Mechanism of High-Rate Cycling Stability of Anthraquinone Cathode for Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
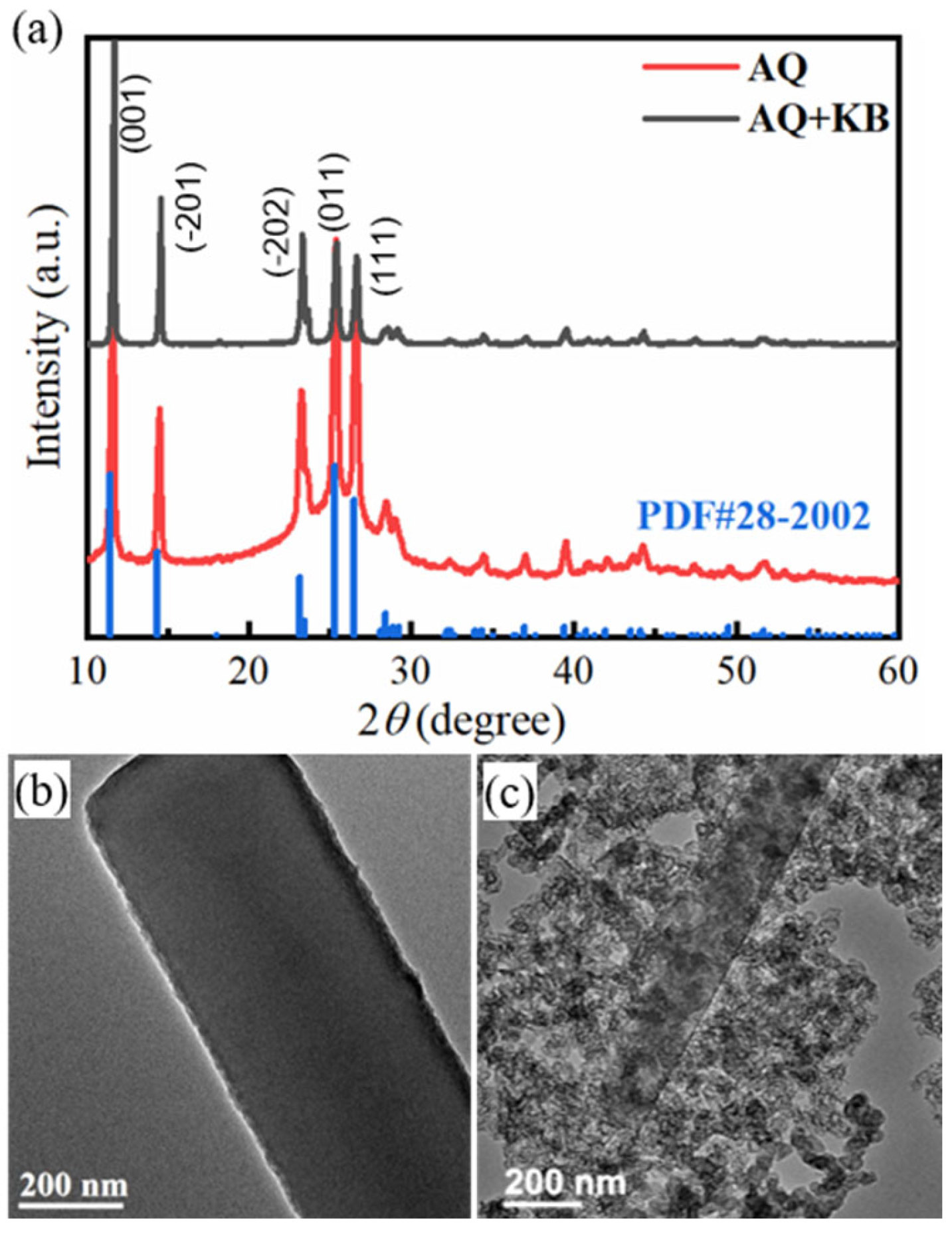
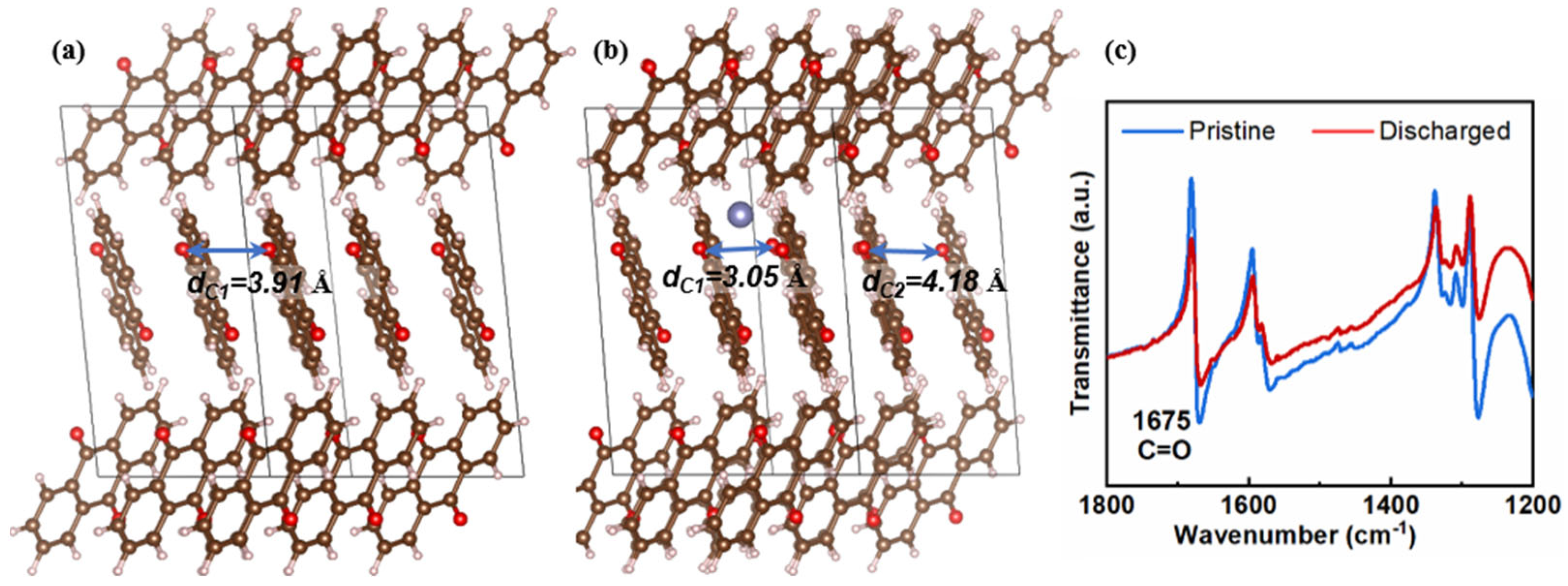
2. Results and Discussion
3. Materials and Methods
3.1. Electrode Preparation
3.2. Characterizations
3.3. Electrochemical Measurements
3.4. Computational Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, G.Z.; Zhou, J.; Pan, A.Q.; Liang, S.Q. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Jia, X.X.; Liu, C.F.; Neale, Z.G.; Yang, J.H.; Cao, G.Z. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.D.; Zhou, G.D.; Robson, M.J.; Yu, J.; Kwok, S.C.T.; Ciucci, F. Hydrated deep eutectic electrolytes for high-performance zn-ion batteries capable of low-temperature operation. Adv. Funct. Mater. 2022, 32, 2109322. [Google Scholar] [CrossRef]
- An, G.-H.; Hong, J.; Pak, S.; Cho, Y.; Lee, S.; Hou, B.; Cha, S. 2D metal zn nanostructure electrodes for high-performance zn ion supercapacitors. Adv. Energy Mater. 2020, 10, 1902981. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Zhang, Y.; Li, Y.; Sun, R.; Wang, Z.; Wang, H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Stor. Mater. 2021, 35, 19–46. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Gao, Y.J.; Li, G.F.; Wang, F.; Chu, J.; Yu, P.; Wang, B.S.; Zhan, H.; Song, Z.P. A high-performance aqueous rechargeable zinc battery based on organic cathode integrating quinone and pyrazine. Energy Stor. Mater. 2021, 40, 31–40. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zheng, K.; Wang, P.P.; Cai, X.; Wang, X.; Gao, D.M.; Yu, D.M.; Chen, C.G.; Liu, Y.P. A new zinc-ion battery cathode with high-performance: Loofah-like lanthanum manganese perovskite. J. Colloid Interface Sci. 2022, 610, 796–804. [Google Scholar] [CrossRef]
- Yoo, G.; Koo, B.-R.; An, G.H. Nano-sized split V2O5 with H2O-intercalated interfaces as a stable cathode for zinc ion batteries without an aging process. Chem. Eng. J. 2022, 434, 134738. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Patil, N.; de la Cruz, C.; Ciurduc, D.; Mavrandonakis, A.; Palma, J.; Marcilla, R. An ultrahigh performance zinc-organic battery using poly(catechol) cathode in zn(TFSI)2-based concentrated aqueous electrolytes. Adv. Energy Mater. 2021, 11, 2100939. [Google Scholar] [CrossRef]
- Han, C.P.; Li, H.F.; Shi, R.Y.; Zhang, T.F.; Tong, J.; Li, J.Q.; Li, B.H. Organic quinones towards advanced electrochemical energy storage: Recent advances and challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Miroshnikov, M.; Divya, K.P.; Babu, G.; Meiyazhagan, A.; Arava, L.M.R.; Ajayan, P.M.; John, G. Power from nature: Designing green battery materials from electroactive quinone derivatives and organic polymers. J. Mater. Chem. A 2016, 4, 12370–12386. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huang, W.; Luo, Z.; Liu, L.; Lu, Y.; Li, Y.; Li, L.; Hu, J.; Ma, H.; Chen, J. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018, 4, eaao1761. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, C.Y.; Zhao, Q.; Niu, Z.Q.; Chen, J. High-performance organic lithium batteries with an ether-based electrolyte and 9,10-anthraquinone (AQ)/CMK-3 cathode. Adv. Sci. 2015, 2, 1500018. [Google Scholar] [CrossRef]
- Guo, C.Y.; Zhang, K.; Zhao, Q.; Peia, L.K.; Chen, J. High-performance sodium batteries with the 9,10-anthraquinone/CMK-3 cathode and an ether-based electrolyte. Chem. Commun. 2015, 51, 10244–10247. [Google Scholar] [CrossRef]
- Werner, D.; Apaydin, D.H.; Portenkirchner, E. An anthraquinone/carbon fiber composite as cathode material for rechargeable sodium-ion vatteries. Batter. Supercaps 2018, 1, 160–168. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Murtaza, I.; Liu, D.; Tan, R.; Zhu, Y.N.; Meng, H. Understanding the mechanism of improvement in practical specific capacity using halogen substituted anthraquinones as cathode materials in lithium batteries. Electrochim. Acta 2017, 224, 622–627. [Google Scholar] [CrossRef]
- Wang, W.; Xu, W.; Cosimbescu, L.; Choi, D.W.; Li, L.Y.; Yang, Z.G. Anthraquinone with tailored structure for a nonaqueous metal-organic redox flow battery. Chem. Commun. 2012, 48, 6669–6671. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Dolhem, F.; Gaubicher, J. Progress in all-organic rechargeable batteries using cationic and anionic configurations: Toward low-cost and greener storage solutions? Curr. Opin. Electrochem. 2018, 9, 70–80. [Google Scholar] [CrossRef]
- Tomai, T.; Hyodo, H.; Komatsu, D.; Honma, I. Analysis of degradation mechanisms in quinone-based electrodes for aqueous electrolyte system via in situ XRD measurements. J. Phys. Chem. C 2018, 122, 2461–2466. [Google Scholar] [CrossRef]
- Liang, Y.L.; Yao, Y. Positioning organic electrode materials in the battery landscape. Joule 2018, 2, 1690–1706. [Google Scholar] [CrossRef]
- Phadke, S.; Cao, M.L.; Anouti, M. Approaches to electrolyte solvent selection for poly-anthraquinone sulfide organic electrode material. ChemSusChem 2018, 11, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Zeng, X.M.; Li, Z.H.; Meng, X.J.; Wei, D.; Liu, T.F.; Ling, M.; Lin, Z.; Liang, C.D. An innovation: Dendrite free quinone paired with ZnMn2O4 for zinc ion storage. Mater. Today Energy 2019, 13, 323–330. [Google Scholar] [CrossRef]
- Yan, L.J.; Zeng, X.M.; Zhao, S.; Jiang, W.; Li, Z.H.; Gao, X.H.; Liu, T.F.; Ji, Z.K.; Ma, T.L.; Ling, M.; et al. 9,10-Anthraquinone/K2CuFe(CN)6: A highly compatible aqueous aluminum-ion full-battery configuration. ACS Appl. Mater. Interfaces 2021, 13, 8353–8360. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]


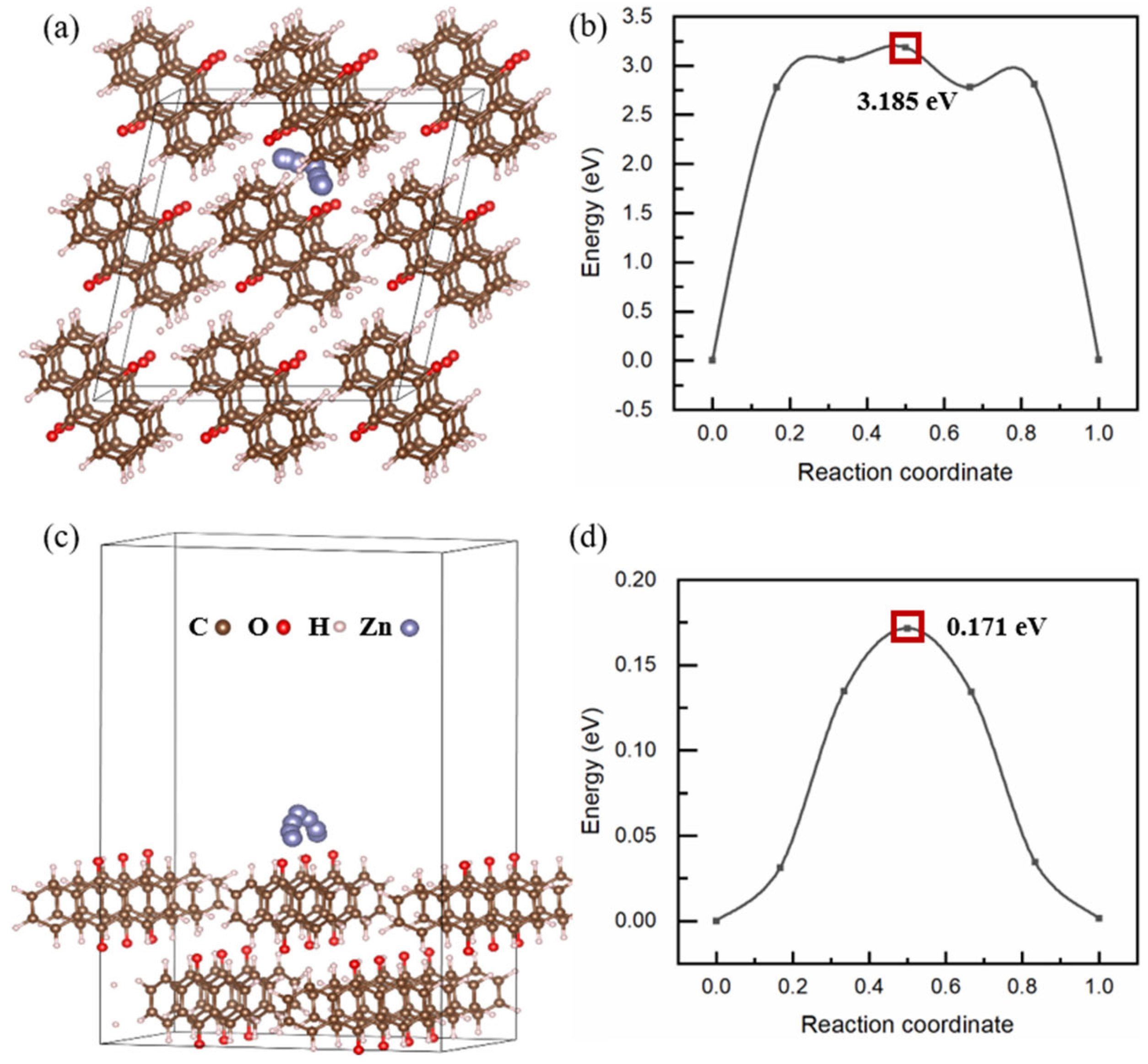
| A Site | B Site | C Site | D Site | |
|---|---|---|---|---|
| Binding energy (eV) | 1.81 | 1.16 | −0.95 | 1.16 |
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Volume (Å3) | |
|---|---|---|---|---|---|---|---|
| AQ | 15.63 | 11.75 | 15.66 | 90 | 102.29 | 90 | 2808.02 |
| Zn inserted AQ | 15.54 | 11.32 | 15.46 | 88.76 | 102.36 | 91.28 | 2654.74 |
| Deformation (%) | −0.58 | −3.66 | −1.28 | −1.38 | 0.07 | 1.42 | −5.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Lai, X.; Chen, W.; Chen, C.; Wan, H.; Sun, D. Mechanism of High-Rate Cycling Stability of Anthraquinone Cathode for Aqueous Zinc-Ion Batteries. Inorganics 2023, 11, 271. https://doi.org/10.3390/inorganics11070271
Chen Q, Lai X, Chen W, Chen C, Wan H, Sun D. Mechanism of High-Rate Cycling Stability of Anthraquinone Cathode for Aqueous Zinc-Ion Batteries. Inorganics. 2023; 11(7):271. https://doi.org/10.3390/inorganics11070271
Chicago/Turabian StyleChen, Qiujie, Xiaoxu Lai, Wenlan Chen, Chi Chen, Houzhao Wan, and Dan Sun. 2023. "Mechanism of High-Rate Cycling Stability of Anthraquinone Cathode for Aqueous Zinc-Ion Batteries" Inorganics 11, no. 7: 271. https://doi.org/10.3390/inorganics11070271
APA StyleChen, Q., Lai, X., Chen, W., Chen, C., Wan, H., & Sun, D. (2023). Mechanism of High-Rate Cycling Stability of Anthraquinone Cathode for Aqueous Zinc-Ion Batteries. Inorganics, 11(7), 271. https://doi.org/10.3390/inorganics11070271






