Hydrothermal Synthesized CoS2 as Efficient Co-Catalyst to Improve the Interfacial Charge Transfer Efficiency in BiVO4
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BiVO4 Photoanode
2.3. Preparation of CoS2 and BiVO4/CoS2 Photoanode
2.4. Structure and Morphology Characterizations
2.5. Electrochemical and Photoelectrochemical Characterization
2.6. Intensity Modulated Photocurrent Spectroscopy (IMPS) Characterization
2.7. Surface Photovoltage Spectroscopy (SPV) Characterization
3. Results and Discussion
3.1. Material and Chemical Characterization
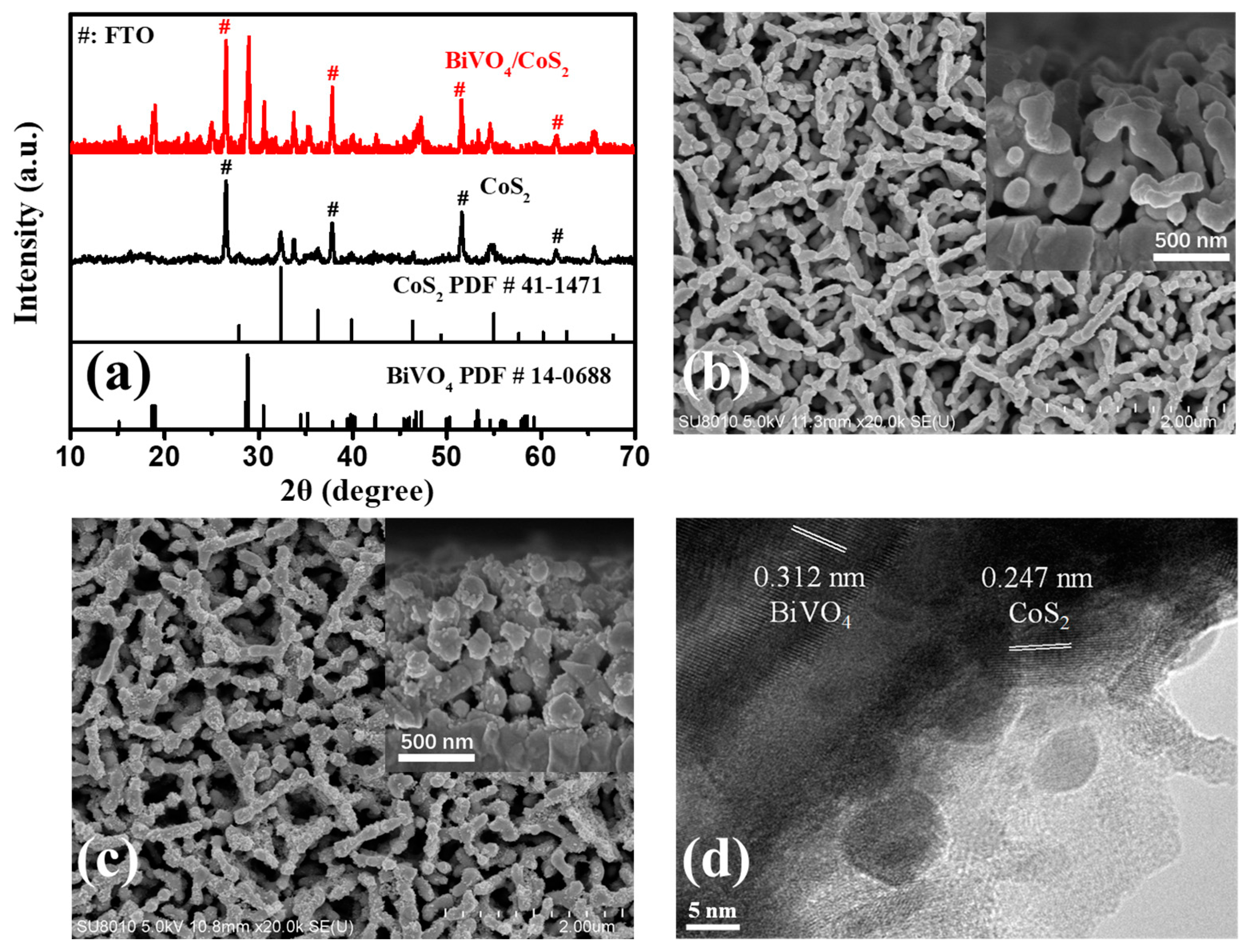
3.1.1. XRD Analysis
3.1.2. SEM and HRTEM Analysis
3.1.3. XPS Analysis
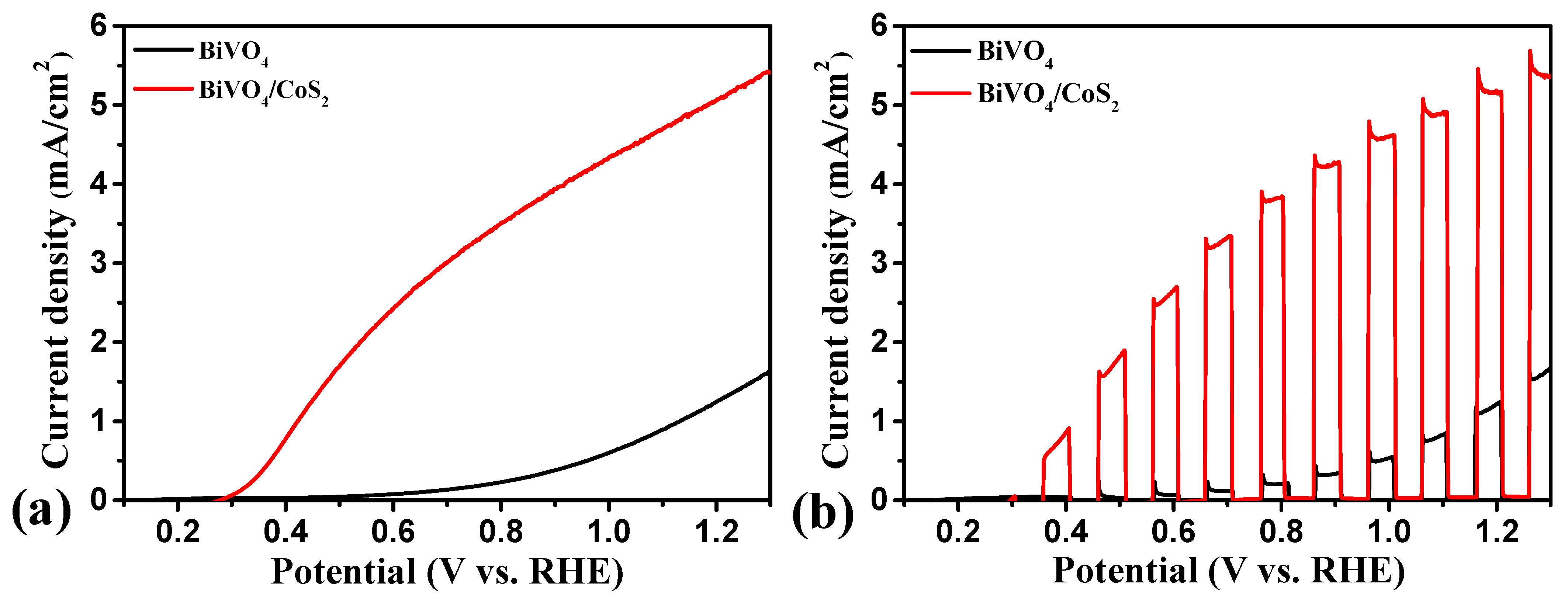
3.2. Photoelectrochemical Water Splitting Performance of the BiVO4/CoS2 Photoanode
3.3. Mechanism Study for the Enhanced Photoelectrochemcial Water Splitting Performance
3.3.1. Optical Absorption Characterization
3.3.2. Bulk Charge Separation Efficiency Analysis
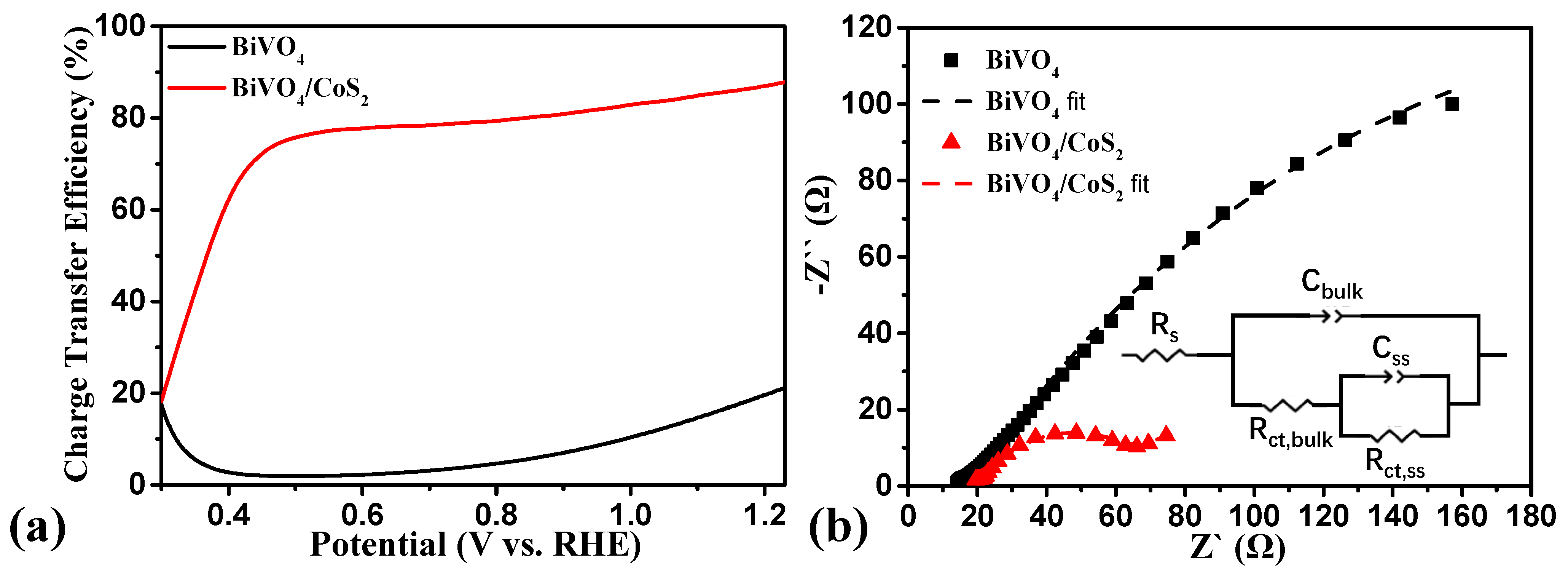
3.3.3. Interfacial Charge Transfer Efficiency Analysis
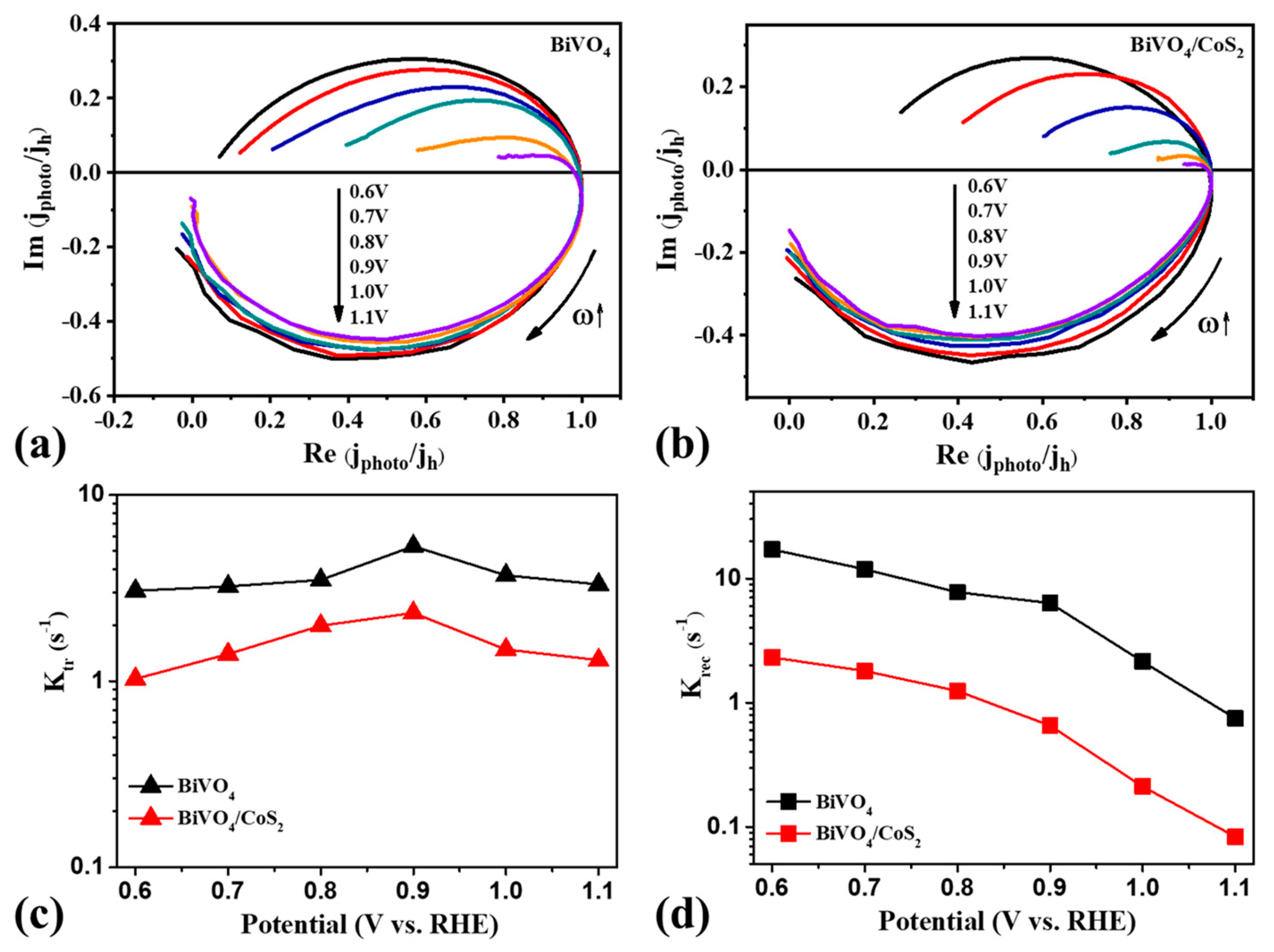
3.3.4. Surface Charge Dynamics Study with Intensity Modulated Photocurrent Spectroscopy
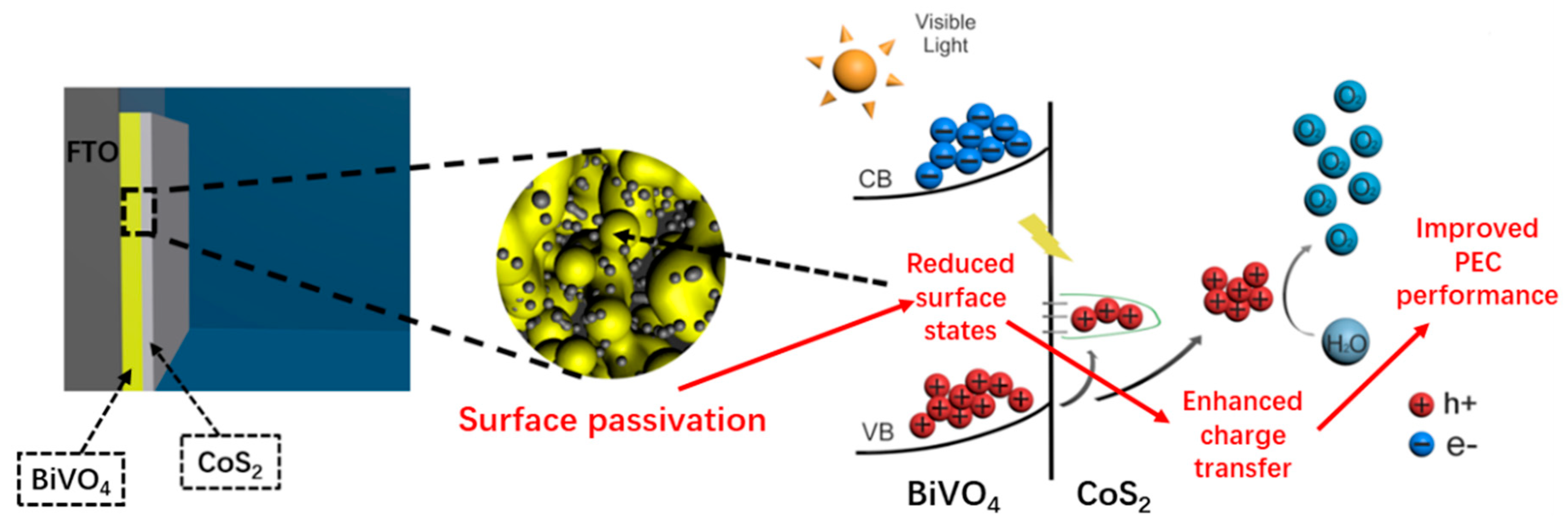
3.3.5. Proposed Mechanism for Enhanced Photoelectrochemical Water Splitting Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. A Realizable Renewable Energy Future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.-F.; Liao, P.-Y.; Zeng, Y.-S.; Wang, Z.; Liu, Z.-Q. Cascaded electron transition in CuWO4/CdS/CdS heterostructure accelerating charge separation towards enhanced photocatalytic activity. Chin. Chem. Lett. 2020, 31, 1516–1519. [Google Scholar] [CrossRef]
- Sarnowska, M.; Bienkowski, K.; Barczuk, P.J.; Solarska, R.; Augustynski, J. Highly Efficient and Stable Solar Water Splitting at (Na)WO3Photoanodes in Acidic Electrolyte Assisted by Non-Noble Metal Oxygen Evolution Catalyst. Adv. Energy Mater. 2016, 6, 1600526. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Lin, L.; Wygant, B.R.; Liu, Y.; Zhu, Y.; Zheng, Y.; Mullins, C.B.; Zhao, Y.; Zhang, X.; et al. Highly efficient photoelectrochemical water splitting from hierarchical WO3/BiVO4 nanoporous sphere arrays. Nano Lett. 2017, 17, 8012–8017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, H.; Liu, Z. Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting. Appl. Catal. B 2017, 201, 84–91. [Google Scholar] [CrossRef]
- Cho, I.S.; Lee, C.H.; Feng, Y.; Logar, M.; Rao, P.M.; Cai, L.; Kim, D.R.; Sinclair, R.; Zheng, X. Codoping titanium dioxide nanowires with tungsten and carbon for enhanced photoelectrochemical performance. Nat. Commun. 2013, 4, 1723. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Chen, S.; Bai, J.; Li, J.; Zhang, Y.; Li, L.; Xia, L.; Rahim, M.; Xu, Q.; et al. Bird-nest structured ZnO/TiO2 as a direct Z-scheme photoanode with enhanced light harvesting and carriers kinetics for highly efficient and stable photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 267, 118599. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Wang, G.; Chang, K.-D.; Ling, Y.; Lin, Y.-K.; Fitzmorris, B.C.; Liu, C.-M.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au Nanostructure-Decorated TiO2 Nanowires Exhibiting Photoactivity Across Entire UV-visible Region for Photoelectrochemical Water Splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef]
- Butburee, T.; Bai, Y.; Wang, H.; Chen, H.; Wang, Z.; Liu, G.; Zou, J.; Khemthong, P.; Lu, G.Q.M.; Wang, L. 2D porous TiO2 single-crystalline nanostructure demonstrating high photo-electrochemical water splitting performance. Adv. Mater. 2018, 30, 1705666. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.-S.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Highly efficient photoelectrochemical water splitting: Surface modification of cobalt-phosphate-loaded Co3O4/Fe2O3 p-n heterojunction nanorod arrays. Adv. Funct. Mater. 2019, 29, 1801902. [Google Scholar] [CrossRef]
- Moradlou, O.; Rabiei, Z.; Banazadeh, A.; Warzywoda, J.; Zirak, M. Carbon quantum dots as nano-scaffolds for α-Fe2O3 growth: Preparation of Ti/CQD@α-Fe2O3 photoanode for water splitting under visible light irradiation. Appl. Catal. B Environ. 2018, 227, 178–189. [Google Scholar] [CrossRef]
- Liao, A.; He, H.; Tang, L.; Li, Y.; Zhang, J.; Chen, J.; Chen, L.; Zhang, C.; Zhou, Y.; Zou, Z. Quasi-Topotactic Transformation of FeOOH Nanorods to Robust Fe2O3 Porous Nanopillars Triggered with a Facile Rapid Dehydration Strategy for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 10141–10146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lyu, M.; Chen, P.; Wang, S.; Wang, L. Energy loss analysis in photoelectrochemical water splitting: A case study of hematite photoanodes. Phys. Chem. Chem. Phys. 2018, 20, 22629–22635. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, S.; Fan, K.; Liu, Z.; Song, B.; Yu, J. MOF-Based Transparent Passivation Layer Modified ZnO Nanorod Arrays for Enhanced Photo-Electrochemical Water Splitting. Adv. Energy Mater. 2018, 8, 1800101. [Google Scholar] [CrossRef]
- Khan, I.; Qurashi, A.; Berdiyorov, G.; Iqbal, N.; Fuji, K.; Yamani, Z.H. Single-step strategy for the fabrication of GaON/ZnO nanoarchitectured photoanode their experimental and computational photoelectrochemical water splitting. Nano Energy 2018, 44, 23–33. [Google Scholar] [CrossRef]
- Han, H.; Karlicky, F.; Pitchaimuthu, S.; Shin, S.H.R.; Chen, A. Highly Ordered N-Doped Carbon Dots Photosensitizer on Metal–Organic Framework-Decorated ZnO Nanotubes for Improved Photoelectrochemical Water Splitting. Small 2019, 15, e1902771. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, P.; Qin, P.; Sun, D.; Zhang, S.; Guo, X.; Zhao, W.; Zhao, D.; Huang, F. Novel black BiVO4/TiO2−x photoanode with enhanced photon absorption and charge separation for efficient and stable solar water splitting. Adv. Energy Mater. 2019, 9, 1901287. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, K.; Huang, J.; Wang, L.; Zhang, M.; Bai, B.; Liu, H.; Wang, Q. Preparation of heterometallic CoNi-MOFs-modified BiVO4: A steady photoanode for improved performance in photoelectrochemical water splitting. Appl. Catal. B 2020, 266, 118513. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Bai, Y.; Yun, J.; Liu, G.; Wang, L. New BiVO4 Dual Photoanodes with Enriched Oxygen Vacancies for Efficient Solar-Driven Water Splitting. Adv. Mater. 2018, 30, e1800486. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Yang, G.; Li, S.; Xiao, N.; Xu, B.; Liu, S.; Qiu, P.; Hao, S.; Ge, L. Fe2TiO5 as an efficient co-catalyst to improve the photoelectrochemical water splitting performance of BiVO4. ACS Appl. Mater. Interfaces 2018, 10, 39713–39722. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, G.; Dai, Y.; Li, X.; Gao, J.; Li, N.; Qiu, P.; Ge, L. Electrodeposited co-substituted LaFeO3 for enhancing the photoelectrochemical activity of BiVO4. ACS Appl. Mater. Interfaces 2020, 12, 17364–17375. [Google Scholar] [CrossRef] [PubMed]
- She, H.; Yue, P.; Ma, X.; Huang, J.; Wang, L.; Wang, Q. Fabrication of BiVO4 photoanode cocatalyzed with NiCo-layered double hydroxide for enhanced photoactivity of water oxidation. Appl. Catal. B 2020, 263, 118280. [Google Scholar] [CrossRef]
- Jia, Q.; Iwashina, K.; Kudo, A. Facile fabrication of an efficient BiVO4 thin film electrode for water splitting under visible light irradiation. Proc. Natl. Acad. Sci. USA 2012, 109, 11564–11569. [Google Scholar] [CrossRef]
- Choi, S.K.; Choi, W.; Park, H. Solar water oxidation using nickel-borate coupled BiVO4 photoelectrodes. Phys. Chem. Chem. Phys. 2013, 15, 6499–6507. [Google Scholar] [CrossRef]
- Abdi, F.F.; Firet, N.; van de Krol, R. Efficient BiVO4 Thin Film Photoanodes Modified with Cobalt Phosphate Catalyst and W-doping. ChemCatChem 2013, 5, 490–496. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, W.; Chen, W.; Zhou, G.; Hsu, P.-C.; Zhang, R.; Liang, Z.; Fan, S.; Zhang, Y.; Cui, Y. Efficient solar-driven water splitting by nanocone BiVO 4 -perovskite tandem cells. Sci. Adv. 2016, 2, e1501764. [Google Scholar] [CrossRef]
- Zhong, D.K.; Gamelin, D.R. Photoelectrochemical water oxidation by cobalt catalyst (“Co−Pi”)/α-Fe2O3 composite photoanodes: Oxygen evolution and resolution of a kinetic bottleneck. J. Am. Chem. Soc. 2010, 132, 4202–4207. [Google Scholar] [CrossRef]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W:BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef]
- Seabold, J.A.; Choi, K.-S. Efficient and Stable Photo-Oxidation of Water by a Bismuth Vanadate Photoanode Coupled with an Iron Oxyhydroxide Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2012, 134, 2186–2192. [Google Scholar] [CrossRef]
- Abdi, F.F.; Savenije, T.J.; May, M.M.; Dam, B.; van de Krol, R. The Origin of Slow Carrier Transport in BiVO4 Thin Film Photoanodes: A Time-Resolved Microwave Conductivity Study. J. Phys. Chem. Lett. 2013, 4, 2752–2757. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Nanostructured bismuth vanadate-based materials for solar-energy-driven water oxidation: A review on recent progress. Nanoscale 2014, 6, 14044–14063. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Choi, K.-S. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy 2018, 3, 53–60. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.S. Elaborately Modified BiVO4Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, e1806938. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Yang, Y.; Cheng, C. 3D WO3/BiVO4/cobalt phosphate composites inverse opal photoanode for efficient photoelectrochemical water splitting. Small 2017, 13, 1603840. [Google Scholar] [CrossRef]
- Zalfani, M.; Hu, Z.-Y.; Yu, W.-B.; Mahdouani, M.; Bourguiga, R.; Wu, M.; Li, Y.; van Tendeloo, G.; Djaoued, Y.; Su, B.-L. BiVO4/3DOM TiO2 nanocomposites: Effect of BiVO4 as highly efficient visible light sensitizer for highly improved visible light photocatalytic activity in the degradation of dye pollutants. Appl. Catal. B 2017, 205, 121–132. [Google Scholar] [CrossRef]
- Kuang, Y.; Jia, Q.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A front-illuminated nanostructured transparent BiVO4 photoanode for >2% efficient water splitting. Adv. Energy Mater. 2016, 6, 1501645. [Google Scholar] [CrossRef]
- Zhong, X.; He, H.; Yang, M.; Ke, G.; Zhao, Z.; Dong, F.; Wang, B.; Chen, Y.; Shi, X.; Zhou, Y. In3+-doped BiVO4 photoanodes with passivated surface states for photoelectrochemical water oxidation. J. Mater. Chem. A 2018, 6, 10456–10465. [Google Scholar] [CrossRef]
- Zhou, M.; Bao, J.; Xu, Y.; Zhang, J.; Xie, J.; Guan, M.; Wang, C.; Wen, L.; Lei, Y.; Xie, Y. Photoelectrodes based upon Mo:BiVO4 inverse opals for photoelectrochemical water splitting. ACS Nano 2014, 8, 7088–7098. [Google Scholar] [CrossRef]
- Byun, S.; Jung, G.; Moon, S.-Y.; Kim, B.; Park, J.Y.; Jeon, S.; Nam, S.-W.; Shin, B. Compositional engineering of solution-processed BiVO4 photoanodes toward highly efficient photoelectrochemical water oxidation. Nano Energy 2018, 43, 244–252. [Google Scholar] [CrossRef]
- Chen, J.S.; Ren, J.; Shalom, M.; Fellinger, T.; Antonietti, M. Stainless Steel Mesh-Supported NiS Nanosheet Array as Highly Efficient Catalyst for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 5509–5516. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, K.; Lokanathan, M.; Kakade, B. Three dimensional flower like cobalt sulfide (CoS)/functionalized MWCNT composite catalyst for efficient oxygen evolution reactions. Appl. Surf. Sci. 2019, 466, 830–836. [Google Scholar] [CrossRef]
- Li, J.; Xia, Z.; Zhang, M.; Zhang, S.; Li, J.; Ma, Y.; Qu, Y. Ce-doped CoS2 pyrite with weakened O2 adsorption suppresses catalyst leaching and stabilizes electrocatalytic H2 evolution. J. Mater. Chem. A 2019, 7, 17775–17781. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, G.; Xu, T.; Wan, P.; Sun, X. A metallic CoS2 nanopyramid array grown on 3D carbon fiber paper as an excellent electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 6306–6310. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Lei, Y.; Yu, H.; Yang, Z.; Zheng, Z.; Wang, D. Ultrathin Fe-NiO nanosheets as catalytic charge reservoirs for a planar Mo-doped BiVO4 photoanode. Chem. Sci. 2018, 9, 8860–8870. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, B.; Li, Z.; Cao, S.; Sun, Y.; Wu, Y.; Gao, Z.; Sun, L. Vertically aligned oxygenated-CoS2–MoS2 heteronanosheet architecture from polyoxometalate for efficient and stable overall water splitting. ACS Catal. 2018, 8, 4612–4621. [Google Scholar] [CrossRef]
- Kong, W.; Luan, X.; Du, H.; Xia, L.; Qu, F. Enhanced electrocatalytic activity of water oxidation in an alkaline medium via Fe doping in CoS2 nanosheets. Chem. Commun. 2019, 55, 2469–2472. [Google Scholar] [CrossRef]
- Hao, J.; Yang, W.; Peng, Z.; Zhang, C.; Huang, Z.; Shi, W. A Nitrogen Doping Method for CoS2 Electrocatalysts with Enhanced Water Oxidation Performance. ACS Catal. 2017, 7, 4214–4220. [Google Scholar] [CrossRef]
- Han, X.; Wu, X.; Deng, Y.; Liu, J.; Lu, J.; Zhong, C.; Hu, W. Ultrafine Pt Nanoparticle-Decorated Pyrite-Type CoS 2 Nanosheet Arrays Coated on Carbon Cloth as a Bifunctional Electrode for Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1800935. [Google Scholar] [CrossRef]
- Feng, C.; Jiao, Z.; Li, S.; Zhang, Y.; Bi, Y. Facile fabrication of BiVO4 nanofilms with controlled pore size and their photoelectrochemical performances. Nanoscale 2015, 7, 20374–20379. [Google Scholar] [CrossRef]
- Madhusudan, P.; Ran, J.; Zhang, J.; Yu, J.; Liu, G. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Appl. Catal. B 2011, 110, 286–295. [Google Scholar] [CrossRef]
- Lee, H.; Wu, X.; Ye, Q.; Wu, X.; Wang, X.; Zhao, Y.; Sun, L. Hierarchical CoS2/Ni3S2/CoNiOx nanorods with favorable stability at 1 A cm−2 for electrocatalytic water oxidation. Chem. Commun. 2019, 55, 1564–1567. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH Nanolayers with Abundant Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 2248–2252. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water Oxidation at Hematite Photoelectrodes: The Role of Surface States. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef]
- Peter, L.M.; Wijayantha, K.G.U.; Tahir, A.A. Kinetics of light-driven oxygen evolution at α-Fe2O3 electrodes. Faraday Discuss. 2012, 155, 309–322. [Google Scholar] [CrossRef]
- Zachaus, C.; Abdi, F.F.; Peter, L.M.; van de Krol, R. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 2017, 8, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.; Peter, L. A generalized theory of intensity modulated photocurrent spectroscopy (IMPS). J. Electroanal. Chem. 1995, 396, 219–226. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Yang, G.; Tian, Z.; Zhu, H.; Ma, L.; Li, X.; Li, N.; Ge, L. Hydrothermal Synthesized CoS2 as Efficient Co-Catalyst to Improve the Interfacial Charge Transfer Efficiency in BiVO4. Inorganics 2022, 10, 264. https://doi.org/10.3390/inorganics10120264
Gao Y, Yang G, Tian Z, Zhu H, Ma L, Li X, Li N, Ge L. Hydrothermal Synthesized CoS2 as Efficient Co-Catalyst to Improve the Interfacial Charge Transfer Efficiency in BiVO4. Inorganics. 2022; 10(12):264. https://doi.org/10.3390/inorganics10120264
Chicago/Turabian StyleGao, Yangqin, Guoqing Yang, Zhijie Tian, Hongying Zhu, Lianzheng Ma, Xuli Li, Ning Li, and Lei Ge. 2022. "Hydrothermal Synthesized CoS2 as Efficient Co-Catalyst to Improve the Interfacial Charge Transfer Efficiency in BiVO4" Inorganics 10, no. 12: 264. https://doi.org/10.3390/inorganics10120264
APA StyleGao, Y., Yang, G., Tian, Z., Zhu, H., Ma, L., Li, X., Li, N., & Ge, L. (2022). Hydrothermal Synthesized CoS2 as Efficient Co-Catalyst to Improve the Interfacial Charge Transfer Efficiency in BiVO4. Inorganics, 10(12), 264. https://doi.org/10.3390/inorganics10120264




