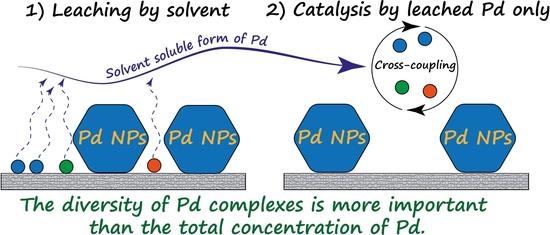
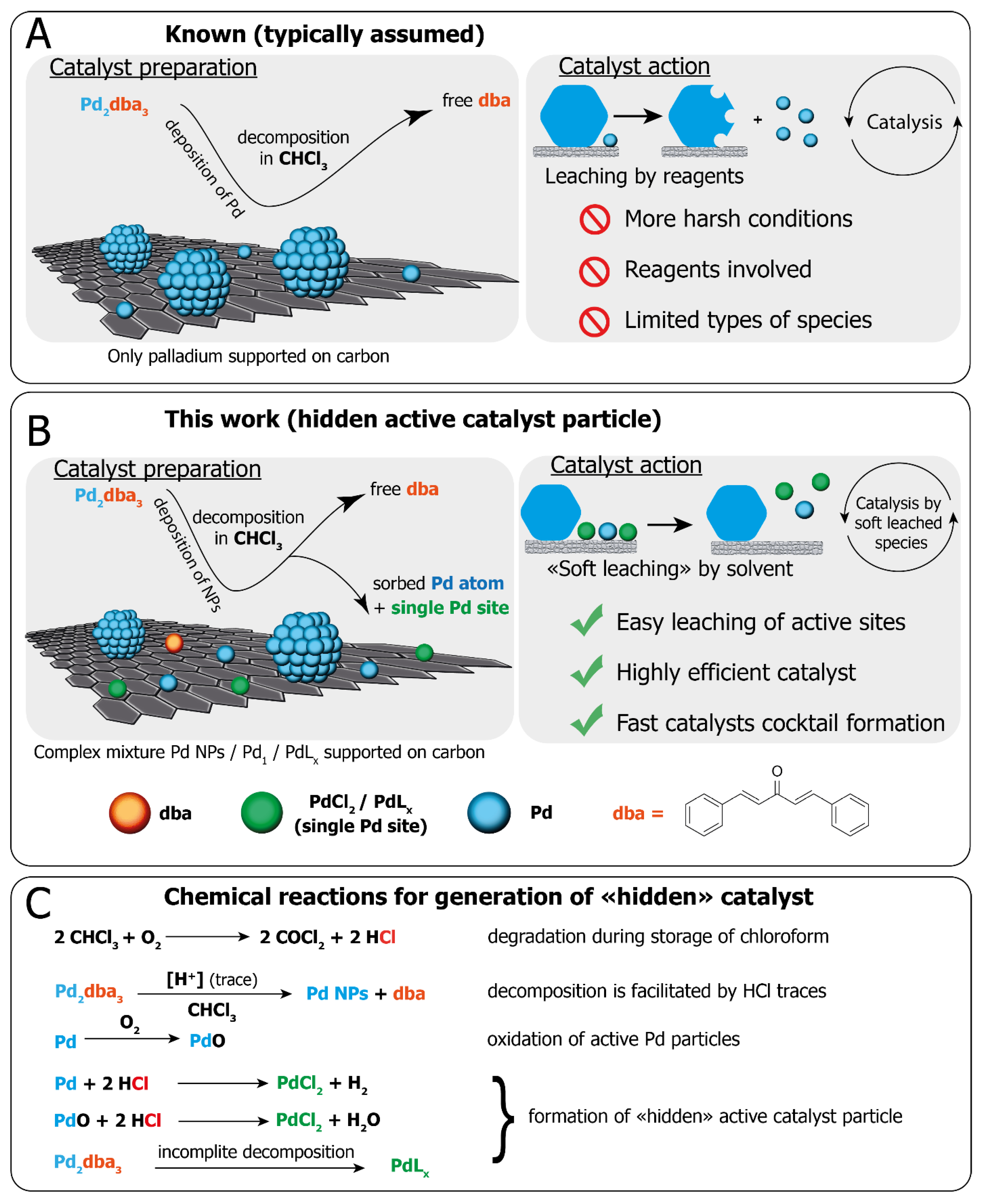
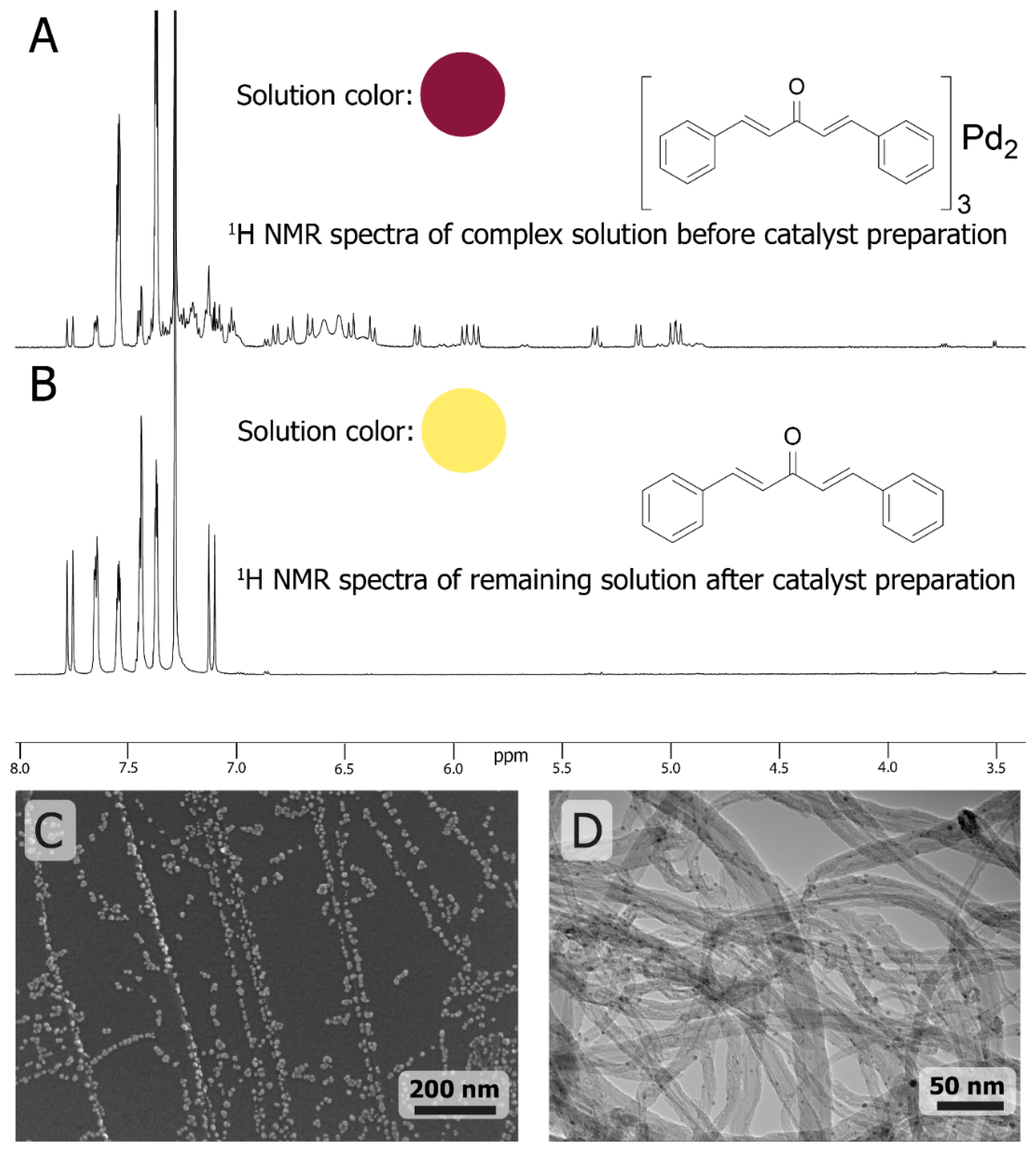
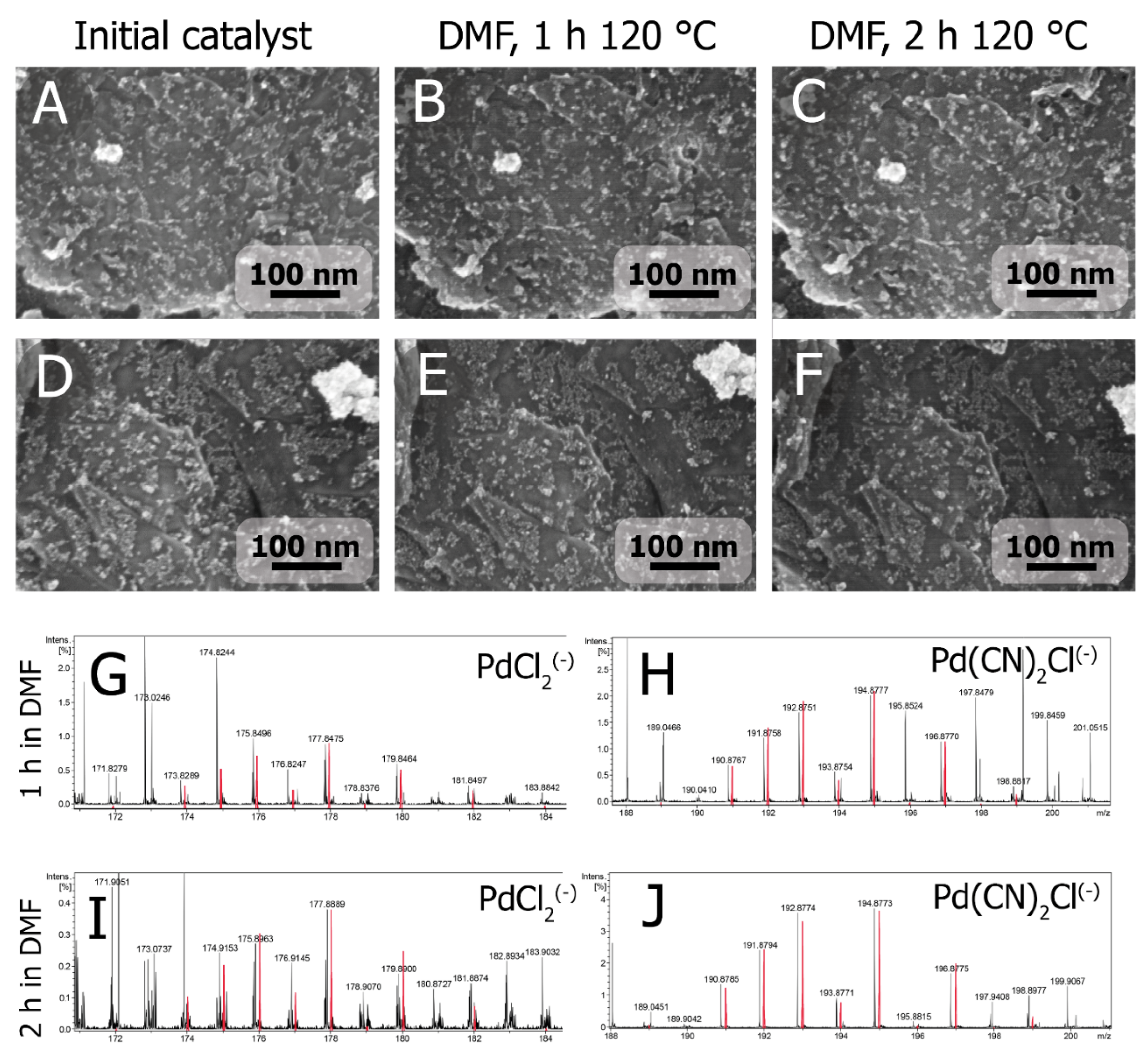
The Fast Formation of a Highly Active Homogeneous Catalytic System upon the Soft Leaching of Pd Species from a Heterogeneous Pd/C Precursor
Abstract
1. Introduction
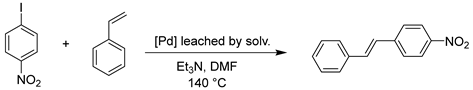
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rana, S.; Biswas, J.P.; Paul, S.; Paik, A.; Maiti, D. Organic synthesis with the most abundant transition metal-iron: From rust to multitasking catalysts. Chem. Soc. Rev. 2021, 50, 243–472. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, Q.; Nishihara, Y. Recent Advances in Transition-metal-catalyzed C-C Bond Formation via C(sp2)-F Bond Cleavage. Chem. Rec. 2021, 21, 3394–3410. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of Organosulfur Compounds Involving S-S Bond Cleavage of Disulfides and Sulfur. Molecules 2020, 25, 3595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Xia, Z.; Wu, C.; Zhang, Z.; Ma, Y.; Qu, Y. Towards highly active Pd/CeO2 for alkene hydrogenation by tuning Pd dispersion and surface properties of the catalysts. Nanoscale 2017, 9, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Song, Z.; Zhang, Z.; Xiao, Y.-S.; Zhang, M.; Hu, X.; Liu, Z.-W.; Qu, Y. Size-Controlled Synthesis of Pd Nanocatalysts on Defect-Engineered CeO2 for CO2 Hydrogenation. ACS Appl. Mater. Interfaces 2021, 13, 24957–24965. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, W.; Liu, X.; Astruc, D. Pd, Rh and Ru nanohybrid-catalyzed tetramethyldisiloxane hydrolysis for H2 generation, nitrophenol reduction and Suzuki–Miyaura cross-coupling. Inorg. Chem. Front. 2022, 9, 1416–1422. [Google Scholar] [CrossRef]
- Liu, Y.; Lopes, R.P.; Lüdtke, T.; Di Silvio, D.; Moya, S.; Hamon, J.-R.; Astruc, D. “Click” dendrimer-Pd nanoparticle assemblies as enzyme mimics: Catalytic o-phenylenediamine oxidation and application in colorimetric H2O2 detection. Inorg. Chem. Front. 2021, 8, 3301–3307. [Google Scholar] [CrossRef]
- Wang, W.; Chamkina, E.S.; Guisasola Cal, E.; Di Silvio, D.; Moro, M.M.; Moya, S.; Hamon, J.-R.; Astruc, D.; Shifrina, Z.B. Ferrocenyl-terminated polyphenylene-type “click” dendrimers as supports for efficient gold and palladium nanocatalysis. Dalton Trans. 2021, 50, 11852–11860. [Google Scholar] [CrossRef]
- Costa, N.J.S.; Vono, L.L.R.; Wojcieszak, R.; Teixiera-Neto, E.; Philippot, K.; Rossi, L.M. One-pot organometallic synthesis of alumina-embedded Pd nanoparticles. Dalton Trans. 2017, 46, 14318–14324. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhang, S.; Qu, Y. Modulated electronic structure of Pd nanoparticles on Mg(OH)2 for selective benzonitrile hydrogenation into benzylamine at a low temperature. Inorg. Chem. Front. 2022, 9, 4899–4906. [Google Scholar] [CrossRef]
- Gómez-Villarraga, F.; De Tovar, J.; Guerrero, M.; Nolis, P.; Parella, T.; Lecante, P.; Romero, N.; Escriche, L.; Bofill, R.; Ros, J.; et al. Dissimilar catalytic behavior of molecular or colloidal palladium systems with a new NHC ligand. Dalton Trans. 2017, 46, 11768–11778. [Google Scholar] [CrossRef]
- Costa, N.J.S.; Guerrero, M.; Collière, V.; Teixeira-Neto, É.; Landers, R.; Philippot, K.; Rossi, L.M. Organometallic Preparation of Ni, Pd, and NiPd Nanoparticles for the Design of Supported Nanocatalysts. ACS Catal. 2014, 4, 1735–1742. [Google Scholar] [CrossRef]
- Nie, W.; Luo, Y.; Yang, Q.; Feng, G.; Yao, Q.; Lu, Z.-H. An amine-functionalized mesoporous silica-supported PdIr catalyst: Boosting room-temperature hydrogen generation from formic acid. Inorg. Chem. Front. 2020, 7, 709–717. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Y.; Liu, S.; Yao, Q.; Qing, S.; Lu, Z.-H. A PdAg-CeO2 nanocomposite anchored on mesoporous carbon: A highly efficient catalyst for hydrogen production from formic acid at room temperature. J. Mater. Chem. A 2019, 7, 21438–21446. [Google Scholar] [CrossRef]
- Peng, W.; Liu, S.; Li, X.; Feng, G.; Xia, J.; Lu, Z.-H. Robust hydrogen production from HCOOH over amino-modified KIT-6-confined PdIr alloy nanoparticles. Chin. Chem. Lett. 2022, 33, 1403–1406. [Google Scholar] [CrossRef]
- Sharma, A.K.; Joshi, H.; Bhaskar, R.; Singh, A.K. Solvent-tailored Pd3P0.95 nano catalyst for amide–nitrile inter-conversion, the hydration of nitriles and transfer hydrogenation of the C=O bond. Dalton Trans. 2019, 48, 10962–10970. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.K. Palladium(II) complexes of N,N-diphenylacetamide based thio/selenoethers and flower shaped Pd16S7 and prismatic Pd17Se15 nano-particles tailored as catalysts for C–C and C–O coupling. Dalton Trans. 2017, 46, 10037–10049. [Google Scholar] [CrossRef]
- Arora, A.; Oswal, P.; Rao, G.K.; Kumar, S.; Singh, A.K.; Kumar, A. Catalytically active nanosized Pd9Te4 (telluropalladinite) and PdTe (kotulskite) alloys: First precursor-architecture controlled synthesis using palladium complexes of organotellurium compounds as single source precursors. RSC Adv. 2021, 11, 7214–7224. [Google Scholar] [CrossRef]
- Vásquez-Céspedes, S.; Betori, R.C.; Cismesia, M.A.; Kirsch, J.K.; Yang, Q. Heterogeneous Catalysis for Cross-Coupling Reactions: An Underutilized Powerful and Sustainable Tool in the Fine Chemical Industry? Org. Process. Res. Dev. 2021, 25, 740–753. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef] [PubMed]
- Zalesskiy, S.S.; Ananikov, V.P. Pd2(dba)3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis. Organometallics 2012, 31, 2302–2309. [Google Scholar] [CrossRef]
- Axet, M.R.; Dechy-Cabaret, O.; Durand, J.; Gouygou, M.; Serp, P. Coordination chemistry on carbon surfaces. Coord. Chem. Rev. 2016, 308, 236–345. [Google Scholar] [CrossRef]
- Bourouina, A.; Meille, V.; de Bellefon, C. About Solid Phase vs. Liquid Phase in Suzuki-Miyaura Reaction. Catalysts 2019, 9, 60. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Ciriminna, R.; Béland, F.; Demma Carà, P. Heterogeneous versus Homogeneous Palladium Catalysts for Cross-Coupling Reactions. Chemcatchem 2012, 4, 432–445. [Google Scholar] [CrossRef]
- Prima, D.O.; Kulikovskaya, N.S.; Galushko, A.S.; Mironenko, R.M.; Ananikov, V.P. Transition metal ‘cocktail’-type catalysis. Curr. Opin. Green Sustain. Chem. 2021, 31, 100502. [Google Scholar] [CrossRef]
- Crabtree, R.H. Resolving Heterogeneity Problems and Impurity Artifacts in Operationally Homogeneous Transition Metal Catalysts. Chem. Rev. 2012, 112, 1536–1554. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “Cocktails” of catalysts and dynamic systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Avanthay, M.; Bedford, R.B.; Begg, C.S.; Böse, D.; Clayden, J.; Davis, S.A.; Eloi, J.-C.; Goryunov, G.P.; Hartung, I.V.; Heeley, J.; et al. Identifying palladium culprits in amine catalysis. Nat. Catal. 2021, 4, 994–998. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. A Chem. 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. Catalytic C–C and C–Heteroatom Bond Formation Reactions: In Situ Generated or Preformed Catalysts? Complicated Mechanistic Picture Behind Well-Known Experimental Procedures. J. Org. Chem. 2013, 78, 11117–11125. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Orlov, N.V.; Zalesskiy, S.S.; Beletskaya, I.P.; Khrustalev, V.N.; Morokuma, K.; Musaev, D.G. Catalytic Adaptive Recognition of Thiol (SH) and Selenol (SeH) Groups Toward Synthesis of Functionalized Vinyl Monomers. J. Am. Chem. Soc. 2012, 134, 6637–6649. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Tan, F.; Zhang, Z.; Chen, X.; Liang, T.; Wu, C. A robust strategy of homogeneously hybridizing silica and Cu3(BTC)2 to in situ synthesize highly dispersed copper catalyst for furfural hydrogenation. Appl. Catal. A 2020, 596, 117518. [Google Scholar] [CrossRef]
- Qian, W.; Lin, L.; Qiao, Y.; Zhao, X.; Xu, Z.; Gong, H.; Li, D.; Chen, M.; Huang, R.; Hou, Z. Ru subnanoparticles on N-doped carbon layer coated SBA-15 as efficient Catalysts for arene hydrogenation. Appl. Catal. A 2019, 585, 117183. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Kashin, A.N.; Ganina, O.G.; Cheprakov, A.V.; Beletskaya, I.P. The Direct Non-Perturbing Leaching Test in the Phosphine-Free Suzuki-Miyaura Reaction Catalyzed by Palladium Nanoparticles. Chemcatchem 2015, 7, 2113–2121. [Google Scholar] [CrossRef]
- Sigeev, A.S.; Peregudov, A.S.; Cheprakov, A.V.; Beletskaya, I.P. The Palladium Slow-Release Pre-Catalysts and Nanoparticles in the “Phosphine-Free” Mizoroki-Heck and Suzuki-Miyaura Reactions. Adv. Synth. Catal. 2015, 357, 417–429. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V. Recyclable Nanostructured Catalytic Systems in Modern Environmentally Friendly Organic Synthesis. Molecules 2010, 15, 4792–4814. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C–C cross-coupling reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Ziółkowski, J.J. Monomolecular, nanosized and heterogenized palladium catalysts for the Heck reaction. Coord. Chem. Rev. 2007, 251, 1281–1293. [Google Scholar] [CrossRef]
- Baumann, C.G.; De Ornellas, S.; Reeds, J.P.; Storr, T.E.; Williams, T.J.; Fairlamb, I.J.S. Formation and propagation of well-defined Pd nanoparticles (PdNPs) during C–H bond functionalization of heteroarenes: Are nanoparticles a moribund form of Pd or an active catalytic species? Tetrahedron 2014, 70, 6174–6187. [Google Scholar] [CrossRef]
- Reay, A.J.; Fairlamb, I.J.S. Catalytic C–H bond functionalisation chemistry: The case for quasi-heterogeneous catalysis. Chem. Commun. 2015, 51, 16289–16307. [Google Scholar] [CrossRef]
- Ellis, P.J.; Fairlamb, I.J.S.; Hackett, S.F.J.; Wilson, K.; Lee, A.F. Evidence for the Surface-Catalyzed Suzuki-Miyaura Reaction over Palladium Nanoparticles: An Operando XAS Study. Angew. Chem. Int. Ed. Engl. 2010, 49, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.W.J.; Ford, M.J.; Schotes, C.; Parker, R.R.; Whitwood, A.C.; Fairlamb, I.J.S. The ubiquitous cross-coupling catalyst system ‘Pd(OAc)2’/2PPh3 forms a unique dinuclear PdI complex: An important entry point into catalytically competent cyclic Pd3 clusters. Chem. Sci. 2019, 10, 7898–7906. [Google Scholar] [CrossRef]
- Fairlamb, I.J.S. Redox-Active NOx Ligands in Palladium-Mediated Processes. Angew. Chem. Int. Ed. Engl. 2015, 54, 10415–10427. [Google Scholar] [CrossRef]
- Zhao, F.; Shirai, M.; Arai, M. Palladium-catalyzed homogeneous and heterogeneous Heck reactions in NMP and water-mixed solvents using organic, inorganic and mixed bases. J. Mol. Catal. A Chem. 2000, 154, 39–44. [Google Scholar] [CrossRef]
- Zhaoa, F.; Murakamib, K.; Shiraia, M.; Arai, M. Recyclable Homogeneous/Heterogeneous Catalytic Systems for Heck Reaction through Reversible Transfer of Palladium Species between Solvent and Support. J. Catal. 2000, 194, 479–483. [Google Scholar] [CrossRef]
- Cantillo, D.; Kappe, C.O. Immobilized Transition Metals as Catalysts for Cross-Couplings in Continuous Flow-A Critical Assessment of the Reaction Mechanism and Metal Leaching. Chemcatchem 2014, 6, 3286–3305. [Google Scholar] [CrossRef]
- Huo, J.; Tessonnier, J.-P.; Shanks, B.H. Improving Hydrothermal Stability of Supported Metal Catalysts for Biomass Conversions: A Review. ACS Catal. 2021, 11, 5248–5270. [Google Scholar] [CrossRef]
- Otor, H.O.; Steiner, J.B.; García-Sancho, C.; Alba-Rubio, A.C. Encapsulation Methods for Control of Catalyst Deactivation: A Review. ACS Catal. 2020, 10, 7630–7656. [Google Scholar] [CrossRef]
- Galushko, A.S.; Gordeev, E.G.; Kashin, A.S.; Zubavichus, Y.V.; Ananikov, V.P. Visualization of catalyst dynamics and development of a practical procedure to study complex “cocktail”-type catalytic systems. Faraday Discuss. 2021, 229, 458–474. [Google Scholar] [CrossRef]
- Fulignati, S.; Antonetti, C.; Licursi, D.; Pieraccioni, M.; Wilbers, E.; Heeres, H.J.; Galletti, A.M.R. Insight into the hydrogenation of pure and crude HMF to furan diols using Ru/C as catalyst. Appl. Catal. A 2019, 578, 122–133. [Google Scholar] [CrossRef]
- Novák, Z.; Szabó, A.; Répási, J.; Kotschy, A. Sonogashira Coupling of Aryl Halides Catalyzed by Palladium on Charcoal. J. Org. Chem. 2003, 68, 3327–3329. [Google Scholar] [CrossRef]
- Köhler, K.; Heidenreich, R.G.; Soomro, S.S.; Pröckl, S.S. Supported Palladium Catalysts for Suzuki Reactions: Structure-Property Relationships, Optimized Reaction Protocol and Control of Palladium Leaching. Adv. Synth. Catal. 2008, 350, 2930–2936. [Google Scholar] [CrossRef]
- Salamatmanesh, A.; Heydari, A. Magnetic nanostructure-anchored mixed-donor ligand system based on carboxamide and N-heterocyclic thiones: An efficient support of CuI catalyst for synthesis of imidazo[1,2-a]pyridines in eutectic medium. Appl. Catal. A 2021, 624, 118306. [Google Scholar] [CrossRef]
- Nikoshvili, L.; Bakhvalova, E.S.; Bykov, A.V.; Sidorov, A.I.; Vasiliev, A.L.; Matveeva, V.G.; Sulman, M.G.; Sapunov, V.N.; Kiwi-Minsker, L. Study of Deactivation in Suzuki Reaction of Polymer-Stabilized Pd Nanocatalysts. Processes 2020, 8, 1653. [Google Scholar] [CrossRef]
- Xu, Z.; Li, M.; Shen, G.; Chen, Y.; Lu, D.; Ren, P.; Jiang, H.; Wang, X.; Dai, B. Solvent Effects in the Preparation of Catalysts Using Activated Carbon as a Carrier. Nanomaterials 2023, 13, 393. [Google Scholar] [CrossRef]
- Yakukhnov, S.A.; Pentsak, E.O.; Galkin, K.I.; Mironenko, R.M.; Drozdov, V.A.; Likholobov, V.A.; Ananikov, V.P. Rapid “Mix-and-Stir” Preparation of Well-Defined Palladium on Carbon Catalysts for Efficient Practical Use. Chemcatchem 2017, 10, 1869–1873. [Google Scholar] [CrossRef]
- Liu, X.; Astruc, D. Development of the Applications of Palladium on Charcoal in Organic Synthesis. Adv. Synth. Catal. 2018, 360, 3426–3459. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Eremin, D.B.; Gordeev, E.G.; Ananikov, V.P. Phantom Reactivity in Organic and Catalytic Reactions as a Consequence of Microscale Destruction and Contamination-Trapping Effects of Magnetic Stir Bars. ACS Catal. 2019, 9, 3070–3081. [Google Scholar] [CrossRef]
- Galushko, A.S.; Boiko, D.A.; Pentsak, E.O.; Eremin, D.B.; Ananikov, V.P. Time-Resolved Formation and Operation Maps of Pd Catalysts Suggest a Key Role of Single Atom Centers in Cross-Coupling. J. Am. Chem. Soc. 2023, 145, 9092–9103. [Google Scholar] [CrossRef] [PubMed]
- Nishchakova, A.D.; Bulusheva, L.G.; Bulushev, D.A. Supported Ni Single-Atom Catalysts: Synthesis, Structure, and Applications in Thermocatalytic Reactions. Catalysts 2023, 13, 845. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Li, Z.; Hong, R.; Zhang, Z.; Wang, H.; Wu, X.; Wu, Z. Single-Atom Catalysts in Environmental Engineering: Progress, Outlook and Challenges. Molecules 2023, 28, 3865. [Google Scholar] [CrossRef]
- Teipel, J.; Gottstein, V.; Hölzle, E.; Kaltenbach, K.; Lachenmeier, D.; Kuballa, T. An Easy and Reliable Method for the Mitigation of Deuterated Chloroform Decomposition to Stabilise Susceptible NMR Samples. Chemistry 2022, 4, 776–785. [Google Scholar] [CrossRef]
- Dann, E.K.; Gibson, E.K.; Blackmore, R.H.; Catlow, C.R.A.; Collier, P.; Chutia, A.; Erden, T.E.; Hardacre, C.; Kroner, A.; Nachtegaal, M.; et al. Structural selectivity of supported Pd nanoparticles for catalytic NH3 oxidation resolved using combined operando spectroscopy. Nat. Catal. 2019, 2, 157–163. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ward, T.R. Recent Advances in the Palladium Catalyzed Suzuki–Miyaura Cross-Coupling Reaction in Water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef]
- Ding, S.; Jiao, N. Direct Transformation of N,N-Dimethylformamide to −CN: Pd-Catalyzed Cyanation of Heteroarenes via C–H Functionalization. J. Am. Chem. Soc. 2011, 133, 12374–12377. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Smirnova, N.S.; Baeva, G.N.; Mashkovsky, I.S.; Gerasimov, E.Y.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. Single-Atom Alloy Pd1Ag10/CeO2–ZrO2 as a Promising Catalyst for Selective Alkyne Hydrogenation. Inorganics 2023, 11, 150. [Google Scholar] [CrossRef]
- Chen, Z.; Vorobyeva, E.; Mitchell, S.; Fako, E.; Ortuño, M.A.; López, N.; Collins, S.M.; Midgley, P.A.; Richard, S.; Vilé, G.; et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 2018, 13, 702–707. [Google Scholar] [CrossRef]
- Eremin, D.B.; Galushko, A.S.; Boiko, D.A.; Pentsak, E.O.; Chistyakov, I.V.; Ananikov, V.P. Toward Totally Defined Nanocatalysis: Deep Learning Reveals the Extraordinary Activity of Single Pd/C Particles. J. Am. Chem. Soc. 2022, 144, 6071–6079. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| Catalyst | Fraction of Leached Pd in Pure DMF in 1 h at 140 °C | Number of Pd-Containing Ions Detected by ESI-MS After Soft Leaching | NMR Conversion of ArI to Product in 5 h, Catalyzed by Only Leached Pd |
| Pd/MWCNTs | 0.54% | 8 (anions) + 6 (cations) | 30% |
| Pd/graphite | 20.38% | 8 (anions) | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galushko, A.S.; Ilyushenkova, V.V.; Burykina, J.V.; Shaydullin, R.R.; Pentsak, E.O.; Ananikov, V.P. The Fast Formation of a Highly Active Homogeneous Catalytic System upon the Soft Leaching of Pd Species from a Heterogeneous Pd/C Precursor. Inorganics 2023, 11, 260. https://doi.org/10.3390/inorganics11060260
Galushko AS, Ilyushenkova VV, Burykina JV, Shaydullin RR, Pentsak EO, Ananikov VP. The Fast Formation of a Highly Active Homogeneous Catalytic System upon the Soft Leaching of Pd Species from a Heterogeneous Pd/C Precursor. Inorganics. 2023; 11(6):260. https://doi.org/10.3390/inorganics11060260
Chicago/Turabian StyleGalushko, Alexey S., Valentina V. Ilyushenkova, Julia V. Burykina, Ruslan R. Shaydullin, Evgeniy O. Pentsak, and Valentine P. Ananikov. 2023. "The Fast Formation of a Highly Active Homogeneous Catalytic System upon the Soft Leaching of Pd Species from a Heterogeneous Pd/C Precursor" Inorganics 11, no. 6: 260. https://doi.org/10.3390/inorganics11060260
APA StyleGalushko, A. S., Ilyushenkova, V. V., Burykina, J. V., Shaydullin, R. R., Pentsak, E. O., & Ananikov, V. P. (2023). The Fast Formation of a Highly Active Homogeneous Catalytic System upon the Soft Leaching of Pd Species from a Heterogeneous Pd/C Precursor. Inorganics, 11(6), 260. https://doi.org/10.3390/inorganics11060260