Can Photobiomodulation Support the Management of Temporomandibular Joint Pain? Molecular Mechanisms and a Systematic Review of Human Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy for the Systematic Review
2.1.1. Research Question
- -
- Is PBM therapy using diode lasers (780–1000 nm) effective in the management of temporomandibular disorders (TMDs) pain?
- -
- What are the appropriate protocol doses investigated till now?
2.1.2. Systematic Search Strategy
- PubMed/Medline electronic database;
- COCHRANE LIBRARY;
- ScienceDirect;
- Scopus;
- Google Scholar.
- Randomized clinical trials (RCT) and clinical trials (CT) published between 1 January 2010 and 18 December 2021;
- Articles published in peer-reviewed journals in the English language;
- Full text;
- Studies that contain diode laser with wavelengths between 780 and 1000 nm;
- Studies that have patients with pain that resulted from any axis of RDC/TMD;
- Studies that contain both genders with age >18.
- Duplicate studies or republished articles;
- Systematic reviews and meta-analysis;
- Patients with a medical history that involves any other diseases (cancers or syndromes in the head and neck region);
- Studies that contain one gender only or focus on specific age groups, such as adolescents or elders;
- Studies that use LEDs or other light sources;
- Studies that use different laser wavelengths;
- Comparative studies that compare PBM with a particular aspect of therapy, such as drugs, exercises, acupuncture, injections … etc.;
- Studies that have patients in pain not related to TMD in particular;
- Patents, degrees, or doctoral theses;
- In vitro studies.
2.1.3. Study Selection and Data Extraction
2.2. Study Quality Assessment
2.2.1. PRISMA Guidelines
2.2.2. Delphi Score
2.2.3. Risk of Bias
3. Results
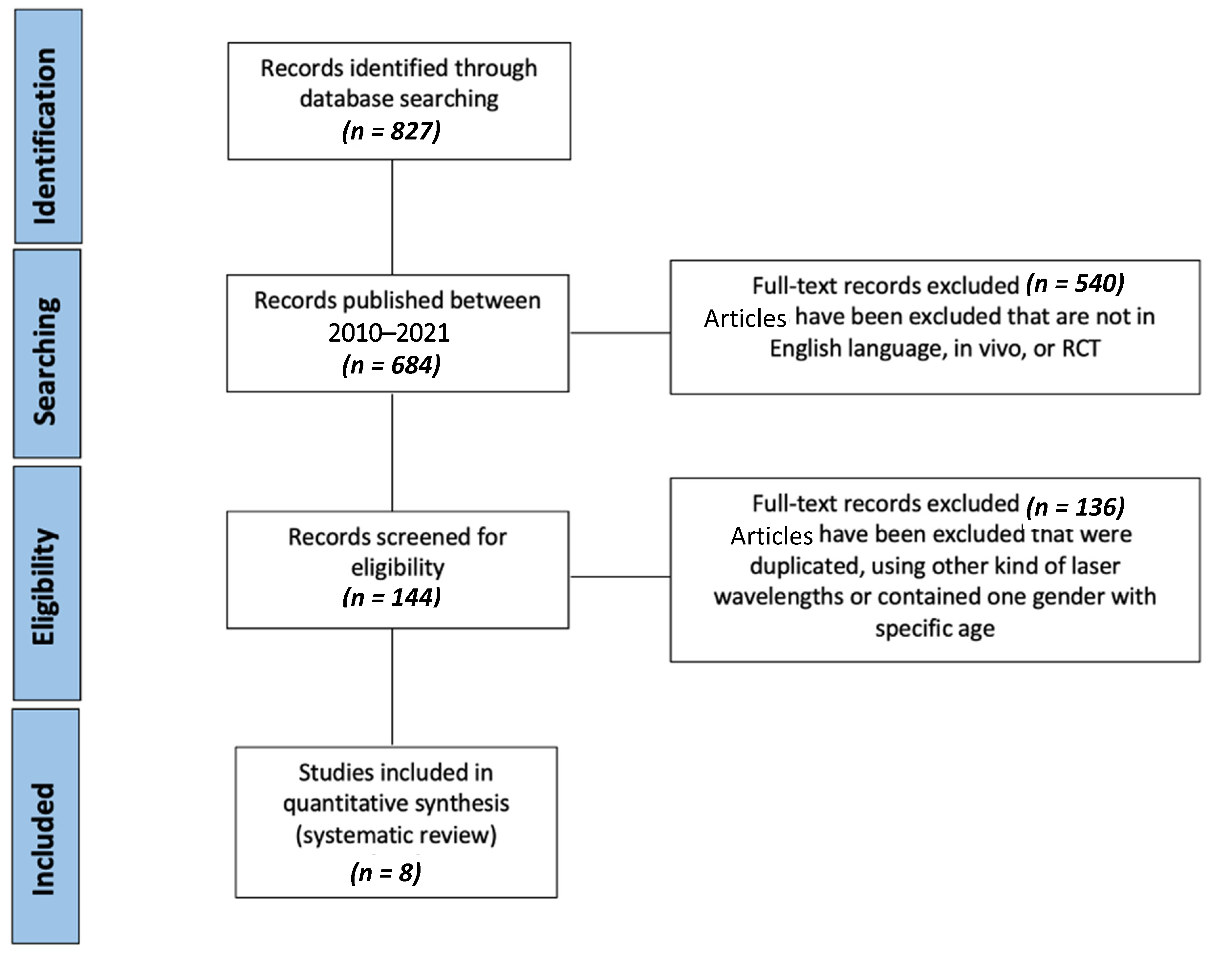
3.1. Literature Search Outcome
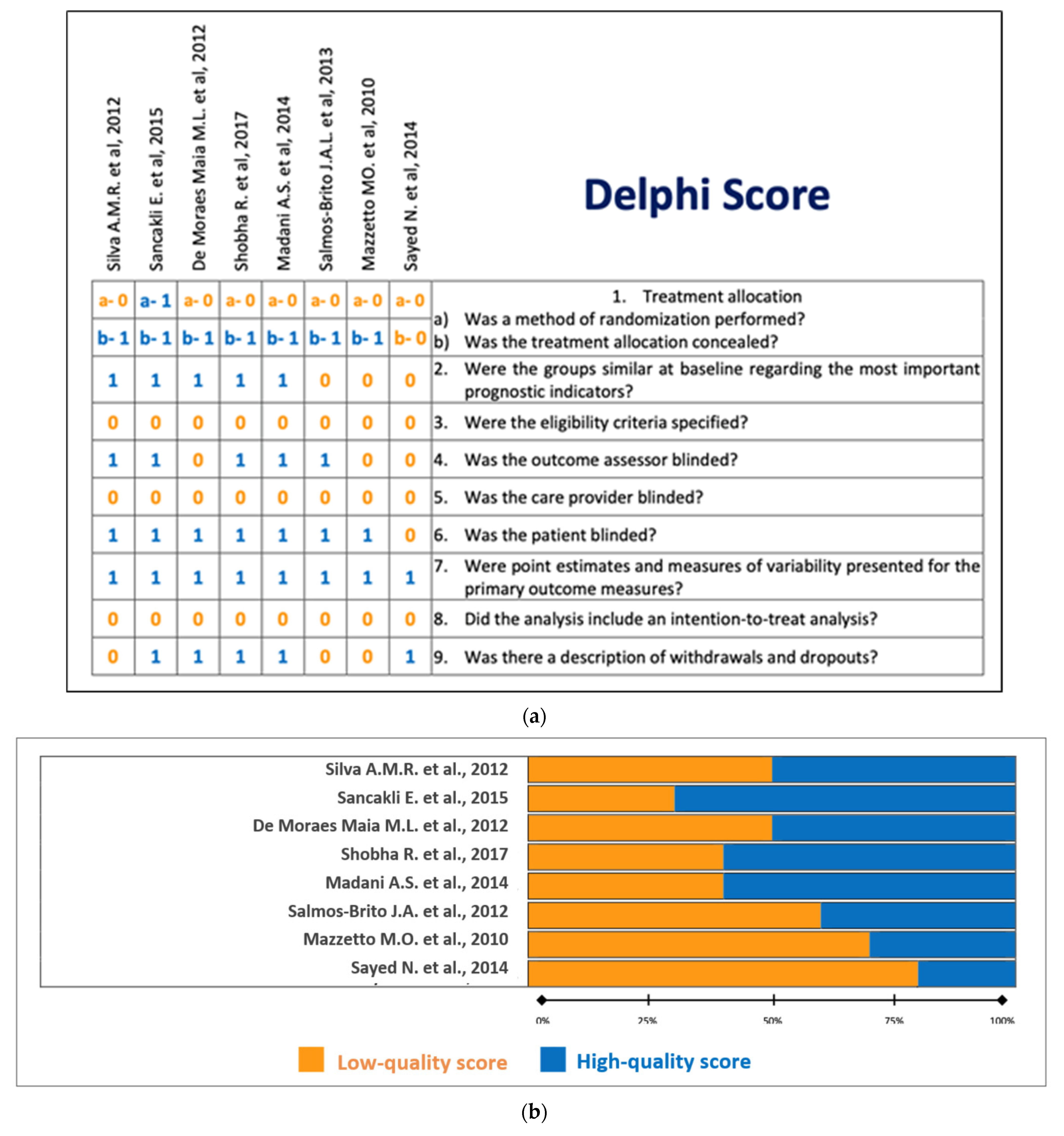
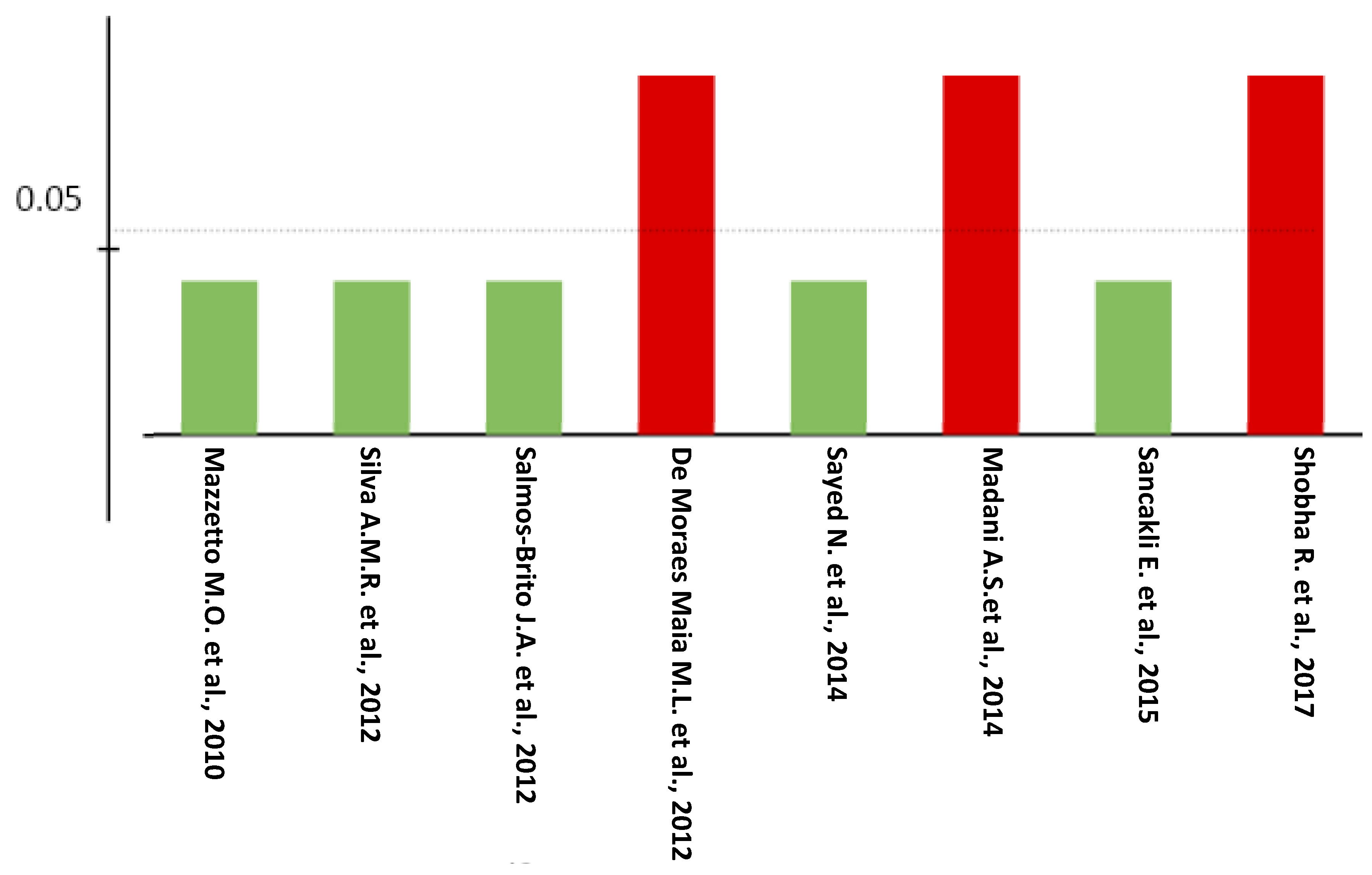
3.2. Delphi Score
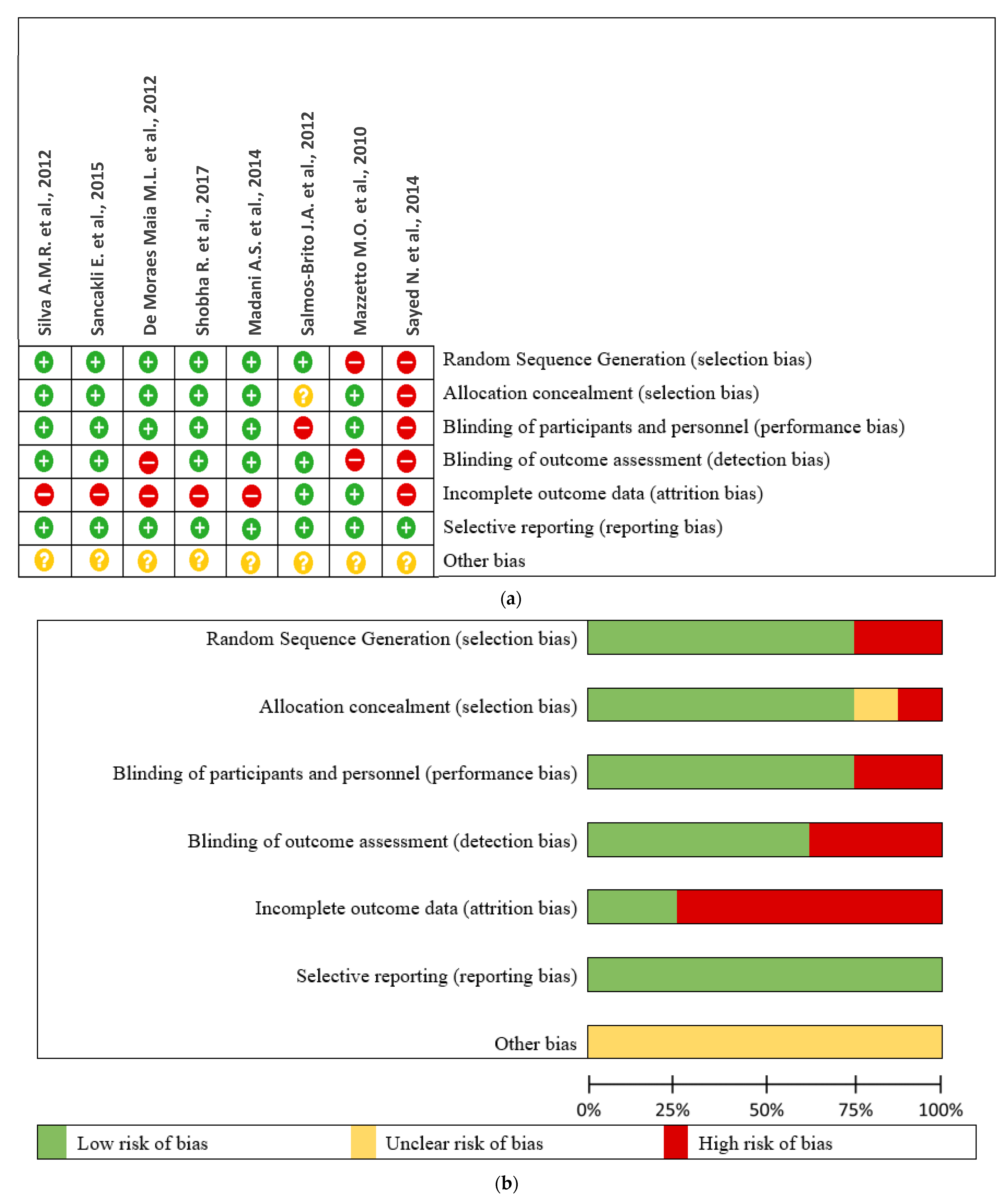
3.3. Risk of Bias
3.4. Study Characteristics
4. Discussion
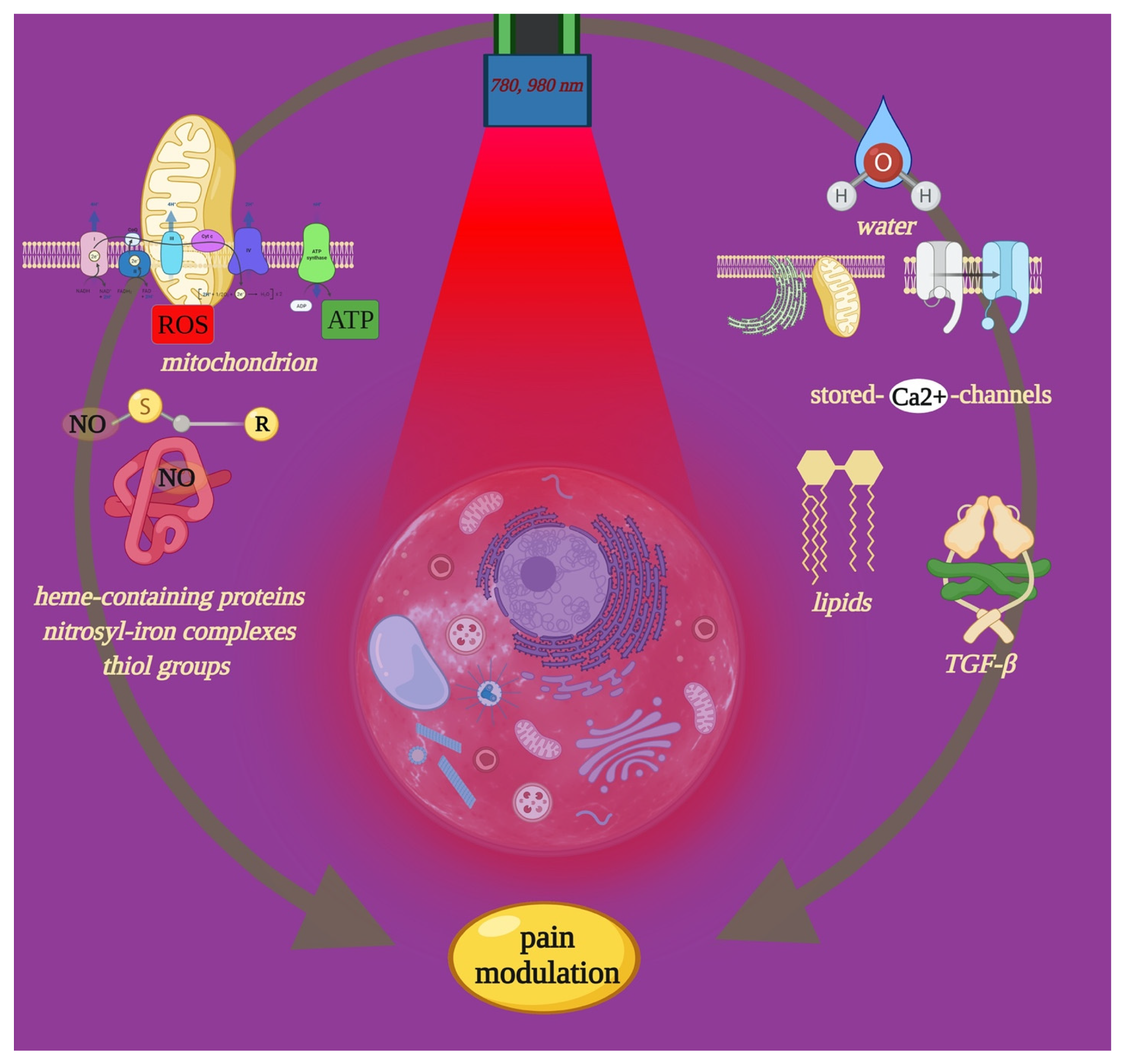
4.1. Effect of PBM-t on Cell Pathways of Pain
4.2. Influencing Pain Recovery through PBM-t
- -
- -
- -
- The number and the position of the tender points: some studies applied the laser treatment directly to the painful trigger points determined by the patient himself during the clinical examination [31,35]. Other studies applied the laser treatment to the painful trigger points determined by the patient himself in addition to other points predetermined by the clinician himself [29,30,32]. Meanwhile, the rest [28,33,34] selected the trigger points following previously published papers to obtain the desired analgesic effect in the TMJ area. In addition, the exact number of tender points was not cited in the clinical trials [29,31,32,35], which leads to confusion on how accurate equal doses were applied between the laser groups in each session.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasad, S.R.; Kumar, N.R.; Shruthi, H.R.; Kalavathi, S.D. Temporomandibular pain. J. Oral. Maxillofac. Pathol. 2016, 20, 272–275. [Google Scholar] [CrossRef]
- Bordoni, B.; Varacallo, M. Anatomy, Head and Neck, Temporomandibular Joint; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Butts, R.; Dunning, J.; Perreault, T.; Mettille, J.; Escaloni, J. Pathoanatomical characteristics of temporomandibular dysfunction: Where do we stand? Narrative review. J. Bodyw. Mov. Ther. 2017, 21, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Steinkeler, A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent. Clin. N. Am. 2013, 57, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G. Management of temporomandibular joint disorders. J. Oral. Biol. Craniofac. Res. 2012, 2, 141–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdel Hamid, M.A.; Hassan, E.A.; Zaied, A.A.; Amaroli, A.; Sorour, N.H. Dose-Dependent Clinical, Radiographic, and Histopathologic Changes of 17β-Estradiol Levels within the Temporomandibular Joint: An Experimental Study in Ovariectomized Dogs. J. Oral. Maxillofac. Surg. 2020, 78, 1304–1313. [Google Scholar] [CrossRef]
- Maia, M.L.; Bonjardim, L.R.; de Quintans, J.S.; Ribeiro, M.A.; Maia, L.G.; Conti, P.C. Effect of Low-Level Laser Therapy on pain levels in patients with temporomandibular disorders: A systematic review. J. Appl. Oral. Sci. 2012, 20, 594–602. [Google Scholar] [CrossRef]
- Amaroli, A.; Colombo, E.; Zekiy, A.; Aicardi, S.; Benedicenti, S.; De Angelis, N. Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation—A Review. Biology 2020, 9, 409. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanism of Photobiomodulation or Low-Level Light Therapy. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation or Low-Level Laser Therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A Narrative Review on Oral and Periodontal Bacteria Microbiota Photobiomodulation, through Visible and Near-Infrared Light: From the Origins to Modern Therapies. Int. J. Mol. Sci. 2022, 25, 1372. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-nm laser therapy with a flat-top handpiece photobiomodulates mitochondria activities of Paramecium primaurelia (Protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and Oxidative Stress: 980 nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxidative Med. Cell Longev. 2021, 3, 6626286. [Google Scholar] [CrossRef]
- Pastore, D.; Greco, M.; Passarella, S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000, 76, 863–870. [Google Scholar] [CrossRef]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 441–449. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef]
- Ferrando, S.; Agas, D.; Mirata, S.; Signore, A.; De Angelis, N.; Ravera, S.; Utyuzh, A.S.; Parker, S.; Sabbieti, M.G.; Benedicenti, S.; et al. The 808 nm and 980 nm infrared laser irradiation affects spore germination and stored calcium homeostasis: A comparative study using delivery hand-pieces with standard (Gaussian) or flat-top profile. J. Photochem. Photobiol. 2019, 199, 111627. [Google Scholar] [CrossRef]
- Jansen, K.; Wu, M.; van der Steen, A.F.; van Soest, G. Photoacoustic imaging of human coronary atherosclerosis in two spectral bands. Photoacoustics 2013, 2, 12–20. [Google Scholar] [CrossRef]
- Amaroli, A.; Marcoli, M.; Venturini, A.; Passalacqua, M.; Agnati, L.F.; Signore, A.; Raffetto, M.; Maura, G.; Benedicenti, S.; Cervetto, C. Near-infrared laser photons induce glutamate release from cerebrocortical nerve terminals. J. Biophotonics 2018, 11, 201800102. [Google Scholar] [CrossRef]
- Bashkatov, A.; Genina, E.; Kochubey, V.; Tuchin, V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Khan, I.; Rahman, S.U.; Tang, E. Accelerated burn wound healing with photobiomodulation therapy involves activation of endogenous latent TGF-β1. Sci. Rep. 2021, 11, 13371. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, 1000097. [Google Scholar] [CrossRef] [PubMed]
- Berger, V.W.; Alperson, S.Y. A general framework for the evaluation of clinical trial quality. Rev. Recent Clin. Trials. 2009, 4, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Savovic, J.; Page, M.; Elbers, R.; Sterne, J. Assessing Risk of Bias in Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019; pp. 205–228. [Google Scholar]
- Bellal, S.; El Feghali, R.; Mehta, A.; Namachivayam, A.; Benedicenti, S. Efficacy of near infrared dental lasers on dentinal hypersensitivity: A meta-analysis of randomized controlled clinical trials. Lasers Med. Sci. 2021, 3, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.A.; Botelho, A.L.; Turim, C.V.; da Silva, A.M. Low level laser therapy as an adjunctive technique in the management of temporomandibular disorders. Cranio 2012, 30, 264–271. [Google Scholar] [PubMed]
- Sancakli, E.; Gökçen-Röhlıg, B.; Balık, A.; Öngül, D.; Kıpırdı, S.; Keskın, H. Early results of low-level laser application for masticatory muscle pain: A double-blind randomized clinical study. BMC Oral. Health 2015, 15, 131. [Google Scholar] [CrossRef]
- De Moraes Maia, M.L.; Ribeiro, M.A.; Maia, L.G.; Stuginski-Barbosa, J.; Costa, Y.M.; Porporatti, A.L.; Conti, P.C.; Bonjardim, L.R. Evaluation of Low-Level Laser Therapy effectiveness on the pain and masticatory performance of patients with myofascial pain. Lasers Med. Sci. 2014, 29, 29–35. [Google Scholar] [CrossRef]
- Shobha, R.; Narayanan, V.S.; Jagadish Pai, B.S.; Jaishankar, H.P.; Jijin, M.J. Low-level laser therapy: A novel therapeutic approach to temporomandibular disorder—A randomized, double-blinded, placebo-controlled trial. Indian J. Dent. Res. 2017, 28, 380–387. [Google Scholar] [CrossRef]
- Madani, A.S.; Ahrari, F.; Nasiri, F.; Abtahi, M.; Tunér, J. Low-level laser therapy for management of TMJ osteoarthritis. Cranio 2014, 32, 38–44. [Google Scholar] [CrossRef]
- Salmos-Brito, J.A.; de Menezes, R.F.; Teixeira, C.E.; Gonzaga, R.K.; Rodrigues, B.H.; Braz, R.; Bessa-Nogueira, R.V.; Gerbi, M.E. Evaluation of Low-Level Laser Therapy in patients with acute and chronic temporomandibular disorders. Lasers Med. Sci. 2013, 28, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mazzetto, M.O.; Hotta, T.H.; Pizzo, R.C. Measurements of jaw movements and TMJ pain intensity in patients treated with GaAlAs laser. Braz. Dent. J. 2010, 21, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Murugavel, C.; Gnanam, A. Management of Temporomandibular Disorders with Low Level Laser Therapy. J. Maxillofac. Oral. Surg. 2014, 13, 444–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sui, B.D.; Xu, T.Q.; Liu, J.W.; Wei, W.; Zheng, C.X.; Guo, B.L.; Wang, Y.Y.; Yang, Y.L. Understanding the role of mitochondria in the pathogenesis of chronic pain. Postgrad. Med. J. 2013, 89, 709–714. [Google Scholar] [CrossRef]
- Hamilton, S.G. ATP and pain. Pain Pract. 2002, 2, 289–294. [Google Scholar] [CrossRef]
- Ryan, L.M.; Rachow, J.W.; McCarty, D.J. Synovial fluid ATP: A potential substrate for the production of inorganic pyrophosphate. J. Rheumatol. 1991, 18, 716–720. [Google Scholar]
- Wang, Z.Q.; Porreca, F.; Cuzzocrea, S. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Zhang, X.; Guo, Z.; Wu, W.; Chen, Z.; Li, J. Reactive Oxygen Species Mediate Low Back Pain by Upregulating Substance P in Intervertebral Disc Degeneration. Oxid. Med. Cell. Longev. 2021, 2021, 6681815. [Google Scholar] [CrossRef]
- Chung, J.M. The role of reactive oxygen species (ROS) in persistent pain. Mol. Interv. 2004, 4, 248–250. [Google Scholar] [CrossRef]
- Passarella, S.; Casamassima, E.; Molinari, S.; Pastore, D.; Quagliariello, E.; Catalano, I.M.; Cingolani, A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984, 175, 95–99. [Google Scholar] [CrossRef]
- Pastore, D.; Greco, M.; Petragallo, V.A.; Passarella, S. Increase in <--H+/e− ratio of the cytochrome c oxidase reaction in mitochondria irradiated with helium-neon laser. Biochem. Mol. Biol. Int. 1994, 34, 817–826. [Google Scholar] [PubMed]
- Gonçalves de Faria, C.M.; Ciol, H.; Salvador Bagnato, V.; Pratavieira, S. Effects of photobiomodulation on the redox state of healthy and cancer cells. Biomed. Opt. Express. 2021, 12, 3902–3916. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Bertola, N.; Pasquale, C.; Bruno, S.; Benedicenti, S.; Ferrando, S.; Zekiy, A.; Arany, P.; Amaroli, A. 808-nm Photobiomodulation Affects the Viability of a Head and Neck Squamous Carcinoma Cellular Model, Acting on Energy Metabolism and Oxidative Stress Production. Biomedicines 2021, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef]
- Fernyhough, P.; Nigel Calcutt, A. Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium 2010, 47, 130–139. [Google Scholar] [CrossRef]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar]
- Amaroli, A.; Benedicenti, A.; Ferrando, S.; Parker, S.; Selting, W.; Gallus, L.; Benedicenti, S. Photobiomodulation by Infrared Diode Laser: Effects on Intracellular Calcium Concentration and Nitric Oxide Production of Paramecium. Photochem. Photobiol. 2016, 92, 854–862. [Google Scholar] [CrossRef]
- Miclescu, A.; Torsten, G. Nitric oxide and pain: ‘Something old, something new’. Acta Anaesthesiol. Scand. 2009, 53, 1107–1120. [Google Scholar] [CrossRef]
- Koch, A.; Zacharowski, K.; Boehm, O. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm. Res. 2007, 56, 32–37. [Google Scholar] [CrossRef]
- Lantero, A.; Tramullas, M.; Díaz, A. Transforming Growth Factor-β in Normal Nociceptive Processing and Pathological Pain Models. Mol. Neurobiol. 2012, 45, 76–86. [Google Scholar] [CrossRef]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The Effects of Photobiomodulation of 808 nm Diode Laser Therapy at Higher Fluence on the in Vitro Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Physiol. 2018, 23, 123. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Baik, K.Y.; Choung, P.H.; Chung, J.H. Pulse frequency dependency of photobiomodulation on the bioenergetic functions of human dental pulp stem cells. Sci. Rep. 2017, 7, 15927. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.P.; Pinheiro, A.L.; Mester, A.R.; Franke, R.P.; Whelan, H.T. Biostimulatory windows in low-intensity laser activation: Lasers, scanners, and NASA’s light-emitting diode array system. J. Clin. Laser Med. Surg. 2001, 19, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hakgüder, A.; Birtane, M.; Gürcan, S.; Kokino, S.; Turan, F.N. Efficacy of Low-Level Laser Therapy in myofascial pain syndrome: An algometric and thermographic evaluation. Lasers Surg. Med. 2003, 33, 339–343. [Google Scholar] [CrossRef]
- Sung, Y.T.; Wu, J.S. The Visual Analogue Scale for Rating, Ranking and Paired-Comparison (VAS-RRP): A new technique for psychological measurement. Behav. Res. 2018, 50, 1694–1715. [Google Scholar] [CrossRef]
- Gupta, U.; Verma, M. Placebo in clinical trials. Perspect. Clin. Res. 2013, 4, 49–52. [Google Scholar] [CrossRef]
- Stocum, D.L.; Roberts, W.E. Part I: Development and Physiology of the Temporomandibular Joint. Curr. Osteoporos Rep. 2018, 16, 360–368. [Google Scholar] [CrossRef]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Bordin-Aykroyd, S.R.; Lynch, E. Systematic Review of Delivery Parameters Used in Dental Photobiomodulation Therapy. Photobiomodul. Photomed. Laser Surg. 2019, 37, 784–797. [Google Scholar] [CrossRef]
| Author/Year | Groups | Number of Patients Gender Age | Number of Application | Points of Application | Scale | Variables | Follow Up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Silva et al. (2012) [28] |
| 30 women 15 men 25–35 years | 2 times/week 5 weeks | Extra-orally: - 1 pt/Anterior Temporalis - 3 pts/Masseter - 5 pts/Condyle - 1 pt/External auditory meatus. | VAS Muscle palpation PPT | Painful symptoms Mandibular movements: (MMO, P, LL, RL) | Baseline After the 1st session After the 5th session After the 10th session After 32 days of completing therapy | The laser Groups showed significant difference compared to placebo group with better outcomes with GL2 that received higher doses. |
| Sancakli et al. (2015) [29] |
| 21 women 9 men 18–60 years | 3 times/week 4 weeks | Extra-orally: 3 pts/Masseter 3 pts/Temporal | VAS Muscle palpation PPT | Pain intensity Mandibular mobility PPT | Baseline End of the therapy | Laser groups showed significant reduction for all variables compared to placebo group. |
| De Moraes Maia et al. (2012) [30] |
| 19 women 2 men Mean of ages 27.76 + 10.44 | 2 times/week 4 weeks | Extra-orally: 5 pts/Masseter 5 pts/Anterior Temporal | VAS | Pain intensity PPT MP | Baseline Weekly End of the therapy 30 days of final session | Laser group did not show significant differences compared to placebo group. |
| Shobha et al. (2017) [31] |
| 31 women 9 men 18–44 years | 2–3 times/week 8 sessions | Extra-orally: Upper joint space Trigger points determined by patients | VAS | Pain MO Joint clicking | Baseline End of treatment 30 days of final session | The laser group did not show significant difference compared to placebo group for all variables. |
| Madani et al. (2014) [32] |
| 19 women 1 man 30–60 years | 3 times/week 4 weeks | Extra-orally: 4 pts/TMJ area Painful muscles | VAS | Pain intensity Joint sounds MO | Baseline After the 6th session After the 12th session One month of final session | The laser group did not show significant difference for all variables compared to placebo group. |
| Salmos-Brito et al. (2012) [33] |
| 50 women 8 men 19–68 years | 2 times/week 6 weeks | Extra-orally: 5 pts/TMJ area | VAS | Pain intensity MMO | Before the LLLT 2 day following final session | The acute TMD group (G1) showed more significant differences for variables compared with chronic TMD group (G2). |
| Mazzetto et al. (2010) [34] |
| 40 patients | 2 times/week 4 weeks | Extra-orally: 5 pts/TMJ area | VAS | Pain intensity Mandibular movements (ML, MO). | Before After each session 7 days of final session 30 days of last session | The laser showed significant improvement compared to placebo group for all variables. |
| Sayed et al. (2014) [35] |
| 9 women 11 men 19–47 years | 3 times/week 2 weeks | Extra-orally: TMJ area Intra-orally: Masseter, Anterior Ramus, Temporalis, Buccal molar area, Pterygoid muscle | VAS Muscle palpation (PPT) | Pain intensity Joint movements Joint sounds Number of tender points | After 1 week After 2 weeks After 1 month of first session After 3 months of first session After 6 months of first session | The laser groups showed improvement for all variables. |
| Study | Wavelength | Power | Tip Diameter | Irradiation Time | Speed of Movement | Tip–Tissue Distance | Delivery Mode | Contact Non-Contact | Energy Density | Power Meter |
|---|---|---|---|---|---|---|---|---|---|---|
| Silva et al. (2012) [28] | 780 nm (GaAlAs) | 70 mW | 5 mm | 30 s 60 s | NM | 0 mm | CW | Contact | 52.5 J/cm2 100 J/cm2 | Yes |
| Sancakli et al. (2015) [29] | 820 nm | 300 mW | 6 mm | 10 s | NM | 2 mm | CW | Non-contact | 3 J/cm2 | Yes |
| De Moraes Maia et al. (2012) [30] | 808 nm (GaAlAs) | 100 mW | NM | 19 s/point | NM | 0 mm | CW | Contact | 70 J/cm2 | Yes |
| Shobha et al. (2017) [31] | 810 nm (GaAlAs) | 100 mW | 300 μm | 60 s | NM | NM | CW | Non-contact | 6 J/cm2 | NM |
| Madani et al. (2014) [32] | 810 nm | 50 mW PP: 80 W | NM | 120 s | NM | 0 mm | SP 1500 Hz 1 μs (Pulse width) | Contact | 3.4 J/cm2 | Yes |
| Salmos-Brito et al. (2012) [33] | 830 nm (GaAlAs) | 40 mW | 6 mm | 60 s | NM | 0 mm | CW | Contact | 8 J/cm2 | Yes |
| Mazzetto et al. (2010) [34] | 830 nm (GaAlAs) | 40 mW | NM | 10 s | NM | 0 mm | CW | Contact | 5 J/cm2 | NM |
| Sayed N et al. (2014) [35] | 904 nm (GaAs) | 0.6 W | NM | 60 s | NM | 0 mm | CW | contact | 4 J/cm2 | NM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsarhan, J.; El Feghali, R.; Alkhudari, T.; Benedicenti, S.; Pasquale, C. Can Photobiomodulation Support the Management of Temporomandibular Joint Pain? Molecular Mechanisms and a Systematic Review of Human Clinical Trials. Photonics 2022, 9, 420. https://doi.org/10.3390/photonics9060420
Alsarhan J, El Feghali R, Alkhudari T, Benedicenti S, Pasquale C. Can Photobiomodulation Support the Management of Temporomandibular Joint Pain? Molecular Mechanisms and a Systematic Review of Human Clinical Trials. Photonics. 2022; 9(6):420. https://doi.org/10.3390/photonics9060420
Chicago/Turabian StyleAlsarhan, Jumana, Rita El Feghali, Thaer Alkhudari, Stefano Benedicenti, and Claudio Pasquale. 2022. "Can Photobiomodulation Support the Management of Temporomandibular Joint Pain? Molecular Mechanisms and a Systematic Review of Human Clinical Trials" Photonics 9, no. 6: 420. https://doi.org/10.3390/photonics9060420
APA StyleAlsarhan, J., El Feghali, R., Alkhudari, T., Benedicenti, S., & Pasquale, C. (2022). Can Photobiomodulation Support the Management of Temporomandibular Joint Pain? Molecular Mechanisms and a Systematic Review of Human Clinical Trials. Photonics, 9(6), 420. https://doi.org/10.3390/photonics9060420







