In Situ Synthesis of Ti:Fe2O3/Cu2O p-n Junction for Highly Efficient Photogenerated Carriers Separation
Abstract
1. Introduction
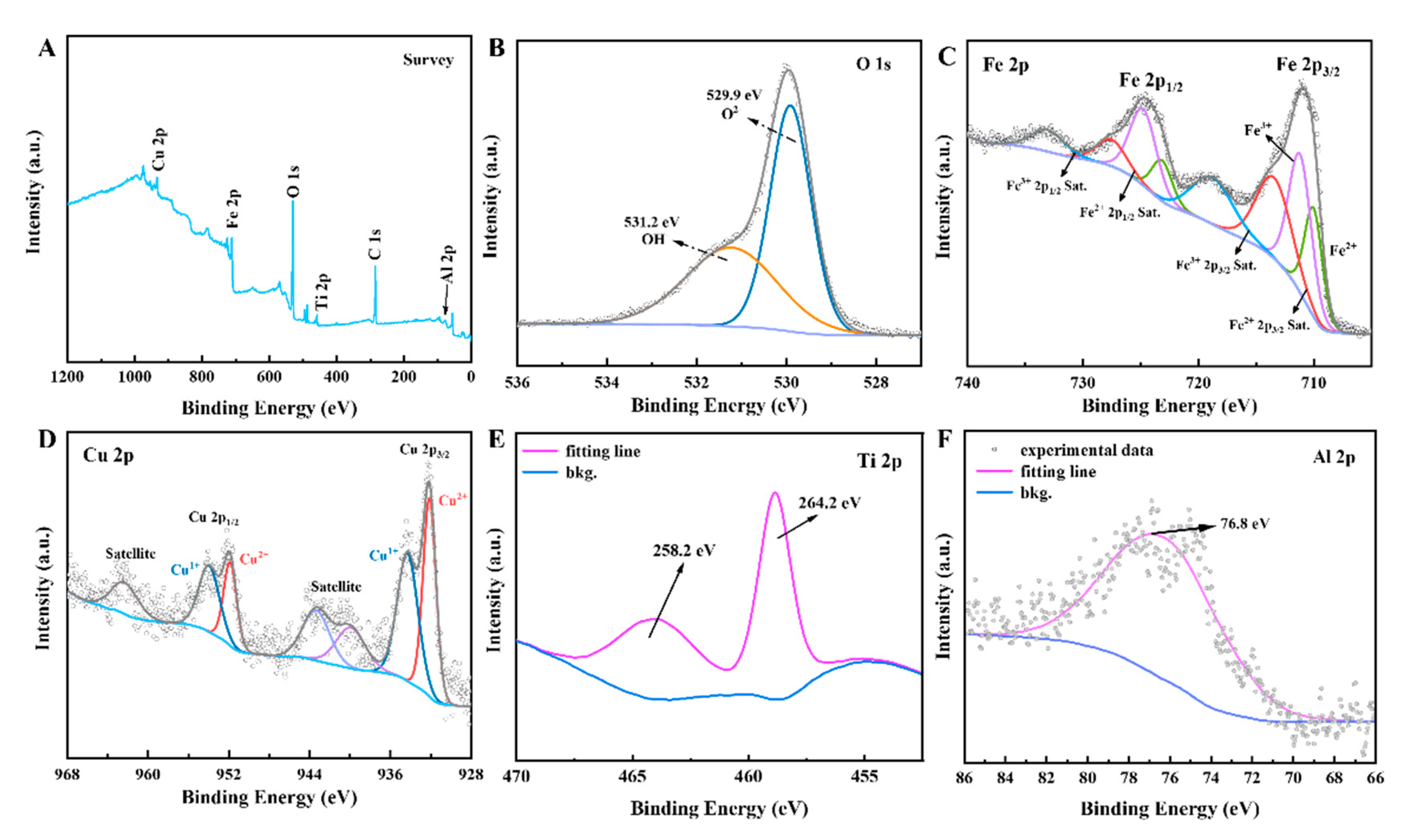
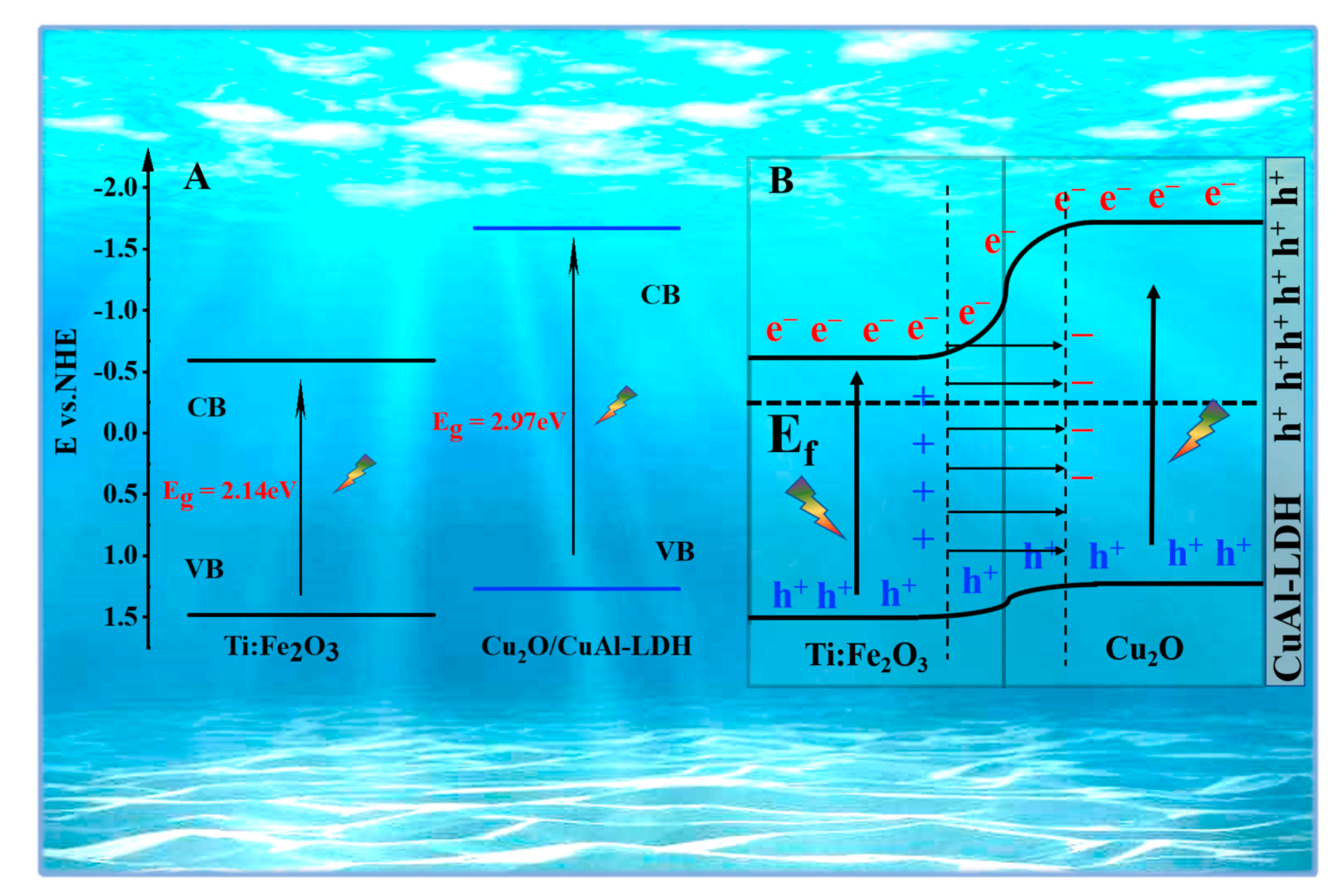
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of Ti:Fe2O3 Photoanode
3.3. Preparation of Ti:Fe2O3/CuAl-LDH Photoanode
3.4. Preparation of Ti:Fe2O3/Cu2O Photoanode
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic Materials for Solar Water Splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Gong, J.; Pu, W.; Yang, C.; Zhang, J. Tungsten and nitrogen co-doped TiO2 electrode sensitized with Fe–chlorophyllin for visible light photoelectrocatalysis. Chem. Eng. J. 2012, 209, 94–101. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Quan, X.; Zhao, Y.; Chen, S. Visible light photoelectrocatalysis with salicylic acid-modified TiO2 nanotube array electrode for p-nitrophenol degradation. J. Hazard. Mater. 2009, 166, 547–552. [Google Scholar] [CrossRef]
- Li, G.; Lian, Z.; Wang, W.; Zhang, D.; Li, H. Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy 2016, 19, 446–454. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Yu, X.; Zhang, J.; Yang, R.; Zhang, X.; Ji, Y.; Wu, M.; Deng, L.; Li, L.; et al. Piezoelectric-Effect-Enhanced Full-Spectrum Photoelectrocatalysis in p–n Heterojunction. Adv. Funct. Mater. 2019, 29, 1807279. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-granados, B.; Muñoz-Portero, M.J.; García-Antón, J. Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets. Chem. Eng. J. 2018, 350, 1114–1124. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, L.; Xiao, C.; Hua, X.; Li, Z.; Pan, B.; Xie, Y. Promoting Photogenerated Holes Utilization in Pore-Rich WO3 Ultrathin Nanosheets for Efficient Oxygen-Evolving Photoanode. Adv. Energy Mater. 2016, 6, 1600437. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, H.; Pei, S.; Xie, S.; You, S. Oxygen-deficient WO3−x nanoplate array film photoanode for efficient photoelectrocatalytic water decontamination. Chem. Eng. J. 2020, 381, 122740. [Google Scholar] [CrossRef]
- Yue, P.; She, H.; Zhang, L.; Niu, B.; Lian, R.; Huang, J.; Wang, L.; Wang, Q. Super-hydrophilic CoAl-LDH on BiVO4 for enhanced photoelectrochemical water oxidation activity. Appl. Catal. B Environ. 2021, 286, 119875. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Wang, Z.; Zhang, X.; Qin, X.; Dai, Y.; Liu, Y.; Wang, P.; Li, Y.; Huang, B. Doping strategy to promote the charge separation in BiVO4 photoanodes. Appl. Catal. B Environ. 2017, 211, 258–265. [Google Scholar] [CrossRef]
- Yu, F.; Li, F.; Yao, T.; Du, J.; Liang, Y.; Wang, Y.; Han, H.; Sun, L. Fabrication and Kinetic Study of a Ferrihydrite-Modified BiVO4 Photoanode. ACS Catal. 2017, 7, 1868–1874. [Google Scholar] [CrossRef]
- Arazas, A.P.R.; Wu, C.-C.; Chang, K.-S. Hydrothermal fabrication and analysis of piezotronic-related properties of BiFeO3 nanorods. Ceram. Int. 2018, 44, 14158–14162. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Liu, X.; Li, Y.; Hu, X.; He, B.; Shu, Z.; Li, Z.; Zhao, Y. Synergistically enhanced charge separation in BiFeO3/Sn:TiO2 nanorod photoanode via bulk and surface dual modifications. Nano Energy 2019, 59, 33–40. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Zhang, Y.; Rui, Q.; Zhang, B.; Shen, Z.; Bi, Y. One-dimensional hematite photoanodes with spatially separated Pt and FeOOH nanolayers for efficient solar water splitting. J. Mater. Chem. A 2017, 5, 17056–17063. [Google Scholar] [CrossRef]
- Grave, D.A.; Yatom, N.; Ellis, D.S.; Toroker, M.C.; Rothschild, A. The “Rust” Challenge: On the Correlations between Electronic Structure, Excited State Dynamics, and Photoelectrochemical Performance of Hematite Photoanodes for Solar Water Splitting. Adv. Mater. 2018, 30, 1706577. [Google Scholar] [CrossRef] [PubMed]
- Mesa, C.A.; Kafizas, A.; Francàs, L.; Pendlebury, S.R.; Pastor, E.; Ma, Y.; Le Formal, F.; Mayer, M.T.; Grätzel, M.; Durrant, J.R. Kinetics of Photoelectrochemical Oxidation of Methanol on Hematite Photoanodes. J. Am. Chem. Soc. 2017, 139, 11537–11543. [Google Scholar] [CrossRef]
- Noh, J.; Li, H.; Osman, O.I.; Aziz, S.G.; Winget, P.; Brédas, J.-L. Impact of Hydroxylation and Hydration on the Reactivity of α-Fe2O3 (0001) and (102) Surfaces under Environmental and Electrochemical Conditions. Adv. Energy Mater. 2018, 8, 1800545. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Triana, C.A.; Patzke, G.R. Hematite Photoanodes for Water Oxidation: Electronic Transitions, Carrier Dynamics, and Surface Energetics. Angew. Chem. Int. Ed. 2021, 60, 18380–18396. [Google Scholar] [CrossRef]
- Li, J.; Cai, L.; Shang, J.; Yu, Y.; Zhang, L. Giant Enhancement of Internal Electric Field Boosting Bulk Charge Separation for Photocatalysis. Adv. Mater. 2016, 28, 4059–4064. [Google Scholar] [CrossRef]
- Dotan, H.; Kfir, O.; Sharlin, E.; Blank, O.; Gross, M.; Dumchin, I.; Ankonina, G.; Rothschild, A. Resonant light trapping in ultrathin films for water splitting. Nat. Mater. 2013, 12, 158–164. [Google Scholar] [CrossRef]
- Cao, D.; Luo, W.; Feng, J.; Zhao, X.; Li, Z.; Zou, Z. Cathodic shift of onset potential for water oxidation on a Ti4+ doped Fe2O3 photoanode by suppressing the back reaction. Energy Environ. Sci. 2014, 7, 752–759. [Google Scholar] [CrossRef]
- Szyja, B.M.; Podsiadły-Paszkowska, A. Helping Thy Neighbor: How Cobalt Doping Alters the Electrocatalytic Properties of Hematite. J. Phys. Chem. Lett. 2020, 11, 4402–4407. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Long, X.; Wei, S.; Wang, T.; Li, F.; Gao, L.; Hu, Y.; Li, S.; Jin, J. Conformally Coupling CoAl-Layered Double Hydroxides on Fluorine-Doped Hematite: Surface and Bulk Co-Modification for Enhanced Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2019, 11, 29799–29806. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Wang, L.; Zhang, Y.; Feng, C.; Zhang, B.; Fu, S.; Guo, H.; Hu, H.; Bi, Y. Synergistic effects of P-doping and a MnO2 cocatalyst on Fe2O3 nanorod photoanodes for efficient solar water splitting. J. Mater. Chem. A 2018, 6, 7021–7026. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, L.; Lou, X.W. Construction of Heterostructured Fe2O3-TiO2 Microdumbbells for Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 15076–15080. [Google Scholar] [CrossRef]
- Noruozi, A.; Nezamzadeh-Ejhieh, A. Preparation, characterization, and investigation of the catalytic property of α-Fe2O3-ZnO nanoparticles in the photodegradation and mineralization of methylene blue. Chem. Phys. Lett. 2020, 752, 137587. [Google Scholar] [CrossRef]
- Wang, N.; Wu, L.; Li, J.; Mo, J.; Peng, Q.; Li, X. Construction of hierarchical Fe2O3@MnO2 core/shell nanocube supported C3N4 for dual Z-scheme photocatalytic water splitting. Sol. Energy Mater. Sol. Cells 2020, 215, 110624. [Google Scholar] [CrossRef]
- Bi, D.; Xu, Y. Synergism between Fe2O3 and WO3 particles: Photocatalytic activity enhancement and reaction mechanism. J. Mol. Catal. A Chem. 2013, 367, 103–107. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Constructing 2D/2D Fe2O3/g-C3N4 Direct Z-Scheme Photocatalysts with Enhanced H2 Generation Performance. Sol. RRL 2018, 2, 1800006. [Google Scholar] [CrossRef]
- Sharma, D.; Upadhyay, S.; Verma, A.; Satsangi, V.R.; Shrivastav, R.; Dass, S. Nanostructured Ti-Fe2O3/Cu2O heterojunction photoelectrode for efficient hydrogen production. Thin Solid Film. 2015, 574, 125–131. [Google Scholar] [CrossRef]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite heterostructures for photoelectrochemical water splitting: Rational materials design and charge carrier dynamics. Energy Environ. Sci. 2016, 9, 2744–2775. [Google Scholar] [CrossRef]
- Wang, L.; Nguyen, N.T.; Zhang, Y.; Bi, Y.; Schmuki, P. Enhanced Solar Water Splitting by Swift Charge Separation in Au/FeOOH Sandwiched Single-Crystalline Fe2O3 Nanoflake Photoelectrodes. ChemSusChem 2017, 10, 2720–2727. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Formation of α-Fe2O3/FeOOH nanostructures with various morphologies by a hydrothermal route and their photocatalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 217–220. [Google Scholar] [CrossRef]
- Quang, N.D.; Hu, W.; Chang, H.S.; Van, P.C.; Viet, D.D.; Jeong, J.-R.; Seo, D.B.; Kim, E.T.; Kim, C.; Kim, D. Fe2O3 hierarchical tubular structure decorated with cobalt phosphide (CoP) nanoparticles for efficient photoelectrochemical water splitting. Chem. Eng. J. 2021, 417, 129278. [Google Scholar] [CrossRef]
- Shakeel, M.; Arif, M.; Yasin, G.; Li, B.; Khan, H.D. Layered by layered Ni-Mn-LDH/g-C3N4 nanohybrid for multi-purpose photo/electrocatalysis: Morphology controlled strategy for effective charge carriers separation. Appl. Catal. B Environ. 2019, 242, 485–498. [Google Scholar] [CrossRef]
- Fei, W.; Song, Y.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of visible-light-active ZnO/ZnFe-LDH heterojunction on Ni foam for pollutants removal with enhanced photoelectrocatalytic performance. Sol. Energy 2019, 188, 593–602. [Google Scholar] [CrossRef]
- Fei, W.; Gao, J.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. A visible-light active p-n heterojunction NiFe-LDH/Co3O4 supported on Ni foam as photoanode for photoelectrocatalytic removal of contaminants. J. Hazard. Mater. 2021, 402, 123515. [Google Scholar] [CrossRef]
- Sharma, P.; Jang, J.-W.; Lee, J.S. Key Strategies to Advance the Photoelectrochemical Water Splitting Performance of α-Fe2O3 Photoanode. ChemCatChem 2019, 11, 157–179. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible Light-Driven α-Fe2O3 Nanorod/Graphene/BiV1–xMoxO4 Core/Shell Heterojunction Array for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Zhou, C.; Waterhouse, G.I.N.; Wang, Z.; Weng, Y.; Zhang, T. Sub-3 nm Ultrafine Cu2O for Visible Light Driven Nitrogen Fixation. Angew. Chem. Int. Ed. 2021, 60, 2554–2560. [Google Scholar] [CrossRef]
- Han, J.-H.; Jia, W.-H.; Liu, Y.; Wang, W.-D.; Zhang, L.-K.; Li, Y.-M.; Sun, P.; Fan, J.; Hu, S.-T. α-Fe2O3/Cu2O composites as catalysts for photoelectrocatalytic degradation of benzotriazoles. Water Sci. Eng. 2022, 15, 200–209. [Google Scholar] [CrossRef]
- Cheng, L.; Tian, Y.; Zhang, J. Construction of p-n heterojunction film of Cu2O/α-Fe2O3 for efficiently photoelectrocatalytic degradation of oxytetracycline. J. Colloid Interface Sci. 2018, 526, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, A.; Nezamzadeh-Ejhieh, A. α-Fe2O3/Cu2O heterostructure: Brief characterization and kinetic aspect of degradation of methylene blue. Phys. B Condens. Matter 2020, 599, 412422. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Chen, D.; Yan, W. An efficient hole transfer pathway on hematite integrated by ultrathin Al2O3 interlayer and novel CuCoOx cocatalyst for efficient photoelectrochemical water oxidation. Appl. Catal. B Environ. 2020, 277, 119197. [Google Scholar] [CrossRef]
- Oku, M.; Hirokawa, K. XPS observation of Fe1−xO at temperatures between 25 and 800 °C. J. Appl. Phys. 1979, 50, 6303–6308. [Google Scholar] [CrossRef]
- Yi, S.-S.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Highly Efficient Photoelectrochemical Water Splitting: Surface Modification of Cobalt-Phosphate-Loaded Co3O4/Fe2O3 p–n Heterojunction Nanorod Arrays. Adv. Funct. Mater. 2019, 29, 1801902. [Google Scholar] [CrossRef]
- He, X.; Shang, C.; Meng, Q.; Chen, Z.; Jin, M.; Shui, L.; Zhang, Y.; Zhang, Z.; Yuan, M.; Wang, X.; et al. Hematite photoanode modified with inexpensive hole-storage layer for highly efficient solar water oxidation. Nanotechnology 2020, 31, 455405. [Google Scholar] [CrossRef]
- Kong, T.-T.; Huang, J.; Jia, X.-G.; Wang, W.-Z.; Zhou, Y.; Zou, Z.-G. Nitrogen-Doped Carbon Nanolayer Coated Hematite Nanorods for Efficient Photoelectrocatalytic Water Oxidation. Appl. Catal. B Environ. 2020, 275, 119113. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Kandiel, T.A.; Ahmed, A.Y.; Kretschmer, I.; Rashwan, F.; Bahnemann, D. Enhanced Photoelectrochemical Water Oxidation on Nanostructured Hematite Photoanodes via p-CaFe2O4/n-Fe2O3 Heterojunction Formation. J. Phys. Chem. C 2015, 119, 5864–5871. [Google Scholar] [CrossRef]
- Wang, L.; Nguyen, N.T.; Huang, X.; Schmuki, P.; Bi, Y. Hematite Photoanodes: Synergetic Enhancement of Light Harvesting and Charge Management by Sandwiched with Fe2TiO5/Fe2O3/Pt Structures. Adv. Funct. Mater. 2017, 27, 1703527. [Google Scholar] [CrossRef]
- Xu, W.; Tian, W.; Meng, L.; Cao, F.; Li, L. Interfacial Chemical Bond-Modulated Z-Scheme Charge Transfer for Efficient Photoelectrochemical Water Splitting. Adv. Energy Mater. 2021, 11, 2003500. [Google Scholar] [CrossRef]


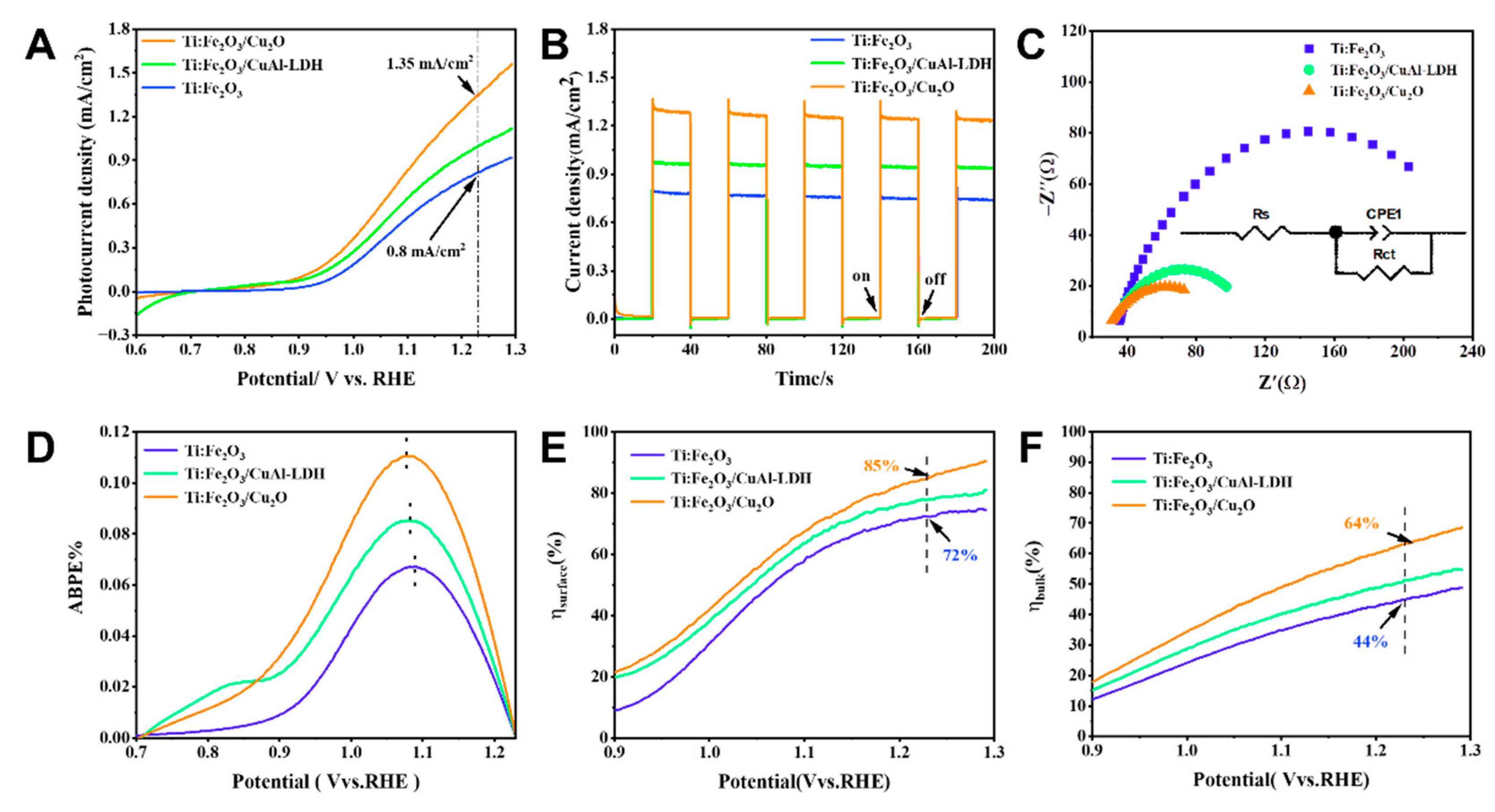


| No. | Sample | Photocurrent Density (1.23 V vs. RHE) |
|---|---|---|
| 1. | Fe2O3 | 0.027 mA/cm2 |
| 2. | Ti:Fe2O3 | 0.80 mA/cm2 |
| 3. | Ti:Fe2O3/CuAl-LDH | 0.92 mA/cm2 |
| 4. | Ti:Fe2O3/Cu2O | 1.35 mA/cm2 |
| Sample | Ef (eV) | EVBM (eV) | ECBM (eV) | Eg (eV) |
|---|---|---|---|---|
| Fe2O3 | −0.42 | 1.48 | −0.59 | 2.07 |
| Cu2O | −0.23 | 1.27 | −1.67 | 2.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Feng, Y.; Zhong, Y.; Ding, H.; Chen, K.; Chen, D. In Situ Synthesis of Ti:Fe2O3/Cu2O p-n Junction for Highly Efficient Photogenerated Carriers Separation. Inorganics 2023, 11, 155. https://doi.org/10.3390/inorganics11040155
Shi T, Feng Y, Zhong Y, Ding H, Chen K, Chen D. In Situ Synthesis of Ti:Fe2O3/Cu2O p-n Junction for Highly Efficient Photogenerated Carriers Separation. Inorganics. 2023; 11(4):155. https://doi.org/10.3390/inorganics11040155
Chicago/Turabian StyleShi, Tie, Yanmei Feng, Yi Zhong, Hao Ding, Kai Chen, and Daimei Chen. 2023. "In Situ Synthesis of Ti:Fe2O3/Cu2O p-n Junction for Highly Efficient Photogenerated Carriers Separation" Inorganics 11, no. 4: 155. https://doi.org/10.3390/inorganics11040155
APA StyleShi, T., Feng, Y., Zhong, Y., Ding, H., Chen, K., & Chen, D. (2023). In Situ Synthesis of Ti:Fe2O3/Cu2O p-n Junction for Highly Efficient Photogenerated Carriers Separation. Inorganics, 11(4), 155. https://doi.org/10.3390/inorganics11040155






