Structural and Spectroscopic Characterization of Co(II) Bis(Benzenedichlorodithiolate): An Intermediate in Hydrogen Evolution Catalysis
Abstract
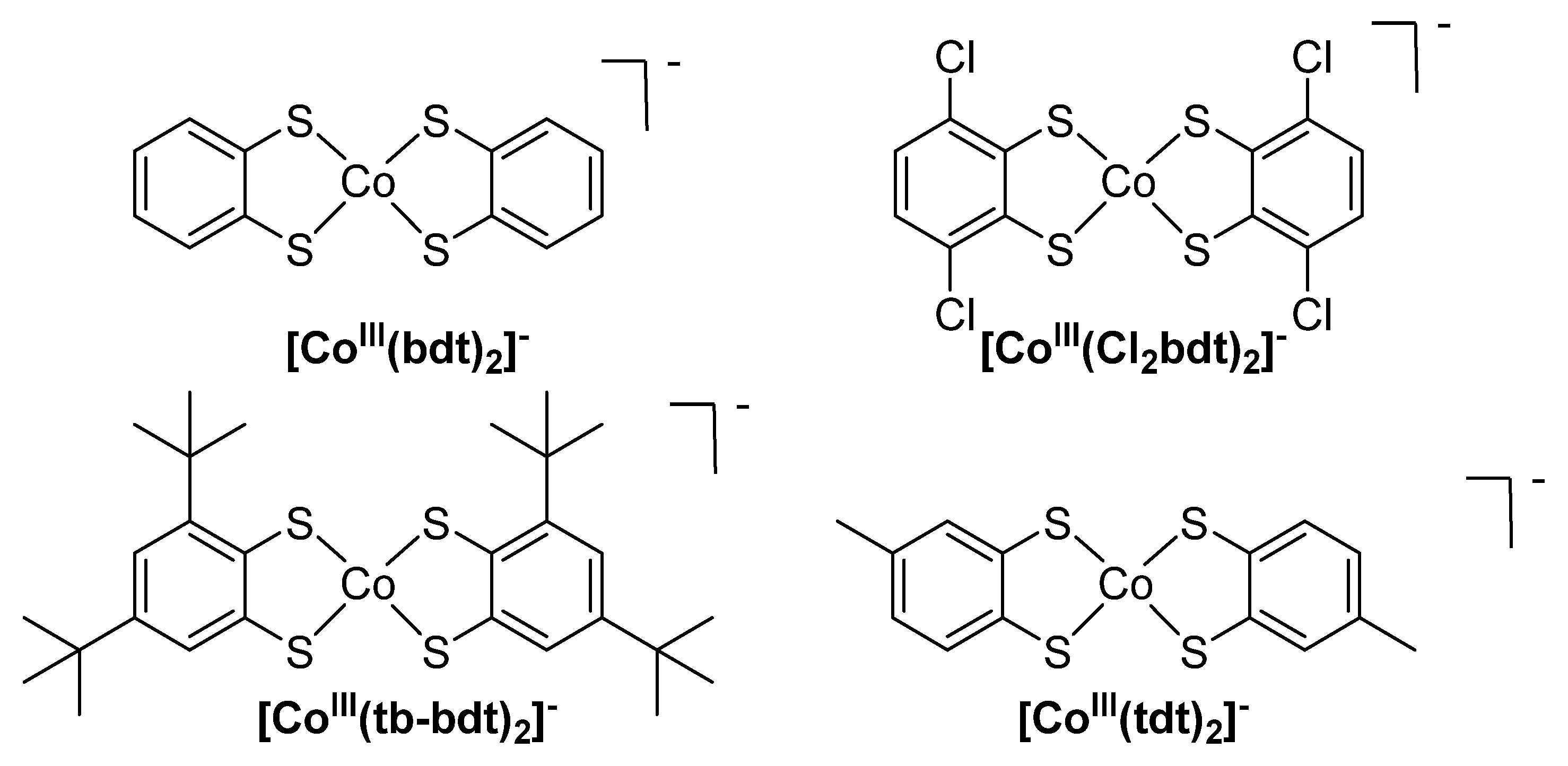
1. Introduction
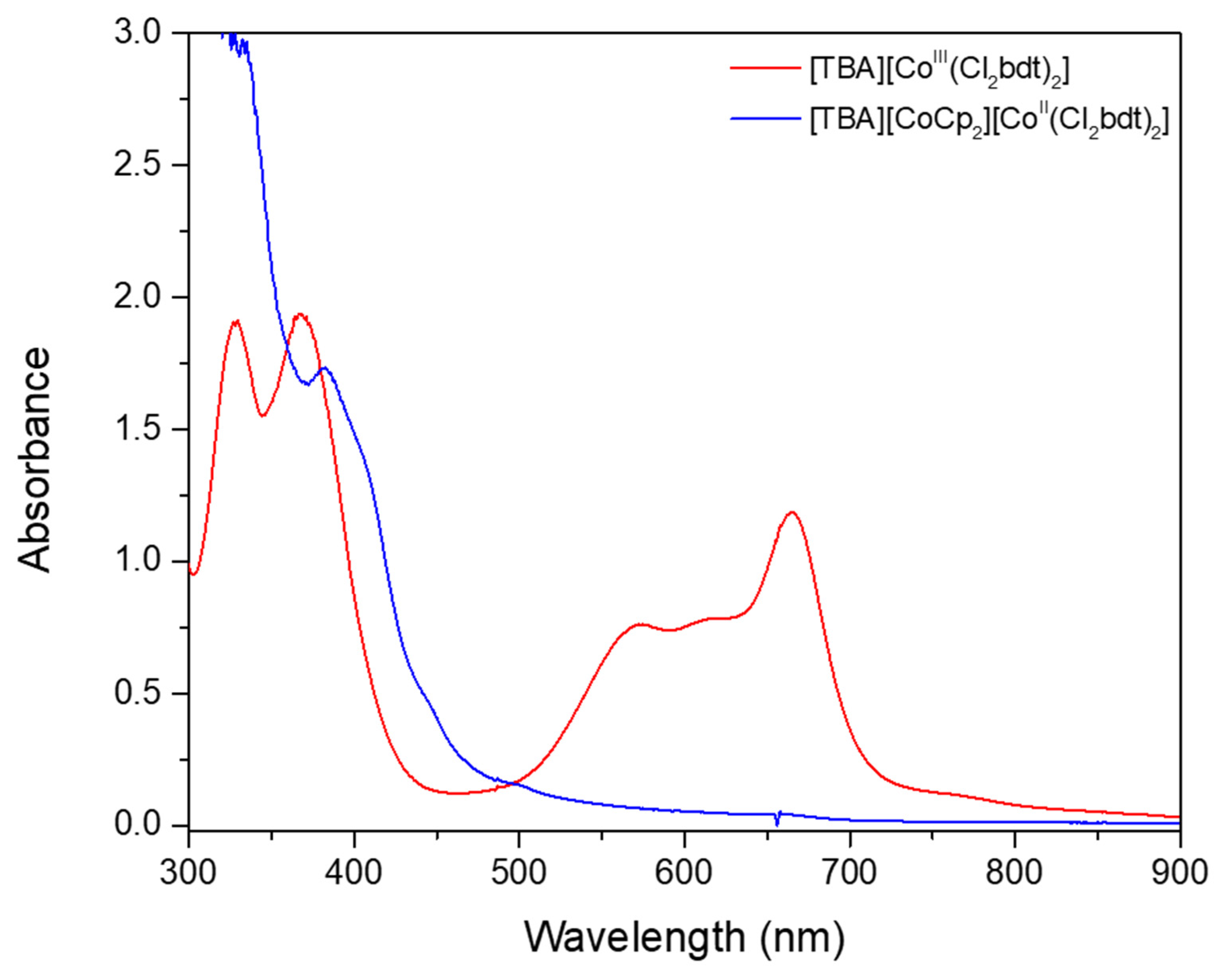
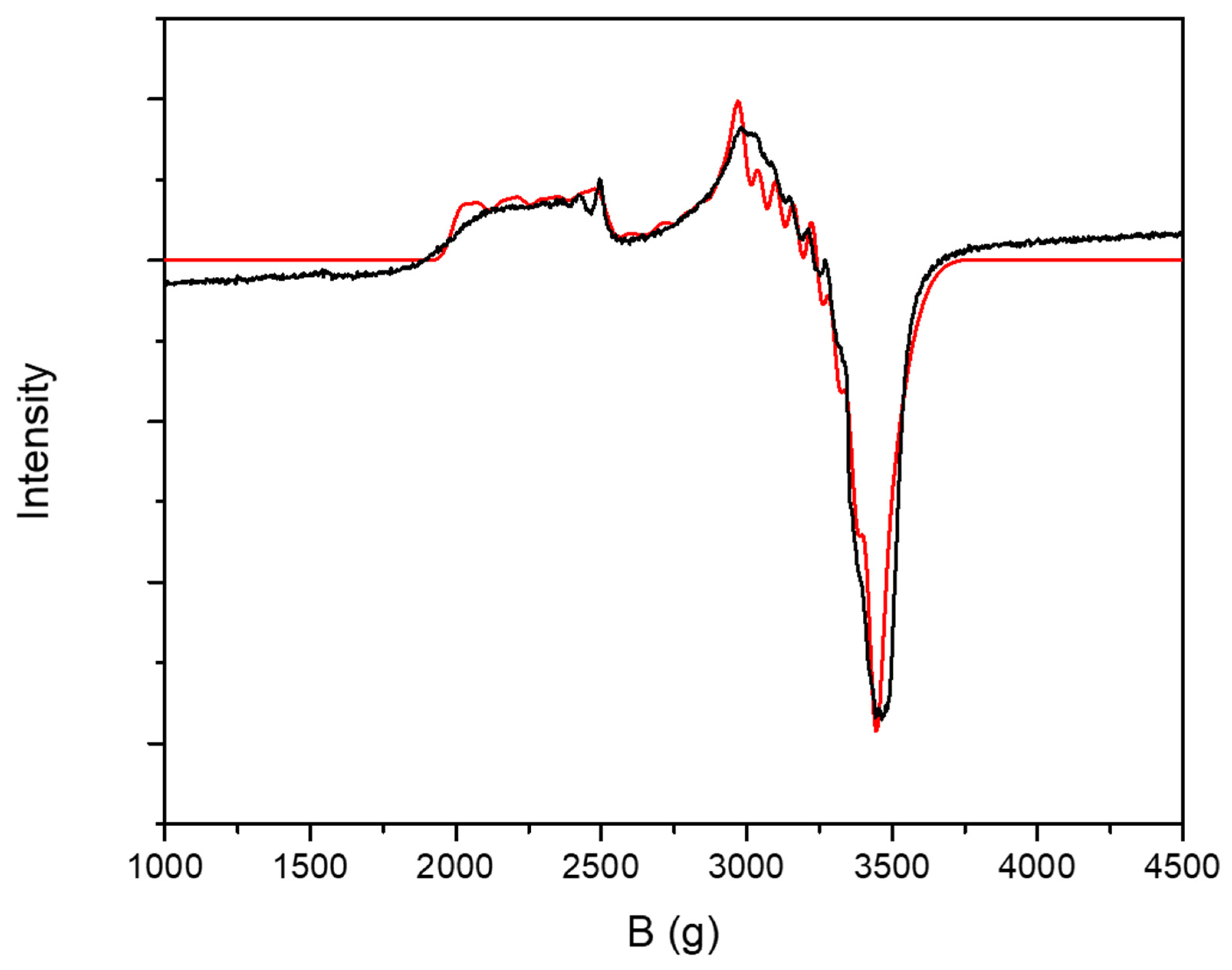
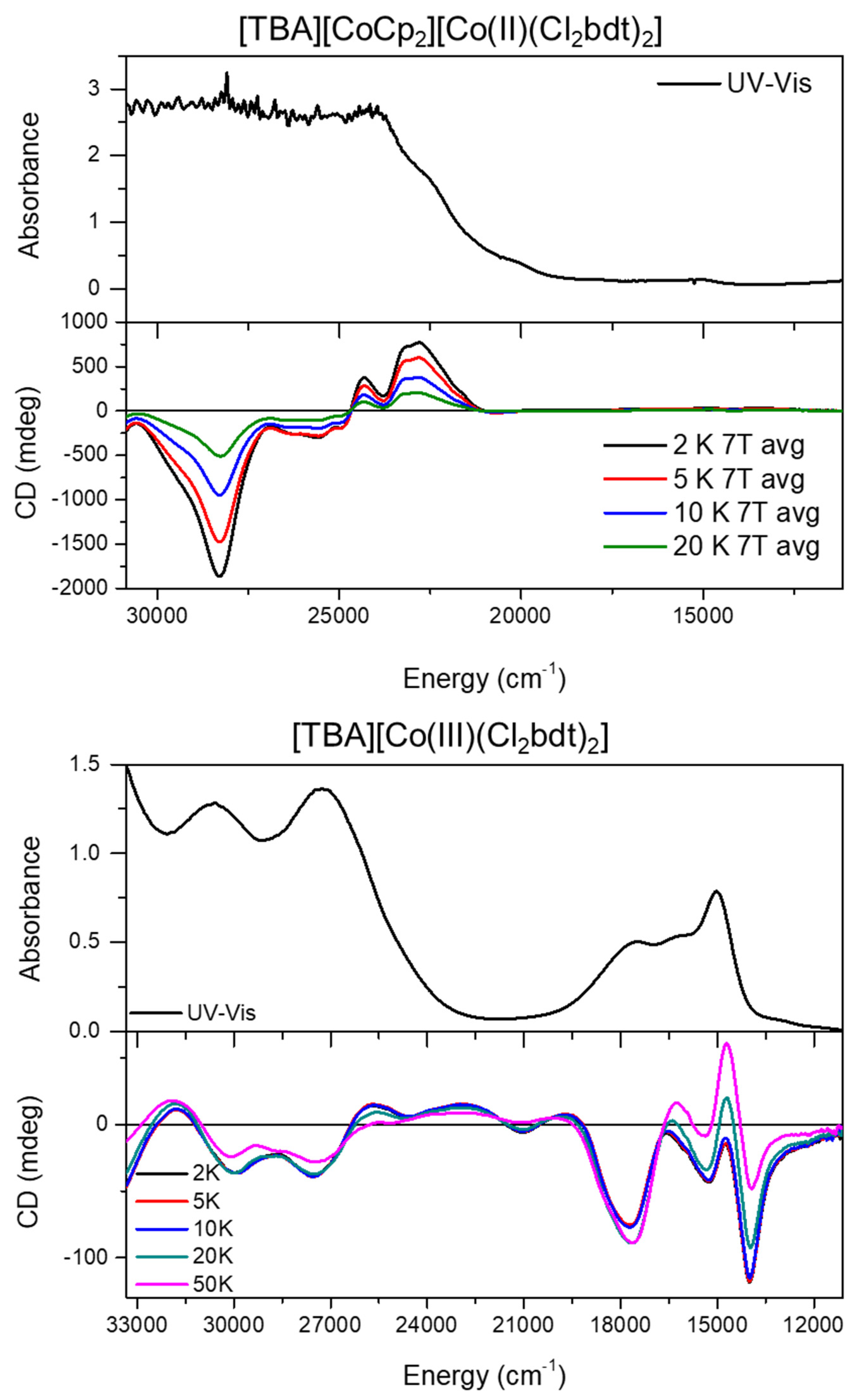
2. Results and Discussion
Synthesis and Characterization of [CoII(Cl2bdt)2]2−
3. Materials and Methods
3.1. General Synthesis and Chemicals
3.2. Reduction Procedures
3.3. Instrumentation
3.4. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McNamara, W.R.; Han, Z.; Alperin, P.J.; Brennessel, W.W.; Holland, P.L.; Eisenberg, R. A Cobalt–Dithiolene Complex for the Photocatalytic and Electrocatalytic Reduction of Protons. J. Am. Chem. Soc. 2011, 133, 15368–15371. [Google Scholar] [CrossRef] [PubMed]
- McNamara, W.R.; Han, Z.; Yin, C.-J.M.; Brennessel, W.W.; Holland, P.L.; Eisenberg, R.; By, E.; Meyer, T.J. Cobalt-Dithiolene Complexes for the Photocatalytic and Electrocatalytic Reduction of Protons in Aqueous Solutions. Proc. Natl. Acad. Sci. USA 2012, 109, 15594–15599. [Google Scholar] [CrossRef] [PubMed]
- Eady, S.C.; Peczonczyk, S.L.; Maldonado, S.; Lehnert, N. Facile Heterogenization of a Cobalt Catalyst via Graphene Adsorption: Robust and Versatile Dihydrogen Production Systems. Chem. Commun. 2014, 50, 8065–8068. [Google Scholar] [CrossRef] [PubMed]
- Eady, S.C.; MacInnes, M.M.; Lehnert, N. A Smorgasbord of Carbon: Electrochemical Analysis of Cobalt–Bis(Benzenedithiolate) Complex Adsorption and Electrocatalytic Activity on Diverse Graphitic Supports. ACS Appl. Mater. Interfaces 2016, 8, 23624–23634. [Google Scholar] [CrossRef] [PubMed]
- Eady, S.C.; MacInnes, M.M.; Lehnert, N. Immobilized Cobalt Bis(Benzenedithiolate) Complexes: Exceptionally Active Heterogeneous Electrocatalysts for Dihydrogen Production from Mildly Acidic Aqueous Solutions. Inorg. Chem. 2017, 56, 11654–11667. [Google Scholar] [CrossRef]
- Downes, C.A.; Marinescu, S.C. Efficient Electrochemical and Photoelectrochemical H2 Production from Water by a Cobalt Dithiolene One-Dimensional Metal-Organic Surface. J. Am. Chem. Soc. 2015, 137, 13740–13743. [Google Scholar] [CrossRef] [PubMed]
- Clough, A.J.; Yoo, J.W.; Mecklenburg, M.H.; Marinescu, S.C. Two-Dimensional Metal-Organic Surfaces for Efficient Hydrogen Evolution from Water. J. Am. Chem. Soc. 2015, 137, 118–121. [Google Scholar] [CrossRef]
- Downes, C.A.; Clough, A.J.; Chen, K.; Yoo, J.W.; Marinescu, S.C. Evaluation of the H2 Evolving Activity of Benzenehexathiolate Coordination Frameworks and the Effect of Film Thickness on H2 Production. ACS Appl. Mater. Interfaces 2018, 10, 1719–1727. [Google Scholar] [CrossRef]
- Downes, C.A.; Marinescu, S.C. Understanding Variability in the Hydrogen Evolution Activity of a Cobalt Anthracenetetrathiolate Coordination Polymer. ACS Catal. 2017, 7, 8605–8612. [Google Scholar] [CrossRef]
- Larson, V.A.; Lehnert, N. Covalent Attachment of Cobalt Bis(Benzylaminedithiolate) to Reduced Graphene Oxide as a Thin Film Electrocatalyst for Hydrogen Production with Remarkable Dioxygen Tolerance. ACS Catal. 2024, 14, 192–210. [Google Scholar] [CrossRef]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef] [PubMed]
- Drosou, M.; Kamatsos, F.; Mitsopoulou, C.A. Recent Advances in the Mechanisms of the Hydrogen Evolution Reaction by Non-Innocent Sulfur-Coordinating Metal Complexes. Inorg. Chem. Front. 2019, 7, 37–71. [Google Scholar] [CrossRef]
- Gray, H.B.; Billig, E. The Electronic Structures of Square-Planar Metal Complexes. III. High-Spin Planar Cobalt(I) and Iron(I). J. Am. Chem. Soc. 1963, 85, 2019–2020. [Google Scholar] [CrossRef]
- Eisenberg, R.; Gray, H.B. Noninnocence in Metal Complexes: A Dithiolene Dawn. Inorg. Chem. 2011, 50, 9741–9751. [Google Scholar] [CrossRef] [PubMed]
- Baker-Hawkes, M.J.; Billig, E.; Gray, H.B. Characterization and Electronic Structures of Metal Complexes Containing Benzene-1,2-Dithiolate and Related Ligands. J. Am. Chem. Soc. 1966, 88, 4870–4875. [Google Scholar] [CrossRef]
- Ray, K.; Begum, A.; Weyhermüller, T.; Piligkos, S.; Van Slageren, J.; Neese, F.; Wieghardt, K. The Electronic Structure of the Isoelectronic, Square-Planar Complexes [FeII(L)2]2- and [CoIII(LBu)2]- (L2- and (LBu)2- = Benzene-1,2-Dithiolates): An Experimental and Density Functional Theoretical Study. J. Am. Chem. Soc. 2005, 127, 4403–4415. [Google Scholar] [CrossRef]
- Benedito, F.L.; Petrenko, T.; Bill, E.; Weyhermüller, T.; Wieghardt, K. Square Planar Bis{3,6-Bis(Trimethylsilyl)Benzene-1,2-Dithiolato}metal Complexes of CrII, CoIII, and RhII: An Experimental and Density Functional Theoretical Study. Inorg. Chem. 2009, 48, 10913–10925. [Google Scholar] [CrossRef]
- Solis, B.H.; Hammes-Schiffer, S. Computational Study of Anomalous Reduction Potentials for Hydrogen Evolution Catalyzed by Cobalt Dithiolene Complexes. J. Am. Chem. Soc. 2012, 134, 15253–15256. [Google Scholar] [CrossRef]
- Letko, C.S.; Panetier, J.A.; Head-Gordon, M.; Tilley, T.D. Mechanism of the Electrocatalytic Reduction of Protons with Diaryldithiolene Cobalt Complexes. J. Am. Chem. Soc. 2014, 136, 9364–9376. [Google Scholar] [CrossRef]
- Zhang, C.; Prignot, E.; Jeannin, O.; Vacher, A.; Dragoe, D.; Camerel, F.; Halime, Z.; Gramage-Doria, R. Efficient Hydrogen Production at PH 7 in Water with a Heterogeneous Electrocatalyst Based on a Neutral Dimeric Cobalt-Dithiolene Complex. ACS Catal. 2023, 13, 2367–2373. [Google Scholar] [CrossRef]
- Panetier, J.A.; Letko, C.S.; Tilley, T.D.; Head-Gordon, M. Computational Characterization of Redox Non-Innocence in Cobalt-Bis (Diaryldithiolene)-Catalyzed Proton Reduction. J. Chem. Theory Comput. 2016, 12, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.T.; Srivatsa, G.S.; Bodini, M.E.; Schaefer, W.P.; Wing, R.M. Redox Chemistry and Spectroscopy of Toluene-3, 4-Dithiol (TDTH2) and of Its M (TDT) 22-/-Complexes with Zinc (II), Copper (II), Nickel (II), Cobalt (II), Iron (II), and Manganese (II). Formation of a Stable Dn-(.Cntdot. SR) Bond upon Oxidation by One Ele. J. Am. Chem. Soc. 1986, 108, 936–942. [Google Scholar] [CrossRef]
- Ray, K. Do S, S’-Coordinated o-Dithiobenzosemiquinonate(1-) Radicals Exist in Coordination Compounds? A Combined Experimental and Computational Study. Ph.D. Thesis, Ruhr-Universität Bochum, Bochum, Germany, 2005. [Google Scholar]
- Cerdeira, A.C.; Afonso, M.L.; Santos, I.C.; Pereira, L.C.J.; Coutinho, J.T.; Rabaça, S.; Simão, D.; Henriques, R.T.; Almeida, M. Synthesis, Structure and Physical Properties of Transition Metal Bis 4-Cyanobenzene-1,2-Dithiolate Complexes [M(Cbdt)2]Z−(M = Zn, Co, Cu, Au, Ni, Pd, Z = 0, 1, 2). Polyhedron 2012, 44, 228–237. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper (I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, τ 4. Dalt. Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Petasis, D.T.; Hendrich, M.P. Quantitative Interpretation of Multifrequency Multimode EPR Spectra of Metal Containing Proteins, Enzymes, and Biomimetic Complexes. Methods Enzymol. 2015, 563, 171–208. [Google Scholar] [CrossRef]
- Chen, K.; Downes, C.A.; Schneider, E.; Goodpaster, J.D.; Marinescu, S.C. Improving and Understanding the Hydrogen Evolving Activity of a Cobalt Dithiolene Metal–Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 16384–16395. [Google Scholar] [CrossRef]

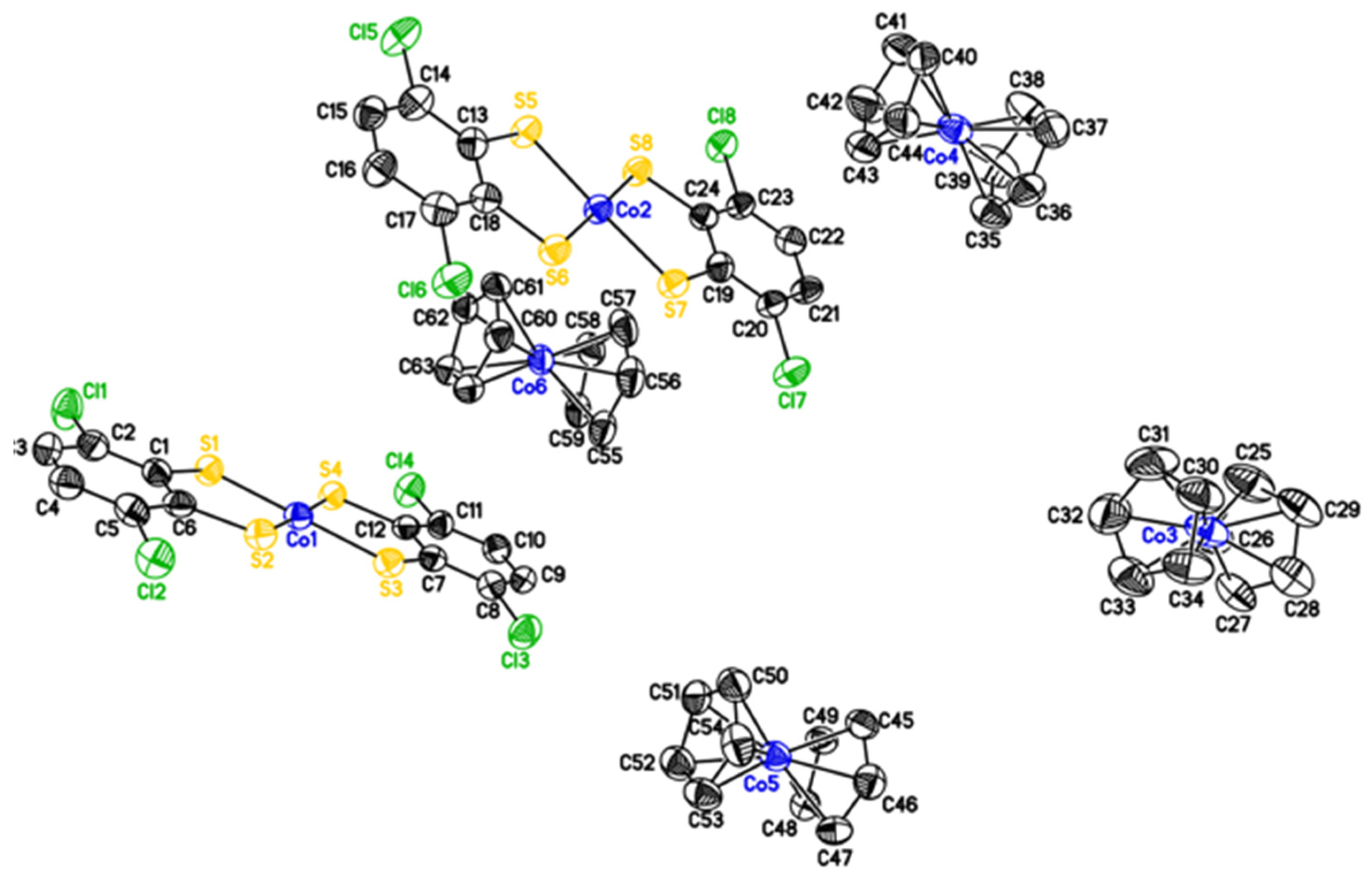

| Bond | Co(II) Catalyst (Å) | Co(III) Catalyst (Å) |
|---|---|---|
| Co-S | 2.182 (4) | 2.164 (5) |
| S-C | 1.749 (7) | 1.750 (3) |
| C-C | 1.395 (14) | 1.393 (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larson, V.A.; Kampf, J.W.; Lehnert, N. Structural and Spectroscopic Characterization of Co(II) Bis(Benzenedichlorodithiolate): An Intermediate in Hydrogen Evolution Catalysis. Inorganics 2024, 12, 75. https://doi.org/10.3390/inorganics12030075
Larson VA, Kampf JW, Lehnert N. Structural and Spectroscopic Characterization of Co(II) Bis(Benzenedichlorodithiolate): An Intermediate in Hydrogen Evolution Catalysis. Inorganics. 2024; 12(3):75. https://doi.org/10.3390/inorganics12030075
Chicago/Turabian StyleLarson, Virginia A., Jeff W. Kampf, and Nicolai Lehnert. 2024. "Structural and Spectroscopic Characterization of Co(II) Bis(Benzenedichlorodithiolate): An Intermediate in Hydrogen Evolution Catalysis" Inorganics 12, no. 3: 75. https://doi.org/10.3390/inorganics12030075
APA StyleLarson, V. A., Kampf, J. W., & Lehnert, N. (2024). Structural and Spectroscopic Characterization of Co(II) Bis(Benzenedichlorodithiolate): An Intermediate in Hydrogen Evolution Catalysis. Inorganics, 12(3), 75. https://doi.org/10.3390/inorganics12030075









