Exploration of Deformation of F-Actin during Macropinocytosis by Confocal Microscopy and 3D-Structured Illumination Microscopy
Abstract
:1. Introduction
2. Materials and Methods
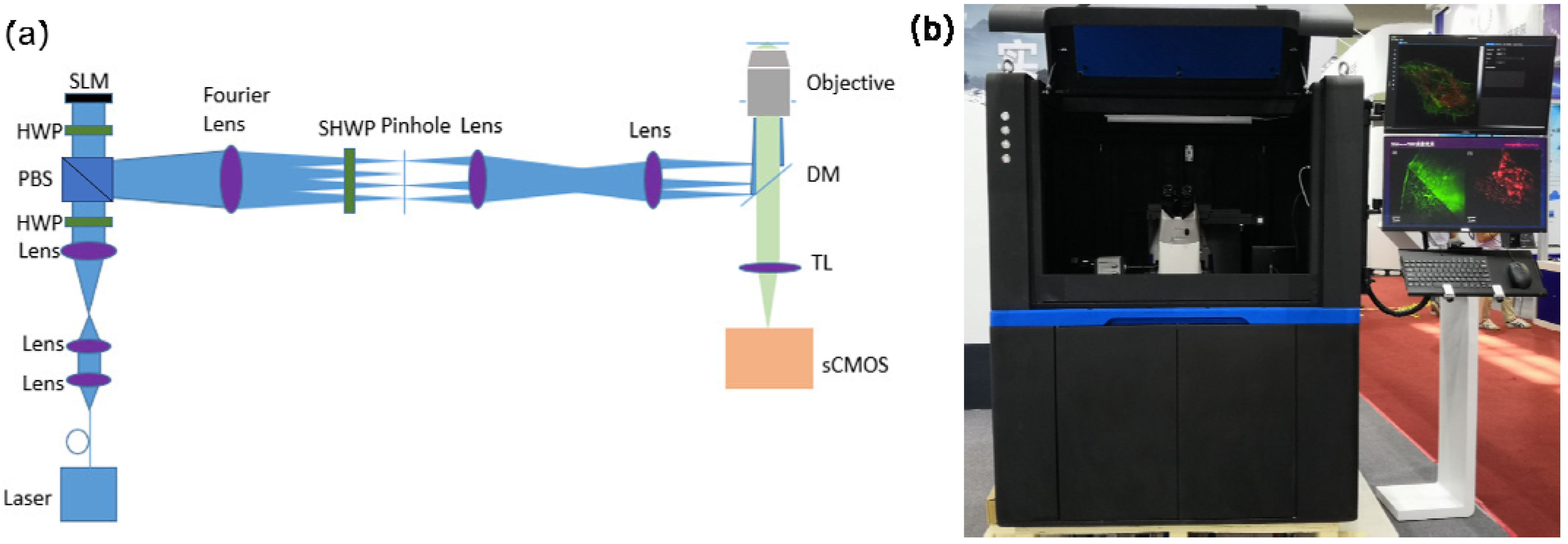
2.1. Brief General Description
2.2. Apparatus
2.3. Cell Culture
2.4. Reagents and Chemicals
2.5. Cell Treatment and Microscopy Imaging
2.5.1. Confocal Microscopy Imaging
2.5.2. ATI-SIM Imaging
3. Results
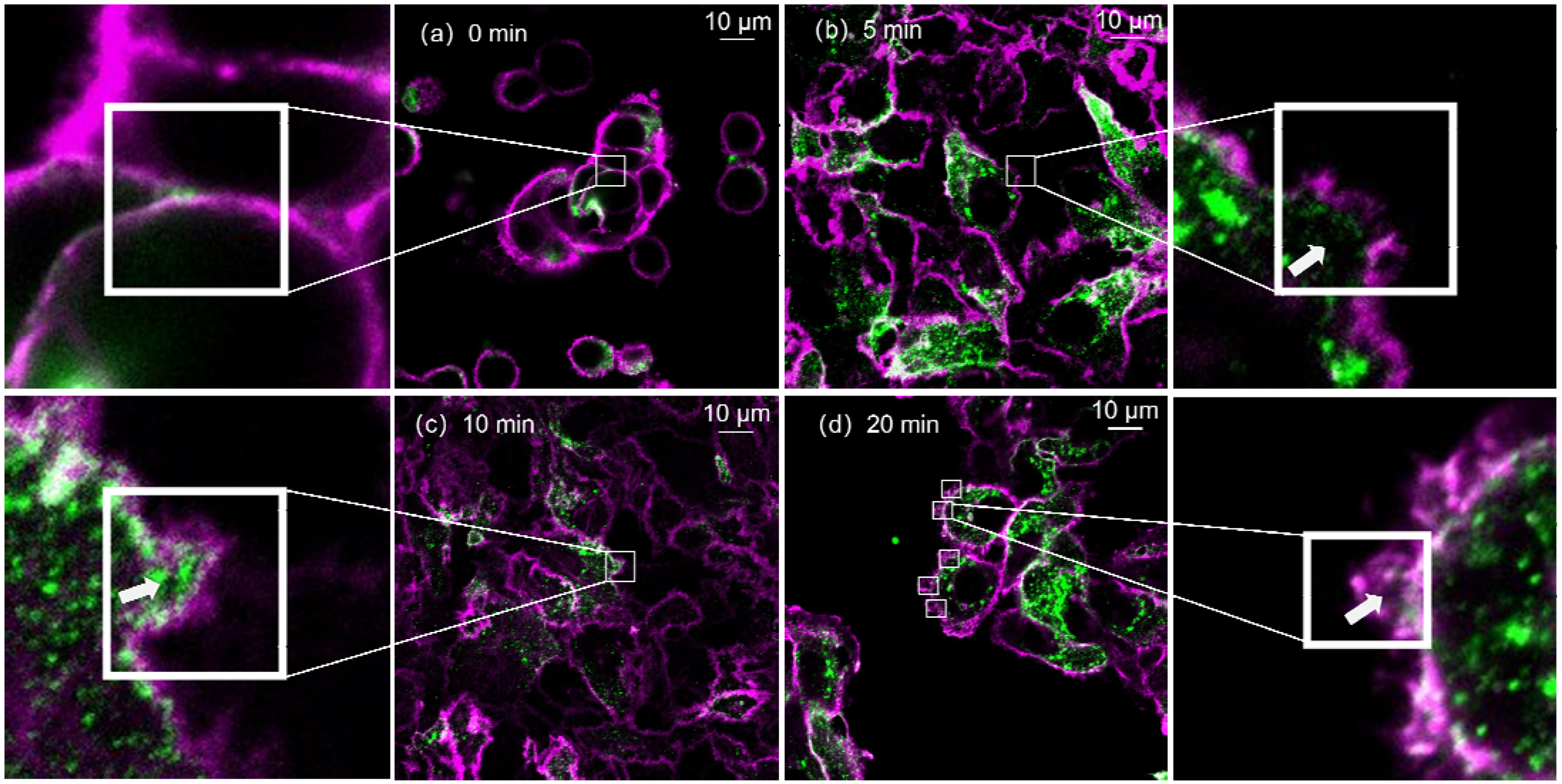
3.1. Time-Series Imaging by Confocal Microscopy
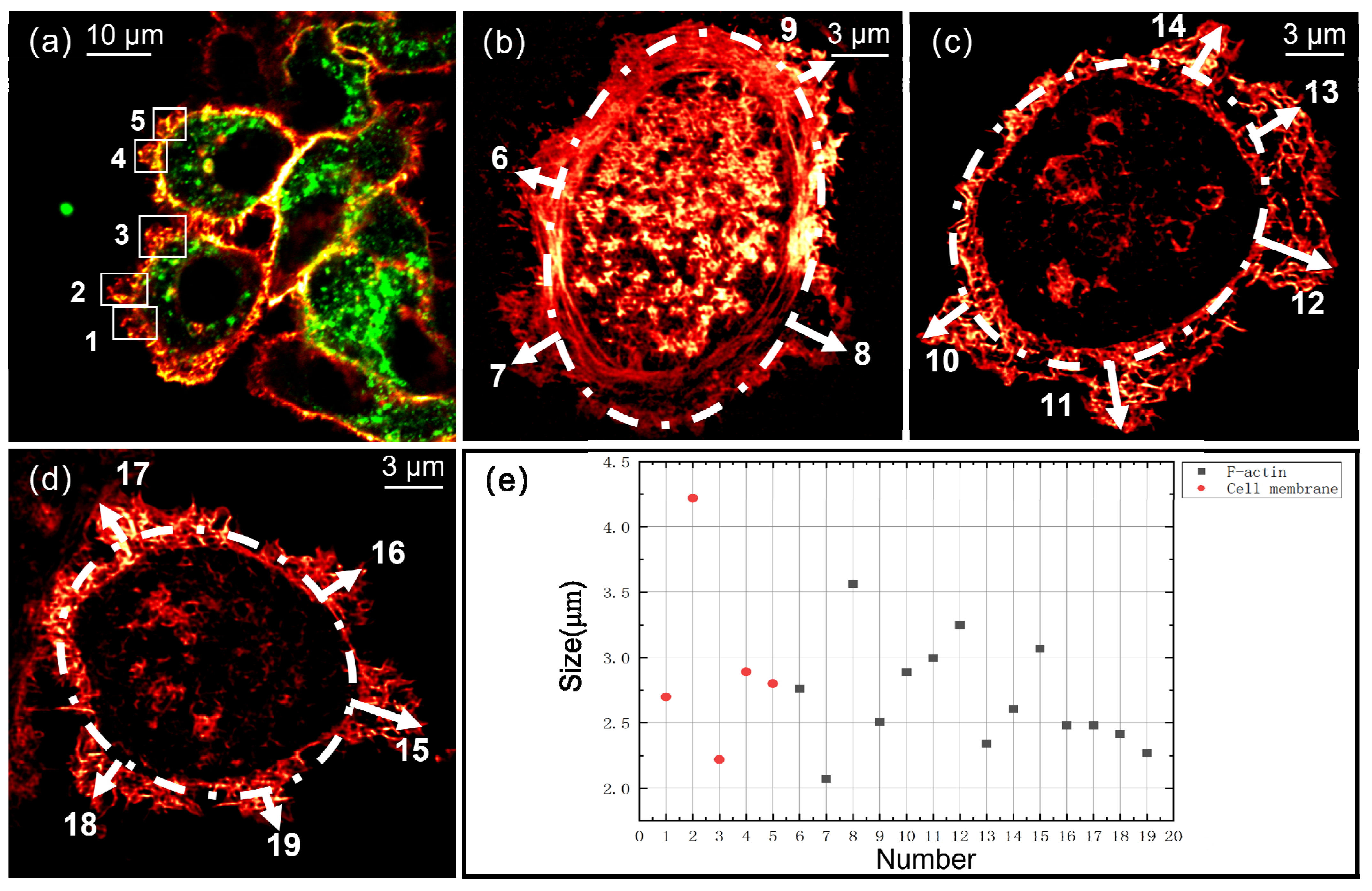
3.2. Super-Resolution Imaging by ATI-SIM
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saheki, Y.; De Camilli, P. Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol. 2012, 4, a005645. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.H. Pinocytosis. Johns Hopkins Hosp. Bull. 1931, 49, 17–27. [Google Scholar]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloomfield, G.; Kay, R.R. Uses and abuses of macropinocytosis. J. Cell Sci. 2016, 129, 2697–2705. [Google Scholar] [CrossRef] [Green Version]
- Buckley, C.M.; King, J.S. Drinking problems: Mechanisms of macropinosome formation and maturation. FEBS J. 2017, 284, 3778–3790. [Google Scholar] [CrossRef] [Green Version]
- Stow, J.L.; Hung, Y.; Wall, A.A. Macropinocytosis: Insights from immunology and cancer. Curr. Opin. Cell Biol. 2020, 65, 131–140. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Charpentier, J.C.; Chen, D.; Lapinski, P.E.; Turner, J.; Grigorova, I.; Swanson, J.A.; King, P.D. Macropinocytosis drives T cell growth by sustaining the activation of mTORC1. Nat. Commun. 2020, 11, 180. [Google Scholar] [CrossRef]
- Toh, W.H.; Louber, J.; Mahmoud, I.S.; Chia, J.; Bass, G.T.; Dower, S.K.; Verhagen, A.M.; Gleeson, P.A. FcRn mediates fast recycling of endocytosed albumin and IgG from early macropinosomes in primary macrophages. J. Cell Sci. 2020, 133, jcs235416. [Google Scholar] [CrossRef] [Green Version]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commisso, C. The pervasiveness of macropinocytosis in oncological malignancies. Philos. Trans. R. Soc. B 2019, 374, 20180153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; Nguyen, T.T.; Ravi, A.; Kubiniok, P.; Finicle, B.T.; Jayashankar, V.; Malacrida, L.; Hou, J.; Robertson, J.; Gao, D.; et al. PTEN Deficiency and AMPK Activation Promote Nutrient Scavenging and Anabolism in Prostate Cancer Cells. Cancer Discov. 2018, 8, 866–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, C.; Hauser, A.D.; Vucic, E.A.; Bar-Sagi, D. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature 2019, 576, 477–481. [Google Scholar] [CrossRef]
- Jiao, Z.; Cai, H.; Long, Y.; Sirka, O.K.; Padmanaban, V.; Ewald, A.J.; Devreotes, P.N. Statin-induced GGPP depletion blocks macropinocytosis and starves cells with oncogenic defects. Proc. Natl. Acad. Sci. USA 2020, 117, 4158–4168. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Liu, Z.; Roche, P.A. Macropinocytosis in phagocytes: Regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front. Physiol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Helenius, A. Virus Entry: Looking Back and Moving Forward. J. Mol. Biol. 2018, 430, 1853–1862. [Google Scholar] [CrossRef]
- Kuroda, M.; Halfmann, P.; Kawaoka, Y. HER2-mediated enhancement of Ebola virus entry. PLoS Pathog. 2020, 16, e1008900. [Google Scholar] [CrossRef]
- Desai, A.S.; Hunter, M.R.; Kapustin, A.N. Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos. Trans. R. Soc. B 2018, 374, 20180156. [Google Scholar] [CrossRef] [Green Version]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin Dynamics, Architecture, and Mechanics in Cell Motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engqvist-Goldstein, A.E.; Drubin, D.G. Actin assembly and endocytosis: From yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003, 19, 287–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.-S.; Lee, S.H.; Sheng, J.; Zhang, Z.; Zhao, W.-D.; Wang, D.; Jin, Y.; Charnay, P.; Ervasti, J.M.; Wu, L.-G. Actin is crucial for all kinetically distinguishable forms of endocytosis at synapses. Neuron 2016, 92, 1020–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.S.; Kay, R.R. The origins and evolution of macropinocytosis. Philos. Trans. R. Soc. B 2019, 374, 20180158. [Google Scholar] [CrossRef]
- Veltman, D.M.; Williams, T.D.; Bloomfield, G.; Chen, B.-C.; Betzig, E.; Insall, R.H.; Kay, R.R. A plasma membrane template for macropinocytic cups. eLife 2016, 5, e20085. [Google Scholar] [CrossRef]
- de Hostos, E.L.; Bradtke, B.; Lottspeich, F.; Guggenheim, R.; Gerisch, G. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 1991, 10, 4097–4104. [Google Scholar] [CrossRef]
- Hacker, U.; Albrecht, R.; Maniak, M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 1997, 110, 105–112. [Google Scholar] [CrossRef]
- Williams, T.D.; Kay, R.R. The physiological regulation of macropinocytosis during Dictyostelium growth and development. J. Cell Sci. 2018, 131, jcs213736. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.J.; Amato, C.; Thomason, P.A.; Insall, R.H. WASP family proteins and formins compete in pseudopod- and bleb-based migration. J. Cell Biol. 2018, 217, 701–714. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Kim, S.; Nahm, M.; Kopke, D.; Kim, J.; Cho, E.; Lee, M.-J.; Lee, M.; Kim, S.H.; Broadie, K.; et al. BMP-dependent synaptic development requires Abi-Abl-Rac signaling of BMP receptor macropinocytosis. Nat. Commun. 2019, 10, 684. [Google Scholar] [CrossRef]
- Junemann, A.; Filic, V.; Winterhoff, M.; Nordholz, B.; Litschko, C.; Schwellenbach, H.; Stephan, T.; Weber, I.; Faix, J. A Diaphanous-related formin links Ras signaling directly to actin assembly in macropinocytosis and phagocytosis. Proc. Natl. Acad. Sci. USA 2016, 113, E7464–E7473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.G.L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranov, M.V.; Olea, R.A.; van den Bogaart, G. Chasing Uptake: Super-Resolution Microscopy in Endocytosis and Phagocytosis. Trends Cell Biol. 2019, 29, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Lang, S.; Wang, H.; Hu, H.; Wang, J.; Gong, Y. Structured illumination microscopy based on asymmetric three-beam interference. J. Innov. Opt. Health Sci. 2021, 14, 2050027. [Google Scholar] [CrossRef]
- Jin, J.; Shen, Y.; Zhang, B.; Deng, R.; Huang, D.; Lu, T.; Sun, F.; Xu, S.; Liang, C. In situ exploration of characteristics of macropinocytosis and size range of internalized substances in cells by 3D-structured illumination microscopy. Int. J. Nanomed. 2018, 13, 5321–5333. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Knecht, D.A. Visualization of Actin Dynamics During Macropinocytosis and Exocytosis. Traffic 2002, 3, 186–192. [Google Scholar] [CrossRef]
- Condon, N.D.; Heddleston, J.M.; Chew, T.-L.; Luo, L.; McPherson, P.S.; Ioannou, M.S.; Hodgson, L.; Stow, J.L.; Wall, A.A. Macropinosome formation by tent pole ruffling in macrophages. J. Cell Biol. 2018, 217, 3873–3885. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhang, Y.; Lang, S.; Gong, Y. Exploration of Deformation of F-Actin during Macropinocytosis by Confocal Microscopy and 3D-Structured Illumination Microscopy. Photonics 2022, 9, 461. https://doi.org/10.3390/photonics9070461
Xu L, Zhang Y, Lang S, Gong Y. Exploration of Deformation of F-Actin during Macropinocytosis by Confocal Microscopy and 3D-Structured Illumination Microscopy. Photonics. 2022; 9(7):461. https://doi.org/10.3390/photonics9070461
Chicago/Turabian StyleXu, Linyu, Yanwei Zhang, Song Lang, and Yan Gong. 2022. "Exploration of Deformation of F-Actin during Macropinocytosis by Confocal Microscopy and 3D-Structured Illumination Microscopy" Photonics 9, no. 7: 461. https://doi.org/10.3390/photonics9070461
APA StyleXu, L., Zhang, Y., Lang, S., & Gong, Y. (2022). Exploration of Deformation of F-Actin during Macropinocytosis by Confocal Microscopy and 3D-Structured Illumination Microscopy. Photonics, 9(7), 461. https://doi.org/10.3390/photonics9070461



