Microporous N- and O-Codoped Carbon Materials Derived from Benzoxazine for Supercapacitor Application
Abstract
1. Introduction
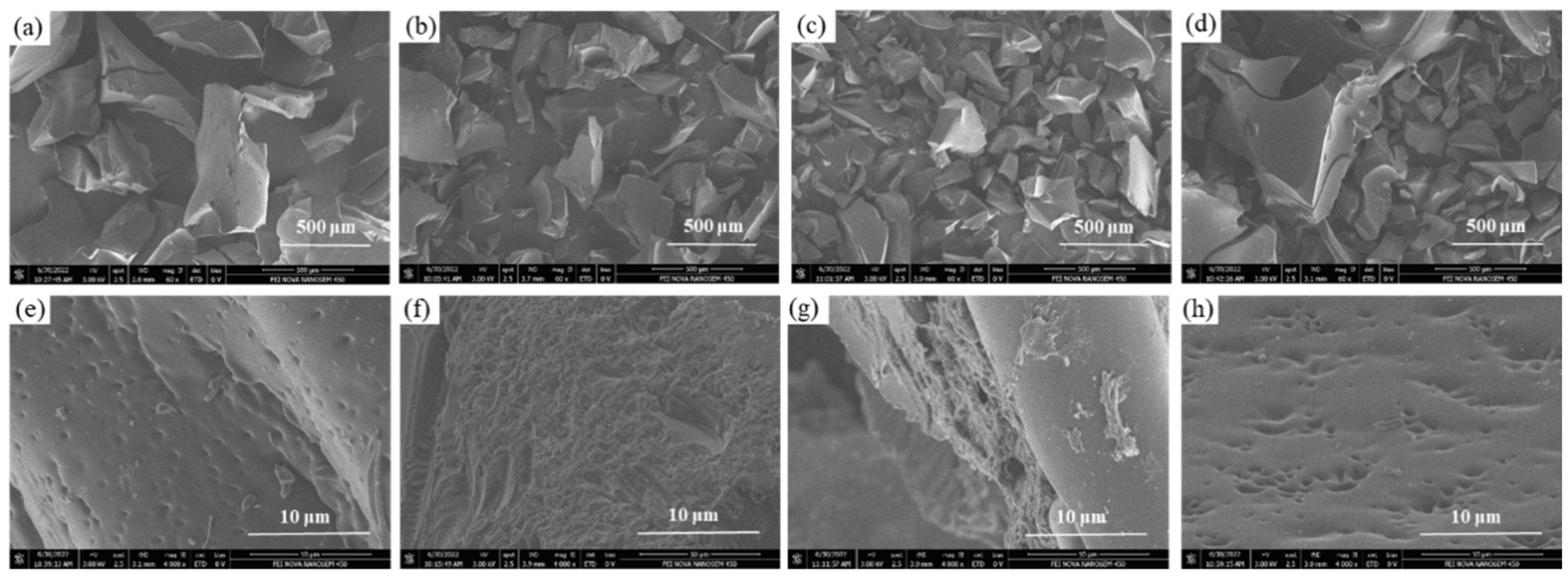
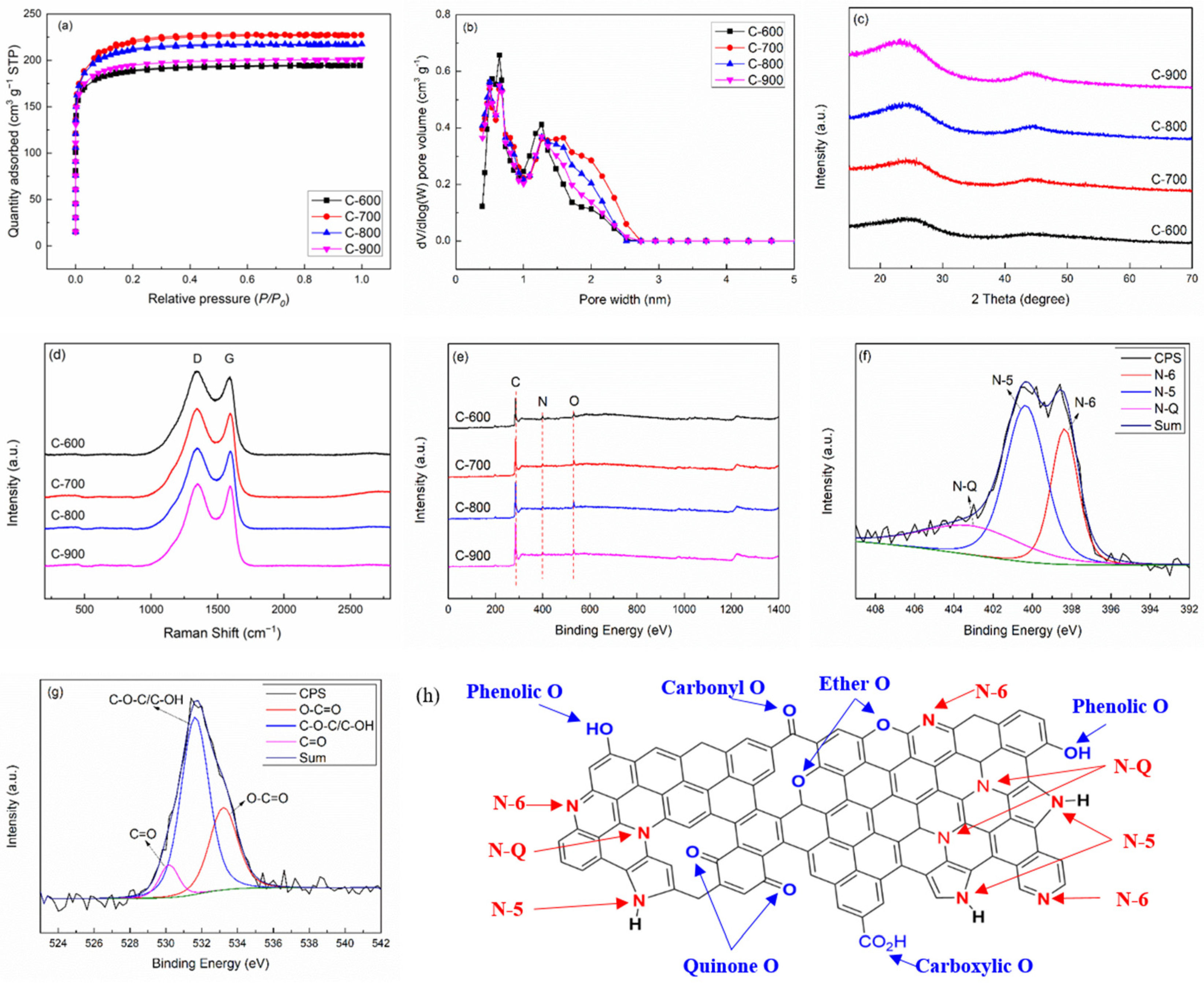
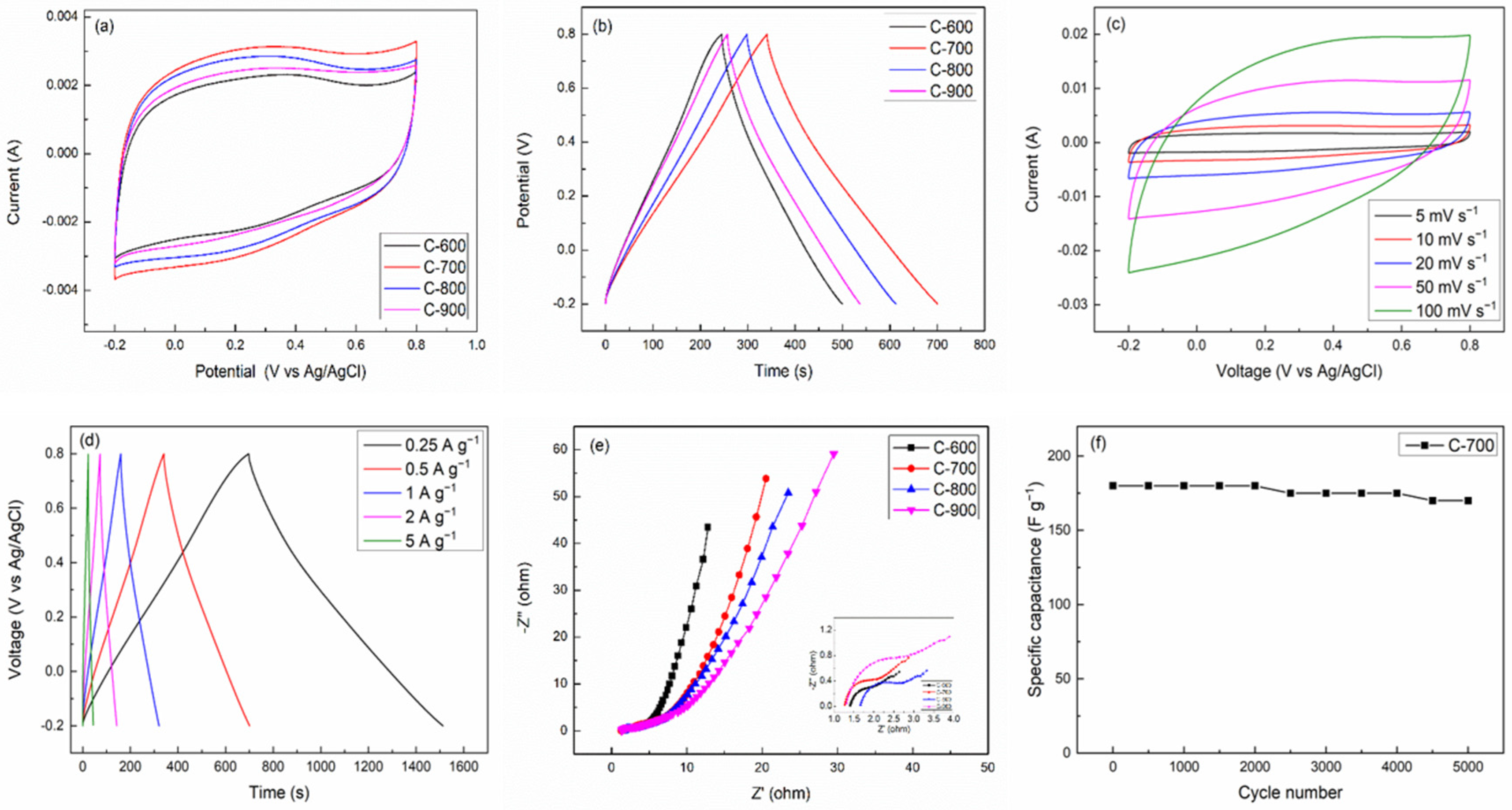
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation
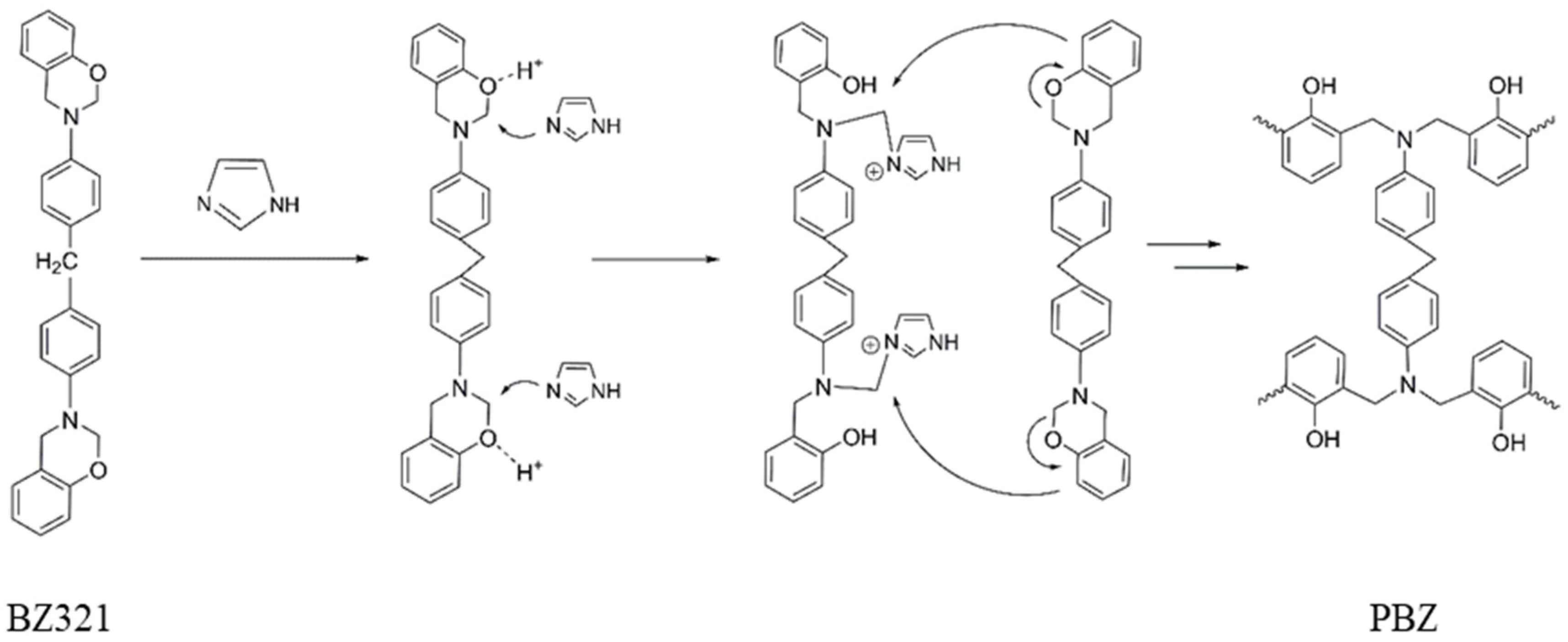
3.2.1. Synthesis of Polybenzoxazine
3.2.2. Preparation of N- and O-Codoped Carbon Materials Derived from PBZ
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Zhou, Q.; Fu, H.; Lian, Y.; Zhang, H. A Fe2(SO4)3-assisted approach towards green synthesis of cuttlefish ink-derived carbon nanospheres for high-performance supercapacitors. J. Colloid Interface Sci. 2023, 638, 695–708. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Guo, J.; Lee, J.; Lin, L.; Diao, G. Carbon nanofibers based on potassium citrate/polyacrylonitrile for supercapacitors. Membranes 2022, 12, 272. [Google Scholar] [CrossRef]
- Liu, T.; Chen, L.; Chen, L.; Tian, G.; Ji, M.; Zhou, S. Layer-by-layer heterostructure of MnO2@reduced graphene oxide composites as high-performance electrodes for supercapacitors. Membranes 2022, 12, 1044. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xin, N.; Liu, Y.; Shi, W. In situ construction of multi-dimensional Co3O4/NiCo2O4 hierarchical flakes on self-supporting carbon substrate with ultra-high capacitance for hybrid supercapacitors. J. Colloid Interface Sci. 2021, 599, 158–167. [Google Scholar] [CrossRef]
- Pang, Z.; Li, G.; Xiong, X.; Ji, L.; Xu, Q.; Zou, X.; Lu, X. Molten salt synthesis of porous carbon and its application in supercapacitors: A review. J. Energy Chem. 2021, 61, 622–640. [Google Scholar] [CrossRef]
- Chen, L.; Hao, C.; Zhang, Y.; Wei, Y.; Dai, L.; Cheng, J.; Zhang, H. Guest ions pre-intercalation strategy of manganese-oxides for supercapacitor and battery applications. J. Energy Chem. 2021, 60, 480–493. [Google Scholar] [CrossRef]
- Yuan, F.; Li, C.; Wu, J.; Liang, Y.; Huang, H.; Xu, S.; Liang, X.; Zhou, W.; Guo, J. Binder-free hybrid cobalt-based sulfide/oxide nanoarrays toward enhanced energy storage performance for hybrid supercapacitors. J. Energy Storage 2023, 63, 106979. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, L.; Zhu, Y.; Wan, Z.; Huang, X.; Yin, J.; Liu, Z.; Zhou, Y.; Xia, Y. A supercapacitor electrode with ultrahigh areal capacity by using loofah-inspired bimetallic selenide-incorporated hierarchical nanowires. J. Alloys Compd. 2023, 943, 169045. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wang, Y.Z.; Tsai, M.Y.; Lin, H.P.; Hsu, C.H. Electrospun benzimidazole-based polyimide membrane for supercapacitor applications. Membranes 2022, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Samuel, E.; Park, C.; Kim, Y.; Lee, H.S.; Yoon, S.S. Bimetallic ZnFe2O4 nanosheets prepared via electrodeposition as binder-free high-performance supercapacitor electrodes. Appl. Surf. Sci. 2021, 559, 149951. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, B.; Guo, A.; Sun, Z.; Zhao, J.; Cui, F.; Yang, X. MnO2 nanoshells/Ti3C2Tx MXene hybrid film as supercapacitor electrode. Appl. Surf. Sci. 2021, 560, 150040. [Google Scholar] [CrossRef]
- Dai, T.; Cai, B.; Yang, X.; Jiang, Y.; Wang, L.; Wang, J.; Li, X.; Lü, W. Asymmetric supercapacitors based on SnNiCoS ternary metal sulfide electrodes. Nanotechnology 2023, 34, 225401. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ding, C.; Wang, X.; Huang, P. Optimized synthesis of N-doped multi-channel carbon derived from fiber-reinforced polyimide composites for supercapacitors. Mater. Lett. 2023, 339, 134036. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, C.; Chu, H.; Qiu, S.; McLeod, J.; She, Z.; Xu, F.; Sun, L.; Zou, Y. Binary Co-Ni oxide nanoparticle-loaded hierarchical graphitic porous carbon for high-performance supercapacitors. J. Mater. Sci. Technol. 2020, 37, 135–142. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Wang, P.; Cong, Z.; Shi, F.; Wei, L.; Wang, K.; Tong, Y. NiCo2S4 combined 3D hierarchical porous carbon derived from lignin for high-performance supercapacitors. Int. J. Biol. Macromol. 2023, 232, 123344. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Xu, A.; Li, W.; Qin, Y. Facile preparation of graphene/polyaniline composite hydrogel film by electrodeposition for binder-free all-solid-state supercapacitor. J. Alloys Compd. 2021, 875, 159931. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Liu, X.; Liu, S.; Zhang, L.; Xu, W.; Yang, H.; Hou, H.; He, S.; Zhao, Y.; et al. Nitrogen, sulfur co-doped hierarchical carbon encapsulated in graphene with ‘‘sphere-in-layer” interconnection for high-performance supercapacitor. J. Colloid Interface Sci. 2021, 599, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Liu, Y.; Yang, J.; Liu, P.; Jiang, Q.; Jiang, F.; Liu, C.; Ding, W.; Xu, J. Heterostructural conductive polymer with multi-dimensional carbon materials for capacitive energy storage. Appl. Surf. Sci. 2021, 558, 149910. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Liu, F.; Han, P.; Qin, G. Wearable pseudocapacitor based on porous MnO2 composite. J. Alloys Compd. 2020, 813, 152089. [Google Scholar] [CrossRef]
- Gu, J.; Wang, H.; Li, S.; Riaz, M.S.; Ning, J.; Pu, X.; Hu, Y. Tuning pyridinic-N and graphitic-N doping with 4,4′-bipyridine in honeycomb-like porous carbon and distinct electrochemical roles in aqueous and ionic liquid gel electrolytes for symmetric supercapacitors. J. Colloid Interface Sci. 2023, 635, 254–264. [Google Scholar] [CrossRef]
- Samal, R.; Bhat, M.; Kapse, S.; Thapa, R.; Late, D.J.; Rout, C.S. Enhanced energy storage performance and theoretical studies of 3D cuboidal manganese diselenides embedded with multiwalled carbon nanotubes. J. Colloid Interface Sci. 2021, 598, 500–510. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, T.; Liu, Z.; Zhong, Q.; Qin, Y. Facile fabrication of binder-free reduced graphene oxide/MnO2/Ni foam hybrid electrode for high-performance supercapacitors. J. Alloys Compd. 2020, 812, 152124. [Google Scholar] [CrossRef]
- Sha, Z.; Zhou, Y.; Huang, F.; Yang, W.; Yu, Y.; Zhang, J.; Wu, S.; Brown, S.A.; Peng, S.; Han, Z.; et al. Carbon fibre electrodes for ultra long cycle life pseudocapacitors by engineering the nano-structure of vertical graphene and manganese dioxide. Carbon 2021, 177, 260–270. [Google Scholar] [CrossRef]
- Fu, F.; Yang, D.; Zhao, B.; Fan, Y.; Liu, W.; Lou, H.; Qiu, X. Boosting capacitive performance of N, S co-doped hierarchical porous lignin-derived carbon via self-assembly assisted template-coupled activation. J. Colloid Interface Sci. 2023, 640, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Liu, A.; Luo, W.; Ma, R.; Yan, L.; Ai, L.; Xu, M.; Wang, L.; Jia, D. Hybrid nanoarchitectonics of coal-derived carbon with oxidationinduced morphology-selectivity for high-performance supercapacitor. J. Colloid Interface Sci. 2023, 639, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, Z.; Chen, X.; Liang, Y.; Zheng, M.; Hu, H.; Dong, H.; Liu, X.; Liu, Y.; Xiao, Y. A mild method to prepare nitrogen-rich interlaced porous carbon nanosheets for high-performance supercapacitors. J. Colloid Interface Sci. 2021, 599, 381–389. [Google Scholar] [CrossRef]
- Lei, X.; Pan, F.; Hua, C.; Wang, S.; Xiong, B.; Liu, Y.; Fu, Z.; Xiang, B.; Lu, Y. Oxide-doped hierarchically porous carbon for high-performance supercapacitor. J. Alloys Compd. 2022, 901, 163624. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Kim, S.C. Heteroatom-enhanced porous carbon materials based on polybenzoxazine for supercapacitor electrodes and CO2 capture. Polymers 2023, 15, 1564. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, X.; Wang, T.; Huang, X.; Dou, J.; Wu, D.; Yu, J.; Wu, S.; Chen, X. S/N-codoped carbon nanotubes and reduced graphene oxide aerogel based supercapacitors working in a wide temperature range. J. Colloid Interface Sci. 2023, 638, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Mo, L.; Wang, F.; Shao, Z. N/O co-doped hierarchically porous carbon with three-dimensional conductive network for high-performance supercapacitors. J. Alloys Compd. 2021, 873, 159705. [Google Scholar] [CrossRef]
- Wang, R.; Lei, W.; Wang, L.; Li, Z.; Chen, J.; Hu, Z. N-doped carbon nanofibrous film with unique wettability, enhanced supercapacitive property, and facile capacity to demulsify surfactant free oil-in-water emulsions. Chem. Res. Chin. Univ. 2021, 37, 436–442. [Google Scholar] [CrossRef]
- Wen, J.; Chen, X.; Huang, M.; Yang, W.; Deng, J. Core-shell-structured MnO2@carbon spheres and nitrogen-doped activated carbon for asymmetric supercapacitors with enhanced energy density. J. Chem. Sci. 2020, 132, 6. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, E.; Huang, Z.; Shen, H.; Tian, Y.; Xiao, C.; Yang, J.; Mao, Z. Preparation of activated carbon from polyaniline by zinc chloride activation as supercapacitor electrodes. J. Solid State Electrochem. 2011, 15, 2667–2674. [Google Scholar] [CrossRef]
- Jaouadi, M.; Marzouki, M.; Hamzaoui, A.H.; Ghodbane, O. Enhanced electrochemical performance of olive stonesderived activated carbon by silica coating for supercapacitor applications. J. Appl. Electrochem. 2022, 52, 125–137. [Google Scholar] [CrossRef]
- Qu, K.; Chen, M.; Wang, W.; Yang, S.; Jing, S.; Guo, S.; Tian, J.; Qi, H.; Huang, Z. Biomass-derived carbon dots regulating nickel cobalt layered double hydroxide from 2D nanosheets to 3D flower-like spheres as electrodes for enhanced asymmetric supercapacitors. J. Colloid Interface Sci. 2022, 616, 584–594. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, Z.; Liu, H. Research progress of low dielectric benzoxazine resin. J. Polym. Sci. Eng. 2018, 1, 234. [Google Scholar] [CrossRef]
- Yu, Z.L.; Qin, B.; Ma, Z.Y.; Huang, J.; Li, S.C.; Zhao, H.Y.; Li, H.; Zhu, Y.B.; Wu, H.A.; Yu, S.H. Superelastic hard carbon nanofiber aerogels. Adv. Mater. 2019, 31, 1900651. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.C. Polybenzoxazine originated N-doped mesoporous carbon ropes as an electrode material for high-performance supercapacitors. J. Alloys Compd. 2018, 750, 384–391. [Google Scholar] [CrossRef]
- Zhang, K.; Shang, Z.; Wu, S.; Wang, J.; Sheng, W.; Shen, X.; Zhu, M. Commercialized benzoxazine resin-derived porous carbon as high performance electrode materials for supercapacitor. J. Inorg. Organomet. Polym. 2017, 27, 1423–1429. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, L.; Luo, J.; Peng, Y.; Ji, Q.; Dai, J.; Zhu, J.; Liu, X. Biobased nitrogen- and oxygen-codoped carbon materials for high-performance supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 2763–2773. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Zhang, H.; Xu, L.; Liu, G. Preparation of benzoxazine-based N-doped mesoporous carbon material and its electrochemical behaviour as supercapacitor. J. Electroanal. Chem. 2020, 868, 114196. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Li, J.; Wang, Y.; Liu, C.; Wang, L.; Li, S.; Song, Y. Preparation and characterization of polybenzoxazine foam with flame retardancy. Polym. Adv. Technol. 2020, 31, 3095–3103. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Tian, G.L.; Nie, J.Q.; Peng, H.J.; Wei, F. Unstacked double-layer templated graphene for high-rate lithium-sulphur batteries. Nat. Commun. 2014, 5, 3410. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ren, P.G.; Dai, Z.; Hou, X.; Ren, F.; Jin, Y.L. Hierarchical porous carbon composite constructed with 1-D CNT and 2-D GNS anchored on 3-D carbon skeleton from spent coffee grounds for supercapacitor. Appl. Surf. Sci. 2021, 558, 149899. [Google Scholar] [CrossRef]
- Liu, W.; Mei, J.; Liu, G.; Kou, Q.; Yi, T.; Xiao, S. Nitrogen-doped hierarchical porous carbon from wheat straw for supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11595–11605. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Liu, G. Synthesis of benzoxazine-based N-doped mesoporous carbons as high-performance electrode materials. Appl. Sci. 2020, 10, 422. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Liu, H.; Xu, W.; Xu, L. Unusual carbon nanomesh constructed by interconnected carbon nanocages for ionic liquid-based supercapacitor with superior rate capability. Chem. Eng. J. 2018, 342, 474–483. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.; Guan, Q.F.; Chen, P.; Wu, Z.Y.; Yu, S.H. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef]
- Guan, X.; Pan, L.; Fan, Z. Flexible, transparent and highly conductive polymer film electrodes for all-solid-state transparent supercapacitor applications. Membranes 2021, 11, 788. [Google Scholar] [CrossRef]
| Sample | SSA (m2 g−1) | Vtotal (cm3 g−1) | Davg (nm) | ID/IG | Element Content (%) | N Species (%) | O Species (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | N-6 | N-5 | N-Q | O-C=O | C-O-C/C-OH | C=O | |||||
| C-600 | 721.77 | 0.301 | 1.668 | 1.07 | 68.61 | 1.86 | 7.32 | 22.21 | 30.13 | 53.65 | 16.22 | 48.04 | 10.53 | 41.43 |
| C-700 | 823.10 | 0.352 | 1.708 | 1.06 | 77.42 | 1.44 | 6.88 | 14.26 | 28.47 | 48.78 | 22.75 | 8.05 | 62.53 | 29.42 |
| C-800 | 802.96 | 0.336 | 1.675 | 1.04 | 78.42 | 1.29 | 6.70 | 13.59 | 26.99 | 43.00 | 30.01 | 18.17 | 61.74 | 20.09 |
| C-900 | 745.05 | 0.311 | 1.670 | 1.03 | 83.96 | 1.07 | 5.41 | 9.56 | 26.53 | 39.25 | 34.22 | 81.00 | 12.60 | 6.40 |
| Sample | Specific Capacitance (F g−1) | ||||
|---|---|---|---|---|---|
| 0.25 (A g−1) | 0.5 (A g−1) | 1 (A g−1) | 2 (A g−1) | 5 (A g−1) | |
| C-600 | 150 | 128 | 120 | 105 | 80 |
| C-700 | 205 | 180 | 160 | 140 | 105 |
| C-800 | 175 | 155 | 140 | 115 | 95 |
| C-900 | 160 | 140 | 130 | 110 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-Y.; Li, Y.-L.; Liu, L.-N.; Xu, Z.-W.; Xie, G.; Wang, Y.; Zhao, F.-G.; Gao, T.; Li, W.-S. Microporous N- and O-Codoped Carbon Materials Derived from Benzoxazine for Supercapacitor Application. Inorganics 2023, 11, 269. https://doi.org/10.3390/inorganics11070269
Li Y-Y, Li Y-L, Liu L-N, Xu Z-W, Xie G, Wang Y, Zhao F-G, Gao T, Li W-S. Microporous N- and O-Codoped Carbon Materials Derived from Benzoxazine for Supercapacitor Application. Inorganics. 2023; 11(7):269. https://doi.org/10.3390/inorganics11070269
Chicago/Turabian StyleLi, Yuan-Yuan, Yu-Ling Li, Li-Na Liu, Zi-Wen Xu, Guanghui Xie, Yufei Wang, Fu-Gang Zhao, Tianzeng Gao, and Wei-Shi Li. 2023. "Microporous N- and O-Codoped Carbon Materials Derived from Benzoxazine for Supercapacitor Application" Inorganics 11, no. 7: 269. https://doi.org/10.3390/inorganics11070269
APA StyleLi, Y.-Y., Li, Y.-L., Liu, L.-N., Xu, Z.-W., Xie, G., Wang, Y., Zhao, F.-G., Gao, T., & Li, W.-S. (2023). Microporous N- and O-Codoped Carbon Materials Derived from Benzoxazine for Supercapacitor Application. Inorganics, 11(7), 269. https://doi.org/10.3390/inorganics11070269






