A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation
Abstract
:1. Introduction
2. Chemical Composition and Structure-Property Relationship of Clays
- (i)
- (ii)
- 2D 2-1 layered clay minerals (organized into six subgroups), formed by alternating layers made by two tetrahedral (Si-based) sheets sandwiching one octahedral (Al-based) sheet in the middle. These sandwich structures are held together by an interlayer made by either water molecules or exchangeable cations (to maintain the electroneutrality of the system), or even both [2,64].
- (i)
- Kaolinite-serpentine subgroup: 1-1 layered structures: This subgroup is characterized by electroneutral layered structures and the absence of cations at the interlayer (with very low CEC, 3–15 cmol/kg). In particular, surface charges of kaolinite derived from the presence of defects (such as isomorphic substitution and broken edges Al-O-Al and Si-O-Si). Tetrahedral and octahedral sheets are held together by either secondary forces (e.g., hydrogen bonding) or water molecules. The kaolinite-serpentine subgroup is characterized by the general chemical formulas Al2Si2O5(OH)4 (for the kaolinite subgroup) and Mg3Si2O5(OH)4 (for the serpentine subgroup) [65]. Examples of clays belonging to this subgroup are kaolinite, halloysite, dickite, nacrite, crysotile, antigorite, lizardite (the latter three belonging to the serpentine subgroup).
- (i)
- Pyrophyllite-talc subgroup: 2-1 layered structures: This subgroup is characterized by electroneutral layered structures and the absence of cations at the interlayer (with very low CEC, below 1 cmol/kg). Weak secondary forces (e.g., van der Waals and/or dipolar interactions) that favor a loss of cohesion between the layers hold tetrahedral sheets together. The pyrophyllite-talc subgroup is characterized by the general chemical formulas Al2Si4O10(OH)2 (for the pyrophyllite subgroup) and Mg3Si4O10(OH)2 (for the talc subgroup) [66].
- (ii)
- Smectite subgroup: 2-1 layered structures: This subgroup is characterized by having octahedral sheets partially substituted, thus generating weak negatively charged layers. In order to balance such negative charge and maintain the electroneutrality of the system, the smectites interlayer region contains miscellaneous cations, together with water molecules. The presence of such an interlayer (made by water molecules and cations) enhances the water affinity of smectites, thus favoring the hydraulic delamination and expansion. Furthermore, smectites show a high ion exchange capacity (i.e., CEC approximately 70–100 cmol/kg). The smectite subgroup is characterized by the chemical formula (Na,Ca)0.33(Al,Mg,Fe,Zn)2Si4O10(OH)2·nH2O [67]. Examples of clays belonging to this subgroup are: montomorillonite, beidellite, laponite, saponite, and hectorite.
- (iii)
- Vermiculite subgroup: 2-1 layered structures. This subgroup is characterized by having both tetrahedral and octahedral sheets partially substituted, thus generating a net negative charge in both layers. In order to balance such net negative charge and maintain the electroneutrality of the system, the vermiculites interlayer region contains two oriented water layers and magnesium cations, thus providing a limited expansion capacity and high ion exchange capacity (i.e., CEC approximately 100–150 cmol/kg). The vermiculite subgroup is characterized by the chemical formula (Mg,Ca)0.3(Mg,Fe)3(Si,Al)4O10(OH)2·4H2O) [68].
- (iv)
- Mica subgroup: 2-1 layered structures: This subgroup is characterized by having the Si-based tetrahedral sheets partially substituted by aluminum atoms, thus generating a charge deficiency in the tetrahedral layers. In order to balance such strong negative charge and maintain the electroneutrality of the system, the micas interlayer region contains potassium cations occupying fixed positions at the tetrahedral sites surface. Such locked structure significantly limits the entry of water and micas ion exchange capacity (i.e., CEC approximately 10–40 cmol/kg). The mica subgroup is characterized by the chemical formula (K,H)Al2(Si,Al)4O10(OH)2·nH2O [69]. Examples of clays belonging to this subgroup are: muskovite, sericite, illite, biotite, and glauconite.
- (v)
- Chlorites subgroup: 2-1 layered structures: This subgroup is characterized by having both tetrahedral and octahedral sheets partially substituted. In order to balance such negative charge and maintain the electroneutrality of the system, the chlorites interlayer region is made by hydroxide sheets, mainly constituted by brucite Mg(OH)2, eventually partially substituted by iron atoms. The presence of hydroxyl functionalities at the interface between tetrahedral sheets and hydroxide-based interlayers induces the formation of hydrogen bonding that hold together the layered structures, generating a locked system characterized by having a poor ion exchange capacity (analogously as in the case of mica subgroup, namely: CEC approximately 10–40 cmol/kg). The chlorite subgroup is characterized by the chemical formula (Mg,Fe)3(Si,Al)4O10(OH)2·((Mg,Fe)3(OH)6) [70].
- (vi)
- Inverted ribbons (palygorskite-sepiolite) subgroup: 2-1 layered structures: This subgroup is characterized by ribbons of 2-1 layered silicates presenting a periodic inversion of the apical oxygen atom in tetrahedral layers extending parallel to the layer directions, forming fibrous clays. These complex structures are characterized by the presence of nanometric channels (parallel-oriented respect to the direction of the layers) containing water molecules weakly bound to the magnesium ions forming the octahedral layers. The presence of these nanochannels guarantees high surface area (SSA higher than approximately 140–320 m2/g) that allows their use as porous systems for advanced applications (e.g, controlled transport and/or release of chemicals, drug-delivery, separation science) [71]. The chemical formulas of palygorskite-sepiolite subgroup are the following, namely: (Mg,Al)2Si4O10OH·4H2O (for the palygorskite subgroup), and Mg4Si6O15(OH)2·6H2O, (for the sepiolite subgroup) [72,73].
- (i)
- Cation exchange capacity (CEC): The CEC corresponds to the amount of cations (expressed in cmol/kg) that can be exchanged with other cations at the surface of clays. The CEC is influenced by the nature and amount of cations at the clays interlayer.
- (ii)
- Interlayer thickness: Depending on the chemical species forming the interlayer, these generate different electrostatic forces (and consequently different degree of attraction) between the different sheets forming the layer structures of clays. These electrostatic forces influence the interlayer thickness.
- (iii)
- Hydration/gel-forming (or swelling) capacity: Mechanisms at the basis of hydration are mostly two: (a) electrical properties of both clay’s inorganic surface and aqueous medium affecting the water molecules orientation at the clay’s surface, and (b) osmosis. Furthermore, both hydration and swelling properties are strongly affected by the nature and the quantity of exchangeable cations present at the interlayer, and these values can be predicted considering the hydration energy of the different ions. In fact, the swelling capability follows the order: Mg > Ca > Li > Na > K [2].
3. Advanced Applications: The Second (Technological) Life of Clays
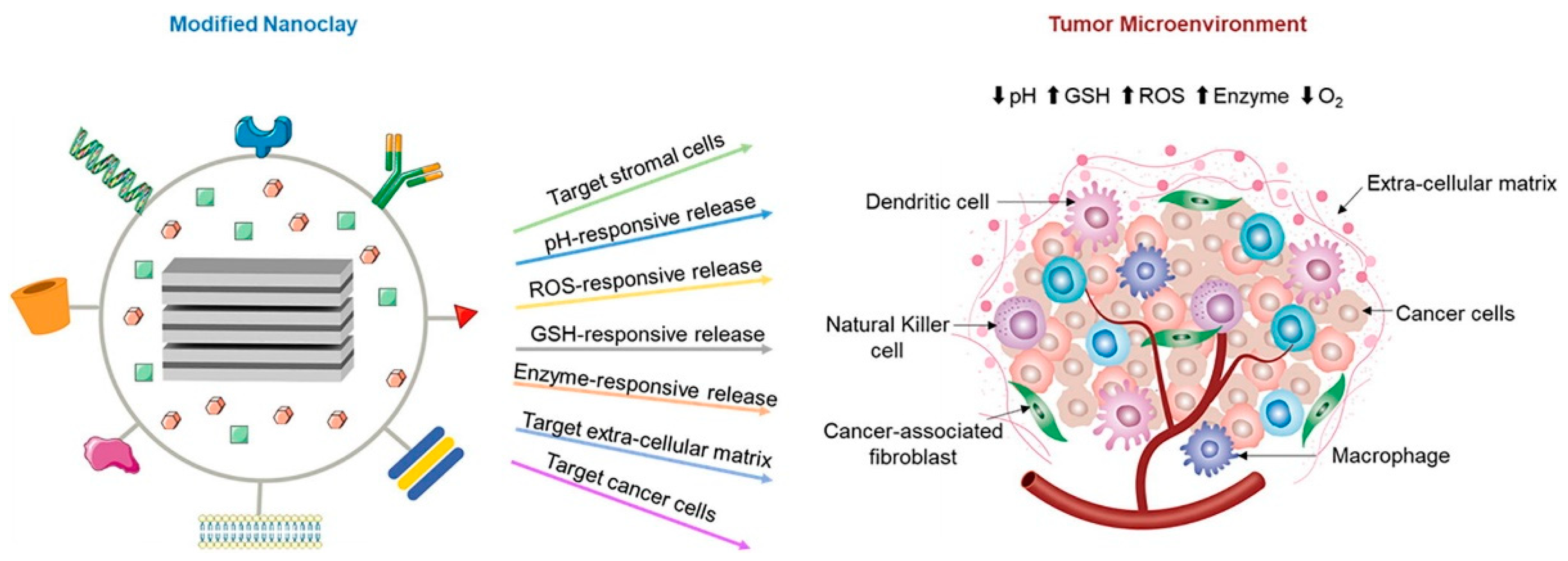
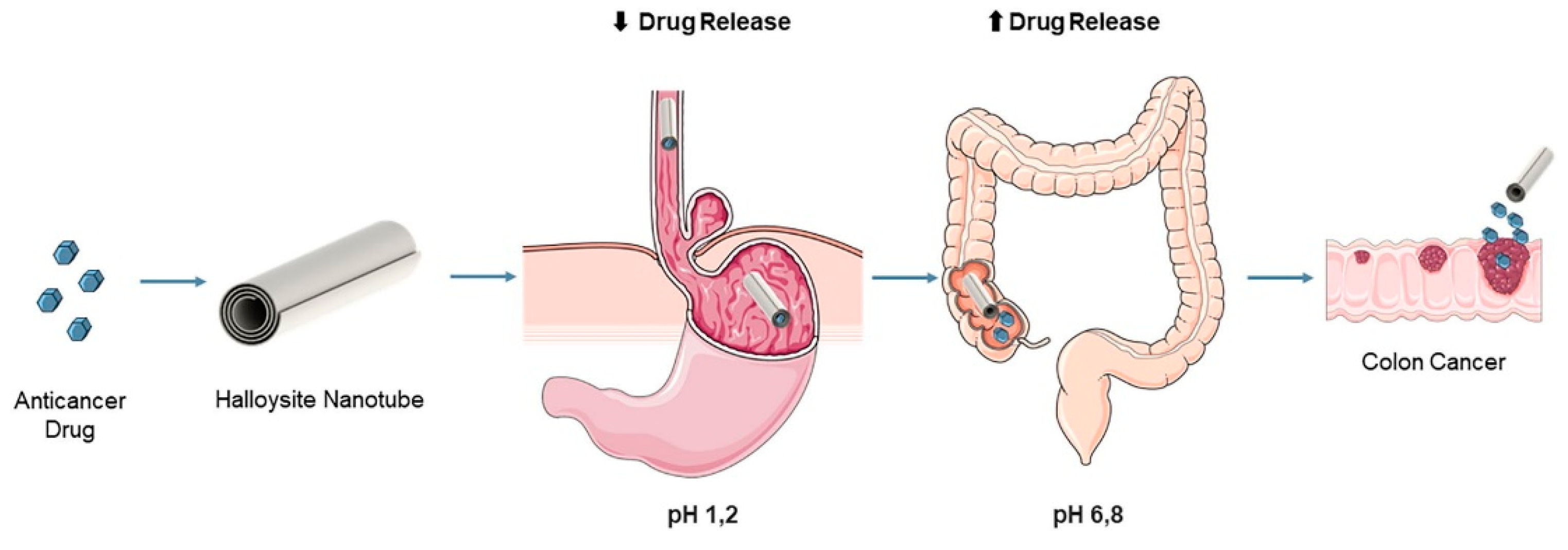
3.1. Biomedical Applications
3.2. Environmental Applications
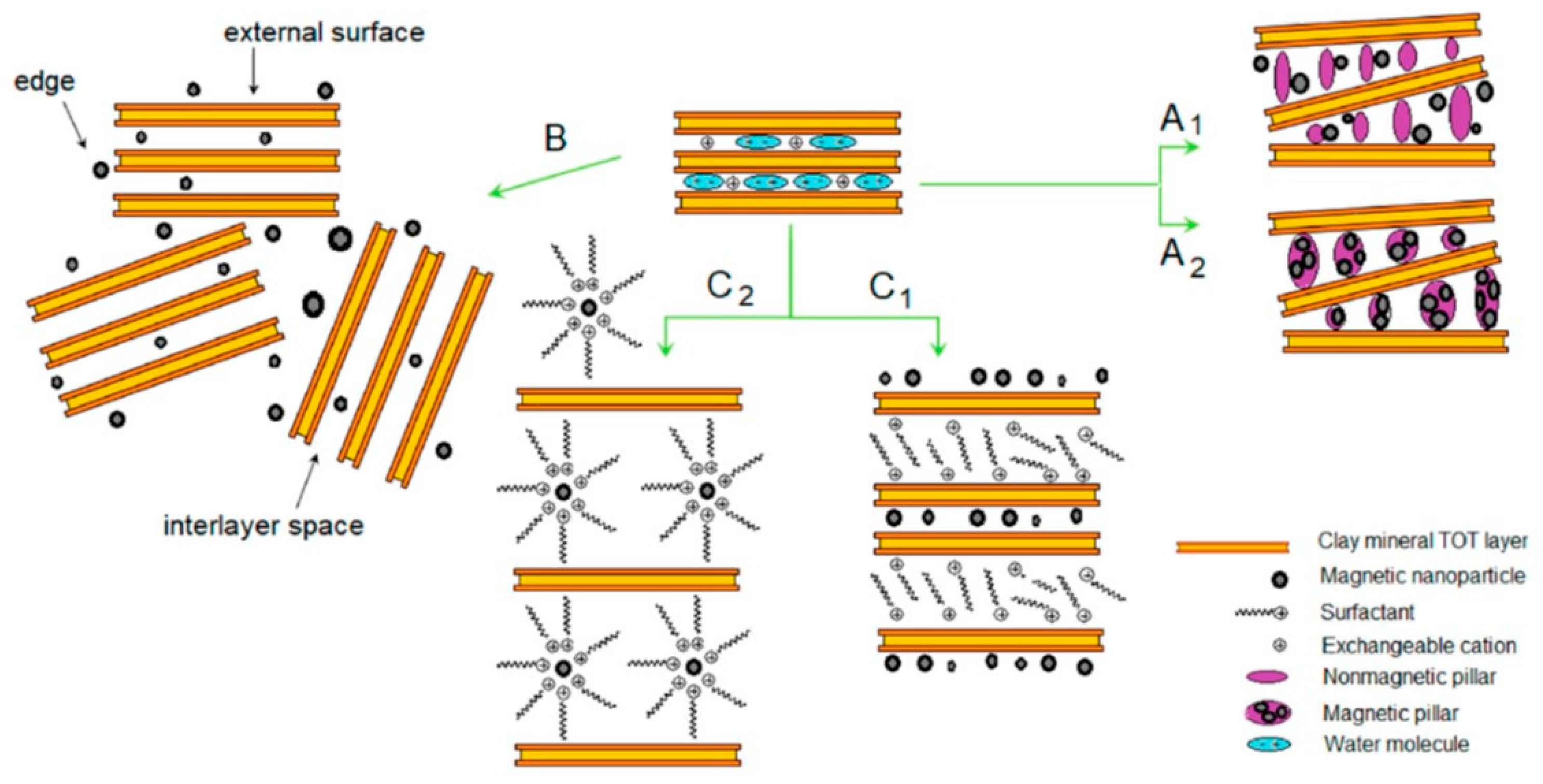
- (i)
- Pillaring: This method consists of introducing a pillar within the structure of the clay by permanently stacking the interlayers, generating a higher porosity [120]. Pillaring is mostly a cationic exchange method in which inorganic species are introduced within the interlayer of clays forming robust oxides strongly bound to the layers of the minerals [17]. In this specific context, the mechanisms proposed are two: either the incorporation of magnetic nanoparticles within the pores of the pillared clays (Figure 4, route A1) or using the magnetic nanoparticles as pillars to expand the interlayer distance of the clays (Figure 4, route A2) [10].
- (ii)
- (iii)
- Intercalation: This method consists in the physical insertion of target chemical species within the interlayers/pores of the clays [122]. In this specific context, the mechanisms proposed are two: either the inclusion of magnetic nanoparticles within a previously surfactants-intercalated clay to facilitate the entrance of the magnetic nanoparticles (Figure 4, route C1) or the direct intercalation of surfactant-stabilized magnetic nanoparticles (Figure 4, route C2) [10].
- (i)
- Cation exchange reaction: This method consists of exchanging the interlayers cations with quaternary alkylammonium cations in aqueous solution.
- (ii)
- Solid-state reaction: This method consists of intercalating organic molecules in dried clays (i.e., in absence of solvents).
3.3. Other Advanced Applications: Additive Manufacturing and Sol-Gel Processes
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of the AIPEA and CMS Nomenclature Committees. Clay Miner. 1995, 30, 257–259. [Google Scholar] [CrossRef]
- Nisticò, R. The importance of surfaces and interfaces in clays for water remediation processes. Surf. Topogr. Metrol. Prop. 2018, 6, 043001. [Google Scholar] [CrossRef]
- Zou, Y.-C.; Mogg, L.; Clark, N.; Bacaksiz, C.; Milanovic, S.; Sreepal, V.; Hao, G.-P.; Wang, Y.-C.; Hopkinson, D.G.; Gorbachev, R.; et al. Ion exchange in atomically thin clays and micas. Nat. Mater. 2021, 20, 1677–1682. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Zhu, Z.; Zhong, Y.; Bando, Y.; Golberg, D.; Yao, J.; Wang, X. The role of geometric sites in 2D materials for energy storage. Joule 2018, 2, 1075–1094. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, M.; Barbalinardo, M.; Riminucci, A.; Belvedere, K.; Boccalon, E.; Sotgiu, G.; Corticelli, F.; Ruani, G.; Zamboni, R.; Aluigi, A.; et al. Magnetic keratic/hydrotalcites sponges as potential scaffolds for tissue regeneration. Appl. Clay Sci. 2021, 297, 106090. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, S.; Scheibel, T. Copolymer/clay nanocomposites for biomedical applications. Adv. Funct. Mater. 2020, 30, 1908101. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, D.; Pereira, I.; Pereira-Silva, M.; Veiga, F.; Hamblin, M.R.; Lvov, Y.; Paiva-Santos, A.C. Emerging role of nanoclays in cancer research, diagnosis, and therapy. Coord. Chem. Rev. 2021, 440, 213956. [Google Scholar] [CrossRef]
- Peralta, M.E.; Ocampo, S.; Funes, I.G.; Onaga Medina, F.; Parolo, M.E.; Carlos, L. Nanomaterials with tailored magnetic properties as adsorbents of organic pollutants from wastewaters. Inorganics 2020, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Manjaiah, K.M.; Datta, S.C.; Yadav, R.K.; Sarkar, B. Inorganically modified clay minerals: Preparation, characterization, and arsenic adsorption in contaminated water and soil. Appl. Clay Sci. 2017, 147, 1–10. [Google Scholar] [CrossRef]
- Beall, G.W. The use of organo-clays in water treatment. Appl. Clay Sci. 2003, 24, 11–20. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Belver, C.; Bedia, J.; Haj Amara, A.B.; Ruiz-Hitzky, E. Reprint of ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 2018, 160, 3–8. [Google Scholar] [CrossRef]
- Es-Sahbany, H.; Hsissou, R.; El Hachimi, M.L.; Allaoui, M.; Nkhili, S.; Elyoubi, M.S. Investigation of the adsorption of heavy metals (Cu, Co, Ni and Pb) in treatment synthetic wastewater using natural clay as a potential adsorbent (Sale-Marocco). Mater. Today Proc. 2021, 45, 7290–7298. [Google Scholar] [CrossRef]
- Li, Z.; Potter, N.; Rasmussen, J.; Weng, J.; Lv, G. Removal of rhodamine 6G with different types of clay minerals. Chemosphere 2018, 202, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, F.; Wang, X.; Cheng, F. Facile preparation of ammonium molybdophosphate/Al-MCM-41 composite material from natural clay and its use in cesium ion adsorption. Eur. J. Inorg. Chem. 2015, 2015, 2125–2131. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and applications for low-cost ceramic membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashad, M.; Logesh, G.; Sabu, U.; Balasubramanian, M. A novel monolithic mullite microfiltration membrane for oil-in-water emulsion separation. J. Membr. Sci. 2021, 620, 118857. [Google Scholar] [CrossRef]
- Elgamouz, A.; Tijani, N. From a naturally occurring-clay mineral to the production of porous ceramic membranes. Microporous Mesoporous Mater. 2018, 271, 52–58. [Google Scholar] [CrossRef]
- Abubakar, M.; Tamin, M.N.; Saleh, M.A.; Uday, M.B.; Ahmad, N. Preparation and characterization of a nigerian mesoporous clay-based membrane for uranium removal from underground water. Ceram. Int. 2016, 42, 8212–8220. [Google Scholar] [CrossRef]
- Yi, H.; Ai, Z.; Zhao, Y.; Zhang, X.; Song, S. Design of 3D-network montmorillonite nanosheet/stearic acid shape-stabilized phase change materials for solar energy storage. Sol. Energy Mater. Sol. Cells 2020, 204, 110233. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.; Wang, C. Investigation of electrochemical performance of montmorillonite clay as Li-ion battery electrode. Sustain. Mater. Technol. 2019, 19, e00086. [Google Scholar] [CrossRef]
- Rajapakse, R.M.G.; Murakami, K.; Bandara, H.M.M.; Rajapakse, R.M.M.Y.; Velauthamurti, K.; Wijeratne, S. Preparation and characterization of electronically conducting polypyrrole-montmorillonite nanocomposite and its potential application as a cathode material for oxygen reduction. Electrochim. Acta 2010, 55, 2490–2497. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, C.H.; Kim, J.S.; Nam, T.U.; Kim, W.-S.; Lee, T.I.; Oh, J.Y. Thermoelectric energy harvesting electronic skin (e-skin) Patch with reconfigurable carbon nanotube clays. Nano Energy 2021, 87, 106156. [Google Scholar] [CrossRef]
- Vaia, R.A.; Price, G.; Ruth, P.N.; Nguyen, H.T.; Lichtenhan, J. Polymer/layered silicate nanocomposites as high performance ablative materials. Appl. Clay Sci. 1999, 15, 67–92. [Google Scholar] [CrossRef]
- Atyia, M.N.; Mahdy, M.G.; Elrahman, M.A. Production and properties of lightweight concrete incorporating lightweight concrete incorporating recycled waste crushed clay bricks. Constr. Build. Mater. 2021, 304, 124655. [Google Scholar] [CrossRef]
- Hassan, A.; Mourad, A.-H.I.; Rashid, Y.; Ismail, N.; Laghari, M.S. Thermal and structural performance of geopolymer concrete containing phase change material encapsulated in expanded clay. Energy Build. 2019, 191, 72–81. [Google Scholar] [CrossRef]
- Madyan, O.A.; Fan, M.; Feo, L.; Hui, D. Physical properties of clay aerogel composites: An overview. Compos. Part B Eng. 2016, 102, 29–37. [Google Scholar] [CrossRef]
- Di Credico, B.; Cobani, E.; Callone, E.; Conzatti, L.; Cristofori, D.; D’Arienzo, M.; Dirè, S.; Giannini, L.; Hanel, T.; Scotti, R.; et al. Size-controlled self-assembly of anisotropic sepiolite fibers in rubber nanocomposites. Appl. Clay Sci. 2018, 152, 51–64. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Huang, B.; Tang, Q. Recent advances in superhydrophobic composites based on clay minerals. Appl. Clay Sci. 2020, 198, 105793. [Google Scholar] [CrossRef]
- Serge, E.J.; Alla, J.P.; Belibi, P.D.B.; Mbadcam, K.J.; Fathima, N.N. Clay/polymer nanocomposites as filler materials for leather. J. Clean. Prod. 2019, 237, 117837. [Google Scholar] [CrossRef]
- Qian, Z.; Hu, G.; Zhang, S.; Yang, M. Preparation and characterization of montmorillonite-silica nanocomposites: A sol-gel approach to modifying clay surfaces. Phys. B Condens. Matter 2008, 403, 3231–3238. [Google Scholar] [CrossRef]
- Meera, K.M.S.; Sankar, R.M.; Murali, A.; Jainsankar, S.N.; Mandal, A.B. Sol-gel network silica/modified montmorillonite clay hybrid nanocomposites for hydrophobic surface coatings. Colloids Surf. B Biointerfaces 2012, 90, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Revelo, C.F.; Colorado, H.A. 3D printing of kaolinite clay ceramics using the Direct Ink Writing (DIW) technique. Ceram. Int. 2018, 44, 5673–5682. [Google Scholar] [CrossRef]
- Chan, S.S.L.; Pennings, R.M.; Edwards, L.; Franks, G.V. 3D printing of clay for decorative architectural applications: Effect of solids volume fraction on rheology and printability. Addit. Manuf. 2020, 35, 101335. [Google Scholar] [CrossRef]
- Njindam, O.R.; Njoya, D.; Mache, J.R.; Mouafon, M.; Messan, A.; Njopwouo, D. Effect of glass powder on the technological properties and microstructure of clay mixture for porcelain stoneware tiles manufacture. Constr. Build. Mater. 2018, 170, 512–519. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Srasra, E.; Zargouni, F. The use of Tunisian Barremian clay in the traditional ceramic industry: Optimization of ceramic properties. Appl. Clay Sci. 2008, 42, 125–129. [Google Scholar] [CrossRef]
- Freyburg, S.; Schwarz, A. Influence of the clay type on the pore structure of structural ceramics. J. Eur. Ceram. Soc. 2007, 27, 1727–1733. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Santalucia, R.; Vacca, T.; Cesano, F.; Martra, G.; Pellegrino, F.; Scarano, D. Few-layered MoS2 nanoparticles covering anatase TiO2 nanosheets: Comparison between ex situ and in situ synthesis approaches. Appl. Sci. 2021, 11, 143. [Google Scholar] [CrossRef]
- Rocha Barreto, I.A.; da Costa, M.L. Viability of Belterra clay, a widespread bauxite cover in the Amazon, as a low-cost raw material for the production of red ceramics. Appl. Clay Sci. 2018, 162, 252–260. [Google Scholar] [CrossRef]
- Lopez-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Lonzano-Morales, V.; Gardi, I.; Nir, S.; Undabeytia, T. Removal of pharmaceuticals from water by clay-cationic starch sorbents. J. Clean. Prod. 2018, 190, 703–711. [Google Scholar] [CrossRef]
- Moussi, B.; Hajjaji, W.; Hachani, M.; Hatira, N.; Labrincha, J.A.; Yans, J.; Jamoussi, F. Numidian clay deposits as raw material for ceramics tile manufacturing. J. Afr. Earth Sci. 2020, 164, 103775. [Google Scholar] [CrossRef]
- Gast, J.; Gundolf, K.; Cesinger, B. Doing business in a green way: A systematic review of the ecological sustainability entrepreneurship literature and future research directions. J. Clean. Prod. 2017, 147, 44–56. [Google Scholar] [CrossRef]
- D’Amato, D.; Droste, N.; Allen, B.; Kettunen, M.; Lahtinen, K.; Korhonen, J.; Leskinen, P.; Matthies, B.D.; Toppinen, A. Green, circular, bio economy: A comparative analysis of sustainability avenues. J. Clean. Prod. 2017, 168, 716–734. [Google Scholar] [CrossRef]
- Mies, A.; Gold, S. Mapping the social dimension of the circular economy. J. Clean. Prod. 2021, 321, 128960. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Toward petroleum-free with plant-based chemistry. Curr. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X.; Liu, Q. A mini review of the specialties of the bio-oils produced from pyrolysis of 20 different biomasses. Renew. Sustain. Energy Rev. 2019, 114, 109313. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrog. Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Tabasso, S.; Ginepro, M.; Tomasso, L.; Montoneri, E.; Nisticò, R.; Francavilla, M. Integrated biochemical and chemical processing of municipal bio-waste to obtain bio based products for multiple uses. The case of soil remediation. J. Clean. Prod. 2020, 245, 119191. [Google Scholar] [CrossRef]
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860–871. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and beneficial impact of biochar for environmental application: A comprehensive review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive plants as potential sustainable feedstock for biochar production and multiple applications: A review. Resour. Conserv. Recycl. 2021, 164, 105204. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, K.; Jin, J.; Gao, B.; Yan, Y.; Han, L.; Wu, F.; Xing, B. Properties of the plant- and manure-derived biochars and their sorption of dibutyl phthalate and phenanthrene. Sci. Rep. 2014, 4, 5295. [Google Scholar] [CrossRef] [Green Version]
- Anceschi, A.; Guerretta, F.; Magnacca, G.; Zanetti, M.; Benzi, P.; Trotta, F.; Caldera, F.; Nisticò, R. Sustainable N-containing biochars obtained at low temperatures as sorbing materials for environmental application: Municipal biowaste-derived substances and nanosponges case studies. J. Anal. Appl. Pyrolysis 2018, 134, 606–613. [Google Scholar] [CrossRef]
- Nisticò, R.; Guerretta, F.; Benzi, P.; Magnacca, G. Chitosan-derived biochars obtained at low pyrolysis temperatures for potential application in electrochemical energy storage devices. Int. J. Biol. Macromol. 2020, 164, 1825–1831. [Google Scholar] [CrossRef]
- Abbasi, A.; Pishvaee, M.S.; Mohseni, S. Third-generation biofuel supply chain: A comprehensive review and future research directions. J. Clean. Prod. 2021, 323, 129100. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- Observatory of Economic Complexity (OEC), Which Countries Exports Clays? Available online: https://oec.world/en/visualize/tree_map/hs92/export/show/all/52508/2019/ (accessed on 10 October 2021).
- Observatory of Economic Complexity (OEC), Which Countries Imports Clays? Available online: https://oec.world/en/visualize/tree_map/hs92/import/show/all/52508/2019/ (accessed on 10 October 2021).
- Martin, R.T.; Bailey, S.W.; Eberl, D.D.; Fanning, D.S.; Guggenheim, S.; Kodama, H.; Pevear, D.R.; Środoń, J.; Wicks, F.J. Report of the Clay Minerals Society Nomenclature Committee: Revised classification of clay materials. Clays Clay Miner. 1991, 39, 333–335. [Google Scholar] [CrossRef]
- Salles, F.; Henry, M.; Douilland, J.-M. Determination of the surface energy of kaolinite and serpentine using PACHA formalism—Comparison with immersion experiments. J. Colloid Interface Sci. 2006, 303, 617–626. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, L.; Pant, R.; Yu, Y.; Wei, Z.; Zhang, G. Effects of interlayer interactions on the nanoindentation behavior and hardness of 2:1 phyllosilicates. Appl. Clay Sci. 2013, 80–81, 267–280. [Google Scholar] [CrossRef]
- Bailey, L.; Lekkerkerker, H.N.W.; Maitland, G.C. Smectite clay–inorganic nanoparticle mixed suspensions: Phase behaviour and rheology. Soft Matter 2015, 11, 222–236. [Google Scholar] [CrossRef] [Green Version]
- Rashad, A.M. Vermiculite as a construction material—A short guide for civil engineer. Constr. Build. Mater. 2016, 125, 53–62. [Google Scholar] [CrossRef]
- De Poel, W.; Vaessen, S.L.; Drnec, J.; Engwerda, A.H.J.; Townsend, E.R.; Pintea, S.; de Jong, A.E.F.; Jankowski, M.; Carlà, F.; Felici, R.; et al. Metal ion-exchange on the muscovite mica surface. Surf. Sci. 2017, 665, 56–61. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, G.; Meng, W.; Wang, P.; Yang, C. Origin of different chlorite occurrences and their effects on tight clastic reservoir porosity. J. Pet. Sci. Eng. 2018, 160, 384–392. [Google Scholar] [CrossRef]
- Damasceno Junior, E.; Ferreira de Almeida, J.M.; do Nascimiento Silva, I.; Moreira de Assis, M.L.; dos Santos, L.M.; Dias, E.F.; Bezerra Aragao, V.E.; Verissimo, L.M.; Fernandes, N.S.; de Silva, D.R. pH-responsive release system of isoniazid using palygorskite as a nanocarrier. J. Drug Deliv. Sci. Technol. 2020, 55, 101399. [Google Scholar] [CrossRef]
- Garcia-Rivas, J.; Sanchez del Rio, M.; Garcia-Romero, E.; Suarez, M. An insight in the structure of a palygorskite from Palygorskaja: Some questions on the standard model. Appl. Clay Sci. 2017, 148, 39–47. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Pina-Zapardiel, R.; Moya, J.S.; Barba, M.F.; Pecharroman, C. The role of magnesium on the stability of crystalline sepiolite structure. J. Eur. Ceram. Soc. 2008, 28, 1763–1768. [Google Scholar] [CrossRef]
- Hong, H.; Churchman, G.J.; Gu, Y.; Yin, K.; Wang, C. Kaolinite-smectite mixed-layer clays in the Jiujiang red soils and their climate significance. Geoderma 2012, 173–174, 75–83. [Google Scholar] [CrossRef]
- Van Ranst, E.; Kips, P.; Mbogoni, J.; Mees, F.; Dumon, M.; Delvaux, B. Halloysite-smectite mixed-layered clay in fluvio-volcanic soils at the southern foot of Mount Kilimanjaro, Tanzania. Geoderma 2020, 375, 114527. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan., C. Basics of Clay Minerals and Their Characteristic Properties. In Clay and Clay Minerals; Morari Do Nascimento, G., Ed.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/online-first/76780 (accessed on 10 October 2021). [CrossRef]
- Thakur, A.; Jaiswal, M.K.; Peak, C.W.; Carrow, J.K.; Gentry, J.; Dolatshahi-Pirouz, A.; Gaharwar, A.K. Injectable shear thinning nanoengineered hydrogels for stem cell delivery. Nanoscale 2016, 8, 12362–12372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered osteoinductive and elastomeric scaffolds for bone tissue enginnering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Hong, J.-H.; Jeong, E.H.; Lee, H.S.; Baik, D.H.; Seo, S.W.; Youk, J.H. Electrospinning of polyurethane/organically modified montmorillonite nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 3171–3177. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSimone, E.; Schacht, K.; Pellert, A.; Scheibel, T. Recombinant spider silk-based bioinks. Biofabrication 2017, 9, 044104. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, R.; Zhang, L.; Cao, X. 4D printing of robust hydrogels consisted of agarose nanofibers and polyacrylamide. ACS Macro Lett. 2018, 7, 442–446. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotube. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Cervini-Silva, J.; Ramirez-Apan, M.T.; Kaufhold, S.; Ufer, K.; Palacios, E.; Montoya, A. Role of bentonite clays on cell growth. Chemosphere 2016, 149, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Abduljauwad, S.N.; Ahmed, H.-u.-R.; Moy, V.T. Melanoma treatment via non-specific adhesion of cancer cells using charged nano-clays in pre-clinical studies. Sci. Rep. 2021, 11, 2737. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Lee, Y.H.; Jian, C.H.; Wong, J.-M.; Shieh, M.-J.; Wang, C.-Y. A study of purified montmorillonite intercalated with 5-fluorouracil as drug carrier. Biomaterials 2002, 9, 1981–1987. [Google Scholar] [CrossRef]
- Yu, M.; Pan, L.; Sun, L.; Li, J.; Shang, J.; Zhang, S.; Liu, D.; Li, W. Supramolecular assemblies constructed from β-cyclodextrin-modified montmorillonite nanosheets as carrier for 5-fluorouracil. J. Mater. Chem. B 2015, 3, 9043–9052. [Google Scholar] [CrossRef]
- Tabasi, H.; Oroojalian, F.; Darroudi, M. Green clay ceramics as potential nanovehicles for drug delivery applications. Ceram. Int. 2021, 47, 31042–31053. [Google Scholar] [CrossRef]
- Zhu, T.; Ma, X.; Chen, R.; Ge, Z.; Xu, J.; Shen, X.; Jia, L.; Zhou, T.; Luo, Y.; Ma, T. Using fluorescently-labeled magnetic nanocomposites as a dual contrast agent for optical and magnetic resonance imaging. Biomater. Sci. 2017, 5, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Gianni, E.; Avgoustakis, K.; Papoulis, D. Kaolinite group minerals: Applications in cancer diagnosis and treatment. Eur. J. Pharm. Biopharm. 2020, 154, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic materials and systems: Domain structure visualization and other characterization techniques for the application in the materials science and biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Jury, W.A.; Vaux Jr., H. The role of science in solving the world’s emerging water problems. Proc. Natl. Acad. Sci. USA 2005, 102, 15715–15720. [Google Scholar] [CrossRef] [Green Version]
- Maier, J.; Palazzo, J.; Geyer, R.; Steigerwald, D.G. How much potable water is saved by wastewater recycling? Quasi-experimental evidence from California. Resour. Conserv. Recycl. 2022, 176, 105948. [Google Scholar] [CrossRef]
- Jain, M.; Khan, S.A.; Sharma, K.; Jadhao, P.R.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.T. Current perspective of innovative strategies for bioremediation of organic pollutants from wastewater. Bioresour. Technol. 2022, 344, 126305. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Khan, S.A.; Pandey, A.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.T. Instructive analysis of engineered carbon materials for potential application in water and wastewater treatment. Sci. Total Environ. 2021, 793, 148583. [Google Scholar] [CrossRef] [PubMed]
- Jaspal, D.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2020, 246, 125788. [Google Scholar] [CrossRef] [PubMed]
- Rostam, A.B.; Taghizadeh, M. Advanced oxidation processes integrated by membrane reactors and bioreactors for various wastewater treatments: A critical review. J. Environ. Chem. Eng. 2020, 8, 104566. [Google Scholar] [CrossRef]
- Polliotto, V.; Pomilla, F.R.; Maurino, V.; Marcì, G.; Bianco Prevot, A.; Nisticò, R.; Magnacca, G.; Paganini, M.C.; Ponce Robles, L.; Perez, L.; et al. Different approaches for the solar photocatalytic removal of micro-contaminants from aqueous environment: Titania vs. hybrid magnetic iron oxides. Catal. Today 2019, 328, 164–171. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Baino, F.; Fabbri, D.; Franzoso, F.; Magnacca, G.; Nisticò, R.; Arques, A. Urban biowaste-derived sensitizing materials for caffeine photodegradation. Environ. Sci. Pollut. Res. 2017, 24, 12599–12607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owodunni, A.A.; Ismail, S. Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment-A review. J. Water Process Eng. 2021, 42, 102096. [Google Scholar] [CrossRef]
- Peralta, M.E.; Martire, D.O.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J. Environ. Chem. Eng. 2021, 9, 104841. [Google Scholar] [CrossRef]
- Nisticò, R.; Tabasso, S.; Magnacca, G.; Jordan, T.; Shalom, M.; Fechler, N. Reactive hypersaline route: One-pot synthesis of porous photoactive nanocomposites. Langmuir 2017, 33, 5213–5222. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Wong, K.C.; Ismail, A.F. Membrane technology: A versatile tool for saline wastewater treatment and resource recovery. Desalination 2022, 521, 115377. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Lofrano, G.; Brown, J. Wastewater management through the ages: A history of mankind. Sci. Total Environ. 2010, 408, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Bentahar, Y.; Hurel, C.; Draoui, K.; Khairoun, S.; Marmier, N. Adsorptive properties of Maroccan clays for the removal of arsenic(V) from aqueous solution. Appl. Clay Sci. 2016, 119, 385–392. [Google Scholar] [CrossRef]
- Veli, S.; Alyuz, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of chromium(VI) from water by clays. Ind. Eng. Chem. Res. 2006, 45, 7232–7240. [Google Scholar] [CrossRef]
- Farrah, H.; Pickering, W.F. pH effects in the adsorption of heavy metal ions by clays. Chem. Geol. 1979, 25, 317–326. [Google Scholar] [CrossRef]
- Priyantha, N.; Bandaranayaka, A. Interaction of Cr(VI) species with thermally treated brick clay. Environ. Sci. Pollut. Res. 2011, 18, 75–81. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.H.; Fiore, S.; Tong, D.S.; Zhang, H.; Li, C.S.; Ji, S.F.; Yu, W.H. Functional magnetic nanoparticle/clay mineral nanocomposites: Preparation, magnetism and versatile applications. Appl. Clay Sci. 2016, 127–128, 143–163. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.P.; Mansur, H.S. Advanced functional nanostructures based on magnetic iron oxide nanomaterials for water remediation: A review. Water Res. 2021, 190, 116693. [Google Scholar] [CrossRef]
- Panda, S.K.; Aggarwal, I.; Kumar, H.; Prased, L.; Kumar, A.; Sharma, A.; Vo, D.-V.N.; Thuan, D.V.; Mishra, V. Magnetite nanoparticles as sorbents for dye removal: A review. Environ. Chem. Lett. 2021, 19, 2487–2525. [Google Scholar] [CrossRef]
- Nisticò, R. Magnetic materials and water treatments for a sustainable future. Res. Chem. Intermed. 2017, 43, 6911–6949. [Google Scholar] [CrossRef]
- Najafi, H.; Farajfaed, S.; Zolgharnian, S.; Mirak, S.H.M.; Asasian-Kolur, N.; Sharifian, S. A comprehensive study in modified-pillared clays as ad adsorbent in wastewater treatment processes. Process Saf. Environ. Prot. 2021, 147, 8–36. [Google Scholar] [CrossRef]
- Nisticò, R. A synthetic guide toward the tailored production of magnetic iron oxide nanoparticles. Bol. Soc. Esp. Cerám. V. 2021, 60, 29–40. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Huang, T.-K.; Wang, Y.-C.; Alamani, B.G.; Lin, J.-J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Magdy, A.; Fouad, Y.O.; Abdel-Aziz, M.H.; Konsowa, A.H. Synthesis and characterization of Fe3O4/kaolin magnetic nanocomposite and its application in wastewater treatment. J. Ind. Eng. Chem. 2017, 56, 299–311. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances form produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, L.; Tizaoui, C.; Geissen, S.-U.; Bousselmi, L. A comparative study on ozone, hydrogen peroxide and UV based advanced oxidation processes for efficient removal of diethyl phthalate in water. J. Hazard. Mater. 2019, 363, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Kurian, M. Advanced oxidation processes and nanomaterials—A review. Clean. Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Sanakousar, F.M.; Vidyasagar, C.C.; Jimenez-Perez, V.M.; Prakash, K. Recent progress on visible-light-driven metal and non-metal doped ZnO nanostructures for photocatalytic degradation of organic pollutants. Mater. Sci. Semicond. Process. 2022, 140, 106390. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Arques, A.; Carlos, L.; Laurenti, E.; Magnacca, G.; Nisticò, R. Innovative sustainable materials for the photoinduced remediation of polluted water. In Sustainable Water and Wastewater Processes; Galanakis, C.M., Agrafioti, E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Chapter 7; pp. 203–238. ISBN 978-0-12-816170-8. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Saini, A.K.; Hosseini-Bandegharaei, A.; Thakur, V.K. Systematic review on applicability of magnetic iron oxides-integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]
- Azzam, M.O.J. Olive mills wastewater treatment using mixed adsorbents of volcanic tuff, natural clay and charcoal. J. Environ. Chem. Eng. 2018, 6, 2126–2136. [Google Scholar] [CrossRef]
- De Paiva, L.B.; Morales, A.R.; Valenzuela Diaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M. Microfiltration of cationic dyes using nano-clay membranes. Ceram. Int. 2017, 43, 15146–15159. [Google Scholar] [CrossRef]
- Blyweert, P.; Nicolas, V.; Fierro, V.; Celzard, A. 3D printing of carbon-based materials: A review. Carbon 2021, 183, 449–485. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, S.; Sehgal, S. 3D printing of engineering materials: A state of the art review. Mater. Today Proc. 2020, 28, 1927–1931. [Google Scholar] [CrossRef]
- Zhang, B.; Goel, A.; Ghalsasi, O.; Anand, S. CAD-based design and pre-processing tools for additive manufacturing. J. Manuf. Syst. 2019, 52, 227–241. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, A. Fused deposition modelling: Current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Anand, M.; Das, A.K. Issues in fabrication of 3D components through DMLS Technique: A review. Opt. Laser Technol. 2021, 139, 106914. [Google Scholar] [CrossRef]
- Nouri, A.; Shirvan, A.R.; Li, Y.; Wen, C. Additive manufacturing of metallic and polymeric load-bearing biomaterials using laser powder bed fusion: A review. J. Mater. Sci. Technol. 2021, 94, 196–215. [Google Scholar] [CrossRef]
- Dowling, L.; Kennedy, J.; O’Shaughnessy, S.; Trimble, D. A review of critical repeatability and reproducibility issues in powder bed fusion. Mater. Des. 2020, 186, 108346. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Simonelli, M.; Perry, L.; Ashcroft, I.; Tuck, C.; Hague, R. 3D printing of aluminium alloys: Additive manufacturing of aluminium alloys using selective laser melting. Prog. Mater. Sci. 2019, 106, 100578. [Google Scholar] [CrossRef]
- Gonzalez-Henriquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Mustapha, K.B.; Metwalli, K.M. A review of fused deposition modelling for 3D printing of smart polymeric materials and composites. Eur. Polym. J. 2021, 156, 110591. [Google Scholar] [CrossRef]
- Xiao, J.; Ji, G.; Zhang, Y.; Ma, G.; Mechtcherine, V.; Pan, J.; Wang, L.; Ding, T.; Duan, Z.; Du, S. Large-scale 3D printing concrete technology: Current status and future opportunities. Cem. Concr. Compos. 2021, 122, 104115. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T. 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material—A review. Colloids Surf. B Biointerfaces 2021, 203, 111726. [Google Scholar] [CrossRef]
- Gao, C.; Lu, C.; Jian, Z.; Zhang, T.; Chen, Z.; Zhu, Q.; Tai, Z.; Liu, Y. 3D bioprinting for fabricating artificial skin tissue. Colloids Surf. B Biointerfaces 2021, 208, 112041. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Esworthy, T.; Miao, S.; Zhou, X.; Lee, S.-J.; Fisher, J.P.; Zhang, L.G. Recent advances in 3D printing: Vascular network for tissue and organ regeneration. Transl. Res. 2019, 211, 46–63. [Google Scholar] [CrossRef]
- Wilms, P.; Daffner, K.; Kern, C.; Gras, S.L.; Schutyser, M.A.I.; Kohlus, R. Formulation engineering of food systems for 3D-printing applications—A review. Food Res. Int. 2021, 148, 110585. [Google Scholar] [CrossRef]
- Chen, Y.; He, S.; Zhang, Y.; Wan, Z.; Copuroglu, O.; Schlangen, E. 3D printing of calcined clay-limestone-based cementitious materials. Cem. Concr. Res. 2021, 149, 106553. [Google Scholar] [CrossRef]
- Long, W.-J.; Lin, C.; Tao, J.L.; Ye, T.-H.; Fang, Y. Printability and particle packing of 3D-printable limestone calcined clay cement composites. Constr. Build. Mater. 2021, 282, 122647. [Google Scholar] [CrossRef]
- Faksawat, K.; Limsuwan, P.; Naemchanthara, K. 3D printing technique of specific bone shape based on raw clay using hydroxyapatite as an additive material. Appl. Clay Sci. 2021, 214, 106269. [Google Scholar] [CrossRef]
- Chikkangoudar, R.N.; Sachidananda, T.G.; Pattar, N. Influence of 3D printing parameters on the dimensional stability of polypropylene/clay printed parts using laser scanning technique. Mater. Today Proc. 2021, 44, 4118–4123. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990; ISBN 9780080571034. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Nisticò, R.; Scalarone, D.; Magnacca, G. Sol-gel chemistry, templating and spin-coating deposition: A combined approach to control in a simple way the porosity of inorganic thin films/coatings. Microporous Mesoporous Mater. 2017, 248, 18–29. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Beland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Lofgreen, J.E.; Ozin, G.A. Controlling morphology and porosity to improve performance of molecularly imprinted sol-gel silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Goebl, J.; Yin, Y. Self-templated synthesis of hollow nanostructures. Nano Today 2009, 4, 494–507. [Google Scholar] [CrossRef]
- Nisticò, R.; Magnacca, G.; Antonietti, M.; Fechler, N. “Salted silica”: Sol-gel chemistry of silica under hypersaline conditions. Z. Anorg. Allg. Chem. 2014, 640, 582–587. [Google Scholar] [CrossRef]
- Pronina, N.; Klauson, D.; Moiseev, A.; Deubener, J.; Krichevskaya, M. Titanium dioxide sol-gel coated expanded clay granules for use in photocatalytic fluidized-bed reactor. Appl. Catal. B Environ. 2015, 178, 117–123. [Google Scholar] [CrossRef]

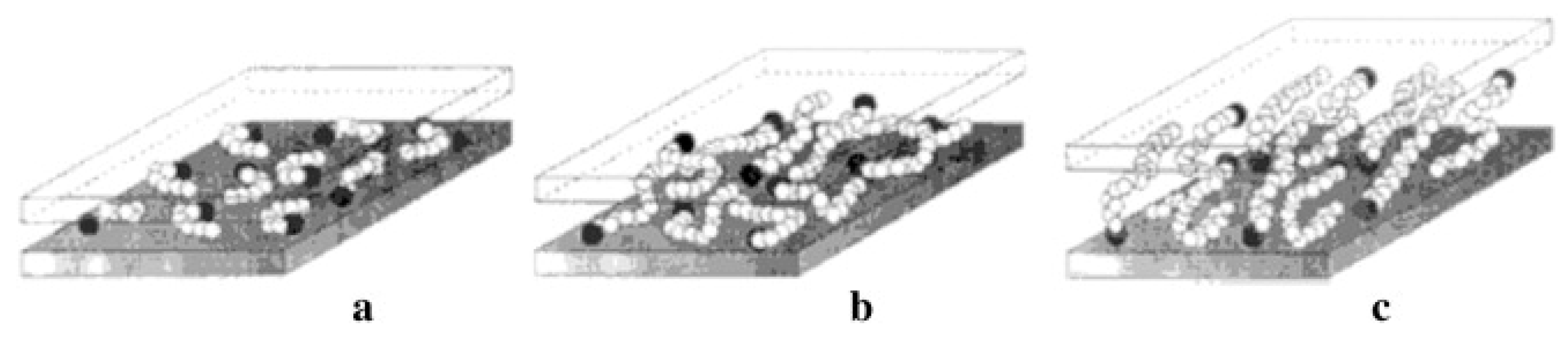
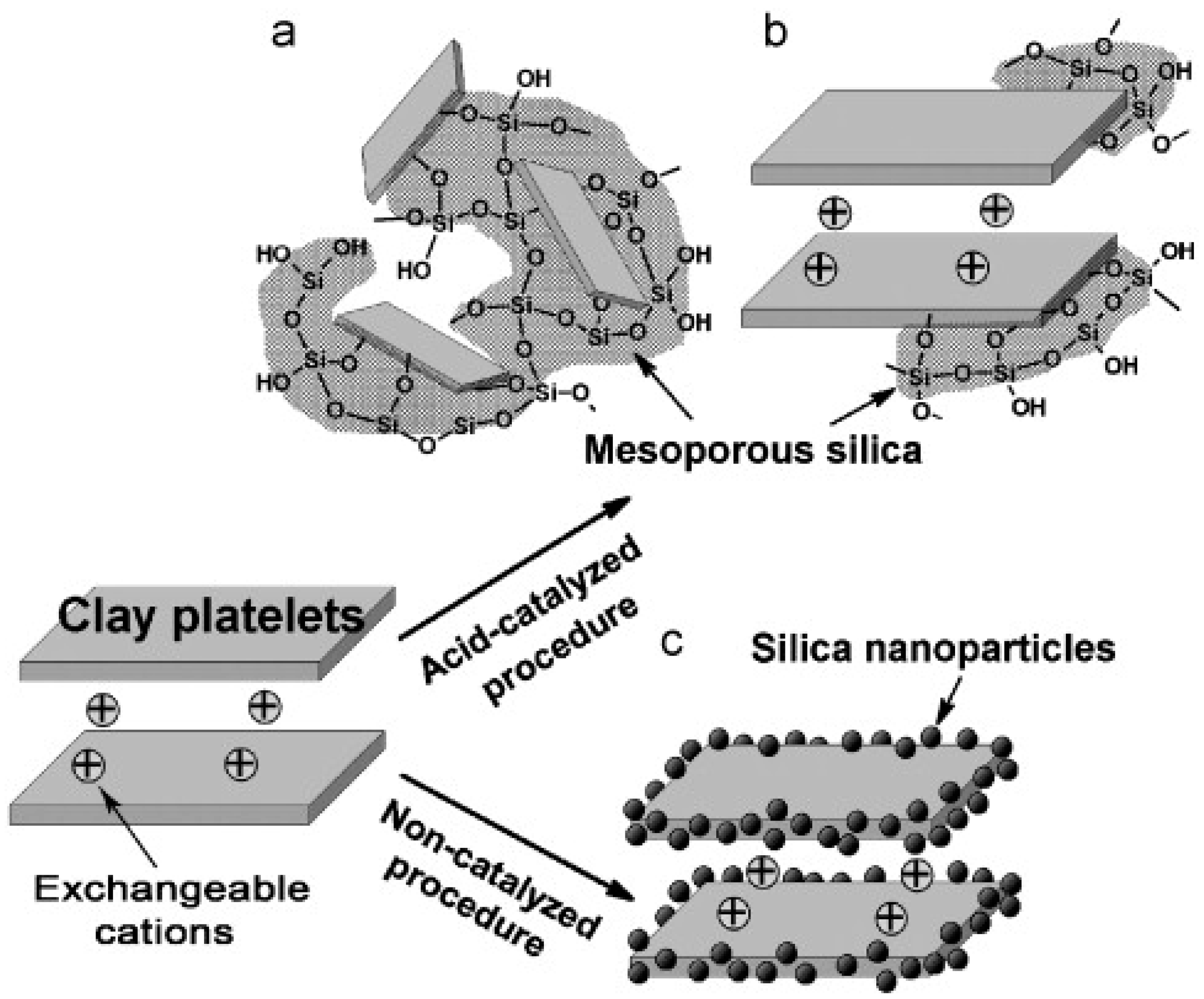
| Clays Subgroups | CEC (cmol/kg) | Interlayer Thickness (Å) | Specific Surface Area (m2/g) | Swelling Capacity |
|---|---|---|---|---|
| Kaolinite-Serpentine | 3–15 | 7 | 5–40 | None |
| Pyrophyllite-Talc | <1 | 9 | 5–40 | None |
| Smectite | 70–100 | 10–11 | 40–800 | High |
| Vermiculite | 100–150 | 12–15 | 500–700 | High |
| Mica | 10–40 | 10–11 | 50–200 | Low |
| Chlorites | 10–40 | 12–15 | 10–60 | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisticò, R. A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation. Inorganics 2022, 10, 40. https://doi.org/10.3390/inorganics10030040
Nisticò R. A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation. Inorganics. 2022; 10(3):40. https://doi.org/10.3390/inorganics10030040
Chicago/Turabian StyleNisticò, Roberto. 2022. "A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation" Inorganics 10, no. 3: 40. https://doi.org/10.3390/inorganics10030040
APA StyleNisticò, R. (2022). A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation. Inorganics, 10(3), 40. https://doi.org/10.3390/inorganics10030040