Construction of Zn0.5Cd0.5S/Bi4O5Br2 Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride
Abstract
1. Introduction
2. Results and Discussion
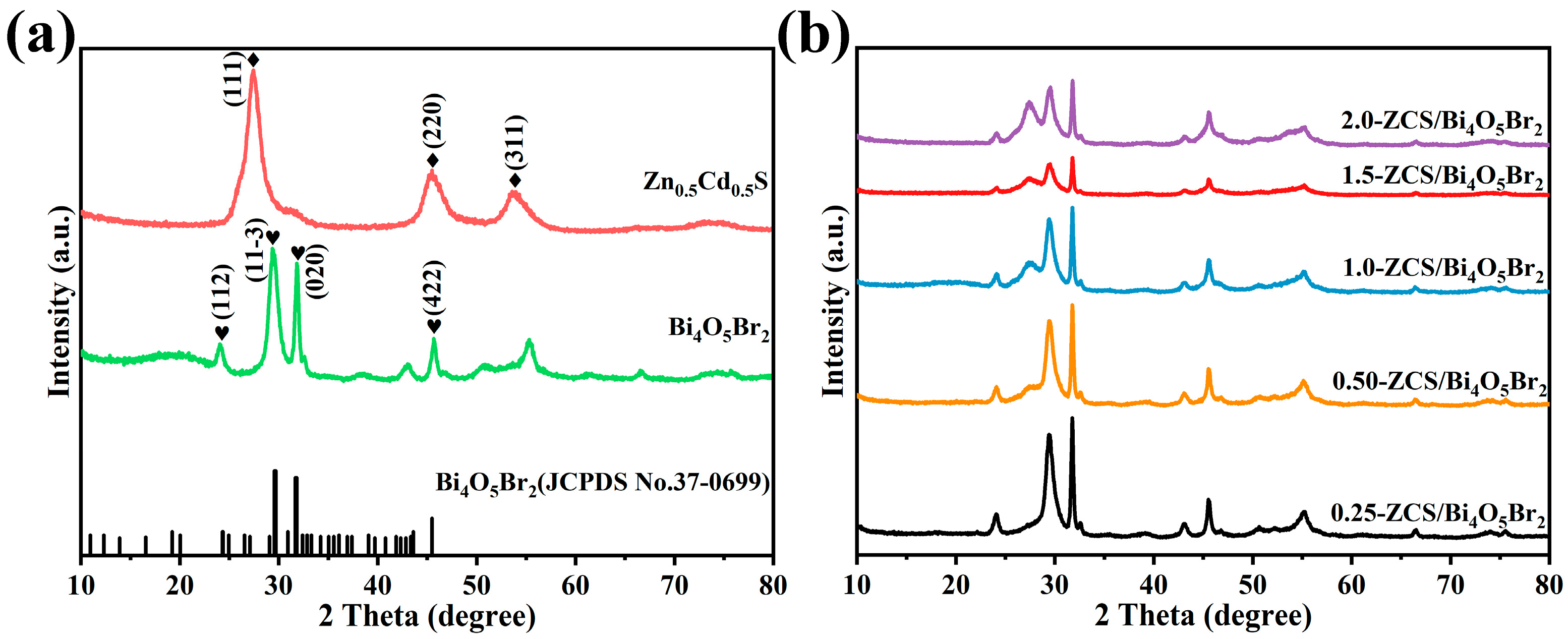
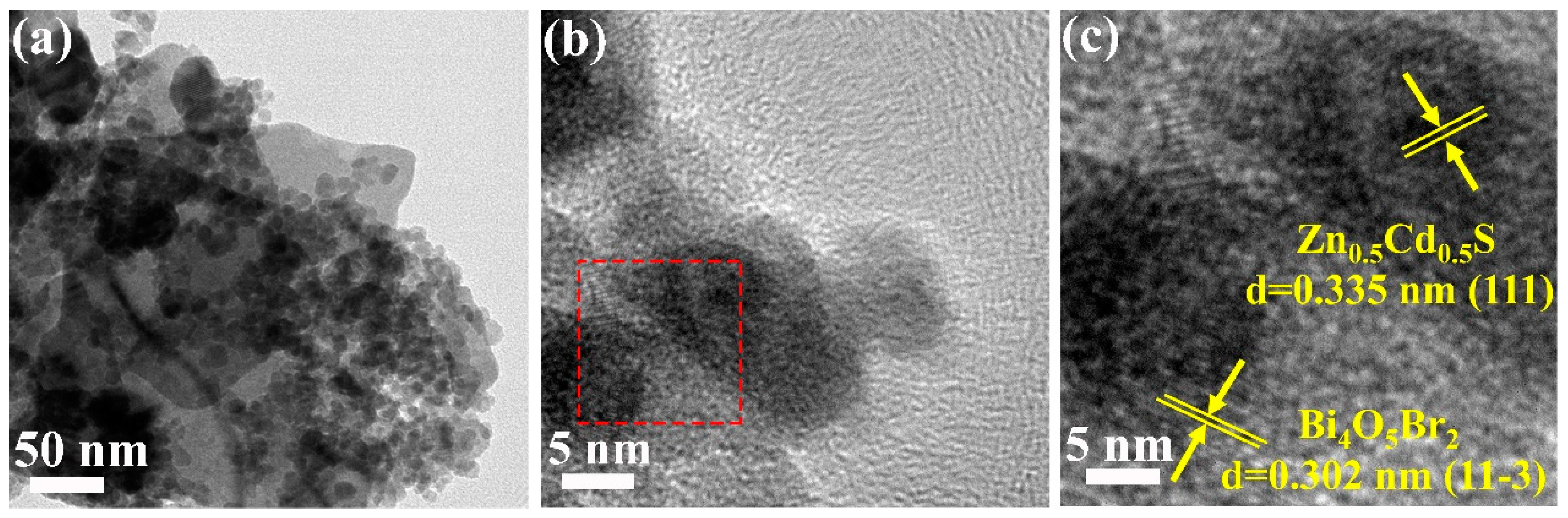
2.1. Material Characterization
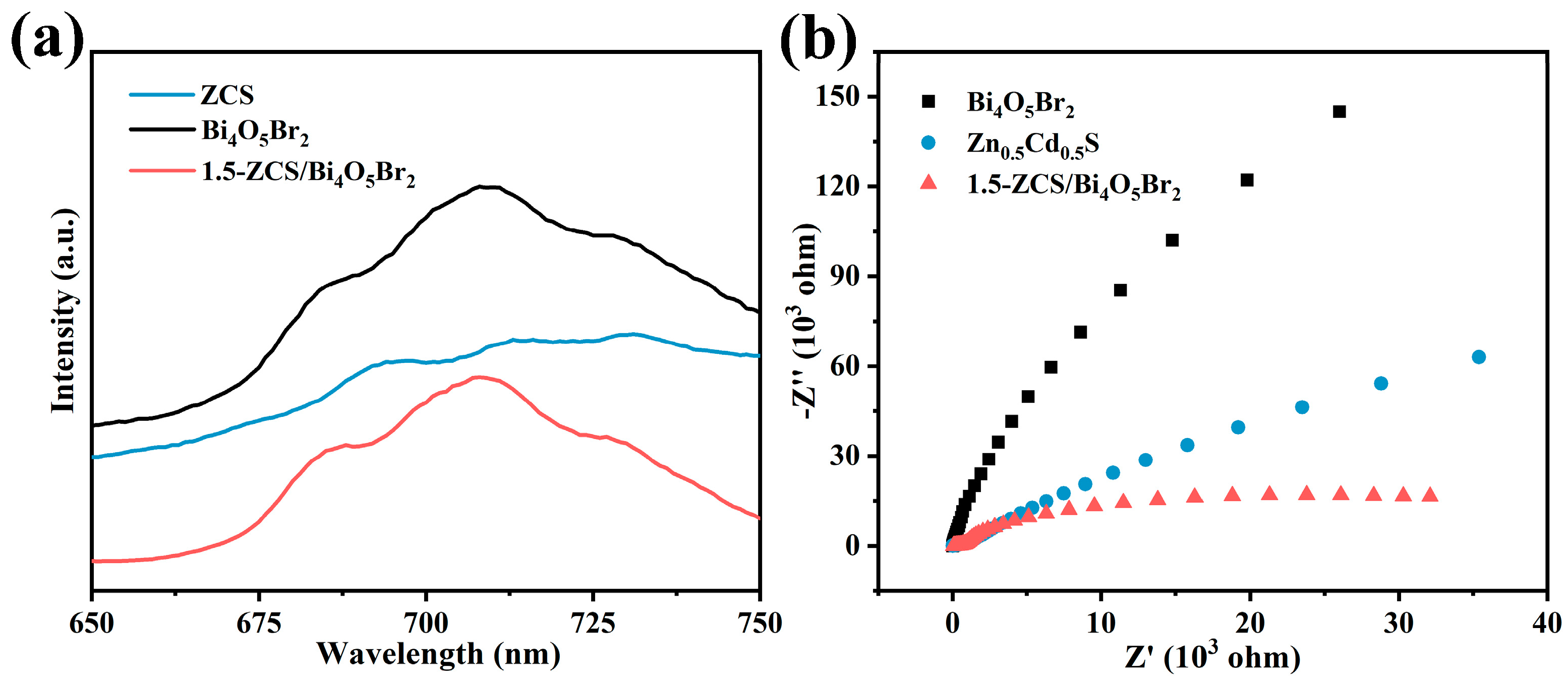
2.2. Optoelectronic Properties
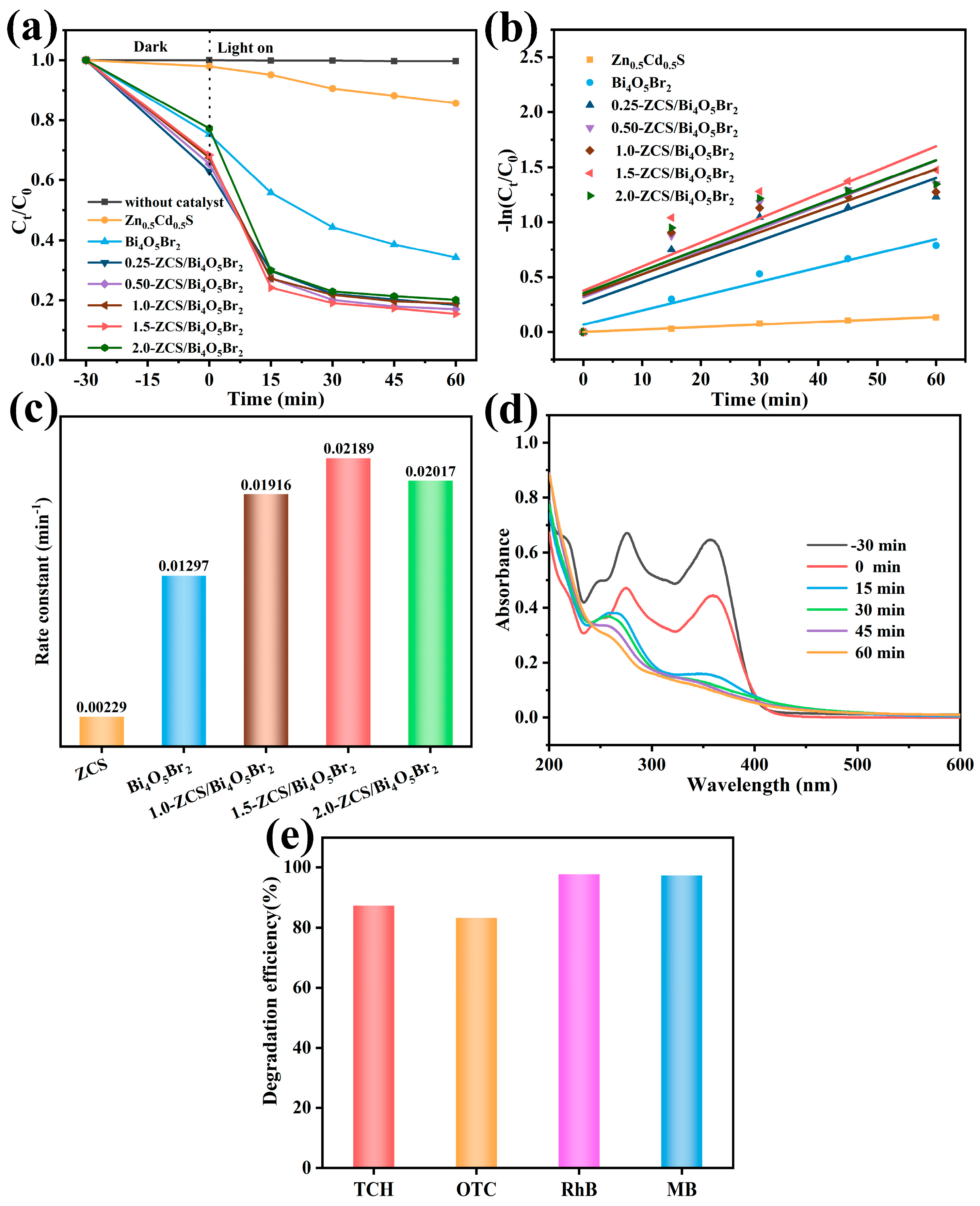
2.3. Photocatalytic Performance
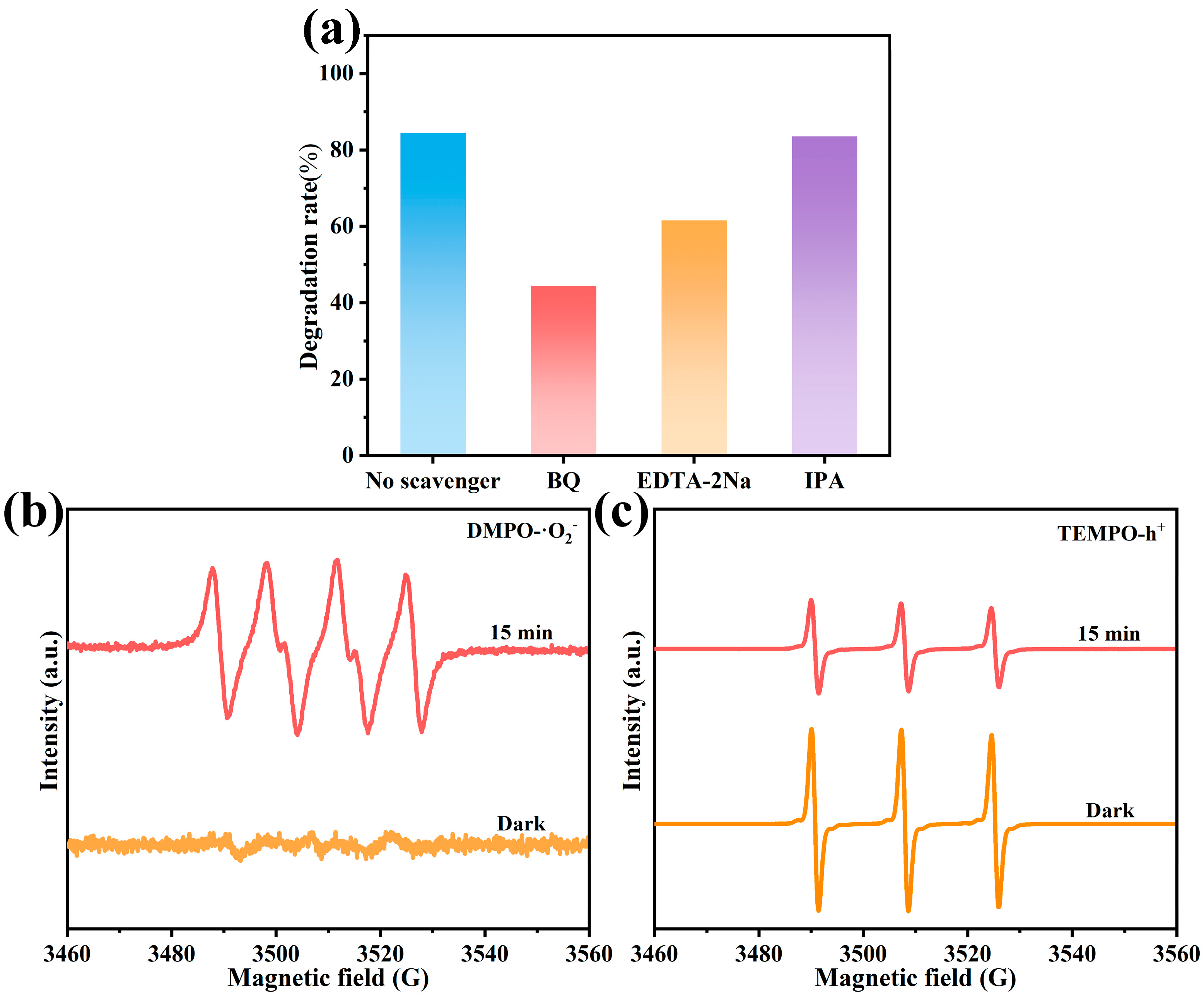
2.4. Analysis of Possible Degradation Pathways
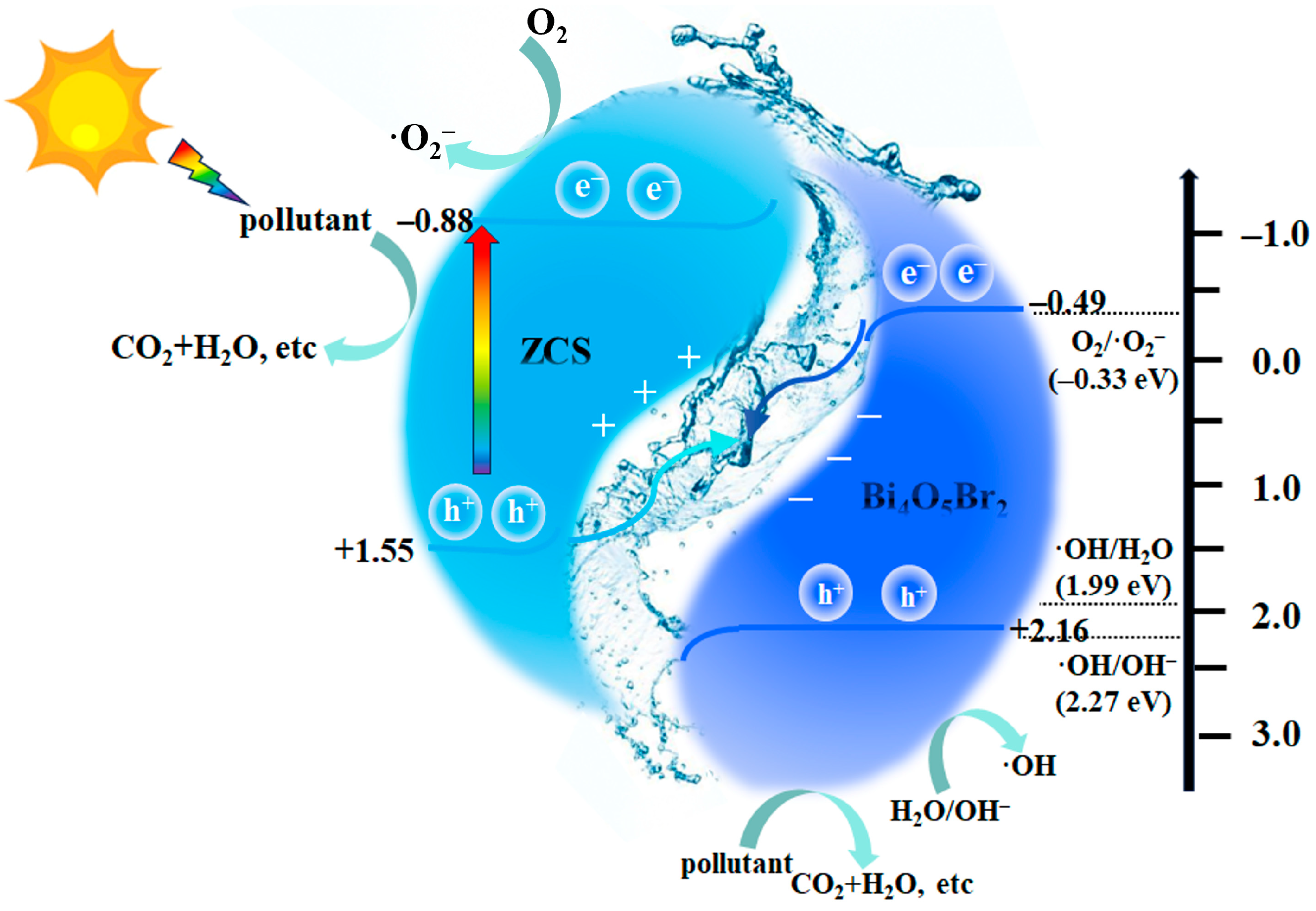
2.5. Photocatalytic Mechanism
3. Experimental Section
3.1. Preparation of Zn0.5Cd0.5S
3.2. Preparation of Bi4O5Br2
3.3. Preparation of Zn0.5Cd0.5S/Bi4O5Br2
3.4. Characterization
3.5. Photocatalytic Performance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Z.Y.; Ma, Y.L.; Zhang, J.T.; Fan, N.S.; Huang, B.C.; Jin, R.C. A critical review of antibiotic removal strategies: Performance and mechanisms. J. Water Process Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Xu, P.; Li, Y.; Jia, X.; Song, H. Fabrication of compressible and underwater superoleophobic carbon/g-C3N4 aerogel for wastewater purification. Mater. Lett. 2019, 254, 210–213. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Kumar, P.S.; Mohamed, R.M.S.R.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of rhodamine B from textile wastewater using nanoparticle photocatalysts: A review for sustainable approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Sharma, K.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Singh, P. Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem. 2019, 78, 1–20. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Rashid, J.; Abbas, A.; Chang, L.C.; Iqbal, A.; Haq, I.U.; Rehman, A.; Awan, S.U.; Arshad, M.; Rafique, M.; Barakat, M.A. Butterfly cluster like lamellar BiOBr/TiO2 nanocomposite for enhanced sunlight photocatalytic mineralization of aqueous ciprofloxacin. Sci. Total Environ. 2019, 665, 668–677. [Google Scholar] [CrossRef]
- Zang, J.; Chen, C.; Chen, X.; Yang, Y. Bi4O5Br2 nanosheets modified by carbon quantum dots: Efficient BPA degradation induced by enhanced absorption and Z-scheme charge transfer. J. Alloys Compd. 2023, 935, 167988. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.; Wang, L.; Ye, L. Bismuth-rich Bi4O5X2 (X = Br, and I) nanosheets with dominant {101} facets exposure for photocatalytic H2 evolution. Chem. Eng. J. 2016, 304, 454–460. [Google Scholar] [CrossRef]
- Mao, D.; Ding, S.; Meng, L.; Dai, Y.; Sun, C.; Yang, S.; He, H. One-pot microemulsion-mediated synthesis of Bi-rich Bi4O5Br2 with controllable morphologies and excellent visible-light photocatalytic removal of pollutants. Appl. Catal. B Environ. 2017, 207, 153–165. [Google Scholar] [CrossRef]
- Zhao, X.; You, Y.; Huang, S.; Wu, Y.; Ma, Y.; Zhang, G.; Zhang, Z. Z-scheme photocatalytic production of hydrogen peroxide over Bi4O5Br2/g-C3N4 heterostructure under visible light. Appl. Catal. B Environ. 2020, 278, 119251. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, X.; Arif, M.; Xia, J.; Fan, H.; Luo, J.; He, G.; Chen, H. Z-scheme Bi4O5Br2/MIL-88B(Fe) heterojunction for boosting visible light catalytic oxidation of tetracycline hydrochloride. Appl. Surf. Sci. 2023, 611, 155667. [Google Scholar] [CrossRef]
- Jin, X.; Lv, C.; Zhou, X.; Xie, H.; Sun, S.; Liu, Y.; Meng, Q.; Chen, G. A bismuth rich hollow Bi4O5Br2 photocatalyst enables dramatic CO2 reduction activity. Nano Energy 2019, 64, 103955. [Google Scholar] [CrossRef]
- Chang, F.; Li, S.; Shi, Z.; Qi, Y.; Liu, D.; Liu, X.; Chen, S. Boosted photocatalytic NO removal performance by S-scheme hierarchical composites WO3/Bi4O5Br2 prepared through a facile ball-milling protocol. Sep. Purif. Technol. 2021, 278, 119662. [Google Scholar] [CrossRef]
- Chang, F.; Yan, W.; Wang, X.; Peng, S.; Li, S.; Hu, X. Strengthened photocatalytic removal of bisphenol a by robust 3D hierarchical n-p heterojunctions Bi4O5Br2-MnO2 via boosting oxidative radicals generation. Chem. Eng. J. 2022, 428, 131223. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Ma, S.; Xing, P.; Hu, X.; Wu, Y.; He, Y. Fabrication of a Z-scheme AgBr/Bi4O5Br2 nanocomposite and its high efficiency in photocatalytic N2 fixation and dye degradation. Inorg. Chem. Front. 2019, 6, 3083–3092. [Google Scholar] [CrossRef]
- Weng, P.; Cai, Q.; Wu, H.; Zhang, L.; Wu, K.; Guo, J. Facile synthesis of flower-like CQDs/S-Bi4O5Br2 composites as a highly efficient visible-light response photocatalyst for ciprofloxacin degradation. J. Mater. Sci. 2022, 57, 1977–1993. [Google Scholar] [CrossRef]
- Wu, D.; Wu, C.; Tan, H. Facile synthesis of novel I-doped Bi4O5Br2 nanosheets with enhanced visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 11090–11095. [Google Scholar] [CrossRef]
- Jia, X.; Shen, Z.; Han, Q.; Bi, H. Rod-like Bi4O5I2/Bi4O5Br2 step-scheme heterostructure with oxygen vacancies synthesized by calcining the solid solution containing organic group. Chin. J. Catal. 2022, 43, 288–302. [Google Scholar] [CrossRef]
- Li, P.; Cao, W.; Zhu, Y.; Teng, Q.; Peng, L.; Jiang, C.; Feng, C.; Wang, Y. NaOH-induced formation of 3D flower-sphere BiOBr/Bi4O5Br2 with proper-oxygen vacancies via in-situ self-template phase transformation method for antibiotic photodegradation. Sci. Total Environ. 2020, 715, 136809. [Google Scholar] [CrossRef]
- Zhang, X.; Zha, X.; Luo, Y.; Liu, T.; Chen, G.; He, X. In2O3 Nanoparticle/Bi4O5Br2 Nanosheet S-Scheme Heterojunctions with Interfacial Oxygen Vacancies for Photocatalytic Degradation of Tetracycline. ACS Appl. Nano Mater. 2023, 6, 11877–11887. [Google Scholar] [CrossRef]
- Qian, X.; Ma, Y.; Arif, M.; Xia, J.; He, G.; Chen, H. Construction of 2D/2D Bi4O5Br2/Bi2WO6 Z-scheme heterojunction for highly efficient photodegradation of ciprofloxacin under visible light. Sep. Purif. Technol. 2023, 316, 123794. [Google Scholar] [CrossRef]
- Li, Y.; Sun, B.; Lin, H.; Ruan, Q.; Geng, Y.; Liu, J.; Wang, H.; Yang, Y.; Wang, L.; Tam, K.C. Efficient visible-light induced H2 evolution from T-CdxZn1−xS/defective MoS2 nano-hybrid with both bulk twinning homojunctions and interfacial heterostructures. Appl. Catal. B Environ. 2020, 267, 118702. [Google Scholar] [CrossRef]
- Song, J.; Sun, R.; Chen, Y.; Sun, D.; Li, X. l-Cysteine assisted synthesis of Zn0.5Cd0.5S solid solution with different morphology, crystal structure and performance for H2 evolution. Int. J. Hydrogen Energy 2018, 43, 18220–18231. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, D.; Li, H.; Kondamareddy, K.K.; Wang, H.; Zhang, B.; Wang, J.; Wu, Q.; Zeng, Y.; Zhang, X.; et al. Enhanced visible Light-Driven photocatalytic hydrogen evolution and stability for noble Metal-Free MoS2/Zn0.5Cd0.5S heterostructures with W/Z phase junctions. Appl. Surf. Sci. 2022, 586, 152770. [Google Scholar] [CrossRef]
- Dong, W.; Cai, T.; Wang, L.; Liu, C.; Chen, H.; Li, W.; Liu, Y.; Xia, X. Optimized removal of antibiotics over Cd0.5Zn0.5S/NiCo-LDH: Constructing a homojunctions-heterojunctions composite photocatalyst. J. Environ. Chem. Eng. 2022, 10, 108624. [Google Scholar] [CrossRef]
- Qi, S.; Wang, D.; Zhao, Y.; Xu, H. Core–shell g-C3N4@Zn0.5Cd0.5S heterojunction photocatalysts with high photocatalytic activity for the degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2019, 30, 5284–5296. [Google Scholar] [CrossRef]
- Madhusudan, P.; Wageh, S.; Al-Ghamdi, A.A.; Zhang, J.; Cheng, B.; Yu, Y. Graphene-Zn0.5Cd0.5S nanocomposite with enhanced visible-light photocatalytic CO2 reduction activity. Appl. Surf. Sci. 2020, 506, 144683. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.-F.; Zhang, X.; Feng, Y.; Li, Y.; Yang, L.; Lu, H.; Yao, J. Constructing Cd0.5Zn0.5S@ZIF-8 nanocomposites through self-assembly strategy to enhance Cr(VI) photocatalytic reduction. J. Hazard. Mater. 2018, 349, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Miao, W.; Zhang, X.; Shi, Y.; Tang, Z.; Shi, H. Insight into the pathway, improvement of performance and photocatalytic mechanism of active carbon/Bi4O5Br2 composite for cefixime and rhodamine B removal. J. Photochem. Photobiol. Chem. 2023, 444, 114892. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.; Xiao, Y.; Jiang, Y.; Zhang, W.; Liu, Y.; Liu, Z.; Zhang, J. Boosting photoelectron transport in Zn0.5Cd0.5S/Sn3O4 heterostructure through close interface contact for enhancing photocatalytic H2 generation and degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2023, 311, 123243. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, Y.; Zhou, E.; Zhang, W.; Liu, Y.; Zhang, J.; Wu, X.; Qi, Q.; Liu, Z. In-suit fabricating an efficient electronic transport channels via S-scheme polyaniline/Cd0.5Zn0.5S heterojunction for rapid removal of tetracycline hydrochloride and hydrogen production. J. Mater. Sci. Technol. 2023, 153, 205–218. [Google Scholar] [CrossRef]
- Li, A.; Pang, H.; Li, P.; Zhang, N.; Chen, G.; Meng, X.; Liu, M.; Liu, X.; Ma, R.; Ye, J. Insights into the critical dual-effect of acid treatment on ZnxCd1−xS for enhanced photocatalytic production of syngas under visible light. Appl. Catal. B Environ. 2021, 288, 119976. [Google Scholar] [CrossRef]
- Madhusudan, P.; Shi, R.; Xiang, S.; Jin, M.; Chandrashekar, B.N.; Wang, J.; Wang, W.; Peng, O.; Amini, A.; Cheng, C. Construction of highly efficient Z-scheme ZnxCd1−xS/Au@g-C3N4 ternary heterojunction composite for visible-light-driven photocatalytic reduction of CO2 to solar fuel. Appl. Catal. B Environ. 2021, 282, 119600. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, C.; Chen, C.; Zhou, H.; Wang, Y. A novel Z-scheme CdS/Bi4O5Br2 heterostructure with mechanism analysis: Enhanced photocatalytic performance. J. Alloys Compd. 2021, 861, 158554. [Google Scholar] [CrossRef]
- Ye, L.; Jin, X.; Liu, C.; Ding, C.; Xie, H.; Chu, K.H.; Wong, P.K. Thickness-ultrathin and bismuth-rich strategies for BiOBr to enhance photoreduction of CO2 into solar fuels. Appl. Catal. B Environ. 2016, 187, 281–290. [Google Scholar] [CrossRef]
- Meng, S.; Bi, Y.; Yan, T.; Zhang, Y.; Wu, T.; Shao, Y.; Wei, D.; Du, B. Room-temperature fabrication of bismuth oxybromide/oxyiodide photocatalyst and efficient degradation of phenolic pollutants under visible light. J. Hazard. Mater. 2018, 358, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liu, Y.; Wang, C.; Lin, W.; Li, S. Novel Cd0.5Zn0.5S/Bi2MoO6 S-scheme heterojunction for boosting the photodegradation of antibiotic enrofloxacin: Degradation pathway, mechanism and toxicity assessment. Sep. Purif. Technol. 2023, 304, 122401. [Google Scholar] [CrossRef]
- Xia, D.; Hu, L.; Wang, Y.; Xu, B.; Liao, Y.; He, C.; Ye, L.; Liang, X.; Ye, Y.; Shu, D. Immobilization of facet-engineered Ag3PO4 on mesoporous Al2O3 for efficient industrial waste gas purification with indoor LED illumination. Appl. Catal. B Environ. 2019, 256, 117811. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Ren, H.; Li, M.; Chen, L. MoS2 nanosheets anchored on porous ZnSnO3 cubes as an efficient visible-light-driven composite photocatalyst for the degradation of tetracycline and mechanism insight. J. Hazard. Mater. 2020, 390, 122158. [Google Scholar] [CrossRef]
- Pei, C.Y.; Chen, Y.G.; Wang, L.; Chen, W.; Huang, G.B. Step-scheme WO3/CdIn2S4 hybrid system with high visible light activity for tetracycline hydrochloride photodegradation. Appl. Surf. Sci. 2021, 535, 147682. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Xu, Y.; You, Y.; Huang, H.; Guo, Y.; Zhang, Y. Bi4NbO8Cl {001} nanosheets coupled with g-C3N4 as 2D/2D heterojunction for photocatalytic degradation and CO2 reduction. J. Hazard. Mater. 2020, 381, 121159. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, Y.; Ji, H.; Huang, S.; Xie, M.; Zhao, Y.; Xu, H.; Li, H. Enhanced long-wavelength light utilization with polyaniline/bismuth-rich bismuth oxyhalide composite towards photocatalytic degradation of antibiotics. J. Colloid Interface Sci. 2019, 537, 101–111. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Dong, S.; Miao, X.; Zhang, M.; Sun, K.; Zhang, Y.; Cao, Z.; Sun, J. Wool-ball-like BiOBr@ZnFe-MOF composites for degradation organic pollutant under visible-light: Synthesis, performance, characterization and mechanism. Opt. Mater. 2022, 131, 112580. [Google Scholar] [CrossRef]
- Luangwanta, T.; Chachvalvutikul, A.; Kaowphong, S. Facile synthesis and enhanced photocatalytic activity of a novel FeVO4/Bi4O5Br2 heterojunction photocatalyst through step-scheme charge transfer mechanism. Colloids Surf. Physicochem. Eng. Asp. 2021, 627, 127217. [Google Scholar] [CrossRef]
- Ma, X.; Chen, K.; Niu, B.; Li, Y.; Wang, L.; Huang, J.; She, H.; Wang, Q. Preparation of BiOCl0.9I0.1/β-Bi2O3 composite for degradation of tetracycline hydrochloride under simulated sunlight. Chin. J. Catal. 2020, 41, 1535–1543. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Xiong, T.; Jiang, G.; Zhang, Y.; Wu, Z.; Dong, F. Activation of amorphous bismuth oxide via plasmonic Bi metal for efficient visible-light photocatalysis. J. Catal. 2017, 352, 102–112. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Wu, K.; Dai, G.; Chen, Q.; Zhou, L.; Zheng, J.; Ma, L.; Li, G.; Wang, W.; et al. Novel Ag-bridged dual Z-scheme g-C3N4/BiOI/AgI plasmonic heterojunction: Exceptional photocatalytic activity towards tetracycline and the mechanism insight. J. Environ. Sci. 2023, 131, 123–140. [Google Scholar] [CrossRef]
- Fei, Q.; Yin, H.; Yuan, C.; Zhang, Y.; Zhao, Q.; Lv, H.; Zhang, Y.; Zhang, Y. Visible-light-driven AgI/Bi4O5I2 S-scheme heterojunction for efficient tetracycline hydrochloride removal: Mechanism and degradation pathway. Chemosphere 2023, 337, 139326. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Ding, T.; Lu, M. Mixed 1T-2H phase MoSe2 as interfacial charge-transfer-bridge to boosting photocatalytic activity of dual Z-scheme AgI/1T-2H MoSe2/Bi4O5Br2 heterojunction. J. Alloys Compd. 2021, 875, 160092. [Google Scholar] [CrossRef]








| Catalyst | Dosage (g/L) | Concentration (mg/L) | Time (min) | Degradation Rate (%) | Reference |
|---|---|---|---|---|---|
| PANI/Bi4O5Br2 | 0.4 | 20 | 240 | 85.7 | [44] |
| BiOBr@ZnFe-MOF | 0.15 | 5 | 90 | 79.2 | [45] |
| Bi4O5I2/Bi4O5Br2 | 0.4 | 20 | 120 | 90.2 | [19] |
| FeVO4/Bi4O5Br2 | 0.5 | 20 | 150 | 88 | [46] |
| BiOCl0.9I0.1/β-Bi2O3 | 0.4 | 20 | 120 | 82.4 | [47] |
| Zn0.5Cd0.5S/Bi4O5Br2 | 0.4 | 20 | 60 | 84.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Shen, J.; Jin, B. Construction of Zn0.5Cd0.5S/Bi4O5Br2 Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. Inorganics 2024, 12, 127. https://doi.org/10.3390/inorganics12050127
Luo L, Shen J, Jin B. Construction of Zn0.5Cd0.5S/Bi4O5Br2 Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. Inorganics. 2024; 12(5):127. https://doi.org/10.3390/inorganics12050127
Chicago/Turabian StyleLuo, Lan, Juan Shen, and Bo Jin. 2024. "Construction of Zn0.5Cd0.5S/Bi4O5Br2 Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride" Inorganics 12, no. 5: 127. https://doi.org/10.3390/inorganics12050127
APA StyleLuo, L., Shen, J., & Jin, B. (2024). Construction of Zn0.5Cd0.5S/Bi4O5Br2 Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. Inorganics, 12(5), 127. https://doi.org/10.3390/inorganics12050127






