Palladium-Catalyzed Cross-Coupling Reaction via C–H Activation of Furanyl and Thiofuranyl Substrates
Abstract
1. Introduction
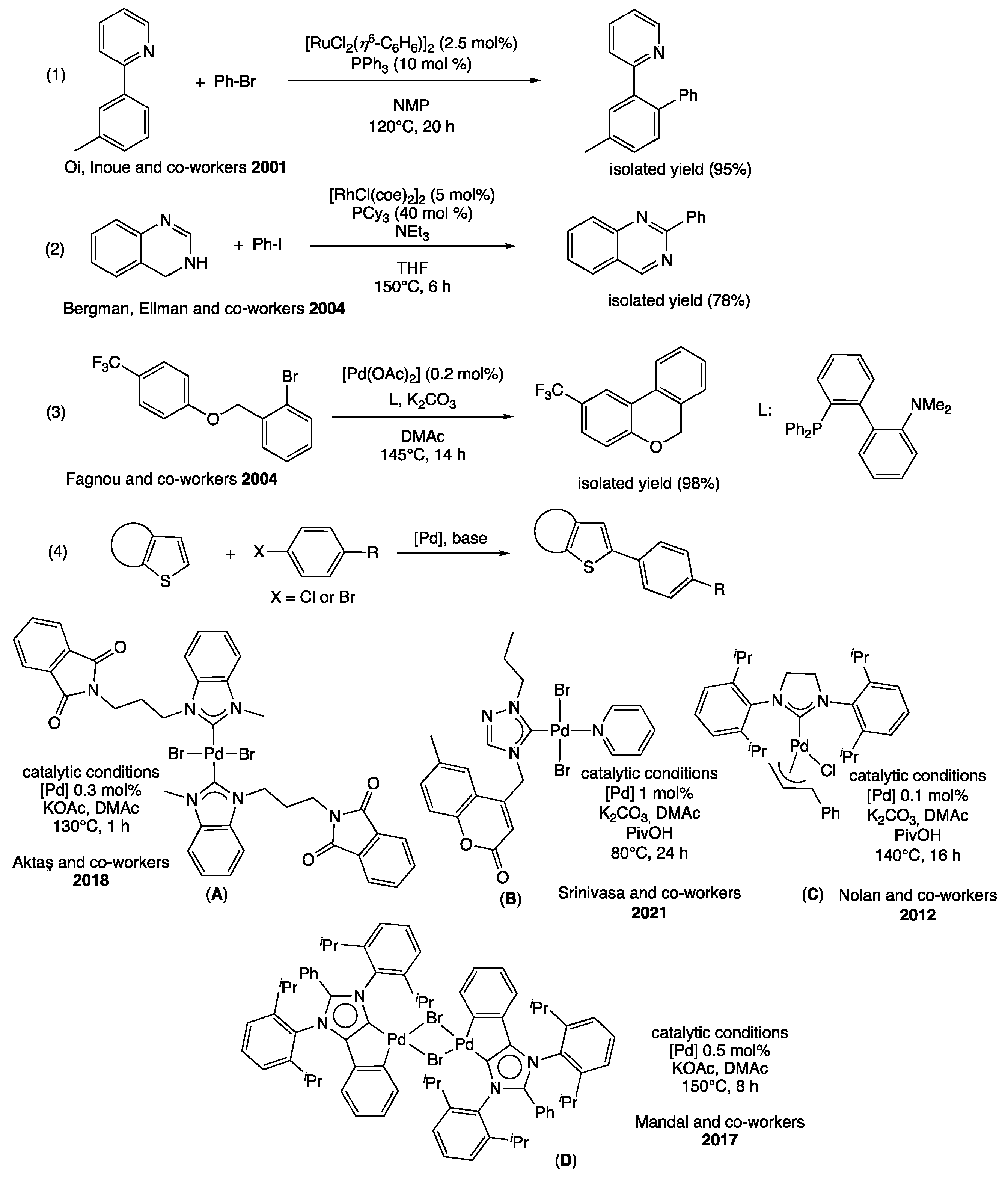
2. Results and Discussion
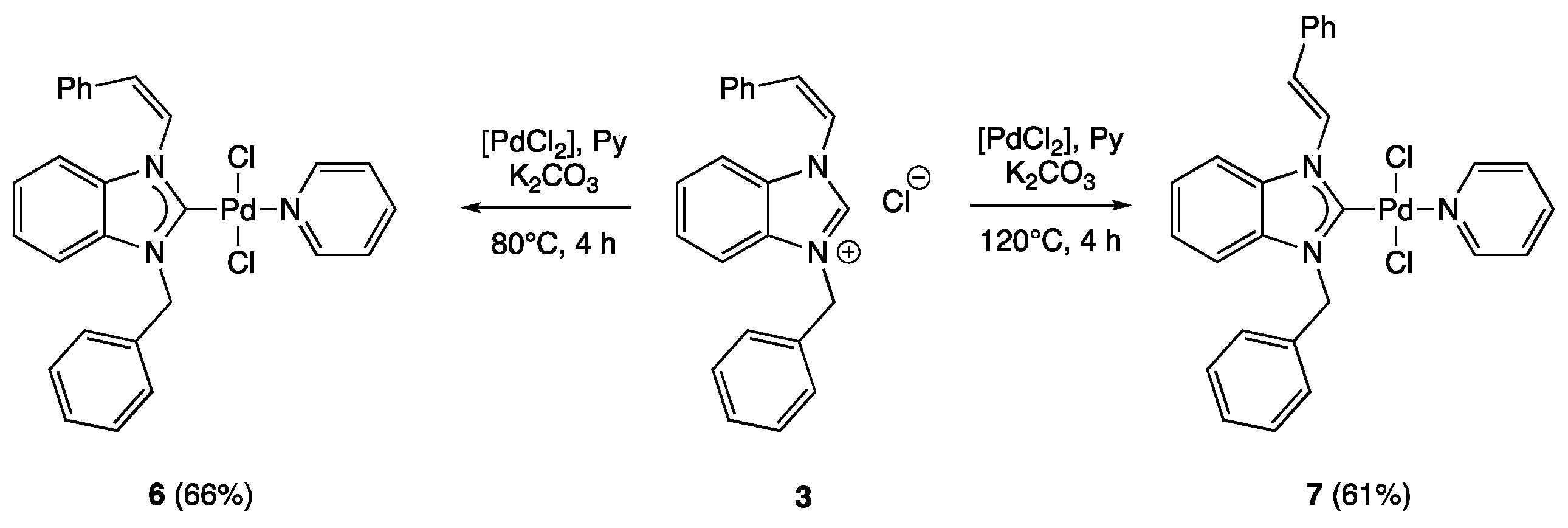
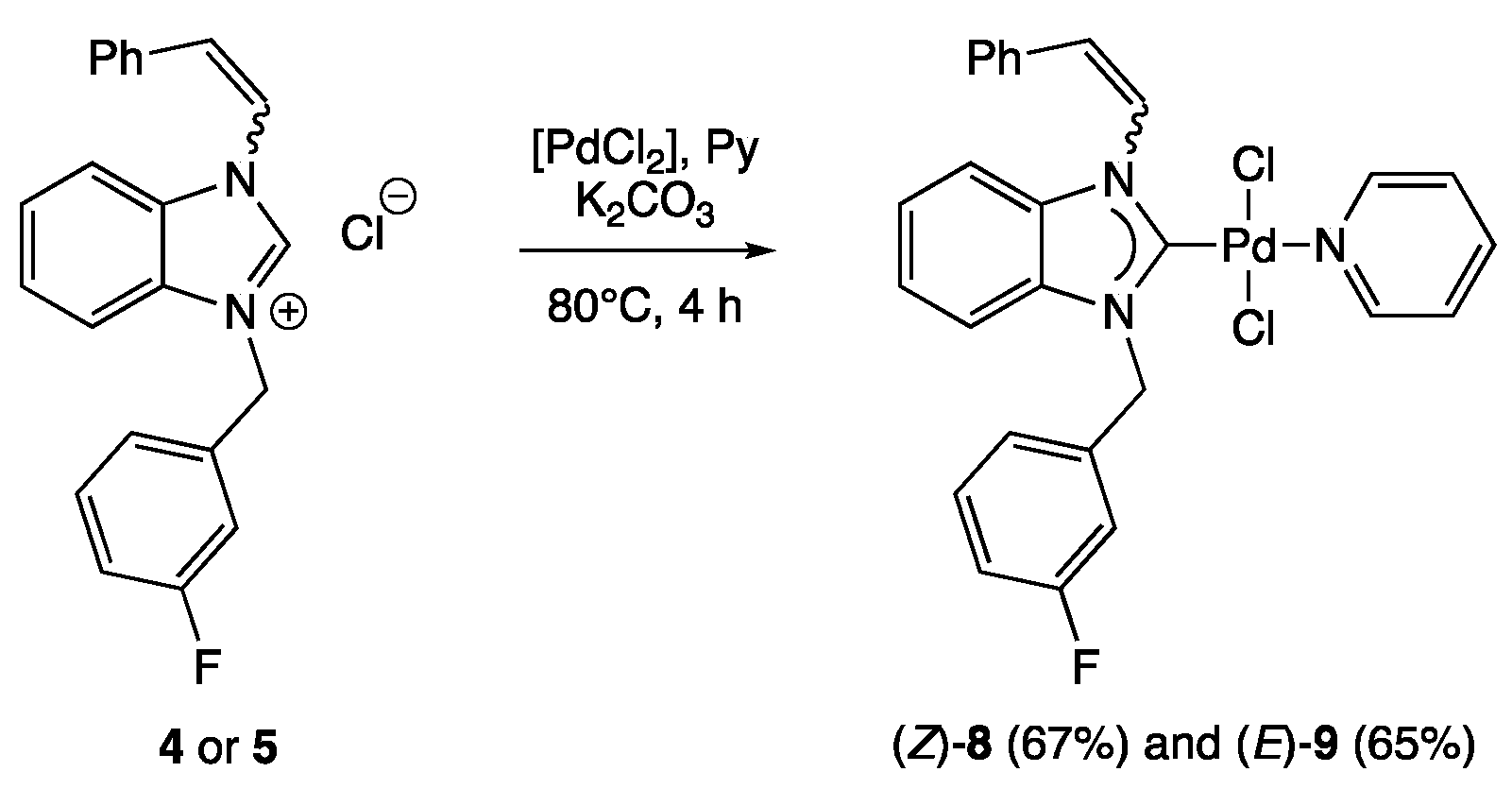
2.1. Synthesis of Palladium(II) Complexes
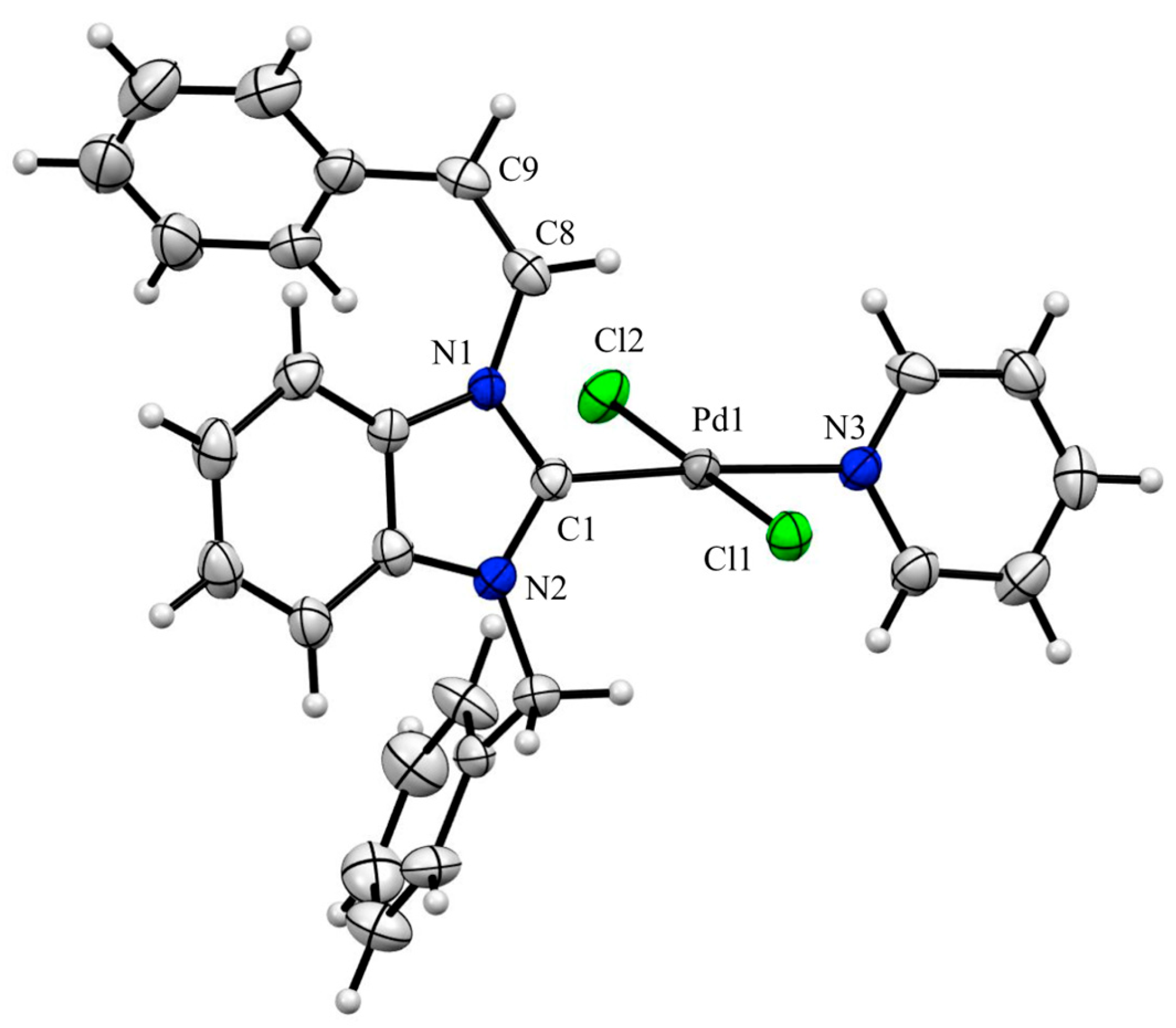
2.2. X-ray Crystal Structure Analysis of Palladium(II) Complexes
2.3. Palladium-Catalyzed C–H Activation
3. Materials and Methods
3.1. The General Procedure for the Synthesis of Benzimidazolium Slats
3.1.1. (Z)-1-Styryl-3-benzyl-benzimidazolium Chloride (3)
3.1.2. (Z)-1-Styryl-3-(3-fluorobenzyl)-benzimidazolium Chloride (4)
3.1.3. (E)-1-Styryl-3-(3-fluorobenzyl)-benzimidazolium Chloride (5)
3.2. The General Procedure for the Synthesis of Palladium(II) Complexes
3.2.1. trans-Dichloro-[(Z)-1-styryl-3-benzyl-benzimidazol-2-yliden]pyridine Palladium(II) (6)
3.2.2. trans-Dichloro-[(E)-1-styryl-3-benzyl-benzimidazol-2-yliden]pyridine Palladium(II) (7)
3.2.3. trans-Dichloro-[(Z)-1-styryl-3-(3-fluorobenzyl)-benzimidazol-2-yliden]pyridine Palladium(II) (8)
3.2.4. trans-Dichloro-[(E)-1-styryl-3-(3-fluorobenzyl)-benzimidazol-2-yliden]pyridine Palladium(II) (9)
3.3. The General Procedure for the Palladium-Catalyzed C–H Activation
3.4. X-ray Crystal Structure Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic carbene complexes in C-H activation reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Rogge, T.; Kaplaneris, N.; Chatani, N.; Kim, J.; Chang, S.; Punji, B.; Schafer, L.L.; Musaev, D.G.; Wencel-Delord, J.; Roberts, C.A.; et al. C-H activation. Nat. Rev. Dis. Primers 2021, 1, 43. [Google Scholar] [CrossRef]
- Dalton, T.; Faber, T.; Glorius, F. C-H activation: Toward sustainability and applications. ACS Cent. Sci. 2021, 7, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Thombal, R.S.; Rubio, P.Y.M.; Lee, D.; Maiti, D.; Lee, Y.R. Modern palladium-catalyzed transformations involving C-H activation and subsequent annulation. ACS Catal. 2022, 12, 5217–5230. [Google Scholar] [CrossRef]
- Oi, S.; Fukita, S.; Hirata, N.; Watanuki, N.; Miyano, S.; Inoue, Y. Ruthenium complex-catalyzed direct ortho arylation and alkenylation of 2-arylpyridines with organic halides. Org. Lett. 2001, 3, 2579–2581. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.C.; Wiedemann, S.H.; Bergman, R.G.; Ellman, J.A. Arylation of heterocycles via rhodium-catalyzed C-H bond functionalization. Org. Lett. 2004, 6, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Campeau, L.-C.; Thansandote, P.; Fagnou, K. High-yielding intramolecular direct arylation reactions with aryl chlorides. Org. Lett. 2005, 7, 1857–1860. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, J.; Mao, P.; Guo, Q. Chelating palladium complexes containing pyridine/pyrimidine hydroxyalkyl di-functionalized N-heterocyclic carbenes: Synthesis, structure, and catalytic activity towards C-H activation. RSC Adv. 2015, 5, 107601–107607. [Google Scholar] [CrossRef]
- Denisov, M.S.; Dmitriev, M.V.; Gorbunov, A.A.; Glushkov, V.A. Complexes of palladium(II) with N-heterocyclic carbenes from adamantylimidazole as precatalysts for thiophene and imidazole arylation. Russ. Chem. Bull. 2019, 68, 2039–2047. [Google Scholar] [CrossRef]
- Glushkov, V.A.; Denisov, M.S.; Gorbunov, A.A.; Myalitzin, Y.A.; Dmitriev, M.V.; Slepukhin, P.A. Adamantanyl-substituted PEPPSI-type palladium(II) N-heterocyclic carbene complexes: Synthesis and catalytic application for CH activation of substituted thiophenes. Chem. Heterocycl. Comp. 2019, 55, 217–228. [Google Scholar] [CrossRef]
- Ma, B.-B.; Lan, X.-B.; Shen, D.-S.; Liu, F.-S.; Xu, C. Direct C-H bond (hetero)arylation of thiazole derivatives at 5-position catalyzed by N-heterocyclic carbene palladium complexes at low catalyst loadings under aerobic conditions. J. Organomet. Chem. 2019, 897, 13–22. [Google Scholar] [CrossRef]
- Campeau, L.-C.; Parisien, M.; Leblanc, M.; Fagnou, K. Biaryl synthesis via direct arylation: Establishment of an efficient catalyst for intramolecular processes. J. Am. Chem. Soc. 2004, 126, 9186–9187. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, H.; Aktaş, A.; Gök, Y.; Sarı, Y. N-Propylphthalimide-substituted bis-(NHC)PdX2 complexes: Synthesis, characterization and catalytic activity in direct arylation reactions. Transit. Met. Chem. 2018, 43, 31–37. [Google Scholar] [CrossRef]
- Gautama, A.; Shahini, C.R.; Siddappa, A.P.; Grzegorz, M.J.; Hemavathi, B.; Ahipa, T.N.; Srinivasa, B. Palladium(II) complexes of coumarin substituted 1,2,4-triazol-5-ylidenes for catalytic C-C cross-coupling and C-H activation reactions. J. Organomet. Chem. 2021, 934, 121540. [Google Scholar] [CrossRef]
- Martin, A.R.; Chartoire, A.; Slawin, A.M.Z.; Nolan, S.P. Extending the utility of [Pd(NHC)(cinnamyl)Cl] precatalysts: Direct arylation of heterocycles. Beilstein J. Org. Chem. 2012, 8, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Sau, S.C.; Sreejyothi, P.; Hota, P.K.; Vardhanapu, P.K.; Vijaykumar, G.; Mandal, S.K. Direct C-H arylation of heteroarenes with aryl chlorides by using an abnormal N-heterocyclic-carbene-palladium catalyst. Eur. J. Org. Chem. 2017, 2017, 1004–1011. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily prepared air- and moisture-stable Pd–NHC (NHC = N-heterocyclic carbene) complexes: A reliable, user-friendly, highly active palladium precatalyst for the Suzuki-Miyaura reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Calimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef] [PubMed]
- Vasu, G.R.P.; Venkata, K.R.M.; Kakarla, R.R.; Ranganath, K.V.S.; Aminabhavi, T.M. Recent advances in sustainable N-heterocyclic carbene-Pd(II)-pyridine (PEPPSI) catalysts: A review. Environ. Res. 2023, 225, 115515. [Google Scholar] [CrossRef]
- Shaughnessy, K.H. Development of palladium precatalysts that efficiently generate LPd(0) active species. Isr. J. Chem. 2020, 60, 180–194. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Szostak, M. Pd-PEPPSI: Pd-NHC precatalyst for Suzuki-Miyaura cross-coupling reactions of amides. J. Org. Chem. 2017, 82, 6638–6646. [Google Scholar] [CrossRef] [PubMed]
- İmik, F.; Yasar, S.; Özdemir, İ. Synthesis and investigation of catalytic activity of phenylene—And biphenylene bridged bimetallic palladium-PEPPSI complexes. J. Organomet. Chem. 2019, 896, 162–167. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zhang, J.; Zhao, Q.; Feliciano, J.; Bisz, E.; Dziuk, B.; Lalancette, R.; Szostak, R.; Szostak, M. Pd-PEPPSI N-heterocyclic carbene complexes from caffeine: Application in Suzuki, Heck, and Sonogashira reactions. Organometallics 2022, 41, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Borah, D.; Saha, B.; Sarma, B.; Das, P. A new PEPPSI type N-heterocyclic carbene palladium(II) complex and its efficiency as a catalyst for Mizoroki-Heck cross-coupling reactions in water. J. Chem. Sci. 2020, 132, 51. [Google Scholar] [CrossRef]
- Organ, M.G.; Abdel-Hadi, M.; Avola, S.; Hadei, N.; Nasielski, J.; O’Brien, C.J.; Valente, C. Biaryls made easy: PEPPSI and the Kumada-Tamao-Corriu reaction. Chem. Eur. J. 2007, 13, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Belowich, M.E.; Hadei, N.; Organ, M.O. Pd-PEPPSI complexes and the Negishi reaction. Eur. J. Org. Chem. 2010, 2010, 4343–4354. [Google Scholar] [CrossRef]
- Erdemir, F.; Celepci, D.B.; Aktaş, A.; Gök, Y. 2-Hydroxyethyl-substituted (NHC)PdI2(pyridine) (Pd-PEPPSI) complexes: Synthesis, characterization and the catalytic activity in the Sonogashira cross-coupling reaction. ChemistrySelect 2019, 4, 5585–5590. [Google Scholar] [CrossRef]
- Reddy, M.V.K.; Anusha, G.; Reddy, P.V.G. Sterically enriched bulky 1,3-bis(N,N′-aralkyl)benzimidazolium based Pd-PEPPSI complexes for Buchwald-Hartwig amination reactions. New J. Chem. 2020, 44, 11694–11703. [Google Scholar] [CrossRef]
- Huang, F.-D.; Xu, C.; Lu, D.-D.; Shen, D.-S.; Li, T.; Liu, F.-S. Pd-PEPPSI-IPentAn promoted deactivated amination of aryl chlorides with amines under aerobic conditions. J. Org. Chem. 2018, 83, 9144–9155. [Google Scholar] [CrossRef]
- He, X.X.; Li, Y.; Ma, B.B.; Ke, Z.; Liu, F.S. Sterically encumbered tetraarylimidazolium carbene Pd-PEPPSI complexes: Highly efficient direct arylation of imidazoles with aryl bromides under aerobic conditions. Organometallics 2016, 35, 2655–2663. [Google Scholar] [CrossRef]
- Nie, B.; Wu, W.; Ren, Q.; Wang, Z.; Zhang, J.; Zhang, Y.; Jiang, H. Access to cycloalkeno[c]-fused pyridines via Pd-catalyzed C(sp2)-H activation and cyclization of N-acetyl hydrazones of acylcycloalkenes with vinyl azides. Org. Lett. 2020, 22, 7786–7790. [Google Scholar] [CrossRef] [PubMed]
- Gokanapalli, A.; Motakatla, V.K.R.; Peddiahgari, V.G.R. Benzimidazole bearing Pd–PEPPSI complexes catalyzed direct C2-arylation/heteroarylation of N-substituted benzimidazoles. Appl. Organomet. Chem. 2020, 34, e5869. [Google Scholar] [CrossRef]
- Bensalah, D.; Mansour, L.; Sauthier, M.; Gurbuz, N.; Özdemir, İ.; Beji, L.; Gatrig, R.; Hamdi, N. Plausible PEPPSI catalysts for direct C-H functionalization of five-membered heterocyclic bioactive motifs: Synthesis, spectral, X-ray crystallographic characterizations and catalytic activity. RSC Adv. 2023, 13, 31386–31410. [Google Scholar] [CrossRef] [PubMed]
- Şahin, N.; Sémeril, D.; Brenner, E.; Matt, D.; Özdemir, İ.; Kaya, C.; Toupet, L. Resorcinarene-functionalised imidazolium salts as ligand precursors for palladium-catalysed Suzuki-Miyaura cross-couplings. ChemCatChem 2013, 5, 1116–1125. [Google Scholar] [CrossRef]
- El-Krim Sandeli, A.; Khiri-Meribout, N.; Benzerka, S.; Boulebd, H.; Gürbüz, N.; Özdemir, N.; Özdemir, İ. Synthesis, structures, DFT calculations, and catalytic application in the direct arylation of five-membered heteroarenes with aryl bromides of novel palladium-N-heterocyclic carbene PEPPSI-type complexes. New J. Chem. 2021, 45, 17878–17892. [Google Scholar] [CrossRef]
- Hamdi, H.; Slimani, I.; Mansour, L.; Alresheedi, F.; Gürbüz, N.; Özdemir, İ. N-Heterocyclic carbene-palladium-PEPPSI complexes and their catalytic activity in the direct C-H bond activation of heteroarene derivatives with aryl bromides: Synthesis, and antimicrobial and antioxidant activities. New J. Chem. 2021, 45, 21248–21262. [Google Scholar] [CrossRef]
- Khan, S.; Buğday, N.; Yasar, S.; Ullah, N.; Özdemir, İ. Pd-N-heterocyclic carbene complex catalysed C-H bond activation of 2-isobutylthiazole at the C5 position with aryl bromides. New J. Chem. 2021, 45, 6281–6292. [Google Scholar] [CrossRef]
- Lasmari, S.; Gürbüz, N.; Boulcina, R.; Özdemir, N.; Özdemir, İ. Synthesis of [PdBr2(benzimidazole-2-ylidene)(pyridine)] complexes and their catalytic activity in the direct C-H bond activation of 2-substituted heterocycles. Polyhedron 2021, 199, 115091. [Google Scholar] [CrossRef]
- Nawaz, Z.; Gürbüz, N.; Zafar, M.N.; Tahir, M.N.; Ashfaq, M.; Karci, H.; Özdemir, İ. Direct arylation (hetero-coupling) of heteroarenes via unsymmetrical palladium-PEPPSI-NHC type complexes. Polyhedron 2021, 208, 115412. [Google Scholar] [CrossRef]
- Kaloğlu, M.; Şahan, M.H.; Düşünceli, S.D.; Özdemir, İ. Synthesis of quinoxaline-linked bis(benzimidazolium) salts and their catalytic application in palladium-catalyzed direct arylation of heteroarenes. Catal. Lett. 2022, 152, 2012–2024. [Google Scholar] [CrossRef]
- Slimani, I.; Boubakri, L.; Özdemir, N.; Mansour, L.; Özdemir, İ.; Gürbüz, N.; Yasar, S.; Sauthier, M.; Hamdi, N. Substituted N-heterocyclic carbene PEPPSI-type palladium complexes with different N-coordinated ligands: Involvement in the direct C-H bond activation of heteroarenes derivatives with aryl bromide and their antimicrobial, anti-inflammatory and antioxidant activities. Inorg. Chim. Acta 2022, 532, 120747. [Google Scholar]
- Munir, N.; Gürbüz, N.; Zafar, M.N.; Evren, E.; Şen, B.; Aygün, M.; Özdemir, İ. Plausible PEPPSI catalysts for direct C-H functionalization of furans and pyrroles. J. Mol. Struct. 2024, 1295, 136679. [Google Scholar] [CrossRef]
- Touj, N.; Bensalah, D.; Mansour, L.; Sauthier, M.; Gürbüz, N.; Özdemir, İ.; Hamdi, N. Synthesis of palladium complexes containing benzimidazole core and their catalytic activities in direct C-H functionalization of five-membered heterocyclic bioactive motifs. J. Mol. Struct. 2024, 1297, 136885. [Google Scholar] [CrossRef]
- Meng, G.; Kakalis, L.; Nolan, S.P.; Szostak, M. A simple 1H NMR method for determining the σ-donor properties of N-heterocyclic carbenes. Tetrahedron Lett. 2019, 60, 378–781. [Google Scholar] [CrossRef]
- Fecher, G.-H.; Kübler, J.; Felser, C. Chirality in the solid state: Chiral crystal structures in chiral and achiral space groups. Materials 2022, 15, 5812. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. Sect. B 2013, 69, 249–259. [Google Scholar] [CrossRef]
- Onar, G.; Gürses, C.; Karatas, M.O.; Balcıoğlu, S.; Akbay, N.; Özdemir, N.; Ates, B.; Alıcı, B. Palladium(II) and ruthenium(II) complexes of benzotriazole functionalized N-heterocyclic carbenes: Cytotoxicity, antimicrobial, and DNA interaction studies. J. Organomet. Chem. 2019, 886, 48–56. [Google Scholar] [CrossRef]
- Reddy, V.P.; Iwasaki, T.; Kambe, N. Synthesis of imidazo and benzimidazo[2,1-a]-isoquinolines by rhodium-catalyzed intramolecular double C-H bond activation. Org. Biomol. Chem. 2013, 11, 2249–2253. [Google Scholar] [CrossRef]
- Kozell, V.; Rahmani, F.; Piermatti, O.; Lanari, D.; Vaccaro, L. A stereoselective organic base-catalyzed protocol for hydroamination of alkynes under solvent-free conditions. Mol. Catal. 2018, 455, 188–191. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


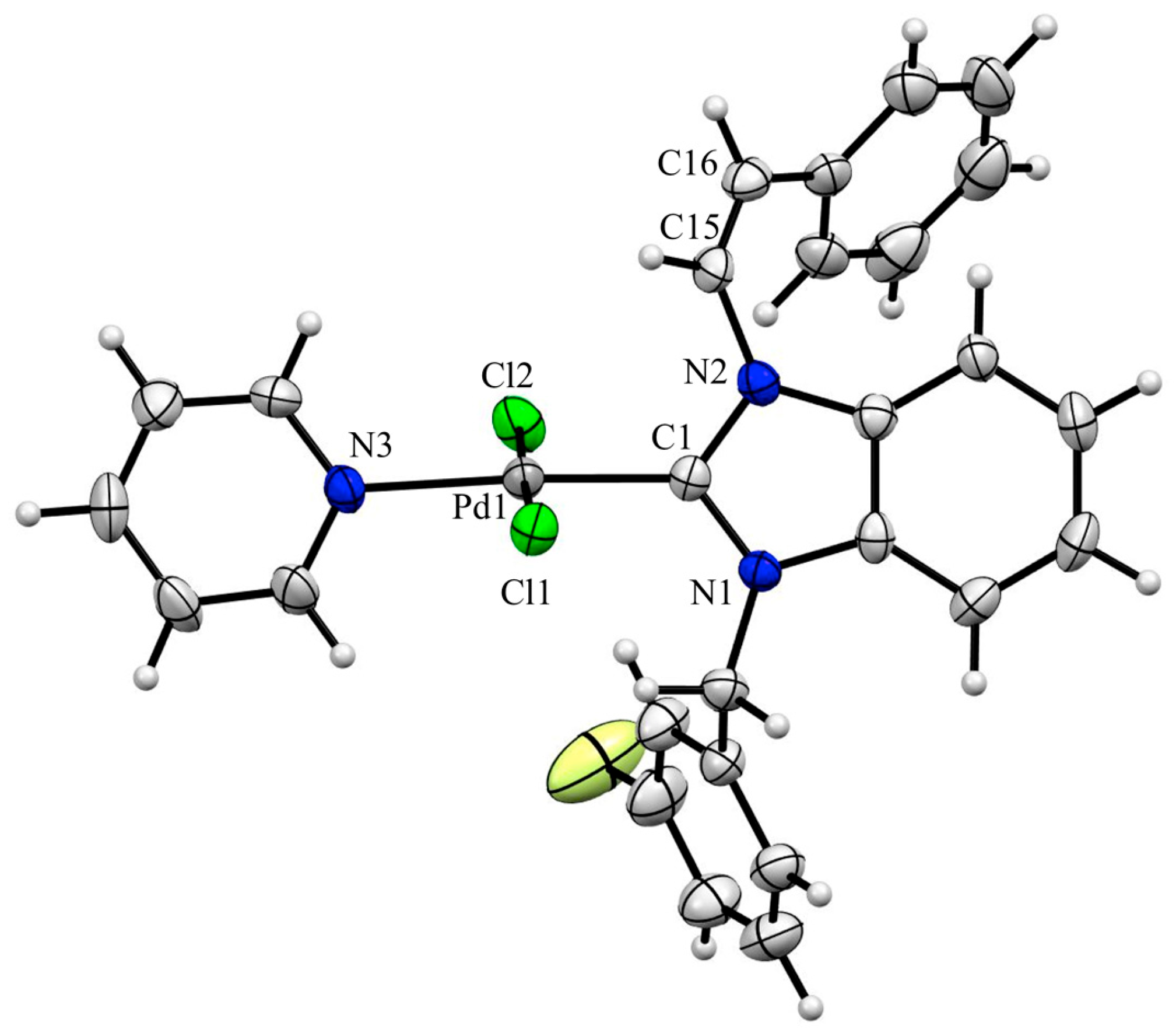
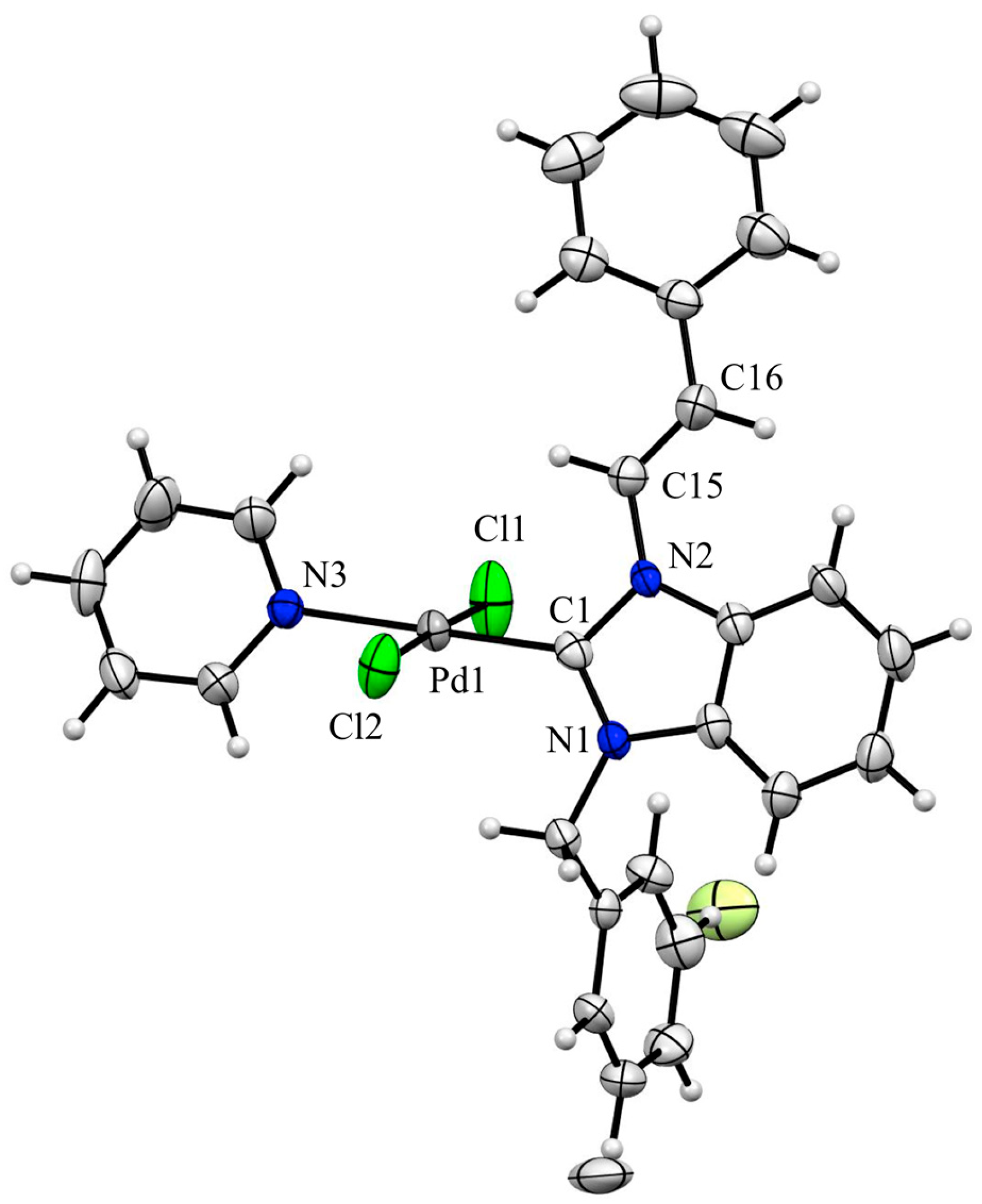
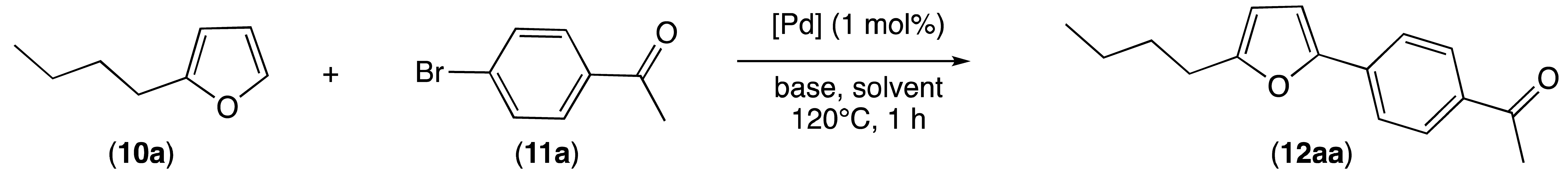 | ||||
| Entry | Complex | Base | Solvent | Conversion (%) 2 |
|---|---|---|---|---|
| 1 | 6 | KOH | DMAc | / |
| 2 | 6 | tBuOK | DMAc | / |
| 3 | 6 | Cs2CO3 | DMAc | 15 |
| 4 | 6 | K2CO3 | DMAc | 21 |
| 5 | 6 | NaOAc | DMAc | 71 |
| 6 | 6 | KOAc | DMAc | 76 |
| 7 | 6 | KOAc | Toluene | 4 |
| 8 | 6 | KOAc | DMSO | 3 |
| 9 | 6 | KOAc | DMF | 54 |
| 10 | 7 | KOAc | DMAc | 79 |
| 11 | 8 | KOAc | DMAc | 62 |
| 12 | 9 | KOAc | DMAc | 54 |
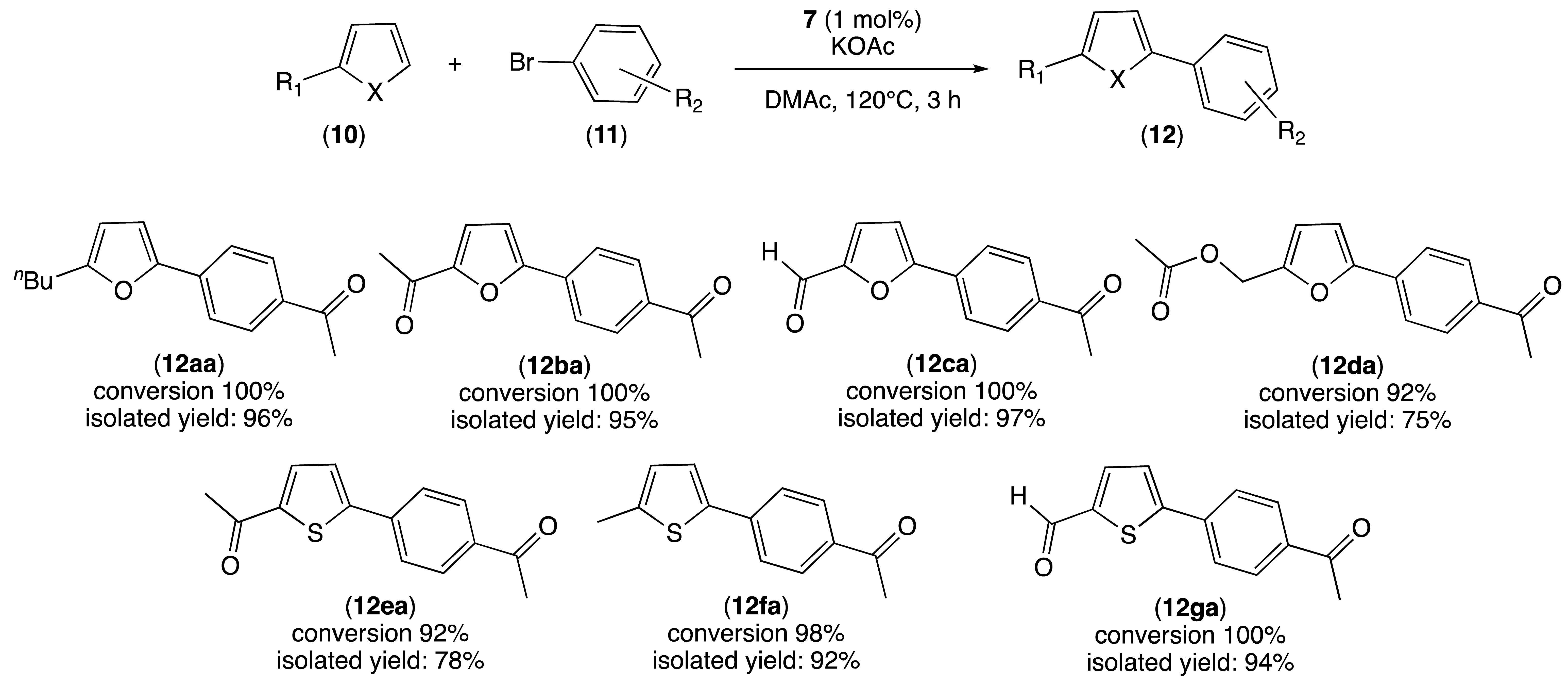 | |||
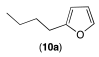 | 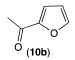 |  | |
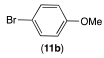 | 12ab conversion: 14% | 12bb conversion: 10% | 12eb conversion: 21% |
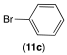 | 12ac conversion: 87% (isolated yield: 83%) | 12bc conversion: 82% (isolated yield: 77%) | 12ec conversion: 100% (isolated yield: 96%) |
 | 12ad conversion: 98% (isolated yield: 92%) | 12bd conversion: 96% (isolated yield: 90%) | 12ed conversion: 94% (isolated yield: 83%) |
 | 12ae conversion: 14% | 12be conversion: 20% | 12ee conversion: 21% |
 | 12af conversion: 7% | 12bf conversion: 21% | 12ef conversion: 23% |
| Complex | 6 | 8 | 9 | |
|---|---|---|---|---|
| CCDC depository | 2343643 | 2343644 | 2343645 | |
| color/shape | colorless/block | yellow/prism | colorless/block | |
| chemical formula | C27H23Cl2N3Pd | C27H22FCl2N3Pd | C27H22FCl2N3Pd | |
| molecular weight (g mol−1) | 566.78 | 584.77 | 584.77 | |
| crystal system | orthorhombic | orthorhombic | monoclinic | |
| space group | P212121 | P212121 | C2/c | |
| unit cell parameters | a (Å) | 9.788(5) | 9.7942(8) | 22.5267(19) |
| b (Å) | 14.022(8) | 14.2926(11) | 16.6782(13) | |
| c (Å) | 18.219(9) | 17.9496(18) | 14.5980(11) | |
| α (°) | 90 | 90 | 90 | |
| β (°) | 90 | 90 | 117.218(4) | |
| γ (°) | 90 | 90 | 90 | |
| volume (Å3) | 2500(2) | 2512.7(4) | 4877.2(7) | |
| Z | 4 | 4 | 8 | |
| D (g cm−3) | 1.506 | 1.546 | 1.593 | |
| μ (mm−1) | 0.976 | 0.979 | 1.009 | |
| Tmin, Tmax | 0.4111, 0.7456 | 0.908, 0.944 | 0.6803, 0.7456 | |
| F(000) | 1144 | 1176 | 2352 | |
| crystal size (mm) | 0.180 × 0.160 × 0.140 | 0.100 × 0.080 × 0.060 | 0.120 × 0.100 × 0.100 | |
| index ranges | −12 ≤ h ≤ 12 | −12 ≤ h ≤ 12 | −29 ≤ h ≤ 29 | |
| −18 ≤ k ≤ 18 | −18 ≤ k ≤ 18 | −21 ≤ k ≤ 21 | ||
| −21 ≤ l ≤ 23 | −20 ≤ l ≤ 23 | −19 ≤ l ≤ 16 | ||
| θ range for data collection (°) | 1.833 ≤ θ ≤ 27.827 | 1.821 ≤ θ ≤ 28.071 | 1.589 ≤ θ ≤ 27.975 | |
| reflections collected | 24,049 | 24,519 | 31,543 | |
| independent/observed | 5925/4777 | 6076/4425 | 5850/4256 | |
| Rint | 0.1000 | 0.0954 | 0.0822 | |
| data/restraints/parameters | 5925/0/298 | 6076/0/307 | 5850/0/316 | |
| goodness-of-fit on F2 | 1.005 | 0.978 | 1.018 | |
| final R indices (I > 2.0 σ(I)) | R1 = 0.0500, wR2 = 0.1157 | R1 = 0.0467, wR2 = 0.0765 | R1 = 0.0525, wR2 = 0.1290 | |
| R indices (all data) | R1 = 0.0710, wR2 = 0.1299 | R1 = 0.0800, wR2 = 0.0879 | R1 = 0.0790, wR2 = 0.1451 | |
| Δρmax, Δρmin (eÅ−3) | 1.379, −0.990 | 0.549, −0.570 | 2.181, −0.945 | |
| Flack parameter | 0.07(4) | −0.04(4) | / | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, N.; Özdemir, İ.; Sémeril, D. Palladium-Catalyzed Cross-Coupling Reaction via C–H Activation of Furanyl and Thiofuranyl Substrates. Inorganics 2024, 12, 175. https://doi.org/10.3390/inorganics12060175
Şahin N, Özdemir İ, Sémeril D. Palladium-Catalyzed Cross-Coupling Reaction via C–H Activation of Furanyl and Thiofuranyl Substrates. Inorganics. 2024; 12(6):175. https://doi.org/10.3390/inorganics12060175
Chicago/Turabian StyleŞahin, Neslihan, İsmail Özdemir, and David Sémeril. 2024. "Palladium-Catalyzed Cross-Coupling Reaction via C–H Activation of Furanyl and Thiofuranyl Substrates" Inorganics 12, no. 6: 175. https://doi.org/10.3390/inorganics12060175
APA StyleŞahin, N., Özdemir, İ., & Sémeril, D. (2024). Palladium-Catalyzed Cross-Coupling Reaction via C–H Activation of Furanyl and Thiofuranyl Substrates. Inorganics, 12(6), 175. https://doi.org/10.3390/inorganics12060175







