XPS and NEXAFS Characterization of Mg/Zn and Mn Codoped Bismuth Tantalate Pyrochlores
Abstract
1. Introduction
2. Experimental Part
3. Results and Discussion
3.1. Phase Composition of the Bi2Zn(Mg)xMn1−xTa2O9.5−Δ
3.2. NEXAFS and XPS of the Bi2Mg(Zn)xMn1−xTa2O9.5−Δ
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanderah, T.A.; Levin, I.; Lufaso, M.W. An Unexpected Crystal-Chemical Principle for the Pyrochlore Structure. Eur. J. Inorg. Chem. 2005, 14, 2895–2901. [Google Scholar] [CrossRef]
- Hiroi, Z.; Yamaura, J.-I.; Yonezawa, S.; Harima, H. Chemical trends of superconducting properties in pyrochlore oxides. Phys. C Supercond. Appl. 2007, 460–462, 20–27. [Google Scholar] [CrossRef]
- Du, H.; Yao, X. Structural trends and dielectric properties of Bi-based pyrochlores. J. Mater. Sci. Mater. Electron. 2004, 15, 613–616. [Google Scholar]
- Bongers, P.F.; Van Meurs, E.R. Ferromagnetism in Compounds with Pyrochlore Structure. J. Appl. Phys. 1967, 38, 944–945. [Google Scholar] [CrossRef]
- Greedan, J.E. Frustrated rare earth magnetism: Spin glasses, spin liquids and spin ices in pyrochlore oxides. J. Alloys Compd. 2006, 408–412, 444–455. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Ellert, O.G.; Popova, E.F.; Kirdyankin, D.I.; Maksimov, Y.V. Weak ferromagnetism in a diamagnetically diluted sublattice of Fe3+ ions in solid solutions Y2 − xFe1 + xTaO7 (x = 0–0.2) with pyrochlore-like structure. Mendeleev Commun. 2023, 33, 519–521. [Google Scholar] [CrossRef]
- Giampaoli, G.; Siritanon, T.; Day, B.; Li, J.; Subramanian, M.A. Temperature in-dependent low loss dielectrics based on quaternary pyrochlore oxides. Prog. Solid State Chem. 2018, 50, 16–23. [Google Scholar] [CrossRef]
- Murugesan, S.; Huda, M.N.; Yan, Y.; Al-Jassim, M.M.; Subramanian, V. Band-Engineered Bismuth Titanate Pyrochlores for Visible Light Photocatalysis. J. Phys. Chem. C 2010, 114, 10598–10605. [Google Scholar] [CrossRef]
- Khaw, C.C.; Tan, K.B.; Lee, C.K. High temperature dielectric properties of cubic bismuth zinc tantalate. Ceram. Intern. 2009, 35, 1473–1480. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- McCauley, R.A. Structural Characteristics of Pyrochlore Formation. J. Appl. Phys. 1980, 51, 290–294. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Noren, L.; Liu, Y.; Withers, R.L.; Wei, X.R.; Elcombe, M.M. The disordered structures and low temperature dielectric relaxation properties of two misplaced-displacive cubic pyrochlores found in the Bi2O3-MO-Nb2O5 (M=Mg,Ni) systems. J. Sol. St. Chem. 2007, 180, 2558–2565. [Google Scholar] [CrossRef]
- Levin, I.; Amos, T.G.; Nino, J.C.; Vanderah, T.A.; Randall, C.A.; Lanagan, M.T. Structural Study of an Unusual Cubic Pyrochlore Bi1.5Zn0.92Nb1.5O6.92. J. Solid State Chem. 2002, 168, 69–75. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Vanderah, T.A.; Pazos, I.M.; Levin, I.; Roth, R.S.; Nino, J.C.; Provenzano, V.; Schenck, P.K. Phase formation, crystal chemistry, and properties in the system Bi2O3–Fe2O3–Nb2O5. J. Solid State Chem. 2006, 179, 3900–3910. [Google Scholar] [CrossRef]
- Vanderah, T.A.; Lufaso, M.W.; Adler, A.U.; Levin, I.; Nino, J.C.; Provenzano, V.; Schenck, P.K. Subsolidus phase equilibria and properties in the system Bi2O3:Mn2O3±x:Nb2O5. J. Solid State Chem. 2006, 179, 3467–3477. [Google Scholar] [CrossRef]
- Vanderah, T.A.; Siegrist, T.; Lufaso, M.W.; Yeager, M.C.; Roth, R.S.; Nino, J.C.; Yates, S. Phase Formation and Properties in the System Bi2O3:2CoO1+x:Nb2O5. Eur. J. Inorgan. Chem. 2006, 2006, 4908–4914. [Google Scholar] [CrossRef]
- Kamba, S.; Porokhonskyy, V.; Pashkin, A.; Bovtun, V.; Petzelt, J.; Nino, J.C.; Trolier-McKinstry, S.; Lanagan, M.T.; Randall, C.A. Anomalous broad dielectric relaxation in Bi1.5Zn1.0Nb1.5O7 pyrochlore. Phys. Rev. B 2002, 66, 054106. [Google Scholar] [CrossRef]
- Ismunandar; Kamiyama, T.; Oikawa, K.; Hoshikawa, A.; Kennedy, B.J.; Kubota, Y.; Kato, K. Static bismuth disorder in Bi2−x(CrTa)O7−y. Mater. Res. Bull. 2004, 39, 553–560. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Ellert, O.G.; Popova, E.F.; Kirdyankin, D.I.; Khramov, E.V.; Maksimov, Y.V. Phase Equilibria in the Sm2O3–Fe2O3–Ta2O5 System: Structural Transitions and Magnetic Properties of the Solid Solution Sm2−xFe1 + xTaO7. Russ. J. Inorg. Chem. 2022, 67, 1695–1705. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Sivkov, D.V.; Abdurakhmanov, I.E. Crystal structure, dielectric and thermal properties of cobalt doped bismuth tantalate pyrochlore. J. Mater. Res. Technol. 2023, 22, 1791–1799. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Kharton, V.V.; Koroleva, A.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Makeev, B.A.; Lebedev, A.M.; et al. Novel Ni-Doped Bismuth–Magnesium Tantalate Pyrochlores: Structural and Electrical Properties, Thermal Expansion, X-ray Photoelectron Spectroscopy, and Near-Edge X-ray Absorption Fine Structure Spectra. ACS Omega 2021, 6, 23262–23273. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Sekushin, N.A.; Nekipelov, S.V.; Kharton, V.V.; Sennikova, Y.D. Cu, Mg Codoped Bismuth Tantalate Pyrochlores: Crystal Structure, XPS Spectra, Thermal Expansion, and Electrical Properties. Inorg. Chem. 2022, 61, 4270–4282. [Google Scholar] [CrossRef]
- Chon, M.P.; Tan, K.B.; Khaw, C.C.; Zainal, Z.; Taufiq-Yap, Y.H.; Chen, S.K.; Tan, P.Y. Subsolidus phase equilibria and electrical properties of pyrochlores in the Bi2O3–CuO–Ta2O5 ternary system. J. Alloys Compd. 2016, 675, 116–127. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Popova, E.F.; Tyurin, A.V.; Khoroshilov, A.V. Synthesis and Thermodynamic Properties of the Ln2CrTaO7 (Ln = Sm, Gd, Y) Pyrochlores. Russ. J. Inorg. Chem. 2021, 66, 1649–1659. [Google Scholar] [CrossRef]
- Youn, H.-J.; Sogabe, T.; Randall, C.A.; Shrout, T.R.; Lanagan, M.T. Phase Relations and Dielectric Properties in the Bi2O3-ZnO-Ta2O5 System. J. Am. Ceram. Soc. 2001, 84, 2557–2562. [Google Scholar] [CrossRef]
- Khaw, C.C.; Tan, K.B.; Lee, C.K.; West, A.R. Phase equilibria and electrical properties of pyrochlore and zirconolite phases in the Bi2O3–ZnO–Ta2O5 system. J. Eur. Ceram. Soc. 2012, 32, 671–680. [Google Scholar] [CrossRef]
- Jusoh, F.A.; Tan, K.B.; Zainal, Z.; Chen, S.K.; Khaw, C.C.; Lee, O.J. Novel pyrochlores in the Bi2O3-Fe2O3-Ta2O5 (BFT) ternary system: Synthesis, structural and electrical properties. J. Mater. Res. Technol. 2020, 9, 11022–11034. [Google Scholar] [CrossRef]
- Tan, P.Y.; Tan, K.B.; Khaw, C.C.; Zainal, Z.; Chen, S.K.; Chon, M.P. Phase equilibria and dielectric properties of Bi3+(5/2)xMg2−xNb3–(3/2)xO14−x cubic pyrochlores. Ceram. Intern. 2014, 40, 4237–4246. [Google Scholar] [CrossRef]
- Tan, P.Y.; Tan, K.B.; Khaw, C.; Zainal, Z.; Chen, S.K.; Chon, M.P. Structural and electrical properties of bismuth magnesium tantalate pyrochlores. Ceram. Intern. 2012, 38, 5401–5409. [Google Scholar] [CrossRef]
- Lebedev, A.M.; Menshikov, K.A.; Nazin, V.G.; Stankevich, V.G.; Tsetlin, M.B.; Chumakov, R.G. NanoPES Photoelectron Beamline of the Kurchatov Synchrotron Radiation Source. J. Surface Investig. X-ray Synchrotron Neutron Tech. 2021, 15, 1039–1044. [Google Scholar] [CrossRef]
- Muktha, B.; Darriet, J.; Madras, G.; Guru Row, T.N. Crystal structures and photocatalysis of the triclinic polymorphs of BiNbO4 and BiTaO4. J. Solid State Chem. 2006, 179, 3919–3925. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rylchenko, E.P.; Makeev, B.A.; Sivkov, D.V.; Korolev, R.I.; Zhuk, N.A. Features of phase formation of pyrochlore-type Bi2Cr1/6Mn1/6Fe1/6Co1/6Ni1/6Cu1/6Ta2O9+Δ. Lett. Mater. 2022, 12, 486–492. [Google Scholar] [CrossRef]
- Allred, A.L.; Rochow, E.G. A scale of electronegativity based on electrostatic force. J. Inorg. Nucl. Chem. 1958, 5, 264–268. [Google Scholar] [CrossRef]
- Chezhina, N.V.; Korolev, D.A.; Fedorova, A.V.; Zhuk, N.A.; Lutoev, V.P.; Makeev, B.A. Structure, magnetic, and electrical properties of bismuth niobates doped with d-elements: XVIII. Magnetic susceptibility and ESR spectra of Bi2BaNb2−2xMn2xO9−δ solid solutions with layered perovskite-like structure. Russ. J. Gen. Chem. 2017, 87, 2525–2532. [Google Scholar] [CrossRef]
- Chezhina, N.V.; Korolev, D.A.; Fedorova, A.V.; Zhuk, N.A.; Shevchuk, S.S.; Nizovtsev, A.N. Structure, magnetic, and electrical properties of bismuth niobates doped with d-elements: XVII. Magnetic properties of Bi5Nb3–3xMn3xO15–δ solid solutions. Russ. J. Gen. Chem. 2017, 87, 2251–2257. [Google Scholar] [CrossRef]
- Khairallah, F.; Glisenti, A. XPS Study of MgO Nanopowders Obtained by Different Preparation Procedures. Surf. Sci. Spectra 2006, 13, 58–71. [Google Scholar] [CrossRef]
- Grissa, R.; Martinez, H.; Cotte, S.; Galipaud, J.; Pecquenard, B.; Cras, F.L. Thorough XPS analyses on overlithiated manganese spinel cycled around the 3V plateau. Appl. Surf. Sci. 2017, 411, 449–456. [Google Scholar] [CrossRef]
- Stranick, M.A. Mn2O3 by XPS. Surf. Sci. Spectra 1999, 6, 39–46. [Google Scholar] [CrossRef]
- Gri, F.; Bigiani, L.; Gasparotto, A.; Maccato, C.; Barreca, D. XPS investigation of F-doped MnO2 nanosystems fabricated by plasma assisted-CVD. Surf. Sci. Spectra 2018, 25, 024004. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Sarma, D.D.; Rao, C.N.R. XPES studies of oxides of second- and third-row transition metals including rare earths. J. Electron Spectrosc. Relat. Phenom. 1980, 20, 25–45. [Google Scholar] [CrossRef]
- Kasatikov, S.; Filatova, E.; Sakhonenkov, S.; Konashuk, A.; Makarov, A. Relationship between Ta Oxidation state and Its Local Atomic Coordination Symmetry in a Wide Range of Oxygen Non-stoichiometry Extent of TaOx. J. Phys. Chem. C 2019, 123, 6849–6860. [Google Scholar] [CrossRef]
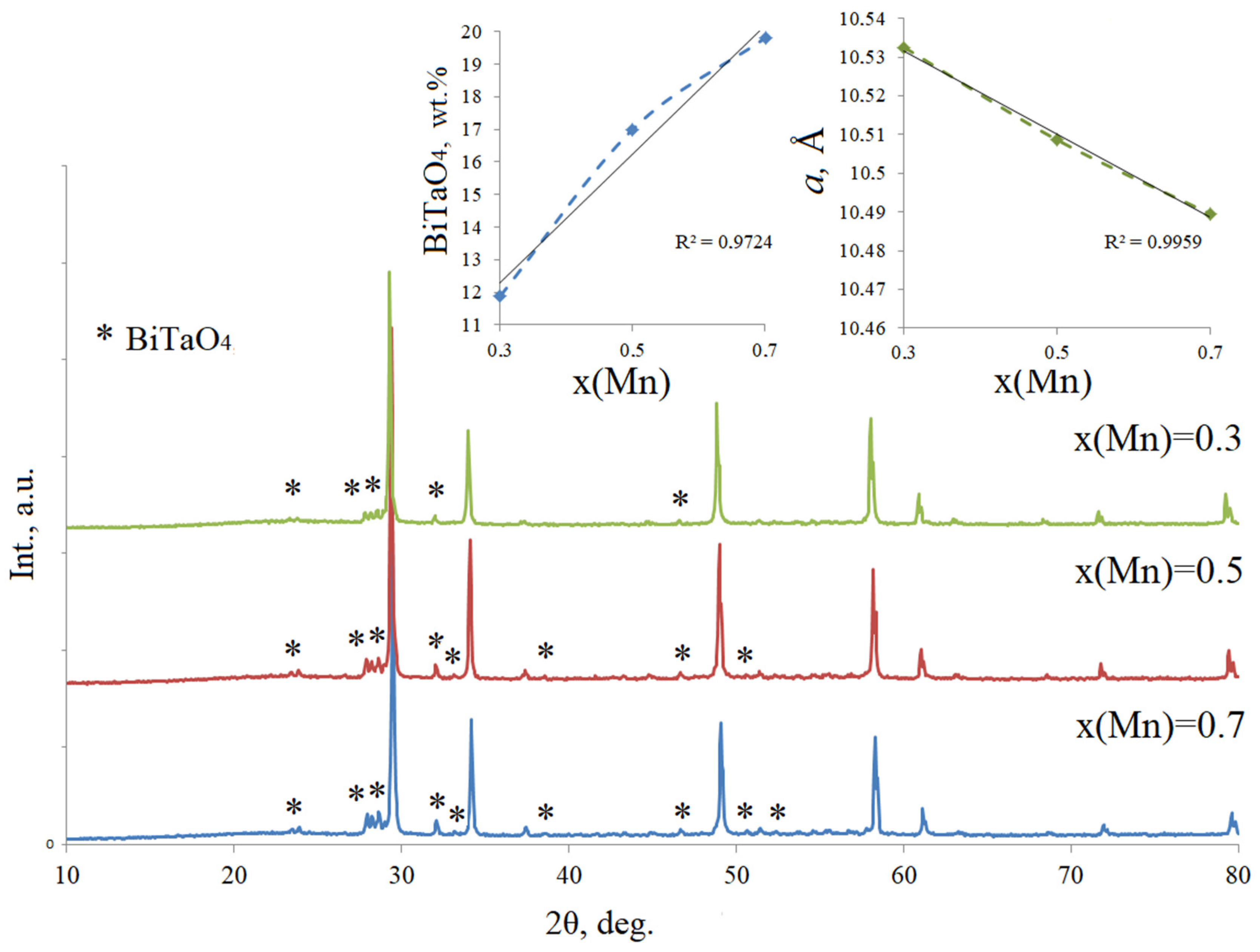

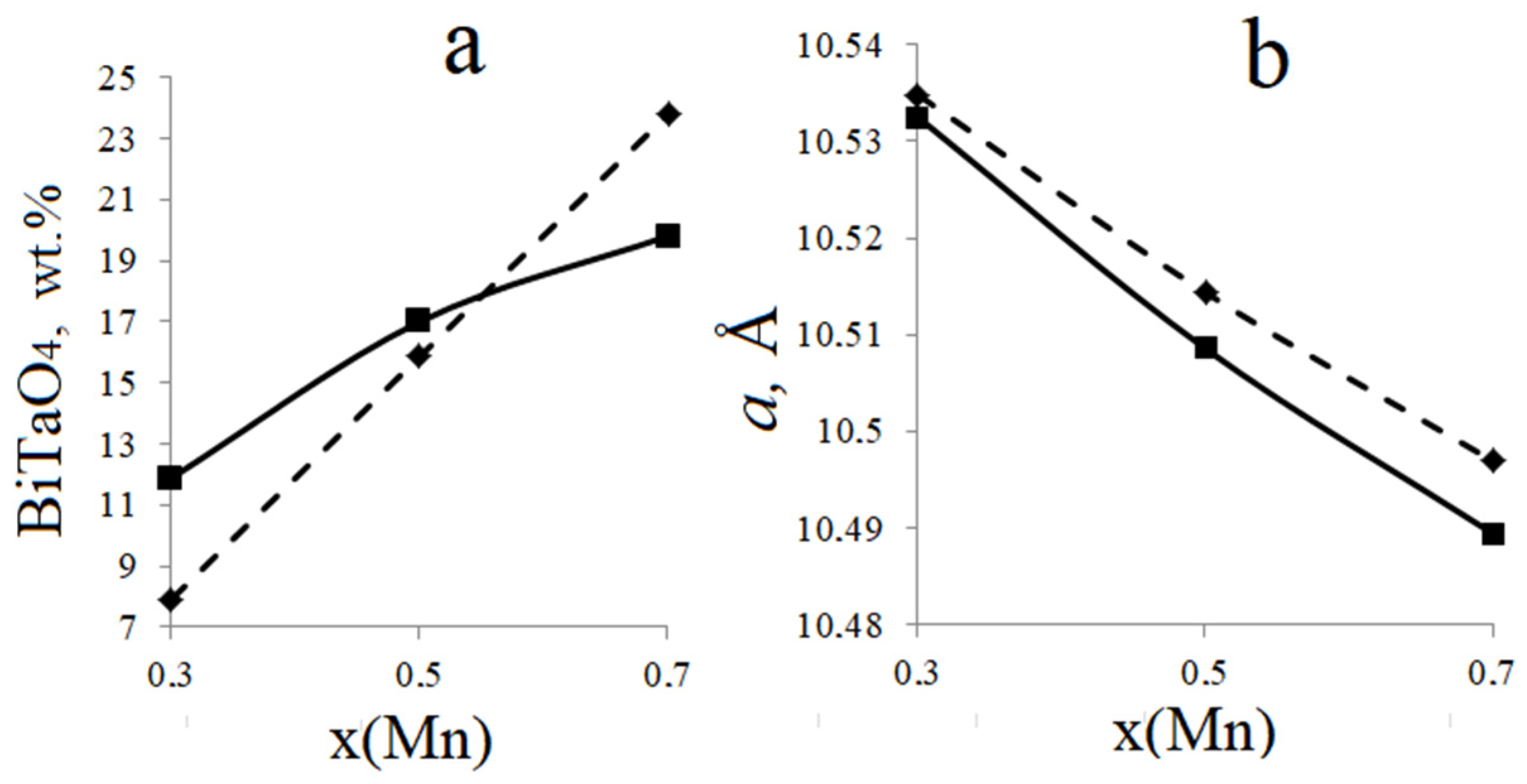
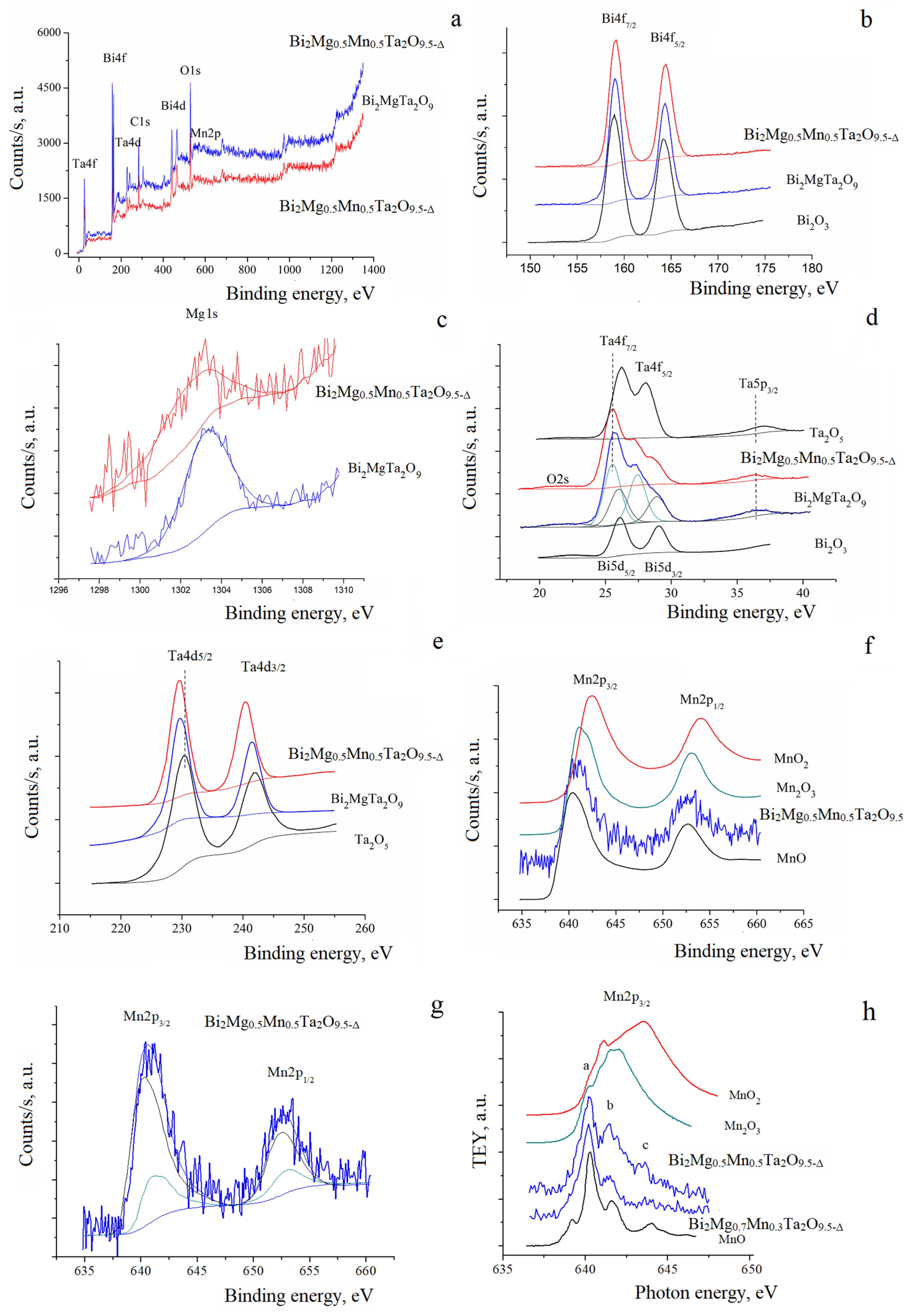

| Chemical Composition of Compounds | ||||
|---|---|---|---|---|
| Peak | Bi2Mg0.5Mn0.5Ta2O9.5−Δ | Bi2MgTa2O9 | Bi2Zn0.3Mn0.7Ta2O9.5−Δ | Bi2Zn0.7Mn0.3Ta2O9.5−Δ |
| Energy (eV) | ||||
| Bi4f7/2 | 158.80 | 159.03 | 158.81 | 158.79 |
| Bi4f5/2 | 164.12 | 164.35 | 164.13 | 164.10 |
| Bi5d5/2 | 25.82 | 26.11 | 25.81 | 25.87 |
| Bi5d3/2 | 28.72 | 29.08 | 28.71 | 28.78 |
| Ta4f7/2 | 25.31 | 25.66 | 25.35 | 25.41 |
| Ta4f5/2 | 27.21 | 27.56 | 27.25 | 27.31 |
| Mg1s | 1302.49 | 1303.19 | ||
| Mn2p3/2 | 640.83 | 640.98 | 640.71 | |
| Mn2p1/2 | 652.39 | 652.75 | 652.51 | |
| Zn2p3/2 | 1021.07 | 1020.98 | ||
| Zn2p1/2 | 1044.10 | 1043.99 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuk, N.A.; Makeev, B.A.; Koroleva, A.V.; Lebedev, A.M.; Petrova, O.V.; Nekipelov, S.V.; Sivkov, V.N. XPS and NEXAFS Characterization of Mg/Zn and Mn Codoped Bismuth Tantalate Pyrochlores. Inorganics 2024, 12, 74. https://doi.org/10.3390/inorganics12030074
Zhuk NA, Makeev BA, Koroleva AV, Lebedev AM, Petrova OV, Nekipelov SV, Sivkov VN. XPS and NEXAFS Characterization of Mg/Zn and Mn Codoped Bismuth Tantalate Pyrochlores. Inorganics. 2024; 12(3):74. https://doi.org/10.3390/inorganics12030074
Chicago/Turabian StyleZhuk, Nadezhda A., Boris A. Makeev, Aleksandra V. Koroleva, Aleksey M. Lebedev, Olga V. Petrova, Sergey V. Nekipelov, and Viktor N. Sivkov. 2024. "XPS and NEXAFS Characterization of Mg/Zn and Mn Codoped Bismuth Tantalate Pyrochlores" Inorganics 12, no. 3: 74. https://doi.org/10.3390/inorganics12030074
APA StyleZhuk, N. A., Makeev, B. A., Koroleva, A. V., Lebedev, A. M., Petrova, O. V., Nekipelov, S. V., & Sivkov, V. N. (2024). XPS and NEXAFS Characterization of Mg/Zn and Mn Codoped Bismuth Tantalate Pyrochlores. Inorganics, 12(3), 74. https://doi.org/10.3390/inorganics12030074






