterogeneous Fenton-like catalysis. Ceram. Int. 2022, 48, 34864–34876. [Google Scholar] [CrossRef]
- Antonio, A.M.; Rosenthal, J.; Bloch, E.D. Electrochemically Mediated Syntheses of Titanium(III)-Based Metal–Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 11383–11387. [Google Scholar] [CrossRef] [PubMed]
- Larasati, L.; Dendy, D.; Lestari, W.W.; Suharbiansah, R.S.R.; Firdaus, M.; Masykur, A.; Wibowo, F.R. Rapid and Facile Electrochemical Synthesis of MIL-101(Fe)-NH2 and Its Curcumin Loading and Release Studies. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4039–4049. [Google Scholar] [CrossRef]
- Pangestu, A.; Lestari, W.W.; Wibowo, F.R.; Larasati, L. Green Electro-Synthesized MIL-101(Fe) and Its Aspirin Detoxification Performance Compared to MOF-808. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1828–1839. [Google Scholar] [CrossRef]
- Wu, W.; Decker, G.E.; Weaver, A.E.; Arnoff, A.I.; Bloch, E.D.; Rosenthal, J. Facile and Rapid Room-Temperature Electrosynthesis and Controlled Surface Growth of Fe-MIL-101 and Fe-MIL-101-NH2. ACS Cent. Sci. 2021, 7, 1427–1433. [Google Scholar] [CrossRef]
- Xuemin, W.; Longxue, L.; Ling, L.; Zihan, Y.; Hongxu, G.; Chen, Z. Ultrasound Assisted Synthesis of Nanoscale NH2-MIL-53(Fe) for the Adsorption of Dye. Chin. J. Struct. Chem. 2021, 40, 42–46. [Google Scholar] [CrossRef]
- Abbasian, M.; Khayyatalimohammadi, M. Ultrasound-assisted synthesis of MIL-88(Fe) conjugated starch-Fe3O4 nanocomposite: A safe antibacterial carrier for controlled release of tetracycline. Int. J. Biol. Macromol. 2023, 234, 123665. [Google Scholar] [CrossRef]
- Shiri, P.; Cui, H.; Zhang, L. Sustainable process intensification: Ultrasonic preparation of MIL-88A for benzoxazole synthesis. Chem. Eng. Process.-Process Intensif. 2024, 201, 109797. [Google Scholar] [CrossRef]
- Le, B.T.; La, D.D.; Nguyen, P.T.H. Ultrasonic-Assisted Fabrication of MIL-100(Fe) Metal–Organic Frameworks as a Carrier for the Controlled Delivery of the Chloroquine Drug. ACS Omega 2022, 8, 1262–1270. [Google Scholar] [CrossRef]
- Mirzaei, D.; Zabardasti, A.; Sadeghi, M.; Yekta, S. Synthesis of the Novel ZSM-5/NiO/MIL-101(Cr) Zeolite Catalyst Nanocomposite and Its Performance for the Sonodegradation of Organic Dyes in Aqueous Solutions. J. Inorg. Organomet. Polym. Mater. 2021, 31, 960–977. [Google Scholar] [CrossRef]
- Al Amery, N.; Abid, H.R.; Al-Saadi, S.; Wang, S.; Liu, S. Facile directions for synthesis, modification and activation of MOFs. Mater. Today Chem. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Valekar, A.H.; Lee, S.-K.; Kim, Y.K.; Cho, K.H.; Jo, D.; Hwang, Y.K.; Yoon, J.W.; Lee, U.H. Facile shaping of flexible MIL-53(Al) for effective separation of propylene over propane. Chem. Eng. J. 2024, 480, 147872. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yu, X.; Shen, X.; Xu, L.; Zhang, Y.; Shi, J.; Gan, L. Alginate/MIL-88B(Fe) derived magnetic biochar bead for non-radical degradation of organic pollutant with low peroxymonosulfate consumption. Chem. Eng. J. 2024, 502, 157892. [Google Scholar] [CrossRef]
- Dapaah, M.F.; Niu, Q.; Yu, Y.-y.; Jia, H.; Cheng, L. Tunning MIL-101(Cr) MOF polymorphism towards efficient adsorption and localized Fenton-like degradation of para-nitroaniline by Fe incorporation. Chem. Eng. J. 2024, 486, 150162. [Google Scholar] [CrossRef]
- An, Y.; Lv, X.; Jiang, W.; Wang, L.; Shi, Y.; Hang, X.; Pang, H. The stability of MOFs in aqueous solutions—Research progress and prospects. Green Chem. Eng. 2024, 5, 187–204. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Teng, C.; Musho, T.D.; van de Burgt, L.; Lochner, E.; Heller, W.T.; Strouse, G.F.; Dudley, G.B.; Stiegman, A.E. Direct Measurement of the Selective Microwave-Induced Heating of Agglomerates of Dipolar Molecules: The Origin of and Parameters Controlling a Microwave Specific Superheating Effect. J. Phys. Chem. B 2021, 125, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Choi, J.Y.; Lu, C.; Reichert, E.; Pham, H.T.B.; Park, J. From 0D to 2D: Microwave-assisted Synthesis of Electrically Conductive Metal-Organic Frameworks with Controlled Morphologies. Chem. Sci. 2025, 16, 3168–3172. [Google Scholar] [CrossRef]
- Wang, H.-C.; Liu, X.; Ma, J.-G.; Cheng, P. Rapid and high-throughput synthesis of diverse MOFs with centrifuge tube grinding strategy. Green Chem. 2024, 26, 7312–7319. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Huang, P.; Lei, B.; Qiao, L.; Li, T.; Lin, K.-Y.A.; Wang, H. Mechanochemical synthesis of biochar encapsulated FeMn nanoparticles with strong metal–carbon interactions for efficient degradation of tetracycline via activating peroxymonosulfate. Chem. Eng. J. 2024, 479, 147525. [Google Scholar] [CrossRef]
- Zelenka, T.; Baláž, M.; Férová, M.; Diko, P.; Bednarčík, J.; Királyová, A.; Zauška, Ľ.; Bureš, R.; Sharda, P.; Király, N. The influence of HKUST-1 and MOF-76 hand grinding/mechanical activation on stability, particle size, textural properties and carbon dioxide sorption. Sci. Rep. 2024, 14, 15386. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. Review—Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar] [CrossRef]
- Cao, Y.-J.; Chen, Z.-Y.; Peng, H.-L.; Chen, H.-P.; Xie, C.-F.; Fan, J.-P. One-step electrochemical synthesis of Zn-MOFs/NF composite electrodes for efficient electrosorption of organic dyes. Sep. Purif. Technol. 2025, 360, 131020. [Google Scholar] [CrossRef]
- Sachdeva, S.; Pustovarenko, A.; Sudhölter, E.J.R.; Kapteijn, F.; de Smet, L.C.P.M.; Gascon, J. Control of interpenetration of copper-based MOFs on supported surfaces by electrochemical synthesis. CrystEngComm 2016, 18, 4018–4022. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.; Boudewijns, T.; Denayer, J.; Binnemans, K.; De Vos, D.; Fransaer, J. High pressure, high temperature electrochemical synthesis of metal–organic frameworks: Films of MIL-100 (Fe) and HKUST-1 in different morphologies. J. Mater. Chem. A 2013, 1, 5827–5830. [Google Scholar] [CrossRef]
- He, G.; Dakhchoune, M.; Zhao, J.; Huang, S.; Agrawal, K.V. Electrophoretic Nuclei Assembly for Crystallization of High-Performance Membranes on Unmodified Supports. Adv. Funct. Mater. 2018, 28, 1707427. [Google Scholar] [CrossRef]
- Al-Kutubi, H.; Gascon, J.; Sudhölter, E.J.R.; Rassaei, L. Electrosynthesis of Metal–Organic Frameworks: Challenges and Opportunities. ChemElectroChem 2015, 2, 462–474. [Google Scholar] [CrossRef]
- Ansari, K.R.; Singh, A.; Younas, M.; Ali, I.H.; Lin, Y. Progress in metal-organic frameworks (MOFs) as multifunctional material: Design, synthesis and anticorrosion performance techniques. Coord. Chem. Rev. 2025, 523, 216294. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Davis, T.P. Handbook of Radical Polymerization; Wiley-Interscience: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Applications of ultrasound to the synthesis of nanoscale metal–organic coordination polymers. Coord. Chem. Rev. 2015, 292, 1–14. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Facile preparation of nanocubes zinc-based metal-organic framework by an ultrasound-assisted synthesis method; precursor for the fabrication of zinc oxide octahedral nanostructures. Ultrason. Sonochemistry 2018, 40, 921–928. [Google Scholar] [CrossRef]
- Suslick, K.S. The sonochemical hot spot. J. Acoust. Soc. Am. 2005, 89, 1885–1886. [Google Scholar] [CrossRef]
- Yan, X.-W.; Hakimifar, A.; Bigdeli, F.; Hanifehpour, Y.; Wang, S.-J.; Liu, K.-G.; Morsali, A.; Joo, S.W. Rapid and Selective Sensing of 2,4,6-Trinitrophenol via a Nano-Plate Zn(II)-Based MOF Synthesized by Ultrasound Irradiation. Crystals 2023, 13, 1344. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, Y.; Yu, P.; Wang, X. Ultrasonic-assisted in situ synthesis of MOF-199 on the surface of carboxylated cellulose fibers for efficient adsorption of methylene blue. RSC Adv. 2024, 14, 15095–15105. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Baluk, M.A.; Pieczyńska, A.; Kroczewska, M.; Łuczak, J.; Matus, K.; Nikiforow, K.; Zaleska-Medynska, A. Efficient method for octahedral NH2-MIL-125 (Ti) synthesis: Fast and mild conditions. Chem. Eng. J. 2024, 492, 152313. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Zhang, X.; Tang, L.; Wang, L.; Wang, Y.; Wu, M. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB. Appl. Catal. B Environ. 2018, 221, 119–128. [Google Scholar] [CrossRef]
- Liu, F.; Cao, J.; Yang, Z.; Xiong, W.; Xu, Z.; Song, P.; Jia, M.; Sun, S.; Zhang, Y.; Zhong, X. Heterogeneous activation of peroxymonosulfate by cobalt-doped MIL-53(Al) for efficient tetracycline degradation in water: Coexistence of radical and non-radical reactions. J. Colloid Interface Sci. 2021, 581, 195–204. [Google Scholar] [CrossRef]
- Yan, J.; Wu, Y.; Huang, M.; Cheng, L.; Pan, Y.; Wu, C.-C.; Yeh, C.-H.; Li, J.-L.; Lin, Y.-D.; Chi, Y.; et al. Iridium(III) Carbene Complexes Featuring Either Metal-to-Ligand Charge Transfer (MLCT) or Through-Space Charge Transfer (TSCT) Blue Luminescence. Angew. Chem. Int. Ed. 2025, 64, e202424694. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Ping, Y.; Zhao, Z.; Guo, D.; Wang, D.; Bu, X. Ru@MIL-125/MnOx metal-organic-framework-based cocatalysts for photocatalytic nitrogen fixation. Appl. Catal. B Environ. Energy 2024, 347, 123781. [Google Scholar] [CrossRef]
- Du, C.; Lv, Y.; Cao, J.; Zhu, H.; Zhang, Y.; Zou, Y.; Peng, H.; Dong, W.; Zhou, L.; Yu, G.; et al. Removal of oxytetracycline from water by S-doped MIL-53(Fe): Synergistic effect of surface adsorption and persulfate activation. Environ. Res. 2023, 239, 116842. [Google Scholar] [CrossRef]
- George, P.; Chaudhari, K.; Chowdhury, P. Influence of cation doping (Li+, Na+, K+) on photocatalytic activity of MIL-53(Fe). J. Mater. Sci. 2018, 53, 11694–11714. [Google Scholar] [CrossRef]
- Jiang, X.; Su, S.; Ren, B.; Qiu, Y.; Wang, S.; Yang, X. Lanthanum-Doped iron MOFs: A sustainable solution for Arsenic(V) and phosphate pollution in water. Sep. Purif. Technol. 2025, 354, 129098. [Google Scholar] [CrossRef]
- Wan, Z.; Xu, X.; Bi, Z.; Jiajia, D.; Li, Y.; Chen, M.; Huang, Z. Gadolinium doping-induced electronic structure optimization of MIL-101-NH2: Efficient adsorption of arsenic (V) and phosphorus and electrochemical regeneration. Sep. Purif. Technol. 2025, 357, 130133. [Google Scholar] [CrossRef]
- Mo, G.; Wang, L.; Luo, J. Controlled thermal treatment of NH2-MIL-125(Ti) for drastically enhanced photocatalytic reduction of Cr(VI). Sep. Purif. Technol. 2021, 277, 119643. [Google Scholar] [CrossRef]
- Thi Nguyen, Q.; Na, J.; Lee, Y.-R.; Baek, K.-Y. Boosting catalytic performance for CO2 cycloaddition under mild condition via amine grafting on MIL-101-SO3H catalyst. J. Environ. Chem. Eng. 2024, 12, 111852. [Google Scholar] [CrossRef]
- Zhao, X.; Han, X.; Li, Z.; Huang, H.; Liu, D.; Zhong, C. Enhanced removal of iodide from water induced by a metal-incorporated porous metal–organic framework. Appl. Surf. Sci. 2015, 351, 760–764. [Google Scholar] [CrossRef]
- Inchongkol, Y.; Saothayanun, T.K.; Adpakpang, K.; Phongsuk, N.; Impeng, S.; Kosasang, S.; Ma, N.; Horike, S.; Bureekaew, S. Tuning Electronic and Proton Transfer Properties on Amino-Functionalized Co-Based MOF for Efficient Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2024, 16, 64638–64645. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Gao, P.; Du, H.; Dong, L. Interfacial Co–O–Fe bonding in novel amorphous NiCo-ZIF@MIL-100 as efficient active sites enabling electrocatalytic water oxidation. J. Mater. Chem. A 2024, 12, 12712–12720. [Google Scholar] [CrossRef]
- Bhuyan, A.; Ahmaruzzaman, M. Design and Development of p-toluenesulfonic acid Functionalized Dual-functional Fe-MOF@SBC Heterogeneous Catalyst for Efficient Biodiesel Production and Photocatalytic Degradation of Metronidazole. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4842–4862. [Google Scholar] [CrossRef]
- Huang, B.; Wang, J.; Xie, D.; Huang, Q.; Wen, D.; Zeng, X.; Lin, D.; Guo, W.; Sun, H.; Xie, F. Surface reconstruction of defect-engineered MIL-88@Fe2O3 p-n heterojunction for enhanced electrocatalytic water and urea oxidation. Chem. Eng. J. 2024, 498, 155006. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, M.; Ma, X.; Xu, L.; Gao, F.; Xiao, J.; Gao, H. Interfacial electronic modulation of CoP-CoO p-p type heterojunction for enhancing oxygen evolution reaction. J. Colloid Interface Sci. 2022, 607, 1343–1352. [Google Scholar] [CrossRef]
- Yang, J.; Liu, T.; Zhou, H.; Cao, W.; Chen, C.; He, X.; Jiang, C.; Li, Y.; Wang, Y. In situ conversion of typical type-I MIL-125(Ti)/BiOBr into type-II heterostructure photocatalyst via MOF self-sacrifice: Photocatalytic mechanism and theoretical study. J. Alloys Compd. 2022, 900, 163440. [Google Scholar] [CrossRef]
- Humayun, M.; Shu, L.; Pi, W.; Xia, H.; Khan, A.; Zheng, Z.; Fu, Q.; Tian, Y.; Luo, W. Vertically grown CeO2 and TiO2 nanoparticles over the MIL53Fe MOF as proper band alignments for efficient H2 generation and 2,4-DCP degradation. Environ. Sci. Pollut. Res. 2022, 29, 34861–34873. [Google Scholar] [CrossRef]
- Li, R.; Nie, J.-H.; Xian, J.-J.; Zhou, J.-W.; Lu, Y.; Miao, M.-P.; Zhang, W.-H.; Fu, Y.-S. Planar Heterojunction of Ultrathin CrTe3 and CrTe2 van der Waals Magnet. ACS Nano 2022, 16, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, L.; Cai, X.; Zheng, X.; Liu, W.; Wang, G.; Tan, D.; Luo, X.; Dong, M. Heterogeneous structures and morphological transitions of composite materials and its applications. Adv. Compos. Hybrid Mater. 2024, 7, 251. [Google Scholar] [CrossRef]
- Lin, Z.-F.; Wang, T.-H.; Venkatesan, P.; Doong, R.-A. Indirect Z-scheme MXene@g-C3N4/MIL-101(Fe) heterojunction for the enhanced visible-light-responsive enrofloxacin photodegradation. Chem. Eng. J. 2025, 505, 159411. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Q.; Wang, P.; Wang, E.; Chen, S.; Shi, Y.; Deng, H.; Liu, A.; Du, H.; Li, Z.; et al. S-scheme POM/MIL-101(Fe) heterojunction for photocatalytic decontamination of Cr(VI). Sep. Purif. Technol. 2025, 361, 131498. [Google Scholar] [CrossRef]
- Xue, B.; Yang, C.; Guo, P.; Wang, T.; Li, G.; Liu, D. The type II MIL-100/BiOBr heterojunctions promote efficient photo-Fenton degradation of tetracycline hydrochloride. Appl. Surf. Sci. 2025, 689, 162552. [Google Scholar] [CrossRef]
- Lu, H.; Deng, C.; Yu, Z.; Zhang, D.; Li, W.; Huang, J.; Bao, T.; Liu, X. Synergistic degradation of fluorene in soil by dielectric barrier discharge plasma combined with P25/NH2-MIL-125(Ti). Chemosphere 2022, 296, 133950. [Google Scholar] [CrossRef]
- Hou, L.; Li, W.; Wu, Z.; Wei, Q.; Yang, H.; Jiang, Y.; Wang, T.; Wang, Y.; He, Q. Embedding ZnCdS@ZnIn2S4 into thiazole-modified g-C3N4 by electrostatic self-assembly to build dual Z-scheme heterojunction with spatially separated active centers for photocatalytic H2 evolution and ofloxacin degradation. Sep. Purif. Technol. 2022, 290, 120858. [Google Scholar] [CrossRef]
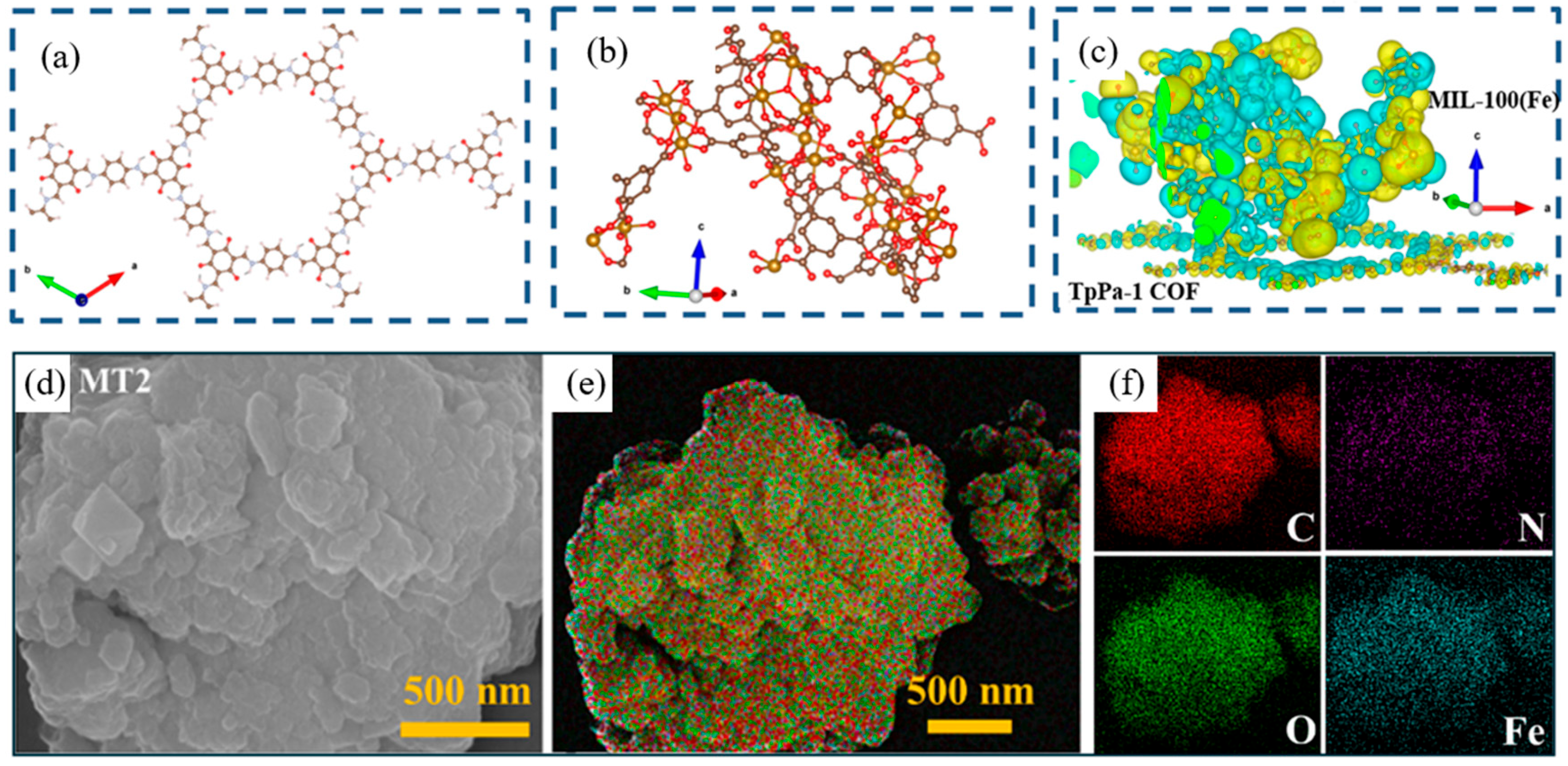
- Li, Q.; Deng, M.; Gao, J.; Liu, A.; Wang, Q.; Wang, E.; Li, Z.; Zhu, H.; Tang, X. Rational design of a novel MIL-100(Fe)/TpPa-1 COF direct Z-scheme heterojunction for photo-self-Fenton removal of antibiotics: Performance and ecotoxicity assessment. Sep. Purif. Technol. 2025, 362, 131722. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Dong, W.; Du, C.; Zhang, Y.; Cao, J.; Jiang, J.; Zhou, L.; Yu, G.; Zou, Y.; Peng, H.; Yan, R.; et al. Enhanced carrier separation and photocatalytic degradation of oxytetracycline via S-scheme MIL-53(Fe)/FeOCl heterojunction composites with peroxydisulfate activation. J. Environ. Chem. Eng. 2025, 13, 115024. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Wu, Y.; Shi, Y.; Liu, Y.; Deng, H.; Chen, S.; Li, Z.; Wang, E.; Zhu, H.; et al. Dual S-scheme heterojunction via MOF-on-MOF strategy for efficient photoelectrocatalytic removal of organic contaminants: Detoxification and mechanism. J. Environ. Sci. 2025, 155, 111–126. [Google Scholar] [CrossRef]
- Du, J.; Ahmad, I.; Ashraf, I.M.; Ahmed, F.B.M.; Aslam, A.; Ali, I.; Mohammad, A.; Khasawneh, M.A. Advancements in dual S-scheme heterojunction systems for photocatalytic applications: A mini-review. Int. J. Hydrogen Energy 2025, 100, 1361–1384. [Google Scholar] [CrossRef]
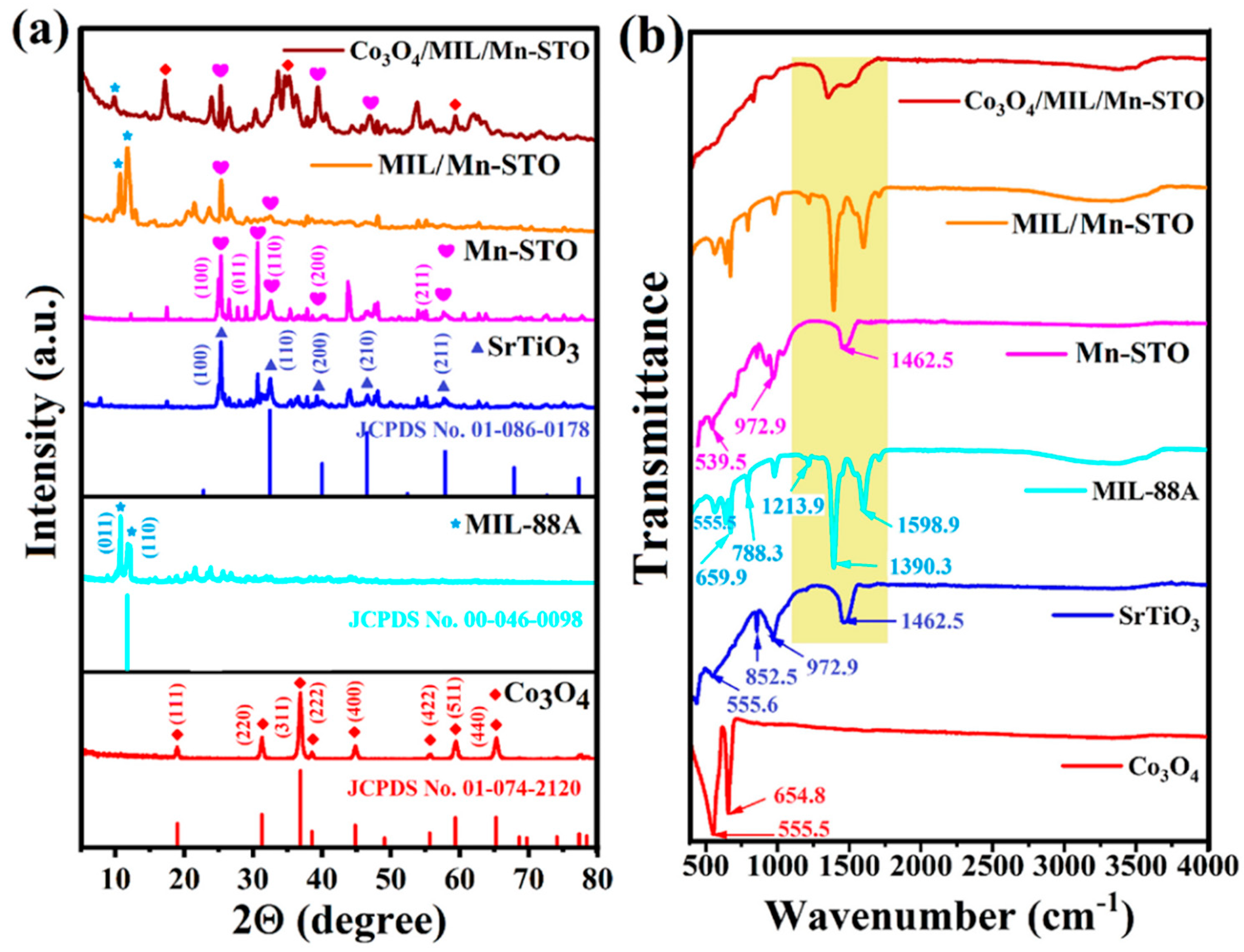
- Patial, S.; Sudhaik, A.; Sonu; Thakur, S.; Van Le, Q.; Ahamad, T.; Singh, P.; Huang, C.-W.; Nguyen, V.-H.; Raizada, P. Synergistic interface engineering in n-p-n type heterojunction Co3O4/MIL/Mn-STO with dual S-scheme multi-charge migration to enhance visible-light photocatalytic degradation of antibiotics. Environ. Res. 2024, 240, 117481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xing, Z.; Su, S.; Song, S.; Li, Z.; Zhou, W. Gear-shaped mesoporous NH2-MIL-53(Al)/CdS P-N heterojunctions as efficient visible-light-driven photocatalysts. Appl. Catal. B Environ. 2021, 291, 120106. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, L.; Huang, S.; Niu, J.; Zhang, Y.; Liao, J.; Dong, G.; Song, D.; Zhou, Q. Metal-organic framework functionalized magnetic Nb2CTX for high enrichment of polychlorinated biphenyls in water prior to gas chromatography tandem mass spectrometry. J. Chromatogr. A 2025, 1740, 465560. [Google Scholar] [CrossRef]
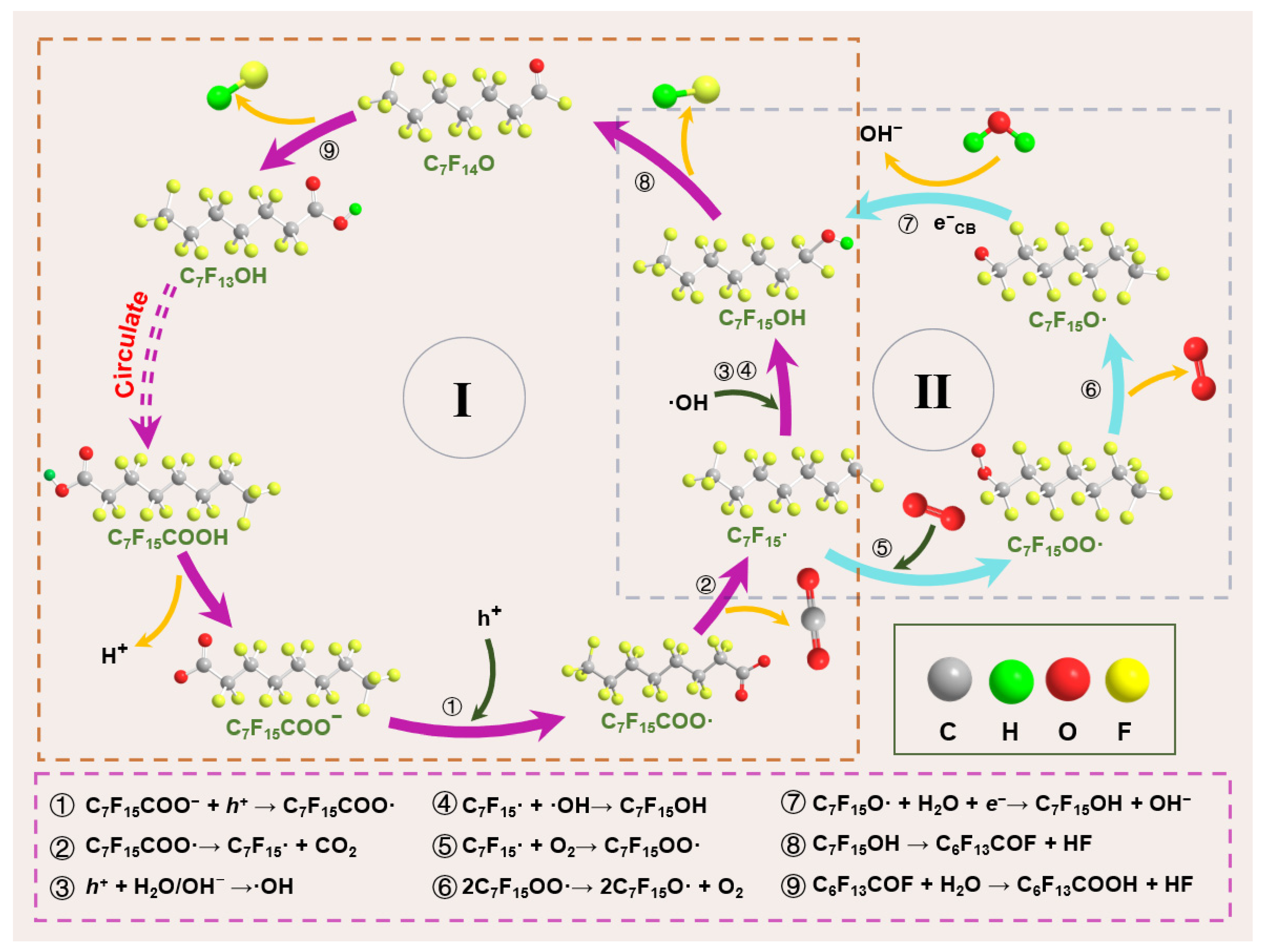
- Su, P.; Zhang, C.; Liu, Y.; Zhang, J.; Djellabi, R.; Wang, R.; Guo, J.; Zhang, R.; Guo, H.; Ding, X.; et al. Boosting PFOA photocatalytic removal from water using highly adsorptive and sunlight-responsive ZIF67/MIL-100(Fe) modified C3N4. J. Environ. Chem. Eng. 2023, 11, 110765. [Google Scholar] [CrossRef]
- Ismail, M.A.; Taki, A.G.; Kumar, S.; Sammen, S.S.; Amari, A.; Bongale, A.; Kisi, O.; Salem, A. Effectiveness of waste-derived MIL type MOFs in removing PFOA and PFAS pollutants for environmental remediation. Sci. Rep. 2025, 15, 9439. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Al-bonayan, A.M.; Alsharief, H.H.; Aljohani, M.; Alshammari, K.F.; Saad, F.A.; Abumelha, H.M.; El-Metwaly, N.M. Efficient removal of carbofuran by sono-photo active CdS@MIL based Ti Framework. Surf. Interfaces 2023, 40, 103133. [Google Scholar] [CrossRef]
- Wen, Y.; Rentería-Gómez, Á.; Day, G.S.; Smith, M.F.; Yan, T.-H.; Ozdemir, R.O.K.; Gutierrez, O.; Sharma, V.K.; Ma, X.; Zhou, H.-C. Integrated Photocatalytic Reduction and Oxidation of Perfluorooctanoic Acid by Metal–Organic Frameworks: Key Insights into the Degradation Mechanisms. J. Am. Chem. Soc. 2022, 144, 11840–11850. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, A.; Bhuiyan, M.A.; Pramanik, B.K. A hybrid LMO MOF catalytic membrane with PMS activation for efficient degradation of pharmaceutical micropollutants and nanoplastics removal. Sep. Purif. Technol. 2025, 360, 130961. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Tseng, Y.-S.; Nguyen, T.-B.; Chen, C.-W.; Bui, X.-T.; Dong, C.-D. Z-scheme TiO2 derived from MIL-125(Ti)/g-C3N5 heterojunction for enhanced photocatalytic degradation of sulfadiazine via peroxymonosulfate activation. J. Environ. Chem. Eng. 2025, 13, 115910. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, T.; Wang, J.; Yu, T.; Kong, C.; Yang, Z.; Zhu, H. Oxygen vacancy-rich BiVO4 modified with mesoporous MIL-88A(Fe) Z-scheme heterojunction for enhanced photocatalytic formaldehyde degradation. Sep. Purif. Technol. 2025, 353, 128581. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, M.; Fan, J.; Feng, R.; Zhou, Y.; Zhang, L. TiO2@MIL-101(Cr) nanocomposites as an efficient photocatalyst for degradation of toluene. Adv. Compos. Hybrid Mater. 2021, 4, 1322–1329. [Google Scholar] [CrossRef]
- Zhu, P.; Hu, Z.; Chen, S. Praseodymium-Doped Cr2O3 Prepared by In Situ Pyrolysis of MIL-101(Cr) for Highly Efficient Catalytic Oxidation of 1,2-Dichloroethane. Molecules 2024, 29, 3417. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, K.; Rao, R.; Chen, J.; Liu, Q.; Yang, Y.; Bi, F.; Wang, Y.; Xu, J.; Liu, N. Synthesis of acidic MIL-125 from plastic waste: Significant contribution of N orbital for efficient photocatalytic degradation of chlorobenzene and toluene. Appl. Catal. B Environ. 2022, 310, 121300. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Chen, X.; Chu, H.; Fu, H.; Wang, C.-C. Robust photocatalytic benzene degradation using mesoporous disk-like N-TiO2 derived from MIL-125(Ti). Chin. J. Catal. 2020, 41, 1186–1197. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Song, G.; Lu, G.; Shi, G.; Wang, Y.; Xie, X.; Sun, J. Ti-O-Mo bond-bridged PMA@MIL-125-NH2 photocatalyst for gas acetone photocatalytic degradation. Appl. Catal. B Environ. Energy 2025, 367, 125112. [Google Scholar] [CrossRef]
- Liu, H.; Feng, J.; Wang, X.; Xu, M.; Deng, Y.; Li, G.; Yu, Y.; An, T. Improving photocatalytic activity and chlorine resistance of carbon nanolayer-wrapped TiO2 nanocomposite catalysts for dichloromethane purification. Environ. Sci. Nano 2025, 12, 2486–2494. [Google Scholar] [CrossRef]
- He, L.; Xu, Y.; Yang, Z.; Lu, X.; Yao, X.; Li, C.; Xu, D.; Wu, C.; Yao, Z. Copper-decorated strategy based on defect-rich NH2-MIL-125(Ti) boosts efficient photocatalytic degradation of methyl mercaptan under sunlight. Environ. Pollut. 2024, 344, 123341. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Chen, Z.; Xie, Y.; Yu, H.; Yuan, S.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Rapid magnetization and removal of microplastics from environment and food based on magnetic metal-organic framework Fe3O4@SiO2@MIL-53(Al). Environ. Sci. Pollut. Res. 2023, 30, 117373–117389. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Long, R.; Liu, C.; Liu, X. Visible-light-driven removal of tetracycline hydrochloride and microplastics (HDPE) by nano flower hybrid heterojunction NH2-MIL-88B(Fe)/MoS2 via enhanced electron-transfer. Sep. Purif. Technol. 2022, 302, 122138. [Google Scholar] [CrossRef]
- Rojas-Guerrero, C.A.; Villanueva-Rodríguez, M.; Guzmán-Mar, J.L.; Hernández-Ramírez, A.; Cedillo-González, E.I.; Longoria Rodríguez, F.E.; Hinojosa-Reyes, L. Solar photocatalytic degradation of polyethylene terephthalate nanoplastics: Evaluation of the applicability of the TiO2/MIL-100(Fe) composite material. J. Environ. Chem. Eng. 2023, 11, 110415. [Google Scholar] [CrossRef]
- Gu, X.; Li, L.; Wu, Y.; Dong, W. Enhancement of microplastics degradation with MIL-101 modified BiOI photocatalyst under light and dark alternated system. J. Environ. Chem. Eng. 2024, 12, 112958. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Dong, J.; Chen, S.; Hu, W.; Zhang, Y.; Wang, H.; Li, Z.; Wang, Z. MX@MIL-125(Ti)-mediated sonocatalytic degradation for the dyes and microplastics. Sep. Purif. Technol. 2024, 337, 126488. [Google Scholar] [CrossRef]
- Su, X.; Dong, Y.; Zhu, Y.; Shi, H. MIL-125-NH2/BNQDs persistent photocatalyst enhanced peroxymonosulfate activation for efficient PET plastics removal. Chem. Eng. J. 2024, 501, 157764. [Google Scholar] [CrossRef]
- Wang, Z.; Adu-Kumi, S.; Diamond, M.L.; Guardans, R.; Harner, T.; Harte, A.; Kajiwara, N.; Klánová, J.; Liu, J.; Moreira, E.G.; et al. Enhancing Scientific Support for the Stockholm Convention’s Implementation: An Analysis of Policy Needs for Scientific Evidence. Environ. Sci. Technol. 2022, 56, 2936–2949. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Q.; Lin, S.; Liang, X.; Khan, S.; Yang, X.; Wang, X. Magnetic Z-scheme CuFe2O4/MIL-101(Fe) toward chlorpyrifos degradation: Photocatalytic mechanism, degradation pathways, and intermediates toxicity evaluation. J. Environ. Chem. Eng. 2023, 11, 110054. [Google Scholar] [CrossRef]
- Madaj, R.; Sobiecka, E.; Kalinowska, H. Lindane, kepone and pentachlorobenzene: Chloropesticides banned by Stockholm convention. Int. J. Environ. Sci. Technol. 2018, 15, 471–480. [Google Scholar] [CrossRef]
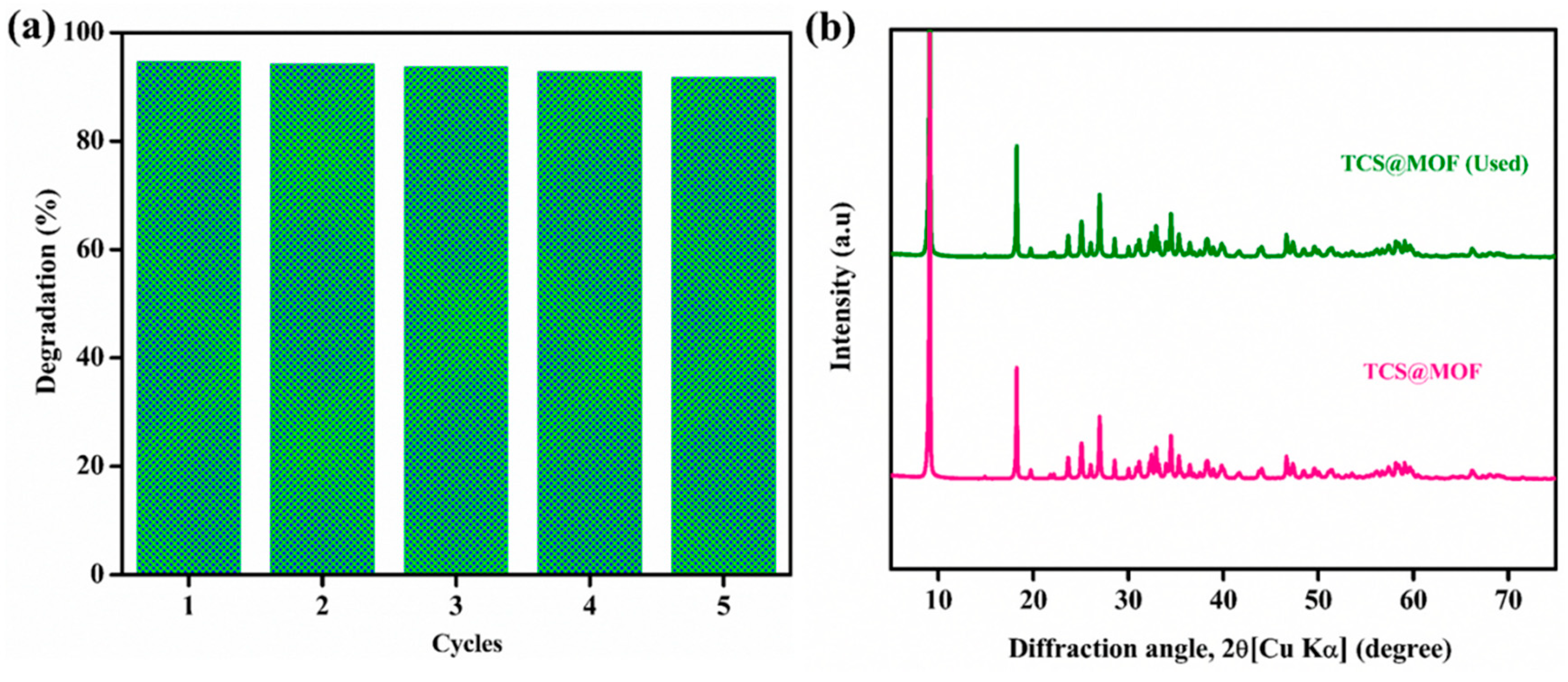
- Keshta, B.E.; Yu, H.; Wang, L. MIL series-based MOFs as effective adsorbents for removing hazardous organic pollutants from water. Sep. Purif. Technol. 2023, 322, 124301. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Darwesh, O.M.; El-Shahat, M. Titanium-based metal-organic framework capsulated with magnetic nanoparticles: Antimicrobial and photocatalytic degradation of pesticides. Microporous Mesoporous Mater. 2023, 354, 112543. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Nikitha, M.; Ramkumar, K.; Meenakshi, S. Immobilization of MIL-88(Fe) anchored TiO2-chitosan(2D/2D) hybrid nanocomposite for the degradation of organophosphate pesticide: Characterization, mechanism and degradation intermediates. J. Hazard. Mater. 2021, 406, 124728. [Google Scholar] [CrossRef]
- Saini, K.; Singh, J.; Malik, S.; Saharan, Y.; Goyat, R.; Umar, A.; Akbar, S.; Ibrahim, A.A.; Baskoutas, S. Metal-Organic Frameworks: A promising solution for efficient removal of heavy metal ions and organic pollutants from industrial wastewater. J. Mol. Liq. 2024, 399, 124365. [Google Scholar] [CrossRef]
- Schlummer, M.; Sölch, C.; Meisel, T.; Still, M.; Gruber, L.; Wolz, G. Emission of perfluoroalkyl carboxylic acids (PFCA) from heated surfaces made of polytetrafluoroethylene (PTFE) applied in food contact materials and consumer products. Chemosphere 2015, 129, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, J.; Xiong, W.; Yang, Z.; Sun, S.; Jia, M.; Xu, Z. In-situ growing of metal-organic frameworks on three-dimensional iron network as an efficient adsorbent for antibiotics removal. Chem. Eng. J. 2020, 392, 124844. [Google Scholar] [CrossRef]
- Kong, Z.; Lu, L.; Zhu, C.; Xu, J.; Fang, Q.; Liu, R.; Shen, Y. Enhanced adsorption and photocatalytic removal of PFOA from water by F-functionalized MOF with in-situ-growth TiO2: Regulation of electron density and bandgap. Sep. Purif. Technol. 2022, 297, 121449. [Google Scholar] [CrossRef]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Eberle, D.; Ball, R.; Boving, T.B. Impact of ISCO treatment on PFAA co-contaminants at a former fire training area. Environ. Sci. Technol. 2017, 51, 5127–5136. [Google Scholar] [CrossRef]
- Babalar, M.; Siddiqua, S.; McIntyre, L.; Ellenor, D.; Usakiewicz, J. Nanoporous dopamine/β-cyclodextrin PES-PMACZ/MOF modified membrane for high-efficiency, low-fouling extraction of microplastics and PCB 209 from synthetic landfill leachate. J. Hazard. Mater. Adv. 2025, 18, 100637. [Google Scholar] [CrossRef]
- Shi, X.; Ren, B.; Jin, X.; Wang, X.C.; Jin, P. Metabolic hazards of pharmaceuticals and personal care products (PPCPs) in sewers. J. Hazard. Mater. 2022, 432, 128539. [Google Scholar] [CrossRef]
- Zhichang, D.; Chunyan, S.; Xiangrui, Z.; Wensong, L.; Xue-Rong, S. The Application of Metal-Organic Frameworks in the Adsorptive Removal of Harmful Species from Aqueous Solutions. Mini-Rev. Org. Chem. 2023, 20, 227–239. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Vinesh, V.; Sabarikirishwaran, P.; Rajkamal, A.; Ashokkumar, M.; Neppolian, B. Investigating the role of ultrasound in improving the photocatalytic ability of CQD decorated boron-doped g-C3N4 for tetracycline degradation and first-principles study of nitrogen-vacancy formation. Carbon 2022, 192, 405–417. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Song, M.; Gu, Z.-Y.; Wang, H.-F.; Yan, X.-P. Probing the Adsorption Characteristic of Metal–Organic Framework MIL-101 for Volatile Organic Compounds by Quartz Crystal Microbalance. Environ. Sci. Technol. 2011, 45, 4490–4496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nie, J.; Huang, H.; He, F.; Wang, K.; Yang, P. Experimental Study on the Adsorption Performance of Metal–Organic Framework MIL-101 (Cr) for Indoor Toluene. Buildings 2025, 15, 2506. [Google Scholar] [CrossRef]
- Montano, L.; Baldini, G.M.; Piscopo, M.; Liguori, G.; Lombardi, R.; Ricciardi, M.; Esposito, G.; Pinto, G.; Fontanarosa, C.; Spinelli, M.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in the Environment: Occupational Exposure, Health Risks and Fertility Implications. Toxics 2025, 13, 151. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, J.; Muhammad, Y.; Hu, P.; Hu, Y.; Chu, Z.; Zhao, Z.; Zhao, Z. Hydrophobic shell structured NH2-MIL(Ti)-125@mesoporous carbon composite via confined growth strategy for ultra-high selective adsorption of toluene under highly humid environment. Chem. Eng. J. 2022, 432, 134340. [Google Scholar] [CrossRef]
- Tu, T.N.; Pham, T.M.; Nguyen, Q.H.; Tran, N.T.; Le, V.N.; Ngo, L.H.; Chang, K.; Kim, J. Metal–organic frameworks for aromatic-based VOC capture. Sep. Purif. Technol. 2024, 333, 125883. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Lu, G.; Xie, H.; Xie, X.; Sun, J. Improving the removal efficiency of oxygenated volatile organic compounds by defective UiO-66 regulated with water. J. Hazard. Mater. 2024, 469, 134055. [Google Scholar] [CrossRef]
- Yu, X.; Hu, Y.; Luan, X.; Jalil Shah, S.; Liu, L.; Li, C.; Ren, Y.; Zhou, L.; Li, J.; Deng, J.; et al. Microwave-assisted construction of MXene/MOF aerogel via N-metal bonds for efficient photodegradation of vapor acetone under high humidity. Chem. Eng. J. 2023, 476, 146878. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Wang, W.-N.; Chen, S.-C. A Novel Sustainable Semiconductor/Metal-organic Framework Coated Electret Filter for Simultaneous Removal of PM2.5 and VOCs. Aerosol Air Qual. Res. 2023, 23, 220445. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, J.; Ge, S.; Tian, L. Enhanced gaseous benzene degradation by bimetallic MIL-101(Fe, Cu) activated persulfate system: Efficiency and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135785. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Deng, J.; Jing, L.; Wang, Z.; Wei, L.; Wei, Z.; Hou, Z.; Tao, J.; Dai, H. The Advancement of Supported Bimetallic Catalysts for the Elimination of Chlorinated Volatile Organic Compounds. Catalysts 2024, 14, 531. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, H.; Ren, M.; Zhang, Y.; Li, Y.; Wang, L.; Chen, J. Low-temperature catalytic degradation of chlorinated aromatic hydrocarbons over bimetallic Ce-Zr/UiO-66 catalysts. Chem. Eng. J. 2021, 414, 128782. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Zhao, Y.; Xu, Z.; Fan, S.; Long, H.; Fan, Q.; Duan, Z.; Zhang, J.; Jin, Z. The anodic chlorine ion repelling mechanisms of Fe/Co/Ni-based nanocatalysts for seawater electrolytic hydrogen production. Nano Energy 2025, 135, 110662. [Google Scholar] [CrossRef]
- Ding, S.; Wu, S.; Wang, P.; Fang, N.; Zhang, Q.; Li, S.; Chu, Y. Structure-selectivity relevance of multiple-component catalysts for CVOCs’ complete oxidation: State-of-the-art and perspectives. Sep. Purif. Technol. 2025, 354, 128964. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Q.; Su, G.; Pang, J.; Sun, B.; Meng, J.; Shi, B. Catalytic degradation of chlorinated volatile organic compounds (CVOCs) over Ce-Mn-Ti composite oxide catalysts. J. Environ. Sci. 2024, 138, 326–338. [Google Scholar] [CrossRef]
- Starkova, J.E.; Borisov, R.S.; Kanateva, A.Y. Recent Advances in the Detection of Sulfur Compounds in Crude Oil and Petroleum Products. J. Anal. Chem. 2024, 79, 2005–2022. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, J.; Liu, Z. Study on adsorption of low-concentration methyl mercaptan by starch-based activated carbon. Chemosphere 2022, 302, 134901. [Google Scholar] [CrossRef]
- McNamara, N.D.; Neumann, G.T.; Masko, E.T.; Urban, J.A.; Hicks, J.C. Catalytic performance and stability of (V) MIL-47 and (Ti) MIL-125 in the oxidative desulfurization of heterocyclic aromatic sulfur compounds. J. Catal. 2013, 305, 217–226. [Google Scholar] [CrossRef]
- Lv, M.; Jiang, B.; Xing, Y.; Ya, H.; Zhang, T.; Wang, X. Recent advances in the breakdown of microplastics: Strategies and future prospectives. Environ. Sci. Pollut. Res. 2022, 29, 65887–65903. [Google Scholar] [CrossRef]
- Zhang, D.; Xing, Y.; Wang, X.; Li, W.; Guo, Y.; Tang, Y.; Zhang, H.; Chen, J.; Jiang, B. The effect of polyvinyl chloride microplastics on soil properties, greenhouse gas emission, and element cycling-related genes: Roles of soil bacterial communities and correlation analysis. J. Hazard. Mater. 2024, 480, 136248. [Google Scholar] [CrossRef]
- Peller, J.R.; Mezyk, S.P.; Shidler, S.; Castleman, J.; Kaiser, S.; Faulkner, R.F.; Pilgrim, C.D.; Wilson, A.; Martens, S.; Horne, G.P. Facile nanoplastics formation from macro and microplastics in aqueous media. Environ. Pollut. 2022, 313, 120171. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Z.; Sun, L.; Sun, Y.; Yang, S.; Qin, Q.; Xue, Y. The bridging role of soil organic carbon in regulating bacterial community by microplastic pollution: Evidence from different microplastic additions. J. Hazard. Mater. 2025, 490, 137761. [Google Scholar] [CrossRef]
- Sharma, V.K.; Ma, X.; Lichtfouse, E.; Robert, D. Nanoplastics are potentially more dangerous than microplastics. Environ. Chem. Lett. 2023, 21, 1933–1936. [Google Scholar] [CrossRef]
- Morreale, M.; La Mantia, F.P. Current Concerns about Microplastics and Nanoplastics: A Brief Overview. Polymers 2024, 16, 1525. [Google Scholar] [CrossRef]
| MOF | The 3D Structure | Ligand | Central Metal Atom | Typical Shape | Ref. |
|---|---|---|---|---|---|
| MIL-53 |  | Terephthalic acid | Fe, Al, Cr | Rod-shaped, rectangular cone-like | [21] |
| MIL-88 |  | Fumarate, terephthalic acid | Fe | Fusiform, hexagonal rod-shaped | [22] |
| MIL-100 |  | Tricarboxylic benzene, trimesic acid | Fe, Al, Cr | Octahedron | [23] |
| MIL-101 |  | Terephthalic acid | Cr, Fe | Octahedron | [24] |
| MIL-125 |  | Terephthalic acid | Ti | Disc/cake, octahedron | [25] |
| Synthesis Method | MIL | Metal Source | Ligand | Synthesis Conditions | Material Properties | Number of Experimental Cycles | Modification Method | Ref |
|---|---|---|---|---|---|---|---|---|
| Solvent synthesis/Hydrothermal synthesis | MIL-53 | Cr(NO3)3·9H2O | H2bdc | 220 °C, 72 h, acidification by adding 40% HF | Specific surface area of 1050 m2/g | Five times | / | [42] |
| MIL-53 | FeCl3·6H2O, Ce(NO3)3·6H2O | H2bdc | 140 °C, 24 h, 10% citric acid instead of DMF | Specific surface area > 1000 m2/g | Eight times | Carbon-based material loading | [43] | |
| MIL-88B | FeCl3·6H2O | H2bdc | 100 °C, 12 h, add NaOH solution to adjust the Ph of the solution | Specific surface area of 35.05 m2/g | Four times | Amino functionalization | [44] | |
| MIL-88A | FeCl3·6H2O | Fumarate | 65 °C, 12 h | / | Five times | Z-type heterojunction | [45] | |
| MIL-100 | FeCl3·6H2O | H3BTC | 95 °C, 18 h | / | Five times | S-type heterojunction | [46] | |
| MIL-101 | Cr(NO3)2 | H2bdc | 210 °C, 10 h | / | / | Postsynthetic modifier | [47] | |
| MIL-125 | C16H36O4Ti | H2bdc | 150 °C, 20 h | Specific surface area of 943.9 m2/g | Five times | Z-type heterojunction | [48] | |
| Microwave-assisted synthesis | MIL-53 | FeCl3·6H2O | H2bdc | 150 °C, 30 min, 300 W | Specific surface area of 96.33 m2/g | Five times | Carbon-based material load | [49] |
| MIL-88B | FeCl3·6H2O | H2bdc | 150 °C, 10 min, 800 W | Specific surface area of 47 m2/g | Four times | / | [50] | |
| MIL-100 | FeCl3·6H2O | H3BTC | 150 °C, 15 min, 800 W | Specific surface area of 487 m2/g | Twenty times | Z-type heterojunction | [51] | |
| MIL-100 | FeCl3·6H2O | H3BTC | 150 °C, 30 min, 300 W | Specific surface area of 1244.62 m2/g | / | Composite modified | [52] | |
| MIL-101 | FeCl3·6H2O | H2bdc | 200 °C, 50 min, 400 W | / | Four times | Type I heterozygous junction | [53] | |
| MIL-101 | FeCl3·6H2O | H2bdc | 200 °C, 50 min, 600 W | Specific surface area of 960.3 m2/g | Four times | Composite modified | [54] | |
| MIL-125 | C12H28O4Ti | H2bdc | 200 °C, 15 min, 600 W | Specific surface area of 1030 m2/g | Four times | Amino functionalization | [55] | |
| Mechanical grinding synthesis | MIL-53 | Al2(SO4)3·18H2O | H2bdc | Ball milling frequency 30 Hz, ball milling time 1 h | Specific surface area of 1143 m2/g | / | / | [21] |
| MIL-53 | AlCl3·6H2O | H2bdc | Ball milling frequency 30 Hz, ball milling time 1 h | Specific surface area of 638.96 m2/g | Four times | Metal ion doping | [56] | |
| MIL-88A | FeCl3·6H2O | Fumarate | Ball milling at 200 °C for 5 h at a rate of 5 °C/min. | Specific surface area of 723.17 m2/g | Three times | / | [57] | |
| MIL-100 | CrCl3·6H2O | H3BTC | Grinding at room temperature for 40 min, heating at 220 °C for 15 h | Specific surface area of 1557 m2/g | / | / | [58] | |
| MIL-100 | Fe(NO3)3·9H2O | H3BTC | Continuous grinding at room temperature for 3–5 h | Specific surface area of 753 m2/g | / | / | [59] | |
| Electrochemical synthesis | MIL-88A | Iron plate electrode | Fumarate | Mixed solution of 50% H2O and 50% ethanol as electrolyte 15 V voltage, 30 min | Specific surface area of 128.5 m2/g | Three times | Magnetic material load | [60] |
| MIL-100 | TiCl4 | H3TATB | −1.20 V potential, 120 °C, 18 h | Specific surface area of 5842 m2/g | / | / | [61] | |
| MIL-101 | TiCl4 | H2BPDC | −1.20 V potential, 120 °C, 18 h | Specific surface area of 3263 m2/g | / | / | [61] | |
| MIL-101 | FeCl3·6H2O | H2BDC-NH2 | TBATFB electrolyte, 15 V, 30 min | Specific surface area of 139 m2/g | / | Amino functionalization | [62] | |
| MIL-101 | Iron plate electrode | H2BDC | TBATFB electrolyte, 15 V, 30 min | Specific surface area of 131 m2/g | / | / | [63] | |
| MIL-101 | FeCl2 | H2BPDC | +0.75 V potential, room temperature, 8 h | Specific surface area of 1918 m2/g | / | Amino functionalization | [64] | |
| Ultrasound-assisted synthesis | MIL-53 | Fe(NO3)3·9H2O | H2BDC-NH2 | 80 °C, 1 h, 305 W | Specific surface area of 179.9 m2/g | / | Amino functionalization | [65] |
| MIL-88 | Fe(NO3)3·9H2O | H2bdc | Ultrasonic treatment 0.5 h, heating at 85 °C for 12 h | / | / | Magnetic material load | [66] | |
| MIL-88A | FeCl3·6H2O | Fumarate | Room temperature, 4 h, 70% power | / | Three times | / | [67] | |
| MIL-100 | FeCl3·6H2O | H3BTC | 150 °C, 10 min, 1080 W, 20.5 kHz | Specific surface area of 1033 m2/g | / | / | [68] | |
| MIL-101 | Cr(NO3)3·9H2O | H2BDC | Room temperature, 1 h, 100 W, 30 kHz | / | Four times | / | [69] |
| Classification of Contaminants | MIL Names | Synthesis Method | Modification Method | Target Contaminants | Initial Contaminant Concentration | Catalytic Degradation Times | Catalytic Degradation Temperature | Efficiency | Environmental Adaptation | Cycling Stability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| POPs | NH2-MIL-53(Al)/CdS | Hydrothermal synthesis | Amino functionalization, heterojunction | Trichlorophenol | 10 mg/L | 180 min | Room temperature | 98.85% | Stable at 410 °C | Five times | [127] |
| Fe3O4@Nb2CTX@NH2–MIL-88 | Solvothermal synthesis | Amino functionalization, heterojunction | Polychlorinated biphenyl | 0.005–50 μg/L | 60 min | 25 °C | 87.60% | / | / | [128] | |
| MIL-100(Fe)@C3N4 | Hydrothermal synthesis | Heterojunction | Perfluorooctanoic acid | 10–150 μg/L | 6 h | Room temperature | 60.50% | / | / | [129] | |
| NH2-MIL-101(Cr) | Solvothermal synthesis | Amino functionalization | Perfluorooctanoic acid | 10–50 mg/L | / | / | 698.4 mg/g | / | Seven times | [130] | |
| CdS@NH2-MIL-125 | Solvothermal synthesis | Amino functionalization, heterojunction | Carbofuran | 10 g/L | 90 min | Room temperature | 98.40% | / | Five times | [131] | |
| NH2-MIL-125 | Hydrothermal synthesis | Amino functionalization | Perfluorooctanoic acid | 100 μg/L | 24 h | 20 °C | 98.9% | / | Three times | [132] | |
| PPCPs | MIL-53(Fe) | Microwave-assisted synthesis | Carbon material loads | Ciprofloxacin | 50–150 mg/L | 60 min | 25 °C | 98.53% | The effect is less in conditions where inorganic ions are present | Five times | [49] |
| Co-MIL-53(Al) | Mechanical grinding synthesis | Doping of elements | Tetracycline hydrochloride | 10–120 mg/L | 30 min | 25 °C | 96.10% | Less effect in the presence of inorganic ions | Four times | [56] | |
| MIL-88A | Ultrasound-assisted synthesis | / | Benzoxazole | / | 1 h | 25 °C | 84%~96% | / | / | [67] | |
| MIL-88A(Fe)/Ti3C2/MoO3 | Solvothermal synthesis | Heterojunction | Tetracycline hydrochloride | 100 mg/L | 120 min | 25 °C | 94.90% | / | / | [45] | |
| Fe3O4@MIL-100(Fe) | Microwave-assisted synthesis | Magnetic material loads | Diclofenac sodium | 60 mg/L | 3 h | 25 °C | 99.40% | // | Five times | [52] | |
| MIL-100(Fe)/TpPa-1 COF | Solvothermal synthesis | Heterojunction | Tetracycline hydrochloride | 20 mg/L | 120 min | Room temperature | 91% | 36 h of continuous catalytic | Five times | [121] | |
| g-C3N4/MIL-101(Fe) | Hydrothermal synthesis | Heterojunction | Enrofloxacin | 10 mg/L | 60 min | 25 °C | 100% | Degradation efficiency above 80% at pH 3 and 9 | Five times | [116] | |
| MIL-100(Fe)/CoFeLMO/PEG | Hydrothermal synthesis | Heterojunction | Ranitidine | 1–10 mg/L | 1 min | Room temperature | 99.50% | Extreme pH conditions maintain 99.5% degradation efficiency | Twenty times | [133] | |
| MIL-100(Fe)/TpPa-1 COF | Solvothermal synthesis | Heterojunction | Tetracycline hydrochloride | 20 mg/L | 120 min | Room temperature | 91% | 36 h of continuous catalytic | Five times | [121] | |
| MIL-125(Ti)/g-C3N5 | Solvothermal synthesis | Heterojunction | Sulfadiazine | 0.04 mM | 15 min | 25 °C | 95.60% | Degradation rate of 64.5% at pH 3 | Five times | [134] | |
| VOCs | MIL-88A(Fe)@BiVO4 | Hydrothermal synthesis | Heterojunction | Formaldehyde | 50 ppm | 120 min | 25 °C | 91% | Slight decrease in degradation efficiency at higher humidity levels | Five times | [135] |
| TiO2@MIL-101(Cr) | Hydrothermal synthesis | Heterojunction | Toluene | / | 120 min | Room temperature | 39.60% | / | / | [136] | |
| Pr/MIL-101(Cr) | Hydrothermal synthesis | Doping of elements | 1,2-dichloroethane | 1000 ppm | / | 290 °C | 95% | Stabilized at 290 °C for 120 h | / | [137] | |
| NH2-MIL-125 | Solvothermal synthesis | Amino functionalization | Methylbenzene | 2000 ppm | 20 min | 25 °C | 96% | High degradation efficiency in the presence of water vapor | Four times | [138] | |
| N/MIL-125(Ti) | Solvothermal synthesis | Doping of elements | Benzene | 0.007% | 480 min | 25 °C | 99.10% | / | Ten times | [139] | |
| PMA@NH2-MIL-125 | Solvothermal synthesis | Amino functionalization, compound modification | Acetone | 400 ppm | / | Room temperature | 78% | / | Five times | [140] | |
| NH2-MIL-125 | Solvothermal synthesis | Carbon material loads | Dichloromethane | 70 ± 1 ppmv | 5 h | Room temperature | 85% | Stabilizes degradation at high humidity | / | [141] | |
| Cu/NH2-MIL-125 | Solvothermal synthesis | Doping of elements | Methyl mercaptan | 100 ppm | 25 min | Room temperature | 100% | / | Five times | [142] | |
| Microplastics | Fe3O4@SiO2@MIL-53(Al) | Hydrothermal synthesis | Heterojunction | Polyvinyl chloride | 1 mg/mL | 10 h | Room temperature | 93.17% | / | Five times | [143] |
| NH2-MIL-88B(Fe)/MoS2 | Solvothermal synthesis | Amino functionalization, heterojunction | Polyethylene | 10 g/L | 48 h | Room temperature | / | High degradation efficiency over the full pH range | Seven times | [144] | |
| TiO2/MIL-100(Fe) | Microwave-assisted synthesis | Heterojunction | Polyethylene terephthalate | 100 mg/L | 5 h | Room temperature | CI = 0.99 | The degradation efficiency was higher at pH 3 | / | [145] | |
| BiOI@MIL-101 | Solvothermal synthesis | Heterojunction | Polyethylene | 1.0 g/L | 6 h | Room temperature | / | / | Five times | [146] | |
| Ti3C2 MXene @MIL-125(Ti) | Hydrothermal synthesis | Heterojunction | Polyethylene | 0.5 g/L | 4 h | Room temperature | 78% | Better removal at low pH | Five times | [147] | |
| MIL-125-NH2/BNQDs | Solvothermal synthesis | Amino functionalization, heterojunction | Polyethylene terephthalate | / | 5 h | Room temperature | 95.71% | The degradation efficiency was higher at pH 3 | Four times | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Jiang, Y.; Li, W.; Su, W.; Xing, Y.; Yu, S.; Li, W.; Guo, Y.; Zhang, D.; Wang, S.; et al. MIL Series in MOFs for the Removal of Emerging Contaminants: Application and Mechanisms. Inorganics 2025, 13, 324. https://doi.org/10.3390/inorganics13100324
Chen Y, Jiang Y, Li W, Su W, Xing Y, Yu S, Li W, Guo Y, Zhang D, Wang S, et al. MIL Series in MOFs for the Removal of Emerging Contaminants: Application and Mechanisms. Inorganics. 2025; 13(10):324. https://doi.org/10.3390/inorganics13100324
Chicago/Turabian StyleChen, Yixiang, Yusheng Jiang, Weiping Li, Wei Su, Yi Xing, Shuyan Yu, Wenxin Li, Ying Guo, Duo Zhang, Shanqing Wang, and et al. 2025. "MIL Series in MOFs for the Removal of Emerging Contaminants: Application and Mechanisms" Inorganics 13, no. 10: 324. https://doi.org/10.3390/inorganics13100324
APA StyleChen, Y., Jiang, Y., Li, W., Su, W., Xing, Y., Yu, S., Li, W., Guo, Y., Zhang, D., Wang, S., Qian, Z., Hong, C., & Jiang, B. (2025). MIL Series in MOFs for the Removal of Emerging Contaminants: Application and Mechanisms. Inorganics, 13(10), 324. https://doi.org/10.3390/inorganics13100324












