Investigation of Photoelectrochemical Performance under the Piezoelectric Effect Based on Different Zinc Oxide Morphologies
Abstract
:1. Introduction
2. Experimental Sections
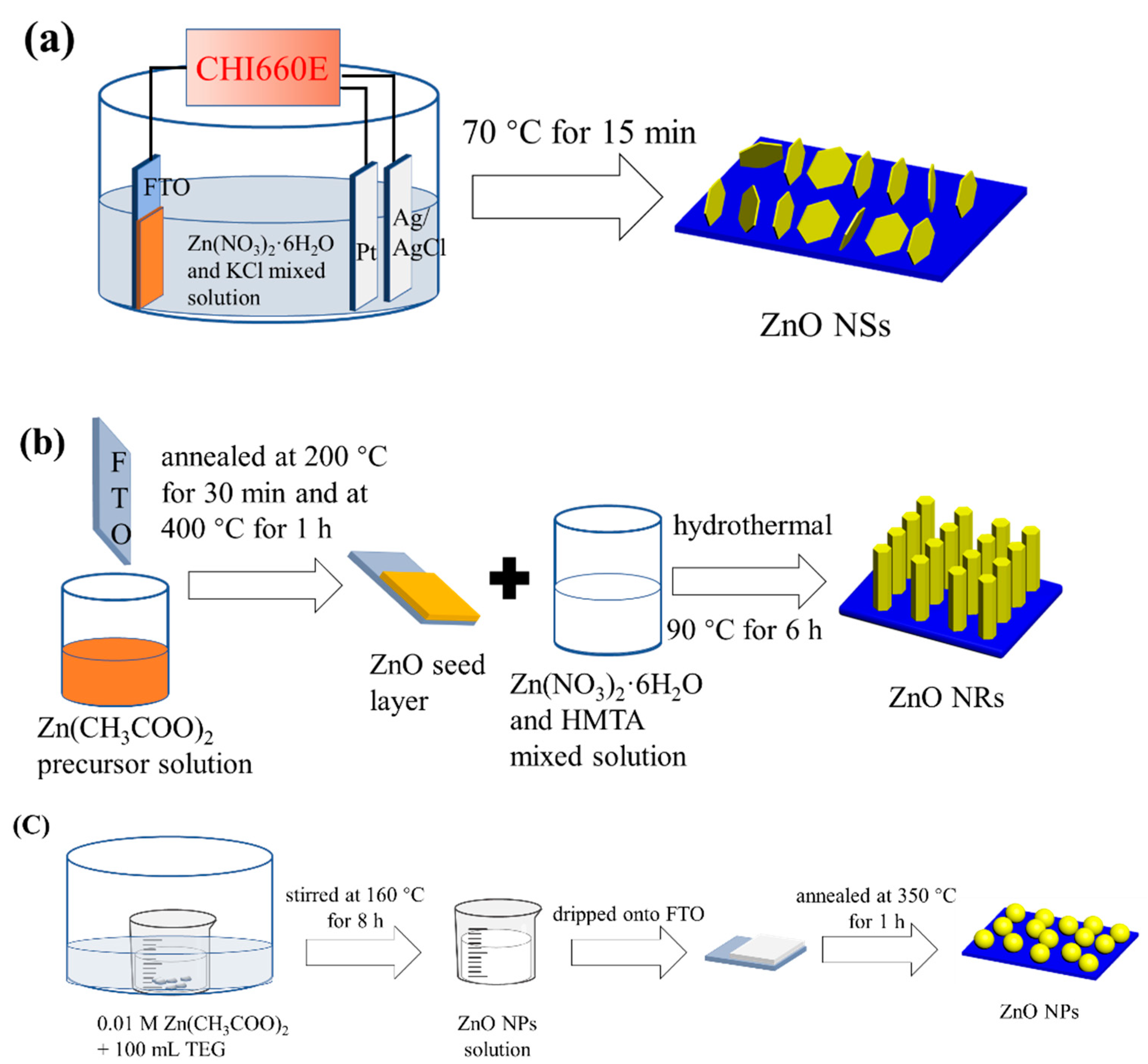
2.1. Preparation of ZnO Nanosheets (ZnO NSs)
2.2. Preparation of ZnO Nanorods (ZnO NRs)
2.3. Preparation of ZnO Nanospheres (ZnO NPs)
2.4. Characterization
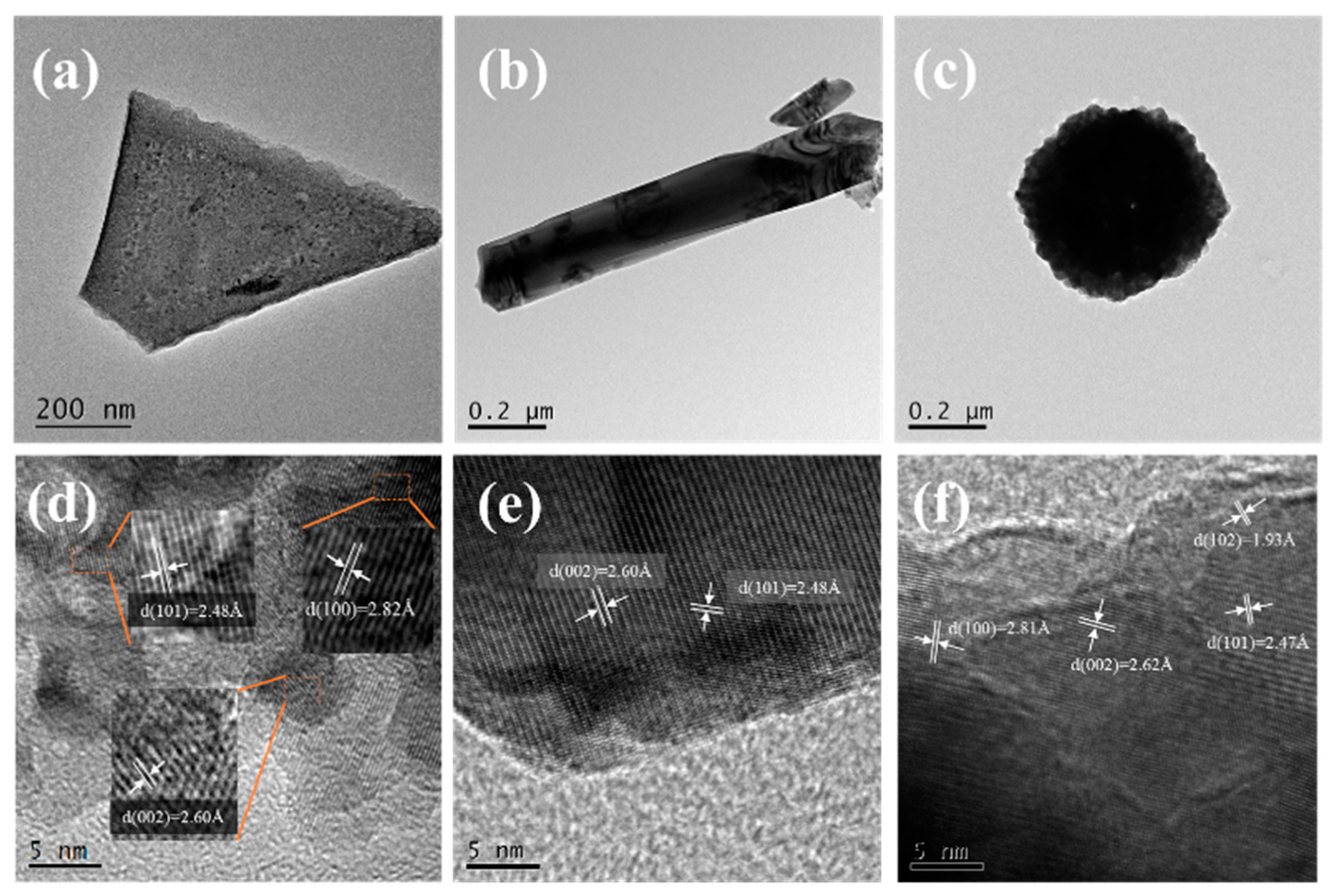
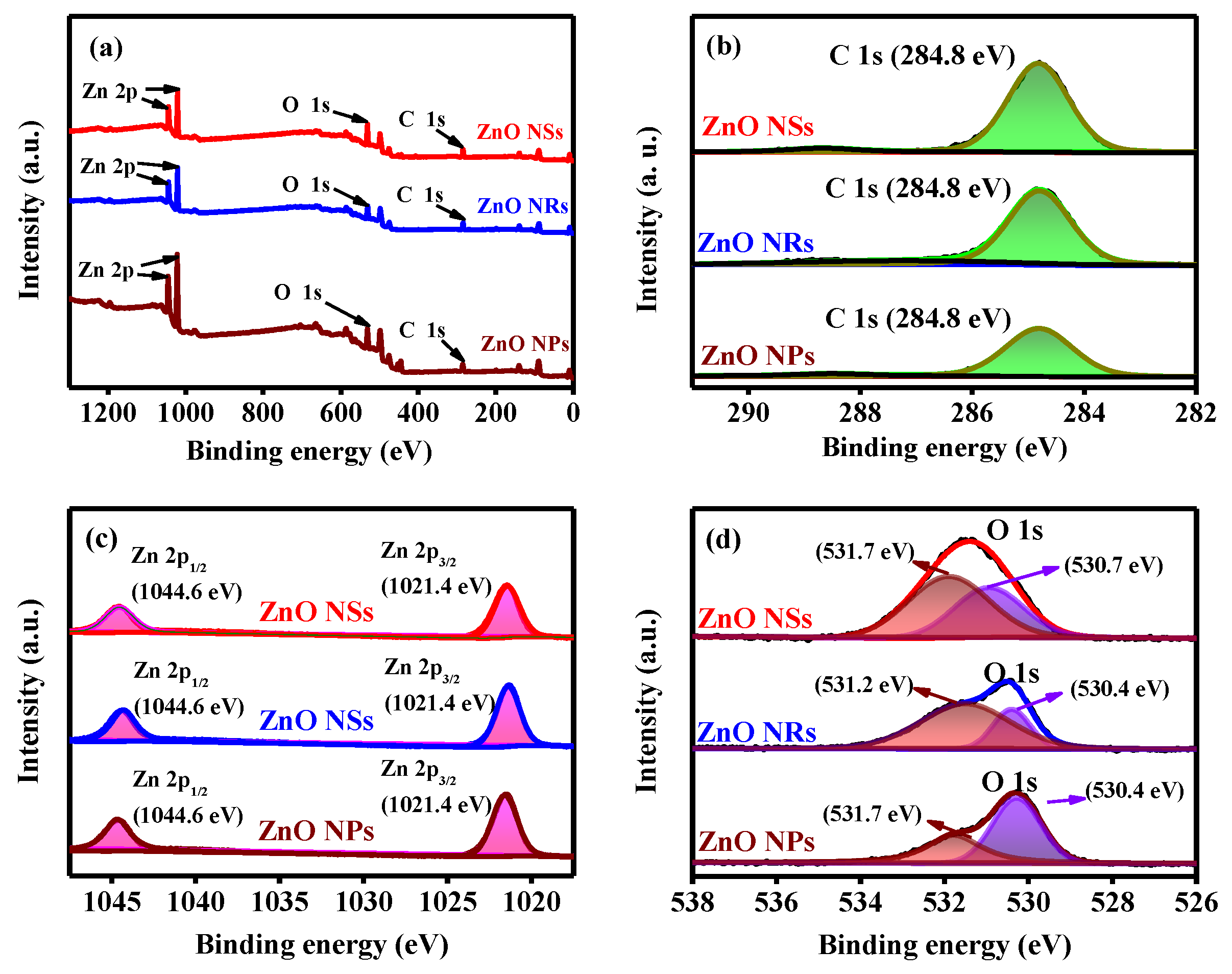
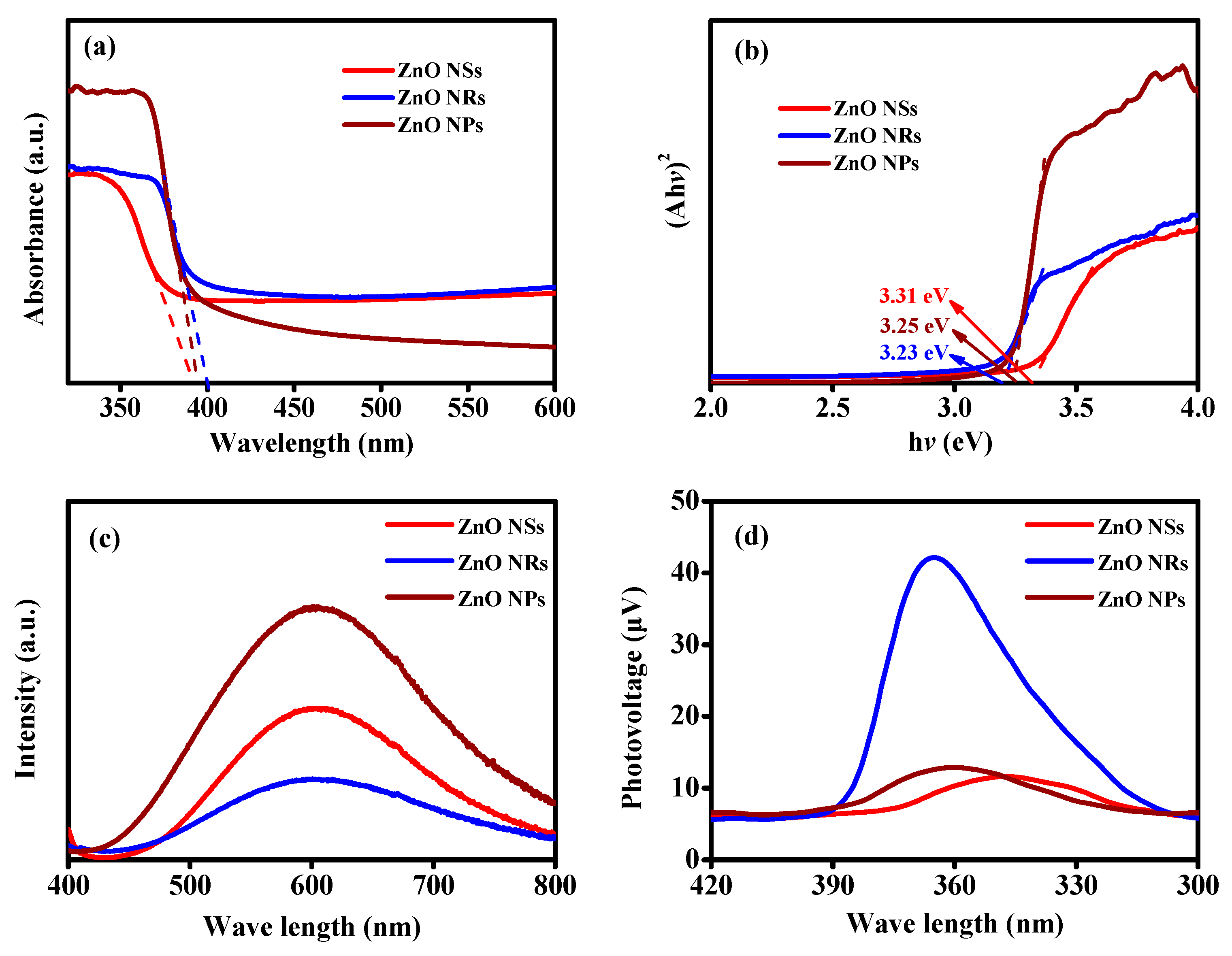
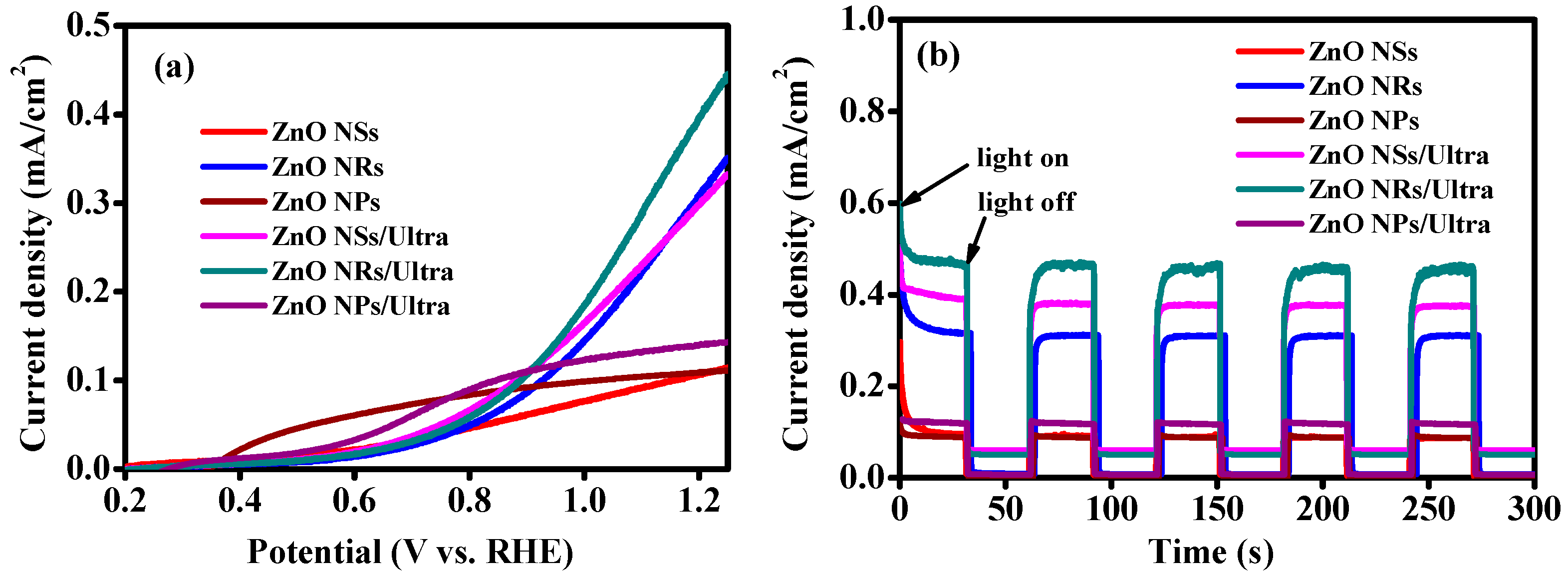
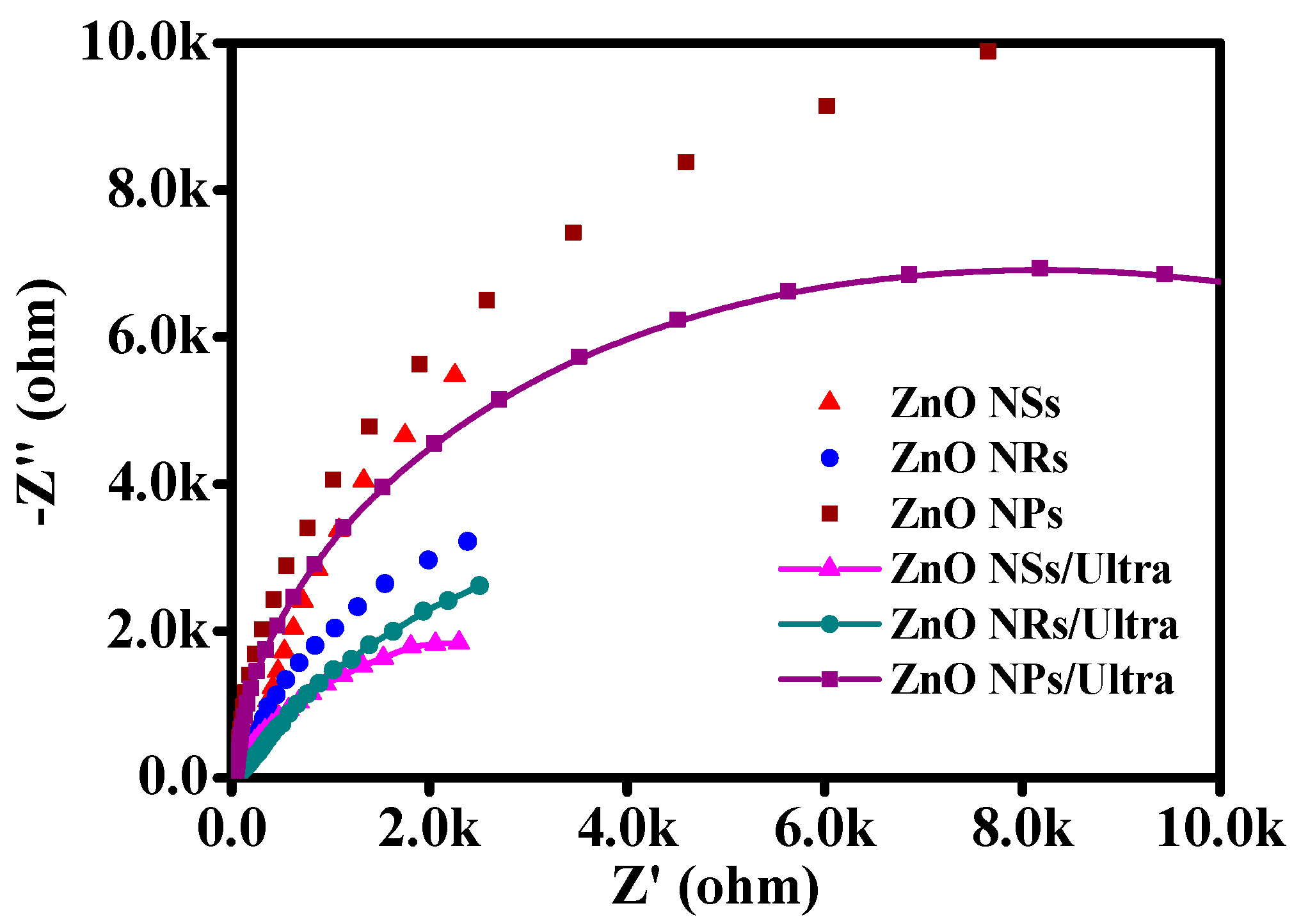
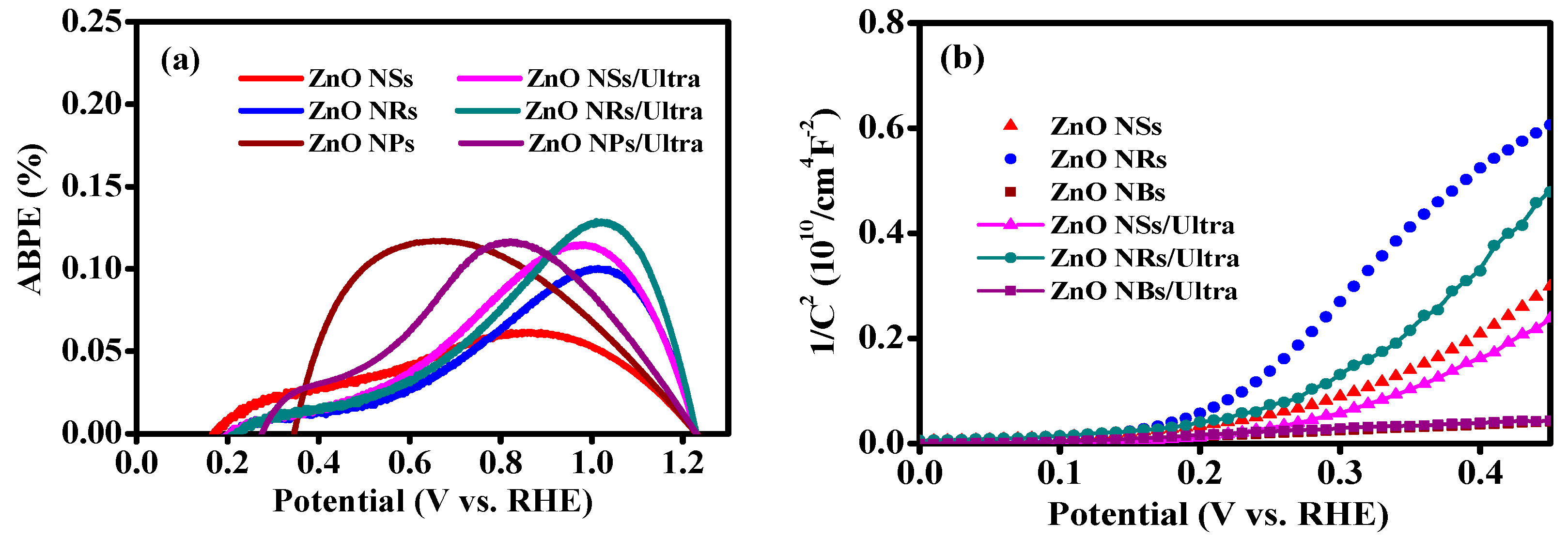
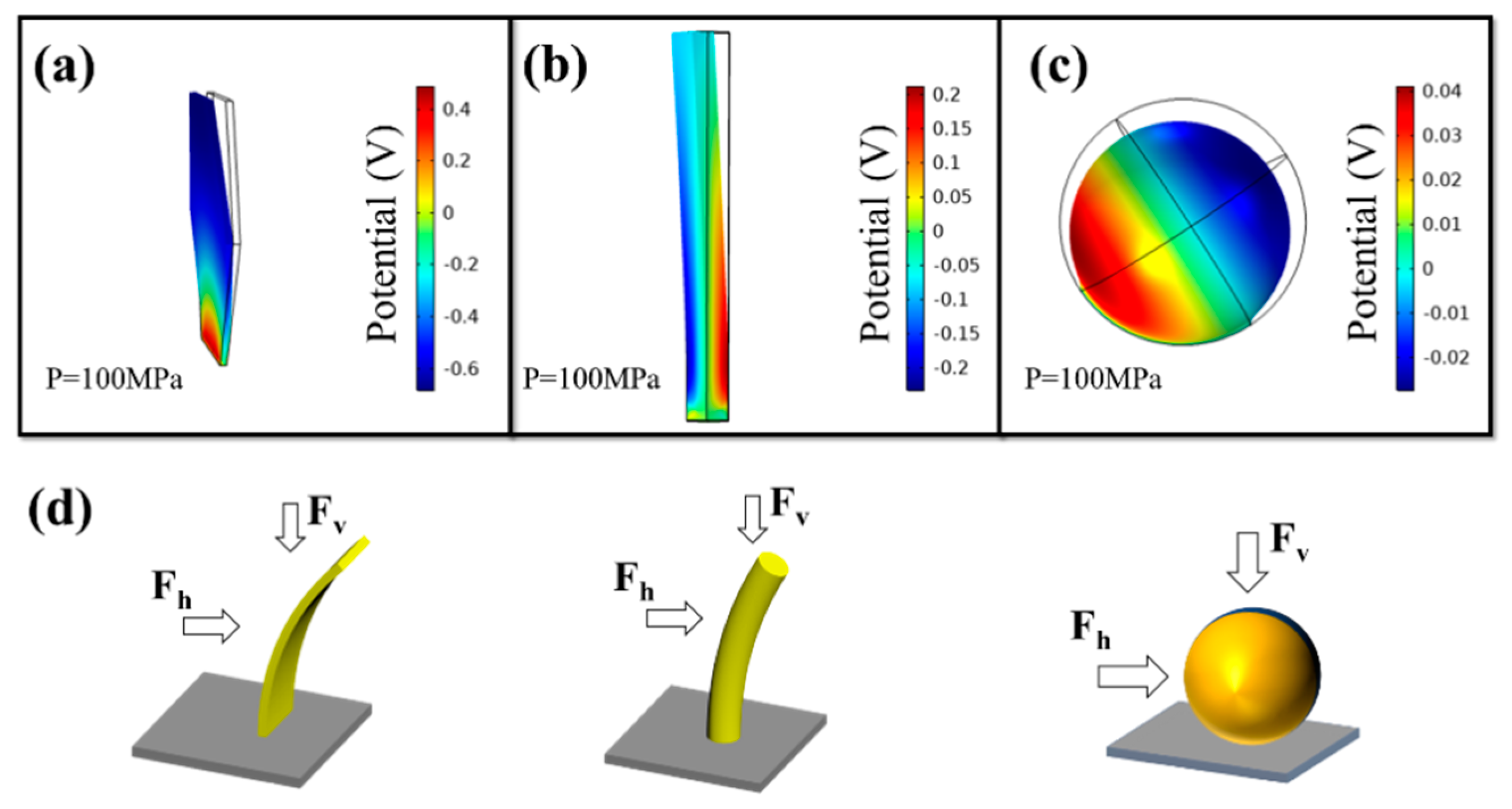
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, Q.J.; Liu, Z.F.; Han, C.C.; Tong, Z.F.; Ma, C.H. CuInS2/Sb2S3 heterostructure modified with noble metal co-catalyst for efficient photoelectrochemical water splitting. J. Alloy. Compd. 2019, 795, 319–326. [Google Scholar] [CrossRef]
- Fang, G.Z.; Liu, Z.F.; Han, C.C. Enhancing the PEC water splitting performance of BiVO4 co-modifying with NiFeOOH and Co-Pi double layer cocatalysts. Appl. Surf. Sci. 2020, 515, 146095. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Zhang, L.C.; Wang, S.B.; Sillanpaa, M.; Sun, H.Q. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B-Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Zhang, S.C.; Liu, Z.F.; Ruan, M.N.; Guo, Z.G.; Lei, E.; Zhao, W.; Zhao, D.; Wu, X.F.; Chen, D.M. Enhanced piezoelectric-effect-assisted photoelectrochemical performance in ZnO modified with dual cocatalysts. Appl. Catal. B-Environ. 2020, 262, 118279. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive tungsten-oxide nanomaterials for water-splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Su, J.Z.; Zhou, J.L.; Guo, L.J. A multistep ion exchange approach for fabrication of porous BiVO4 nanorod arrays on transparent conductive substrate. ACS Sustain. Chem. Eng. 2016, 4, 4492–4497. [Google Scholar] [CrossRef]
- Wang, G.M.; Wang, H.Y.; Ling, Y.C.; Tang, Y.C.; Yang, X.Y.; Fitzmorris, R.C.; Wang, C.C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.F.; Zhou, M.; Wu, P.D.; Wei, J.D. Enhanced photoelectrochemical water splitting performance of alpha-Fe2O3 nanostructures modified with Sb2S3 and cobalt phosphate. J. Alloy. Compd. 2018, 74, 2918–2927. [Google Scholar]
- Wang, P.; Liu, Z.F.; Chen, D.; Zhang, S.C.; Fang, G.Z.; Han, C.C.; Cheng, Z.W.; Tong, Z.F. An unassisted tandem photoelectrochemical cell based on p- and n-Cu2O Photoelectrodes. Catal. Lett. 2021, 151, 1976–1983. [Google Scholar] [CrossRef]
- Toghraie, D.; Mahmoudi, M.; Akbari, O.A.; Pourfattah, F.; Heydari, M. The effect of using water/CuO nanofluid and L-shaped porous ribs on the performance evaluation criterion of microchannels. J. Therm. Anal. Calorim. 2019, 135, 145–159. [Google Scholar] [CrossRef]
- Ma, H.P.; Zhang, J.; Liu, Z.F. Efficient tungsten oxide/bismuth oxyiodide core/shell photoanode for photoelectrochemical water splitting. Appl. Surf. Sci. 2017, 423, 63–70. [Google Scholar] [CrossRef]
- Berglund, S.P.; Abdi, F.F.; Bogdanoff, P.; Chernseddine, A.; Friedrich, D.; van de Krol, R. Comprehensive evaluation of CuBi2O4 as a photocathode material for photoelectrochemical water splitting. Chem. Mater. 2016, 28, 4231–4242. [Google Scholar] [CrossRef]
- Jiang, C.R.; Moniz SJ, A.; Wang, A.Q.; Zhang, T.; Tang, J.W. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, S.W.; Han, D.D.; Liu, T.Y.; Wang, G.M.; Li, Y. Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Li, C.L.; Cao, Q.; Wang, F.Z.; Xiao, Y.Q.; Li, Y.B.; Delaunay, J.J.; Zhu, H.W. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Lee, S.T.; Yang, S.H.; Kang, Z.H. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 1409–1419. [Google Scholar] [CrossRef]
- Shen, S.H.; Jiang, J.G.; Guo, P.H.; Kronawitter, C.X.; Mao, S.S.; Guo, L.J. Effect of Cr doping on the photoelectrochemical performance of hematite nanorod photoanodes. Nano Energy 2012, 1, 732–741. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yan, J.; Yang, S.Y.; Xu, Y.H.; Cai, X.; Li, X.; Zhang, X.C.; Peng, F.; Fang, Y.P. Electrodeposition of Cu2O/g-C3N4 heterojunction film on an FTO substrate for enhancing visible light photoelectrochemical water splitting. Chin. J. Catal. 2017, 38, 365–371. [Google Scholar] [CrossRef]
- Li, Y.T.; Liu, Z.F.; Guo, Z.G.; Ruan, M.N.; Li, X.F.; Liu, Y.L. Efficient WO3 photoanode modified by Pt layer and plasmonic Ag for enhanced charge separation and transfer to promote photoelectrochemical performances. ACS Sustain. Chem. Eng. 2019, 7, 12582–12590. [Google Scholar] [CrossRef]
- Liu, Z.R.; Yu, X.; Li, L.L. Piezopotential augmented photo- and photoelectro-catalysis with a built-in electric field. Chin. J. Catal. 2020, 41, 534–549. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, G.; Wang, Z.Y.; Yu, Z.C.; Wang, K.Y.; Wu, Y.C. Recent progress on piezoelectric materials for renewable energy conversion. Nano Energy 2020, 77, 105180. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.C.; Chen, Y.; Wang, P.H.; Wang, J.Y.; Zhang, X.W.; Zou, J.J.; Wang, Z.L. Advances in piezo-phototronic effect enhanced photocatalysis and photoelectrocatalysis. Adv. Energy Mater. 2020, 10, 2000214. [Google Scholar] [CrossRef]
- You, J.K.; Liu, Z.F.; Guo, Z.G.; Ruan, M.N.; Yan, W.G. Doping of W ions to modulate the polarization intensity of Bi2WO6 for efficient piezoelectric-photoelechochemical water splitting. ACS Appl. Energy Mater. 2022, 5, 11472–11482. [Google Scholar] [CrossRef]
- Shi, J.; Starr, M.B.; Xiang, H.; Hara, Y.; Anderson, M.A.; Seo, J.H.; Ma, Z.Q.; Wang, X.D. Interface engineering by piezoelectric potential in ZnO-based photoelectrochemical anode. Nano Lett. 2011, 11, 5587–5593. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, S.; Khare, N. Piezo-phototronic and plasmonic effect coupled Ag-NaNbO3 nanocomposite for enhanced photocatalytic and photoelectrochemical water splitting activity. Renew. Energy 2021, 163, 1569–1579. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Liu, Z.F.; Guo, Z.G.; Ruan, M.N.; Yan, W.G. The collaborative mechanism of surface S-vacancies and piezoelectric polarization for boosting CdS photoelectrochemical performance. Chem. Eng. J. 2022, 433, 133226. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Q.; Wang, H.G.; Rong, J.C.; Lei, E.; Dai, Y.J. Enhanced catalytic performance of Ag2O/BaTiO3 heterostructure microspheres by the piezo/pyro-phototronic synergistic effect. Nano Energy 2020, 73, 104783. [Google Scholar] [CrossRef]
- Goel, S.; Kumar, B. A review on piezo-/ferro-electric properties of morphologically diverse ZnO nanostructures. J. Alloy. Compd. 2020, 816, 152491. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Babu, B.; Reddy, C.V.; Reddy, I.N.; Reddy, K.R.; Rao, M.C.; Aminabhavi, T.M.; Cho, M.; Kim, D.; Shim, J. ZnO nanosheets-decorated Bi2WO6 nanolayers as efficient photocatalysts for the removal of toxic environmental pollutants and photoelectrochemical solar water oxidation. J. Environ. Manag. 2020, 265, 110504. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Ghosh, S.; Sharma, A.; Kumar, D.; Nguyen, V.H.; Vo DV, N.; Pham, T.D.; Kumar, P. Vertically aligned ZnO nanorods for photoelectrochemical water splitting application. Mater. Lett. 2020, 277, 128295. [Google Scholar] [CrossRef]
- Li, J.L.; Jin, B.J.; Jiao, Z.B. Rationally embedded zinc oxide nanospheres serving as electron transport channels in bismuth vanadate/zinc oxide heterostructures for improved photoelectrochemical efficiency. J. Colloid Interface Sci. 2021, 592, 127–134. [Google Scholar] [CrossRef]
- Hong, K.S.; Xu, H.F.; Konishi, H.; Li, X.C. Direct water splitting through vibrating piezoelectric microfibers in water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Gao, R.J.; Zhang, Y.C.; Pan, L.; Huang, C.Y.; Liu, K.; Chang, X.Y.; Zhang, X.W.; Zou, J.J. Polarization-enhanced direct Z-scheme ZnO-WO3-x nanorod arrays for efficient piezoelectric-photoelectrochemical water splitting. Appl. Catal. B-Environ. 2019, 259, 118079. [Google Scholar] [CrossRef]
- Jézéquel, D.; Guenot, J.; Jouini, N.; Fiévet, F. Submicrometer zinc oxide particles: Elaboration in polyol medium and morphological characteristics. J. Mater. Res. 1995, 10, 77–83. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G.Z. Aggregation of ZnO nanocrystallites for high conversion efficiency in dye-sensitized solar cells. Angew. Chem. Int. Ed. 2008, 47, 2402–2406. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D.; Han, C.C.; Wang, P.; Tong, Z.F.; Tan, B.H.; Liu, Z.F. The synergistic effect of CuBi2O4 and Co-Pi: Improving the PEC activity of BiVO4-based composite materials. New J. Chem. 2022, 46, 2971–2979. [Google Scholar] [CrossRef]
- Skompska, M.; Zarebska, K. Electrodeposition of ZnO nanorod arrays on transparent conducting substrates-a review. Electrochim. Acta 2014, 127, 467–488. [Google Scholar] [CrossRef]
- Messai, Z.; Ouennoughi, Z.; Devers, T.; Mouet, T.; Harel, V.; Konstantinov, K.; Bouguechal, N. Growth and characteristics of ZnO nano-aggregates electrodeposited onto p-Si(111). Appl. Surf. Sci. 2010, 257, 616–621. [Google Scholar] [CrossRef]
- Laudise, R.A.; Ballman, A.A. Hydrothermal synthesis of zinc oxide and zinc sulfide. J. Phys. Chem. 1960, 64, 688–691. [Google Scholar] [CrossRef]
- Tena-Zaera, R.; Elias, J.; Wang, G.; Lévy-Clément, C. Role of chloride ions on electrochemical deposition of ZnO nanowire arrays from O2 reduction. J. Phys. Chem. C 2007, 111, 16706–16711. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Tan, D.H.; Montano, M.; Goldberger, J.; Somorjai, G.; Yang, P.D. General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett. 2005, 5, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef] [Green Version]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sberveglieri, G.; Tondello, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Barreca, D.; Ferrucci, A.P.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E. Temperature-controlled synthesis and photocatalytic performance of ZnO nanoplatelets. Chem. Vap. Depos. 2007, 13, 618–625. [Google Scholar] [CrossRef]
- Bekermann, D.; Gasparotto, A.; Barreca, D.; Devi, A.; Fischer, R.A.; Kete, M.; Lavrenčič, Š.U.; Lebedev, O.I.; Maccato, C.; Van Tendeloo, G. ZnO nanorod arrays by plasma-enhanced CVD for light-activated functional applications. ChemPhysChem 2010, 11, 2337–2340. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, J.; Wen, Y.W.; Shan, B.; Chen, R. Surface modification of LaFeO3 by Co-Pi electrochemical deposition as an efficient photoanode under visible light. RSC Adv. 2016, 6, 26192–26198. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Hossain, M.F.; Takahashi, T. Self-assembled hematite (alpha-Fe2O3) nanotube arrays for photoelectrocatalytic degradation of azo dye under simulated solar light irradiation. Appl. Catal. B-Environ. 2010, 95, 423–429. [Google Scholar] [CrossRef]
- Burstein, E. Anomalous optical absorption limit in InSb. Phys. Rev. 1954, 93, 632. [Google Scholar] [CrossRef]
- Lai, F.C.; Lin, L.M.; Gai, R.Q.; Lin, Y.Z.; Huang, Z.G. Determination of optical constants and thicknesses of In2O3:Sn films from transmittance data. Thin Solid Film. 2007, 515, 7387–7392. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Chen, Y.C.; Lin, Y.G. Novel ZnO/Fe2O3 Core-Shell Nanowires for Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2015, 7, 14157–14162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Chen, R.L.; Han, C.C.; Wang, P.; Tong, Z.F.; Tan, B.H.; Huang, Y.Z.; Liu, Z.F. Copper phosphide decorated g-C3N4 catalysts for highly efficient photocatalytic H2 evolution. J. Colloid Interface Sci. 2022, 610, 126–135. [Google Scholar] [CrossRef]
- Lim, T.; Ico, G.; Jung, K.; Bozhilov, K.N.; Nam, J.; Martinez-Morales, A.A. Crystal growth and piezoelectric characterization of mechanically stable ZnO nanostructure arrays. Crystengcomm 2018, 20, 5688–5694. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.Y.; Yan, X.Q.; Kang, Z.; Liang, Q.J.; Liao, X.Q.; Zhang, Y. Band alignment engineering for improved performance and stability of ZnFe2O4 modified CdS/ZnO nanostructured photoanode for PEC water splitting. Nano Energy 2016, 24, 25–31. [Google Scholar] [CrossRef]
- Fang, G.Z.; Liu, Z.F.; Han, C.C.; Ma, X.G.; Lv, H.; Huang, C.Y.; Cheng, Z.W.; Tong, Z.F.; Wang, P. The CoNiO2 as a novel water oxidation cocatalyst to enhance PEC water splitting performance of BiVO4. Chem. Commun. 2020, 56, 9158–9161. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Satsangi, V.R.; Shrivastav, R.; Kant, R.; Dass, S. Effect of morphology and impact of the electrode/electrolyte interface on the PEC response of Fe2O3 based systems—Comparison of two preparation techniques. RSC Adv. 2020, 10, 42256–42266. [Google Scholar] [CrossRef]
- Khan, S.U.; Perini, J.A.L.; Hussain, S.; Khan, H.; Khan, S.; Zanoni, M.V.B. Electrochemical preparation of Nb2O5 nanochannel photoelectrodes for enhanced photoelectrocatalytic performance in removal of RR120 dye. Chemosphere 2020, 257, 127164. [Google Scholar] [CrossRef]
- Li, H.D.; Sang, Y.H.; Chang, S.J.; Huang, X.; Zhang, Y.; Yang, R.S.; Jiang, H.D.; Liu, H.; Wang, Z.L. Enhanced ferroelectric-nanocrystal-based hybrid photocatalysis by ultrasonic-wave-generated piezophototronic effect. Nano Lett. 2015, 15, 2372–2379. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D.H. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, R.; Han, C.; Tan, B.; Fu, Q.; Liu, Z. Investigation of Photoelectrochemical Performance under the Piezoelectric Effect Based on Different Zinc Oxide Morphologies. Inorganics 2023, 11, 11. https://doi.org/10.3390/inorganics11010011
Wang D, Liu R, Han C, Tan B, Fu Q, Liu Z. Investigation of Photoelectrochemical Performance under the Piezoelectric Effect Based on Different Zinc Oxide Morphologies. Inorganics. 2023; 11(1):11. https://doi.org/10.3390/inorganics11010011
Chicago/Turabian StyleWang, Dong, Rui Liu, Changcun Han, Baohua Tan, Qian Fu, and Zhifeng Liu. 2023. "Investigation of Photoelectrochemical Performance under the Piezoelectric Effect Based on Different Zinc Oxide Morphologies" Inorganics 11, no. 1: 11. https://doi.org/10.3390/inorganics11010011
APA StyleWang, D., Liu, R., Han, C., Tan, B., Fu, Q., & Liu, Z. (2023). Investigation of Photoelectrochemical Performance under the Piezoelectric Effect Based on Different Zinc Oxide Morphologies. Inorganics, 11(1), 11. https://doi.org/10.3390/inorganics11010011






