Facile Synthesis of Nb-Doped CoTiO3 Hexagonal Microprisms as Promising Anode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
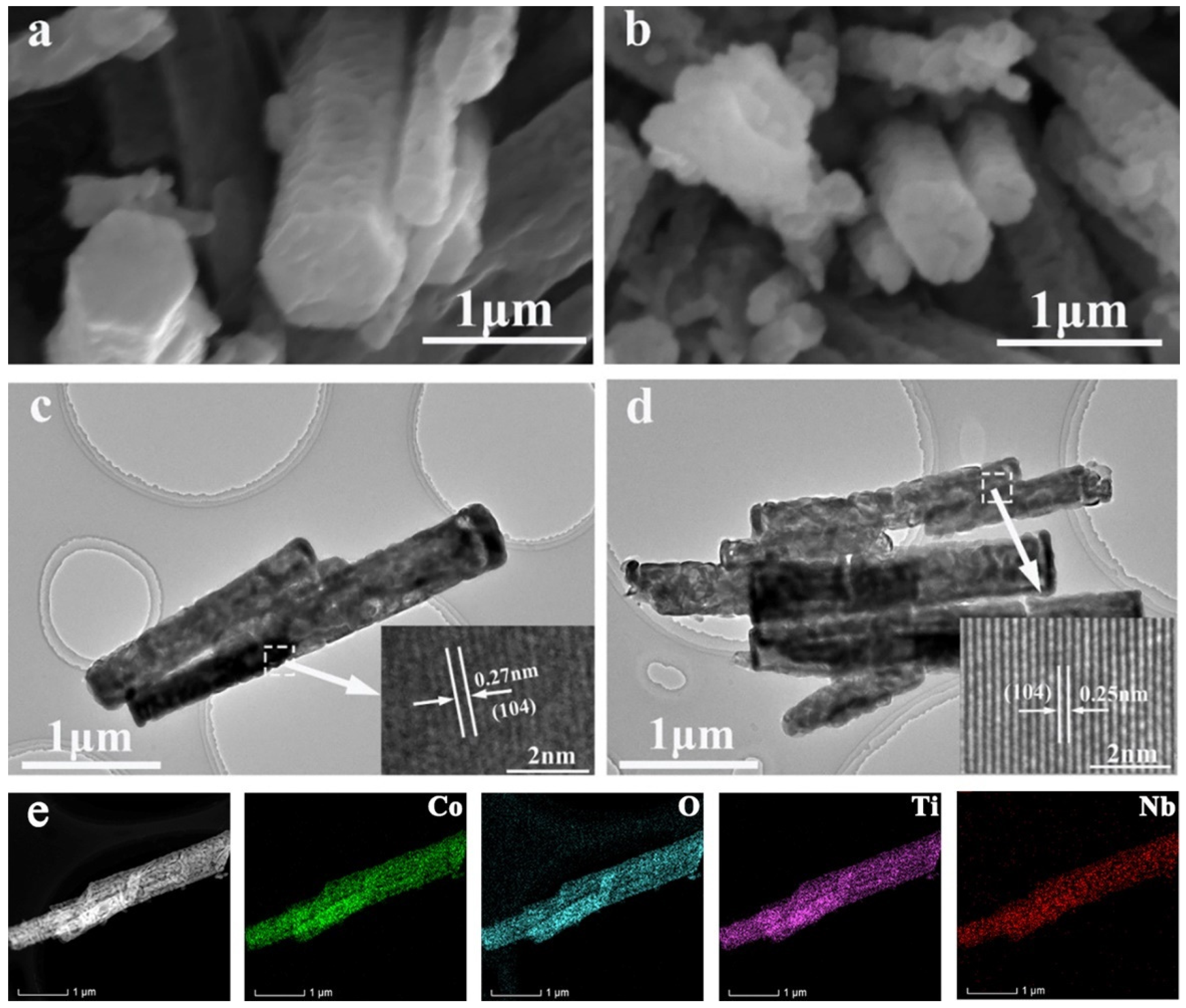
2. Results and Discussion
3. Conclusions
4. Materials and Methods
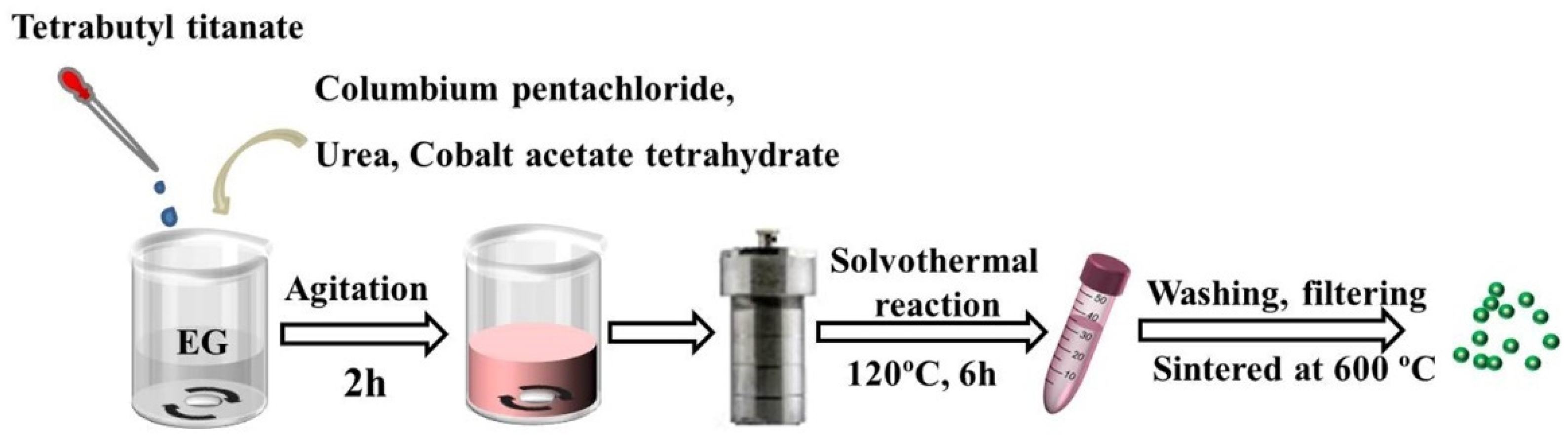
4.1. Materials Preparation
4.2. Materials Characterizations
4.3. Electrochemical Tests
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, R.; Wang, X.; Chen, Z.; Seh, Z.W.; Wang, L.; Sun, Y. Promises and challenges of the practical implementation of prelithiation in lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2101565. [Google Scholar] [CrossRef]
- Alam, M.W.; Al Qahtani, H.S.; Albalawi, H.; Aamir, M.; Bilal, M.; Ahmad Mir, T.; Souayeh, B.; Zaidi, N. Enhanced electrodes for supercapacitor applications prepared by hydrothermal-assisted nano sheet-shaped MgCo2O4@ZnS. Crystals 2022, 12, 822. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar]
- Liu, S.Y.; Fan, C.Y.; Wang, H.C.; Zhang, J.P.; Wu, X.L. Electrochemical in situ formation of a stable Ti-based skeleton for improved Li-storage properties: A case study of porous CoTiO3 nanofibers. Chem.-Eur. J. 2017, 23, 8712–8718. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tang, H.; Li, L.; Hu, Q.; Zhang, L.; Xue, H.; Pang, H. Hierarchically nanostructured transition metal oxides for lithium-ion batteries. Adv. Sci. 2018, 5, 1700592. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zheng, G.; Yang, X.; Zhang, D.; Zhang, Y.; Yan, S.; You, L.; Hou, S.; Huang, Z. Application of different carbon-based transition metal oxide composite materials in lithium-ion batteries. J. Electroanal. Chem. 2021, 898, 115652. [Google Scholar] [CrossRef]
- Avvaru, V.S.; Fernandez, I.J.; Feng, W.; Hinder, S.J.; Rodríguez, M.C.; Etacheri, V. Extremely pseudocapacitive interface engineered CoO@3D-NRGO hybrid anodes for high energy/power density and ultralong life lithium-ion batteries. Carbon 2021, 171, 869–881. [Google Scholar] [CrossRef]
- Xu, G.; Wang, G.-Y.; Zhang, X.-F.; Deng, Z.-P.; Huo, L.-H.; Gao, S. Biotemplate synthesis of mesoporous α-Fe2O3 hierarchical structure with assisted pseudocapacitive as an anode for long-life lithium ion batteries. Ceram. Int. 2021, 47, 3772–3779. [Google Scholar] [CrossRef]
- Wang, G.; Leng, X.; Han, S.; Shao, Y.; Wei, S.; Liu, Y.; Lian, J.; Jiang, Q. How to improve the stability and rate performance of lithium-ion batteries with transition metal oxide anodes. J. Mater. Res. 2017, 32, 16–36. [Google Scholar] [CrossRef]
- Ma, F.-X.; Yu, L.; Xu, C.-Y.; Lou, X.W.D. Self-supported formation of hierarchical NiCo2O4 tetragonal microtubes with enhanced electrochemical properties. Energy Environ. Sci. 2016, 9, 862–866. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L. Recent efforts in design of TiO2(B) anodes for high-rate lithium-ion batteries: A review. J. Power Sources 2019, 442, 227225. [Google Scholar] [CrossRef]
- Paul, S.; Rahman, M.A.; Sharif, S.B.; Kim, J.-H.; Siddiqui, S.-E.-T.; Hossain, M.A.M. TiO2 as an anode of high-performance lithium-ion batteries: A comprehensive review towards practical application. Nanomaterials 2022, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-D.; Zhang, T.-T.; Lu, H.; Masese, T.; Yamamoto, K.; Liu, R.-Q.; Lin, X.-J.; Feng, X.-M.; Liu, X.-M.; Wang, D.; et al. Grain-boundary-rich mesoporous NiTiO3 micro-prism as high tap-density, super rate and long life anode for sodium and lithium ion batteries. Energy Storage Mater. 2018, 13, 329–339. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Z.; Hu, Z.; Lu, X.; Zhang, S.; Huang, L.; Zheng, Y.; Li, H. Designing Uniformly Layered FeTiO3 assemblies consisting of fine nanoparticles enabling high-performance quasi-solid-state sodium-ion capacitors. Front. Chem. 2020, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ding, W.; Zhao, W.; Zhao, W.; Hong, Z.; Huang, F. In situ growth enabling ideal graphene encapsulation upon mesocrystalline MTiO3 (M = Ni, Co, Fe) nanorods for stable lithium storage. ACS Energy Lett. 2017, 2, 659–663. [Google Scholar] [CrossRef]
- Cui, D.; Li, Y.; Chen, Y.; Li, Y.; Hai, Z.; Xu, H.; Xue, C. Synthesis of CoTiO3 nanoparticles with enhanced photocatalytic degradation. Micro Nano Lett. 2019, 14, 840–844. [Google Scholar] [CrossRef]
- Wei, J.; Han, D.; Bi, J.; Gong, J. Fe-doped ilmenite CoTiO3 for antibiotic removal: Electronic modulation and enhanced activation of peroxymonosulfate. Chem. Eng. J. 2021, 423, 130165. [Google Scholar] [CrossRef]
- Guiet, A.; Huan, T.N.; Payen, C.; Porcher, F.; Mougel, V.; Fontecave, M.; Corbel, G. Copper-substituted NiTiO3 ilmenite-type materials for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 31038–31048. [Google Scholar] [CrossRef]
- Siemons, M.; Simon, U. Gas sensing properties of volume-doped CoTiO3 synthesized via polyol method. Sens. Actuators B 2007, 126, 595–603. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A review on design strategies for carbon based metal oxides and sulfides nanocomposites for high performance Li and Na ion battery anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar]
- Li, J.; Wang, D.; Zhou, J.; Hou, L.; Gao, F. MOF-derived in situ synthesized carbon-coated ilmenite cobalt titanate nanocrystalline, high-stability lithium-ion batteries. J. Alloys Compd. 2019, 793, 247–258. [Google Scholar] [CrossRef]
- Li, M.; Xiao, X.; Liu, Y.; Zhang, W.; Zhang, Y.; Chen, L. Ternary perovskite cobalt titanate/graphene composite material as long-term cyclic anode for lithium-ion battery. J. Alloys Compd. 2017, 700, 54–60. [Google Scholar] [CrossRef]
- Ouyang, B.; Chen, T.; Qin, R.; Liu, P.; Fan, X.; Wang, J.; Liu, W.; Liu, K. Bimetal–organic-framework derived CoTiO3/C hexagonal micro-prisms as high-performance anode materials for metal ion batteries. Mater. Chem. Front. 2021, 5, 5760–5768. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, W.; Zhang, Y.; Gao, Y.; Zeng, X.; Liu, L. Rational design of FeTiO3/C hybrid nanotubes: Promising lithium ion anode with enhanced capacity and cycling performance. Chem. Commun. 2020, 56, 12640–12643. [Google Scholar] [CrossRef]
- Xia, X.; Chao, D.; Zhang, Y.; Zhan, J.; Zhong, Y.; Wang, X.; Wang, Y.; Shen, Z.X.; Tu, J.; Fan, H.J. Generic synthesis of carbon nanotube branches on metal oxide arrays exhibiting stable high-rate and long-cycle sodium-ion storage. Small 2016, 12, 3048–3058. [Google Scholar] [CrossRef]
- Levartovsky, Y.; Chakraborty, A.; Kunnikuruvan, S.; Maiti, S.; Grinblat, J.; Talianker, M.; Major, D.T.; Aurbach, D. Enhancement of structural, electrochemical, and thermal properties of high-energy density Ni-rich LiNi0.85Co0.1Mn0.05O2 cathode materials for Li-ion batteries by niobium doping. ACS Appl. Mater. Interfaces 2021, 13, 34145–34156. [Google Scholar] [CrossRef]
- Lee, J.S.; Jo, M.S.; Saroha, R.; Jung, D.S.; Seon, Y.H.; Lee, J.S.; Kang, Y.C.; Kang, D.W.; Cho, J.S. Hierarchically well-developed porous graphene nanofibers comprising N-doped graphitic C-coated cobalt oxide hollow nanospheres as anodes for high-rate Li-ion batteries. Small 2020, 16, 2002213. [Google Scholar] [CrossRef]
- Bai, X.; Li, T.; Wei, C.; Sun, Y.-K.; Qi, Y.-X.; Zhu, H.-L.; Lun, N.; Bai, Y.-J. Enhancing the long-term cyclability and rate capability of Li4Ti5O12 by simple copper-modification. Electrochim. Acta 2015, 155, 132–139. [Google Scholar] [CrossRef]
- Zhou, G.W.; Lee, D.-G.; Kim, Y.-H.; Kim, C.-W.; Kang, Y.-S. Preparation and spectroscopic characterization of ilmenite-type CoTiO3 Nanoparticles. J. Korean Chem. Soc. 2006, 27, 368–372. [Google Scholar]
- Sekhar, M.C.; Reddy, B.P.; Prakash, B.P.; Park, S.-H. Effects of annealing temperature on phase transformation of CoTiO3 nanoparticles and on their structural, optical, and magnetic properties. J. Supercond. Novel Magn. 2020, 33, 407–415. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Segall, M.D.; Shah, R.; Pickard, C.J.; Payne, M.C. Population analysis of plane-wave electronic structure calculations of bulk materials. Phys. Rev. B 1996, 54, 16317–16320. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Gaddam, N.; Banerjee, A.N.; Nallapureddy, R.R.; Kumar, Y.A.; Joo, S.W. Facile construction and controllable design of CoTiO3@Co3O4/NCNO hybrid heterojunction nanocomposite electrode for high-performance supercapacitors. Electrochim. Acta 2022, 407, 139868. [Google Scholar] [CrossRef]
- Li, S.-C.; Yu, J.-G. A well-designed CoTiO3 coating for uncovering and manipulating interfacial compatibility between LiCoO2 and Li1.3Al0.3Ti1.7(PO4)3 in high temperature zone. Appl. Surf. Sci. 2020, 526, 146601. [Google Scholar] [CrossRef]
- Lu, J.; Jia, N.; Cheng, L.; Liang, K.; Huang, J.; Li, J. rGO/CoTiO3 nanocomposite with enhanced gas sensing performance at low working temperature. J. Alloys Compd. 2018, 739, 227–234. [Google Scholar] [CrossRef]
- Tan, T.; Du, Y.; Cao, A.; Sun, Y.; Zha, G.; Lei, H.; Zheng, X. The resistive switching characteristics of Ni-doped HfOx film and its application as a synapse. J. Alloys Compd. 2018, 766, 918–924. [Google Scholar] [CrossRef]
- Jiang, H.; Zang, C.; Zhang, Y.; Wang, W.; Yang, C.; Sun, B.; Shen, Y.; Bian, F. 2D MXene-derived Nb2O5/C/Nb2C/gC3N4 heterojunctions for efficient nitrogen photofixation. Catal. Sci. Technol. 2020, 10, 5964–5972. [Google Scholar] [CrossRef]
- Jia, X.; Wei, L.; Xu, L.; Hu, Y.; Guo, H.; Li, Y. Nb5+ doped Li1.20Mn0.54Ni0.13Co0.13O2 with Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) surface modification as advanced cathode material for Li-ion batteries. J. Alloys Compd. 2020, 832, 154986. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Liu, Z.; Leung, K.; Chen, L.-Q.; Lu, P.; Qi, Y. Connecting the irreversible capacity loss in Li-ion batteries with the electronic insulating properties of solid electrolyte interphase (SEI) components. J. Power Sources 2016, 309, 221–230. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, W.; Hu, J.; Su, Y.; Kong, X.; Uemura, S.; Kusunose, T.; Feng, Q. Lithium ion battery anode of mesocrystalline CoTiO3/TiO2 nanocomposite with extremely enhanced capacity. ACS Appl. Energy Mater. 2021, 4, 13646–13656. [Google Scholar] [CrossRef]
- Wang, F.; Zhuo, H.-Y.; Han, X.; Chen, W.-M.; Sun, D. Foam-like CoO@N,S-codoped carbon composites derived from a well-designed N,S-rich Co-MOF for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 22964–22969. [Google Scholar] [CrossRef]
- Ren, Q.-Q.; Yu, F.-D.; Zhang, S.-W.; Yin, B.-S.; Wang, Z.-B.; Ke, K. Enhanced electrochemical performance by size-dependent SEI layer reactivation of NiCo2O4 anodes for lithium ion batteries. Electrochim. Acta 2019, 297, 1011–1017. [Google Scholar] [CrossRef]
- Sun, M.; Sheng, X.; Li, S.; Cui, Z.; Li, T.; Zhang, Q.; Xie, F.; Wang, Y. Construction of porous CoTiO3 microrods with enhanced performance as lithium-ion battery anode. J. Alloys Compd. 2022, 926, 166809. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Zhi, L.; Cao, Q.; Maier, J.; Müllen, K. Nanographene-constructed hollow carbon spheres and their favorable electroactivity with respect to lithium storage. Adv. Mater. 2010, 22, 838–842. [Google Scholar] [CrossRef]
- Li, T.; Gulzar, U.; Bai, X.; Monaco, S.; Longoni, G.; Prato, M.; Marras, S.; Dang, Z.; Capiglia, C.; Proietti Zaccaria, R. Surface and interface engineering of anatase TiO2 anode for sodium-ion batteries through Al2O3 surface modification and wise electrolyte selection. J. Power Sources 2018, 384, 18–26. [Google Scholar] [CrossRef]
- Tang, L.-B.; Xiao, B.; An, C.-S.; Li, H.; He, Z.-J.; Zheng, J.-C. VPO4@C/graphene microsphere as a potential anode material for lithium-ion batteries. Ceram.Int. 2018, 44, 14432–14438. [Google Scholar] [CrossRef]
- Li, L.; Han, E.; Zhu, L.; Qiao, S.; Du, C.; Liu, H. Effect of Zr doping and Al-Zr co-doping on LiNi0.5Co0.25Mn0.25O2 for lithium-ion batteries. Solid State Ionics 2020, 346, 115220. [Google Scholar] [CrossRef]




| Sample | Rs (Ω) | RSEI (Ω) | Rct (Ω) |
|---|---|---|---|
| S0 | 9.3 | 24.6 | 22.1 |
| S1 | 11.1 | 26.7 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Yu, G.; Song, M.; Zhang, Q.; Li, Y.; Bai, X. Facile Synthesis of Nb-Doped CoTiO3 Hexagonal Microprisms as Promising Anode Materials for Lithium-Ion Batteries. Inorganics 2023, 11, 10. https://doi.org/10.3390/inorganics11010010
Li T, Yu G, Song M, Zhang Q, Li Y, Bai X. Facile Synthesis of Nb-Doped CoTiO3 Hexagonal Microprisms as Promising Anode Materials for Lithium-Ion Batteries. Inorganics. 2023; 11(1):10. https://doi.org/10.3390/inorganics11010010
Chicago/Turabian StyleLi, Tao, Gengchen Yu, Minghui Song, Qi Zhang, Yifan Li, and Xue Bai. 2023. "Facile Synthesis of Nb-Doped CoTiO3 Hexagonal Microprisms as Promising Anode Materials for Lithium-Ion Batteries" Inorganics 11, no. 1: 10. https://doi.org/10.3390/inorganics11010010
APA StyleLi, T., Yu, G., Song, M., Zhang, Q., Li, Y., & Bai, X. (2023). Facile Synthesis of Nb-Doped CoTiO3 Hexagonal Microprisms as Promising Anode Materials for Lithium-Ion Batteries. Inorganics, 11(1), 10. https://doi.org/10.3390/inorganics11010010








