Abstract
Low-level laser therapy (LLLT) has become an important part of the therapeutic process in various diseases. However, despite the broad use of LLLT in everyday clinical practice, the full impact of LLLT on cell life processes has not been fully understood. This paper presents the current state of knowledge concerning the mechanisms of action of LLLT on cells. A better understanding of the molecular processes occurring within the cell after laser irradiation may result in introducing numerous novel clinical applications of LLLT and potentially increases the safety profile of this therapy.
1. Introduction
Since the laser device was constructed in 1960 by Theodor Maiman, every year, an increasing number of medical applications appear. Lasers are distinguished from other light sources by their coherence, polarization, and monochromaticity; therefore, they can transmit a wide range of energy. The device generates laser irradiation by the external energy supply using the phenomenon of stimulated emission. There are many ways of classifying laser devices, including: the spectrum of the emitted radiation, the active medium (semiconductor, gas, liquid, and solid), the nature of the work (pulsed or continuous), and the range of power and energy emitted by the device. Recently, growing attention in the medical field has been focused on low-level energy laser irradiation. Radiation used in this therapy refers to the use of wavelengths from 500 nm up to 1200 nm and power from 1 mW to 500 mW, resulting in relatively low specific energy density (0.05 J/cm2–50 J/cm2). In clinical practice, low-level laser therapy (LLLT) was introduced by E. Master in the second half of the 1960s [1]. Since that time, this type of radiation has been successfully used in cardiology, hematology, dermatology, surgery, orthopedics, and other clinical specialties [2].
The effect of laser radiation on cells is referred to as photobiomodulation (PBM). The basis of this phenomenon is strongly connected with the influence of laser light on cell organelles as well as the biochemical processes carried out inside them (Figure 1 and Figure 2). It needs to be emphasized that photobiomodulation (PBM) is a broader concept referring to different kinds of light sources, not only to coherent laser light (e.g., visible, near-infrared (NIR), infrared (IR)). The North American Association for Photobiomodulation Therapy (NAALT)) and the World Association for Laser Therapy (WALT) define [3] PBM as “the therapeutic use of light absorbed by endogenous chromophores, triggering non-thermal, non-cytotoxic, biological reactions through photochemical or photophysical events, leading to physiological changes”.
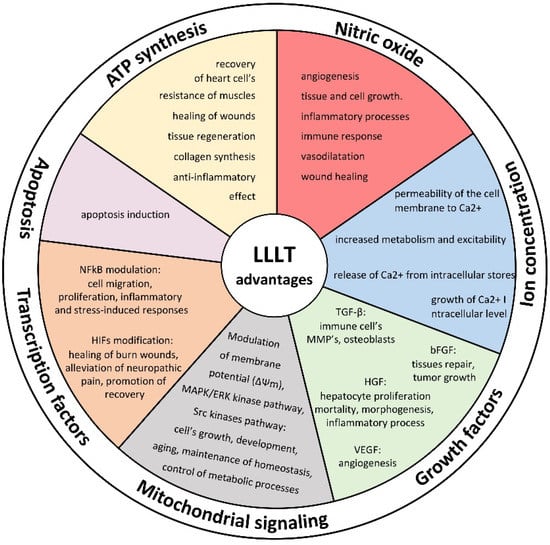
Figure 1.
Mechanism of LLLT action on cell activity and potential advantages of laser therapy on cell biology.
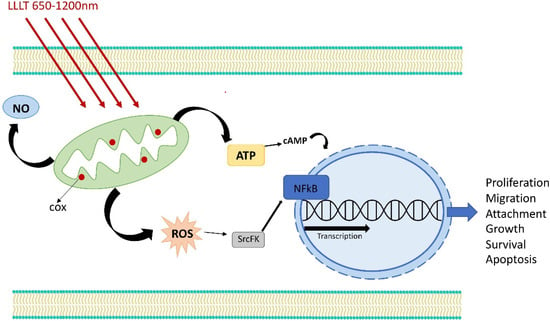
Figure 2.
Mechanism of LLLT action on cell activity.
The interpretation of published studies is difficult and can often be confusing. This fact is inextricably linked with methodological issues. Available studies refer to various light sources with different radiation parameters, including coherence, wavelength, polarization, power, energy density, etc. Additionally, the biological effect of light therapy is often heterogeneous and strongly depends on the light parameters set and the specific cell response.
Two main LLLT features seem to have the strongest impact on cell biology. First is the wavelength. Wavelengths from 600 nm up to 1070 nm have the greatest impact on the promotion of cell proliferation. This phenomenon is probably related to the absorption or interference of light beyond this range. Light with shorter wavelengths is strongly absorbed by hemoglobin, while longer wavelengths are absorbed by water [4]. The second important factor is energy density. In general, lower energy density (0.05 J/cm2–10 J/cm2) promotes cell proliferation, while higher energy density (above 50 J/cm2) enhances apoptotic processes. The mutual transition of both phenomena has a continuous nature. This biphasic response is also known as the “Arndt–Schulz law”.
Additionally, even other parameters of LLLT seem to have an impact on its therapeutic effect. Yet, a full discussion regarding this issue is beyond the scope of this manuscript. As a short example, we can use the laser operating mode. Although continuous-wave (CW) irradiation has been a gold standard in LLLT, some studies have suggested that pulse wave (PW) [5] may be superior to CW, particularly in regenerative applications. However, no strong evidence supports this theory [5], and therefore, future studies focused on this topic are necessary. Other, more specific effects on cell life processes are discussed in the next part of the manuscript.
Study Aim
This paper aims to review the impact of LLLT on the basic intracellular process and discusses the effect of LLLT on individual intracellular information transmission pathways. To avoid misleading comparisons, the data contained in this manuscript are strictly limited to those directly related to low-level laser therapy. All studies referring to other light sources were excluded from this study.
2. ATP Synthesis
Mitochondria are dynamic organelles that play a critical role in energetic metabolism and intracellular signaling [6]. The process of oxidative phosphorylation leads to the formation of high-energy ATP (adenosine triphosphate). When energy is consumed in metabolic processes, it converts to either adenosine diphosphate (ADP) or adenosine monophosphate (AMP). ATP synthesis in cells occurs primarily in mitochondria, which transport high-energy electrons from substrates through a series of protein complexes called the electron transfer chain (ETC). One of the most important proteins in this pathway is cytochrome c—a hemoprotein that acts as an electron transporter in the respiratory chain. It is capable of oxidation and reduction reactions; however, it does not directly bind oxygen [7]. It transfers electrons between Complexes III and IV. In addition, it also plays an important role in apoptosis [8].
Low-level laser therapy irradiation of cell cultures has been shown to result in an immediate and long-lasting increase in ATP production [9] and in the upregulation of mitochondrial function with a therapeutic window for wavelengths from 650 nm to 1200 nm [10]. This effect is closely related to the influence of laser irradiation on the aforementioned mitochondrial cytochrome c oxidase (Cox) [11]. The stimulated ATP synthesis is caused by the increased activity of Cox. Cox is a phosphorylated enzyme controlled mainly by allosteric ATP inhibition, depending on the ATP/ADP ratio. However, there is another, non-“classical” control pathway via increased mitochondrial membrane potential (ΔΨm), and the production [12] of ROS and LLLT has a major impact on this control pathway [8]. It is postulated that the increased ATP synthesis after laser irradiation is directly involved in biological effects, including: improvement in the function and recovery of rat hearts stored in the cold [13], increase in the resistance of muscles to fatigue during intense physical exercise [14,15], healing of burn wounds [16], improvement in depressive behaviors [17], improvement in tissue regeneration and collagen synthesis [18], anti-inflammatory effects [19], and many others. It is noteworthy that the biologically beneficial effect of increased ATP synthesis after LLLT irradiation depends not only on the intracellular ATP amount but is also associated with increased amounts of extracellular ATP [20]. Evidence suggests that the effect of increased ATP synthesis is closely related to the irradiation parameters and that an inappropriate choice of laser light properties may trigger the opposite effect [21]. This biphasic response follows the “Arndt–Schulz law” and was demonstrated in our previous study [22].
3. Retrograde Mitochondrial Signaling
Because of the paramount role of the nucleus compared to other organelles, including mitochondria, the mechanisms of anterograde regulation and communication have been well studied and described for decades. However, retrograde regulatory mechanisms constitute the subject of more recent, intensive studies. In this reverse information transmission pathway, changes in the functional state of the organelle affect cell activity via changes in gene expression. Retrograde regulation affects various cellular activities, including the processes of growth and development, aging, the maintenance of homeostasis, and the control of appropriate metabolic processes [23]. Mitochondrial retrograde signaling is initially defined by the change in mitochondrial membrane potential (ΔΨm), and later, other changes occur—particularly in other secondary elements of mitochondrial retrograde signaling (ROS, pH, nitric oxide—NO) [24]. The increase in the mitochondrial membrane potential (ΔΨm) after LLLT is among the best-demonstrated influences of laser light on cell function. This effect is also observed due to other mentioned mitochondrial retrograde signaling mechanisms such as ROS, pH, and NO [6]. It has been proven that this mechanism is responsible for the pro-proliferative effect of LLLT. Low-level laser therapy causes the phosphorylation of tyrosine kinase receptors (TPKR) due to the abovementioned changes in retrograde mitochondrial signaling, a stimulatory effect on the MAPK/ERK kinase signaling pathway, the activity of which leads to increased cell proliferation [25]. LLLT stimulates proliferation not only via this signaling pathway but also by retrograde mitochondrial signaling. Increased membrane potential is involved in the regulation of melanoma cell proliferation induced by ΔΨm/ATP/cAMP/JNK/AP-1 [26]. ROSs are one of the elements of mitochondrial retrograde signaling that directly affect proliferation. There is no doubt that the production of ROS is stimulated by LLLT and that they act as important secondary messengers regulating the activity of various protein kinases. The Src kinases are a known target of ROS [27] and play a critical role in regulating fundamental cellular processes, including cell proliferation, migration, and commitment. LLLT has been shown to induce bio-stimulatory effects by activating the Src tyrosine kinase by increasing the ROS level [28]. The production of ROS triggered by LLLT also leads to an activation of the transcription factor nuclear factor kappa B (NF-kB) [29], which modulates the expression of target genes involved in cell growth, survival, and death. An example of a prolonged cell growth effect could be observed in the chondrocyte population [30], which may be associated with accelerated bone healing after LLLT. Changes in cell signaling pathways and their clinical impact are presented in Figure 3.
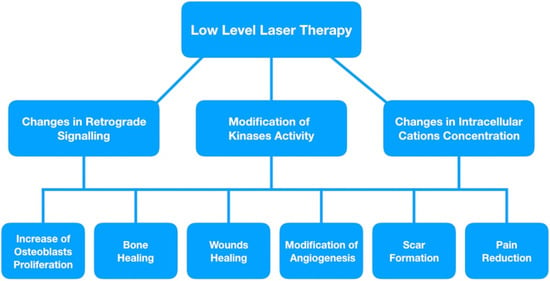
Figure 3.
Changes in cell signaling process and its clinical application.
4. Nitric Oxide
Nitric oxide (NO) and its role as a cellular transmitter has been the subject of numerous studies [31]. It has been shown to promote angiogenesis [32], inextricably linked with tissue and cell growth. Nitric oxide modulates inflammatory processes and the immune response of cells. This effect is achieved through multiple mechanisms which affect cell signaling systems such as cGMP and G-protein, but also the cAMP, JAK/STAT, or MAPK-dependent signal transduction pathways. NO may also modulate transcription factors’ activity and the expression of other mediators of inflammation [33,34]. Nitric oxide is known to have a twofold nature regarding a tumor’s biology and might simultaneously have pro-oncogenic and anticancer properties. The multidirectional nature of this signal molecule under different conditions can be associated with the effect of time and concentration which affects different phenotypes’ expression [35]. There is no doubt that low-energy laser irradiation increases the production of NO in the in vitro models [36], which is also observed in the in vivo experimental models [37,38], including in humans [39]. The exact mechanism of this phenomenon has not been fully understood. However, it is suggested that several different processes are involved. As previously mentioned, LLLT has a strong activating influence on the mitochondrial respiration chain, and, through the cytochrome C oxidase, LLLT increases the production of NO. The molecular basis of this phenomenon is founded on competition between O2 and NO for the active center in the enzymes of the mitochondrial respiratory chain [40]. LLLT, by stimulating the activity of the cytochrome C oxidase complex, increases the release of NO from the active sites of the enzyme. This phenomenon, occurring after laser irradiation, is referred to as the “NO hypothesis” [41]. This is not the only pathway by which LLLT increases NO production. Another potential mechanism is the influence of low-level laser therapy on the induction, expression, and activation of nitric oxide synthesis. The literature suggests that activation may be contributed by the kinase pathway PI3K/eNOS [42]. However, it seems that the mechanism responsible for increased NO synthesis under the influence of LLLT is far more complicated. Clinical trials carried out in human models showed that intravenous LLLT significantly decreases the expression of arginase and EGFR. The downregulation of arginase and the increase in the metabolism of L-arginine by NOS, which induces NO production, may be one of the mechanisms of the vasodilatation and acceleration of wound healing that occurs after laser therapy [43]. On the other hand, Lindgård A. et al. [44] postulate that laser irradiation resulted in elevated levels of NO but had no effect on the iNOS or eNOS activity. They indicate that irradiation at 634 nm releases NO, possibly from a preformed store, and additionally reduces the production of intracellular ROS. Confirmation of this theory may be the study by Mittermayr R. et al [45]. The authors suggest that laser-irradiation-induced NO release from NO–Hb complexes may be a novel concept and may explain the abovementioned [22] reduction in the aggregative potential of human platelets after blood irradiation.
5. Modulation of Ion Concentrations
Calcium ions are an important link in intracellular signal transductions. They are involved in many intracellular processes, and changes in their concentration affect the activity and viability of all cell cultures. LLLT has been shown to increase the permeability of the cell membrane to calcium, leading to an increase in its intracellular level [46,47]. Another mechanism responsible for the increase in calcium levels after LLLT is related to the increased release of Ca2+ from intracellular stores [48]. An experimental study by Lavi et al. [49] suggests that, within the appropriate radiation range, the increase in the calcium level is directly proportional to the increase in the radiant energy density, measured in J/cm2, as well as proportional to the increase in reactive oxygen species generation. The authors also suggest a direct relationship between the intracellular calcium concentration and the levels of ROS. They assume that the mechanism of calcium channel activation is directly related to the production of ROS under the influence of visible electromagnetic radiation. What needs to be emphasized is that laser irradiation increases intracellular calcium levels not only by a ROS-dependent mechanism. Furthermore, LLLT, via the increased production of ATP, may activate multiple subtypes of nucleotide (purinergic) P2 receptors, resulting in the increased level of intracellular calcium levels [50,51]. It is noteworthy that calcium-related signaling pathways include mitochondrial calcium signaling, calcium-sensitive adenylyl cyclase, calcium-sensitive enzymes such as protein kinase C (PKC), calcium-dependent kinase II (CamKII), and extracellular calcium-sensitive receptor (CaSR). Their enhanced activation leads to various changes in the basic activity of the cells and can be summarized as increased metabolism and excitability. This effect affects various cell cultures—myocytes [52], mast cells [53], fibroblasts [54], or reproductive cells [46]. Low-level laser therapy has an impact on the local concentration of sodium and potassium ions. Laser irradiation in a dose-dependent manner alters the ATPase activity of the membrane ion pumps. Depending on the radiation parameters used, an increase or decrease in the Na(+), K(+) ATPase activity is observed [55]. Even though the exact mechanism of this phenomenon has not been fully understood, one study suggests that it may be responsible for the analgesic effect of LLLT [56]. Figure 4 shows the basic changes in ion concentration triggered by LLLT.
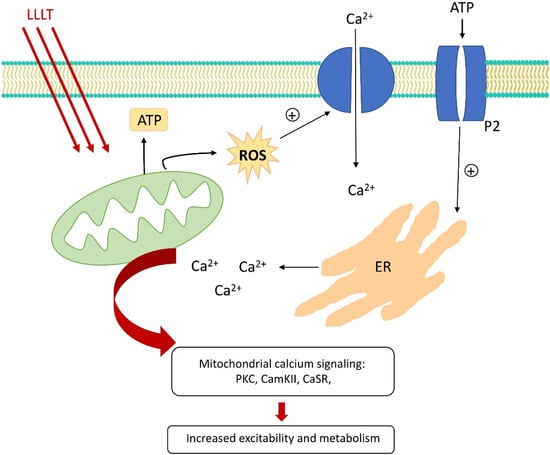
Figure 4.
Changes in ion concentration after LLLT.
6. Growth Factor Release
Low-level laser therapy affects the activity of several growth factors. This molecular effect is widely used in the clinical application of LLLT. Figure 5 shows basic post-LLLT changes in growth factor activity.
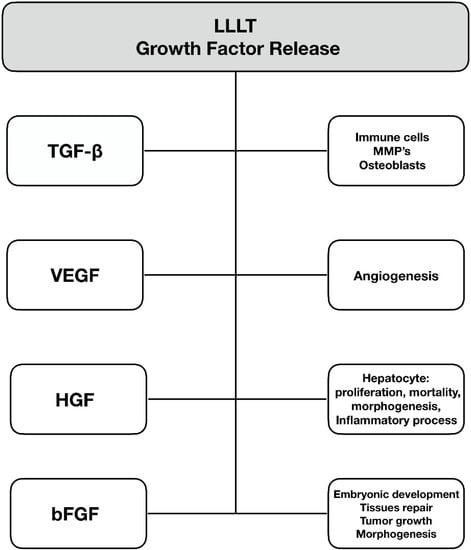
Figure 5.
Changes in growth factor activity after low-level laser therapy.
6.1. Transforming Growth Factor-Beta
Transforming growth factor-beta (TGF-β) is not a single-molecule cytokine but rather a multipotential group of factors consisting of multiple isozymes. Its activation leads to increased activity of a specific TGF-β-related kinase, which activates a signaling cascade that, in the end, causes changes in regulatory protein levels. TGF-β also affects the transcription of several target genes that play a role in the differentiation, chemotaxis, proliferation, and activation of many immune cells [57]. This cytokine plays an important role in collagen production by inducing the expression of extracellular matrix components and inhibiting their degradation by inhibiting matrix metalloproteinases (MMPs) [58]. A large body of data suggests that LLLT accelerates bone healing due to the increased proliferation of osteoblasts [59,60]. Enhanced regeneration of connective tissue following laser irradiation is also associated with increased collagen synthesis through activation of the TGF-β/SMAD pathway [61].
Additionally, LLLT via the TGF-β signaling pathway can suppress the immune response. These properties of laser irradiation have already been utilized as a part of therapy for chronic kidney disease [62], tendon injury [63], hypothyroidism [64], scars, and keloids [65]. On the other hand, we have recently shown that the intravascular use of LLLT decreases TGF-β1 and FGF-2 levels in patients undergoing coronary intervention, which may reduce the neointima formation [66].
6.2. Vascular Endothelial Growth Factor (VEGF)
Vascular endothelial growth factor (VEGF) is a protein that plays a critical role in angiogenesis. It initiates cell migration along with the initial invasion of endothelial cells and the formation of the vascular lumen. Additionally, it promotes the junction process between the new vessel segments and within the existing old ones [67]. VEGF and its activity are directly involved in the pathogenesis of the recovery process from many diseases, such as traumatic injuries to the central nervous system, bone and muscle injuries, rheumatoid arthritis, age-related macular degeneration (AMD), diabetic retinopathy, psoriasis, hair loss, and the whole spectrum of cardiovascular disease [68,69]. There is convincing data on the increased local activity of VEGF after LLLT, which translates into the process of healing and regeneration [70,71]. This effect is probably not permanent and depends on radiation parameters and exposure [72]. Although the side effects after LLLT are hardly observable, still, the safety concerns are not unfounded. Increased activity of VEGF plays an important role in the etiopathogenesis of many tumors, and anti-VEGF therapy is well established in routine clinical practice in oncology or ophthalmology [73]. Increased proliferative potential of the various cells after courses of LLLT via the VEGF pathways implies the necessity of future research, particularly investigating the impact of LLLT on tumor biology.
6.3. Hepatocyte Growth Factor (HGF)
Hepatocyte growth factor (HGF) is a pleiotropic cytokine consisting of an α-chain and a β-chain. The biological response is triggered by the Met receptor, which affects signaling molecules such as PI3K and MAPK proteins [74]. These proteins have a wide range of activities that affect cell proliferation, mortality, morphogenesis, and the inflammatory process, and they influence apoptosis [75,76]. Originally, HGF was characterized as a mitogenic protein associated with hepatocytes, but we now know that its role extends to many other cells. Its multipotent action leads to many therapeutic applications in various disease models, such as liver cirrhosis, cholestasis, peptic ulcers, acute kidney injury, chronic kidney disease, coronary artery disease and myocardial infarction, emphysema, cerebral ischemia, and skeletal muscle and skin injury [77,78]. LLLT has been shown to enhance the synthesis of HGF and may thus be a supportive therapeutic strategy for the listed diseases [79,80].
6.4. Basic Fibroblast Growth Factor (bFGF)
The basic fibroblast growth factor (bFGF) is responsible for cell growth and survival [81]. It is involved in embryonic development, morphogenesis, tissue repair, tumor growth, and invasion. It is postulated to play a crucial role in wound healing—it acts as a chemoattractant for endothelial cells and fibroblasts [82]. This protein is also involved in granulation tissue formation and re-epithelialization, as well as angiogenesis [83,84]. Due to the multiple roles of bFGF, it plays an important role in the development of numerous diseases, including Paget’s disease [85], Alzheimer’s disease [86], malignancies and precancerous lesions [87], nerve injury [88], and Crohn’s disease [89]. There are many studies suggesting that fibroblast growth factor synthesis is more intense after irradiation of a broad spectrum of wavelengths than after low-level laser therapy [90]. On the other hand, some convincing data suggest an opposite effect [66,91], which most likely depends on many factors, and more studies are required to clarify this issue. A similar dual-response effect after irradiation occurs with insulin-like growth factor I [92,93].
7. Activation of Transcription Factors
Laser irradiation also affects cell biology by modulating transcription factors. One of the best-known transcription factors, whose activity is changed by LLLT is nuclear factor-kappa B (NFkB). NFkB regulates many cellular functions (migration, proliferation, inflammatory and stress-induced responses). This protein complex remains in a non-active state, and under the influence of stimulating factors, it turns into the active state [94]. This change occurs without any additional protein synthesis, allowing classification in the “rapid-acting” primary transcription factors category. There are many well-known inducers of NFkB activation, such as tumor necrosis factor-alpha, ROS, bacterial lipopolysaccharides, interleukins, and low-level laser therapy [29,95]. In the case of LLLT stimulation, we observe a typical reaction consistent with the Arndt–Schulz law. The appropriate dose of radiation leads to the activation of the enzyme and increases the proliferative and anti-inflammatory potential [96,97]. However, exceeding the radiation dose led to increased oxidative stress and an over-abundant activation of NF-κB [98].
Another transcription factor activity that is modified under the impact of low-energy laser irradiation is the hypoxia-induced factor (HIF). HIF is a small protein associated with a cell’s response to hypoxia. In hypoxic conditions, HIF activation led to the upregulation of several genes, such as glycolysis enzymes, which allow ATP synthesis in an oxygen-independent manner. Probably, the therapeutic effect of LLLT is partly achieved by changing the level of HIFs. Furthermore, Gupta et al. [99] suggest that modification of HIF activity (upregulation) under laser light might be involved in enhancing the healing of burn wounds. The same effect of increased activity of HIFs was observed by Cury et al. [100]. They prove that LLLT can promote angiogenesis in ischemic skin flaps. What is interesting is that LLLT does not only increase the activity of HIFs. There is evidence that it can alleviate neuropathic pain and promote functional recovery in rats with chronic constriction injury by decreasing the activity of HIFs [101]. It is noteworthy that LLLT may also have a dark side—some data suggest that LLLT can promote aggressive proliferation in cancer cells by activating the Akt/Hypoxia Inducible Factor-1α pathway [102].
8. Influence on Apoptosis
The mechanism of action of low-level laser therapy is not only related to enhancing cellular metabolism and proliferation, but after application at higher doses, it also shows the ability to induce apoptosis. The exact mechanism of this phenomenon has not been fully understood; however, it might be indirectly related to the production of reactive oxygen species (ROS). On the other hand, laser irradiation activates the 3β glycogen synthase kinase (GSK3β), which triggers apoptosis [103]. Interestingly, there is another ROS-related mechanism promoting apoptosis—by the Akt/GSK3β pathway [104]. It is noteworthy that LLLT irradiation can induce both proliferation (low energy density—0.8 J/cm2) and apoptosis (higher energy density—60 J/cm2) by changing the activity of particular kinases, such as C-kinase [105]. This fact points at the quantitative rather than qualitative nature of promotion of proliferation/apoptosis process after LLLT irradiation. Probably, the direct “effector” through which laser-induced apoptosis is induced is the caspase 3 [106], which is a key mediator of programmed cell death and is involved in the breakdown of many of its essential proteins [107]. Even though the mechanism of apoptosis induced by low-level laser therapy is not fully understood, the majority of authors agree that the differentiating factor between pro-proliferative and apoptotic effects is the energy provided by the laser. Interestingly, this process is continuous and mutually permeates—the anti-apoptotic pathways are activated even during irradiation with high energy density. A good representation of this situation is the impact of laser irradiation on the ROS/Src/Stat3 pathway, the activation of which inhibits apoptosis [103]. It should be emphasized that in the case of various cell types, the borderline at which there occurs an advantage of apoptotic processes over the pro-proliferative is variable. The specific cell line type, in combination with the recently described [108] effective use of fractional descriptions of energy transfer as a tool dedicated to the modification of cell biological functions, can open a completely new field of precise LLLT selected for specific medical indications.
9. Conclusions
Low-level laser therapy has nowadays become an important part of the therapeutic process in everyday clinical practice. Despite numerous studies conducted so far, the full impact of LLLT on cell life processes has not been fully understood. This paper has presented the current state of knowledge concerning the mechanisms of action of LLLT on cells. Even though the available data suggest a good safety profile for low-level laser therapy, some concerns regarding the impact on the biology of tumors are actually due to the increased proliferation potential following LLLT. It seems that a complete understanding of the molecular processes occurring within the cell after laser irradiation may result in introducing numerous novel clinical applications for LLLT, after appropriately addressing the safety concerns.
Author Contributions
Conceptualization, P.R. and A.W.; methodology, P.R. and A.W.; software, S.W.; validation, P.R., A.W., S.W. and A.D.; formal analysis, P.R., S.W., M.L., A.D. and A.W.; investigation, P.R., S.W., M.L., A.D. and A.W.; resources, P.R., S.W., M.L., A.D. and A.W.; data curation, P.R., S.W., M.L., A.D. and A.W.; writing—original draft preparation, P.R.; writing—review and editing, P.R., S.W., M.L., A.D. and A.W.; visualization, P.R. and S.W.; supervision, P.R., M.L., A.D. and A.W.; project administration, P.R. and A.W.; funding acquisition, P.R. and A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mester, E.; Szende, B.; Gärtner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Piotr, R.; Doroszko, A.; Derkacz, A. The Use of Low-Level Energy Laser Radiation in Basic and Clinical Research. Adv. Clin. Exp. Med. 2014, 23, 835–842. [Google Scholar]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Supportive Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, S.Y.; Tam, V.C.W.; Ramkumar, S.; Khaw, M.L.; Law, H.K.W.; Lee, S.W.Y. Review on the Cellular Mechanisms of Low-Level Laser Therapy Use in Oncology. Front. Oncol. 2020, 10, 1255. [Google Scholar] [CrossRef]
- Hashmi, J.T.; Huang, Y.Y.; Sharma, S.K.; Kurup, D.B.; de Taboada, L.; Carroll, J.D.; Hamblin, M.R. Effect of pulsing in low-level light therapy. Lasers Surg. Med. 2010, 42, 450–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balderas, P.M.D.O. Mitochondria-plasma membrane interactions and communication. J. Biol. Chem. 2021, 297, 101164. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Photobiomodulation Therapy Attenuates Hypoxic-Ischemic Injury in a Neonatal Rat Model. J. Mol. Neurosci. 2018, 65, 514–526. [Google Scholar]
- Huang, Y.Y.; Chen, A.C.H.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-Response 2009, 7. [Google Scholar] [CrossRef]
- Karu, T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Huettemann, M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion 2015, 24, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, W.; Yang, X.; Hicks, G.L.; Lanzafame, R.J.; Wang, T. Photo-irradiation improved functional preservation of the isolated rat heart. Lasers Surg. Med. 1997, 20, 332–339. [Google Scholar] [CrossRef]
- Kasprzyk-Kucewicz, T.; Szurko, A.; Stanek, A.; Sieroń, K.; Morawiec, T.; Cholewka, A. Usefulness in Developing an Optimal Training Program and Distinguishing between Performance Levels of the Athlete’s Body by Using of Thermal Imaging. Int. J. Environ. Res. Public. Health 2020, 17, 5698. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; de Sousa, M.V.P.; Huang, Y.Y.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med. Sci. 2015, 30, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Gupta, A.; Keshri, G.K.; Verma, S.; Sharma, S.K.; Singh, S.B. Photobiomodulatory effects of superpulsed 904 nm laser therapy on bioenergetics status in burn wound healing. J. Photochem. Photobiol. B Biol. 2016, 162, 77–85. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Yang, Y.; Tucker, D.; Lu, Y.; Xin, N.; Zhang, G.; Yang, L.; Li, J.; Du, X.; et al. Low-level laser irradiation improves depression-like behaviors in mice. Mol. Neurobiol. 2017, 54, 4551–4559. [Google Scholar] [CrossRef]
- Chen, M.-H.; Huang, Y.-C.; Sun, J.-S.; Chao, Y.-H.; Chen, M.-H. Second messengers mediating the proliferation and collagen synthesis of tenocytes induced by low-level laser irradiation. Lasers Med. Sci. 2015, 30, 263–272. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Grygorczyk, R.; Shen, X.; Schwarz, W. Modulation of extracellular ATP content of mast cells and DRG neurons by irradiation: Studies on underlying mechanism of low-level-laser therapy. Mediat. Inflamm. 2015, 2015, 630361. [Google Scholar] [CrossRef]
- Amaroli, A.; Benedicenti, A.; Ravera, S.; Parker, S.; Selting, W.; Panfoli, I.; Benedicenti, S. Short-pulse neodymium: Yttrium–aluminium garnet (Nd: YAG 1064 nm) laser irradiation photobiomodulates mitochondria activity and cellular multiplication of Paramecium primaurelia (Protozoa). Eur. J. Protistol. 2017, 61, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Doroszko, A.; Szahidewicz-Krupska, E.; Rola, P.; Dobrowolski, P.; Skomro, R.; Szymczyszyn, A.; Mazur, G.; Derkacz, A. Low-level laser irradiation exerts antiaggregative effect on human platelets independently on the nitric oxide metabolism and release of platelet activation markers. Oxidative Med. Cell. Longev. 2017, 2017, 6201797. [Google Scholar] [CrossRef] [PubMed]
- Mielecki, J.; Gawroński, P.; Karpiński, S. Retrograde Signaling: Understanding the Communication between Organelles. Int. J. Mol. Sci. 2020, 21, 6173. [Google Scholar] [CrossRef] [PubMed]
- Ježek, P.; Holendová, B.; Plecitá-Hlavatá, L. Redox Signaling from Mitochondria: Signal Propagation and Its Targets. Biomolecules 2020, 10, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefer, G.; Oron, U.; Irintchev, A.; Wernig, A.; Halevy, O. Skeletal muscle cell activation by low-energy laser irradiation: A role for the MAPK/ERK pathway. J. Cell. Physiol. 2001, 187, 73–80. [Google Scholar] [CrossRef]
- Hu, W.-P.; Wang, J.-J.; Yu, C.-L.; Lan, C.-C.E.; Chen, G.-S.; Yu, H.-S. Helium–neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J. Investig. Dermatol. 2007, 187, 2048–2057. [Google Scholar]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, D.; Gao, X. Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J. Cell. Physiol. 2008, 217, 518–528. [Google Scholar] [CrossRef]
- Chen, A.C.-H.; Arany, P.R.; Huang, Y.-Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar]
- De Luca, F. Role of Nuclear Factor Kappa B (NF-κB) in Growth Plate Chondrogenesis. Pediatric Endocrinol. Rev. 2016, 13, 720–730. [Google Scholar]
- Jakubiak, G.K.; Cieślar, G.; Stanek, A. Nitrotyrosine, Nitrated Lipoproteins, and Cardiovascular Dysfunction in Patients with Type 2 Diabetes: What Do We Know and What Remains to Be Explained? Antioxidants 2022, 11, 856. [Google Scholar] [CrossRef]
- Duda, D.G.; Fukumura, D.; Jain, R.K. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation. J. Physiol. Pharm. 2003, 54, 469–487. [Google Scholar]
- Stanek, A.; Cholewka, A.; Wielkoszyński, T.; Romuk, E.; Sieroń, K.; Sieroń, A. Increased Levels of Oxidative Stress Markers, Soluble CD40 Ligand, and Carotid Intima-Media Thickness Reflect Acceleration of Atherosclerosis in Male Patients with Ankylosing Spondylitis in Active Phase and without the Classical Cardiovascular Risk Factors. Oxid. Med. Cell. Longev. 2017, 2017, 9712536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp-Scheinpflug, C.; Forsythe, I.D. Nitric Oxide Signaling in the Auditory Pathway. Front Neural Circuits. 2021, 15, 114. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef]
- Mitchell, U.H.; Mack, G.L. Low-level laser treatment with near-infrared light increases venous nitric oxide levels acutely: A single-blind, randomized clinical trial of efficacy. Am. J. Phys. Med. Rehabil. 2013, 92, 151–156. [Google Scholar] [CrossRef]
- Tuby, H.; Maltz, L.; Oron, U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg. Med. 2006, 38, 682–688. [Google Scholar] [CrossRef]
- Samoilova, K.A.; Zhevago, N.A.; Petrishchev, N.N.; Zimin, A.A. Role of nitric oxide in the visible light-induced rapid increase of human skin microcirculation at the local and systemic levels: II. healthy volunteers. Photomed. Laser Surg. 2008, 26, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Kalendo, G.S. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem. Photobiol. Sci. 2004, 3, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hung, H.S.; Hsu, S.H. Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibly via PI3K signal pathway. Lasers Surg. Med. 2008, 40, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kazemikhoo, N.; Sarafnejad, A.F.; Ansari, F.; Mehdipour, P. Modifying effect of intravenous laser therapy on the protein expression of arginase and epidermal growth factor receptor in type 2 diabetic patients. Lasers Med. Sci. 2016, 31, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Lindgård, A.; Hultén, L.M.; Svensson, L.; Soussi, B. Irradiation at 634 nm releases nitric oxide from human monocytes. Lasers Med. Sci. 2007, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, R.; Osipov, A.; Piskernik, C.; Haindl, S.; Dungel, P.; Weber, C.; Vladimirov, Y.A.; Redl, H.; Kozlov, A.V. Blue laser light increases perfusion of a skin flap via release of nitric oxide from hemoglobin. Mol. Med. 2007, 13, 22. [Google Scholar] [CrossRef]
- Lubart, R.; Friedmann, H.; Sinyakov, M.; Cohen, N.; Breitbart, H. Changes in calcium transport in mammalian sperm mitochondria and plasma membranes caused by 780 nm irradiation. Lasers Surg. Med. 1997, 21, 493–499. [Google Scholar] [CrossRef]
- Abdel-Magied, N.; Elkady, A.A.; Fattah, S.M.A. Effect of Low-Level Laser on Some Metals Related to Redox State and Histological Alterations in the Liver and Kidney of Irradiated Rats. Biol Trace Elem. Res. 2020, 194, 410–422. [Google Scholar] [CrossRef]
- Harraz, O.F.; Jensen, L.J. Vascular calcium signalling and ageing. J. Physiol. 2021, 599, 5361–5377. [Google Scholar] [CrossRef]
- Migliario, M.; Sabbatini, M.; Mortellaro, C.; Renò, F. Near infrared low-level laser therapy and cell proliferation: The emerging role of redox sensitive signal transduction pathways. J. Biophotonics 2018, 11, e201800025. [Google Scholar] [CrossRef]
- Kalthof, B.; Bechem, M.; Flocke, K.; Pott, L.; Schramm, M. Kinetics of ATP-induced Ca2+ transients in cultured pig aortic smooth muscle cells depend on ATP concentration and stored Ca2+. J. Physiol. 1993, 466, 245–262. [Google Scholar]
- Kitajima, S.; Ozaki, H.; Karaki, H. Role of different subtypes of P2 purinoceptor on cytosolic Ca2+ levels in rat aortic smooth muscle. Eur. J. Pharmacol. 1994, 266, 263–267. [Google Scholar] [CrossRef]
- Yang, W.-Z.; Chen, J.-Y.; Yu, J.-T.; Zhou, L.-W. Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem. Photobiol. 2007, 83, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Cellular Signalling and Photobiomodulation in Chronic Wound Repair. Int. J. Mol. Sci. 2021, 22, 11223. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, J.; Zavodnik, L.; Zavodnik, I.; Buko, V.; Lapshyna, A.; Bryszewska, M. Effect of low-intensity (3.75–25 J/cm2) near-infrared (810 nm) laser radiation on red blood cell ATPase activities and membrane structure. J. Clin. Laser Med. Surg. 2004, 22, 111–117. [Google Scholar] [CrossRef] [PubMed]
- De Lima, H.S.; Rigos, C.F.; Tedesco, A.C.; Ciancaglini, P. Biostimulation of Na, K-ATPase by low-energy laser irradiation (685 nm, 35 mW): Comparative effects in membrane, solubilized and DPPC: DPPE-liposome reconstituted enzyme. J. Photochem. Photobiol. B Biol. 2007, 89, 22–28. [Google Scholar] [CrossRef]
- Lopatina, E.V.; Yachnev, I.L.; Penniyaynen, V.A.; Plakhova, V.B.; Podzorova, S.A.; Shelykh, T.N.; Rogachevsky, I.V.; Butkevich, I.P.; Mikhailenko, V.A.; Kipenko, A.V.; et al. Modulation of signal-transducing function of neuronal membrane Na+, K+-ATPase by endogenous ouabain and low-power infrared radiation leads to pain relief. Med. Chem. 2012, 8, 33–39. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Dijke, P.T. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [Green Version]
- Nouruzian, M.; Alidoust, M.; Bayat, M.; Bayat, M.; Akbari, M. Effect of low-level laser therapy on healing of tenotomized Achilles tendon in streptozotocin-induced diabetic rats. Lasers Med. Sci. 2013, 28, 399–405. [Google Scholar] [CrossRef]
- Pyo, S.-J.; Song, W.-W.; Kim, I.-R.; Park, B.-S.; Kim, C.-H.; Shin, S.-H.; Chung, I.-K.; Kim, Y.-D. Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med. Sci. 2013, 28, 543–550. [Google Scholar] [CrossRef]
- Fallahnezhad, S.; Piryaei, A.; Darbandi, H.; Amini, A.; Ghoreishi, S.K.; Jalalifirouzkouhi, R.; Bayat, M. Effect of low-level laser therapy and oxytocin on osteoporotic bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2018, 119, 983–997. [Google Scholar] [CrossRef]
- Dang, Y.; Liu, B.; Liu, L.; Ye, X.; Bi, X.; Zhang, Y.; Gu, J. The 800-nm diode laser irradiation induces skin collagen synthesis by stimulating TGF-β/Smad signaling pathway. Lasers Med. Sci. 2011, 26, 837. [Google Scholar] [CrossRef] [PubMed]
- Schardong, J.; Falster, M.; Sisto, I.R.; Barbosa, A.P.O.; Normann, T.C.; De Souza, K.S.; Jaroceski, G.; Bozzetto, C.B.; Baroni, B.M.; Plentz, R.D.M. Photobiomodulation therapy increases functional capacity of patients with chronic kidney failure: Randomized controlled trial. Lasers Med. Sci. 2021, 36, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.R.; Vieira, C.P.; Oliveira, L.P.; Marques, P.P.; Almeida, M.D.S.; Pimentel, E.R. Low-level laser therapy modulates pro-inflammatory cytokines after partial tenotomy. Lasers Med. Sci. 2016, 31, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Höfling, D.B.; Chavantes, M.C.; Acencio, M.M.; Cerri, G.G.; Marui, S.; Yoshimura, E.M.; Chammas, M.C. Effects of Low-Level Laser Therapy on the Serum TGF-β1 Concentrations in Individuals with Autoimmune Thyroiditis. Photomed. Laser Surg. 2014, 32, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Barolet, D.; Boucher, A. Prophylactic low-level light therapy for the treatment of hypertrophic scars and keloids: A case series. Lasers Surg. Med. 2010, 42, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Derkacz, A.; Protasiewicz, M.; Rola, P.; Podgorska, K.; Szymczyszyn, A.; Gutherc, R.; Poręba, R.; Doroszko, A. Effects of intravascular low-level laser therapy during coronary intervention on selected growth factors levels. Photomed. Laser Surg. 2014, 32, 582–587. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Karaman, S.; Leppänen, V.-M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef] [Green Version]
- Stanek, A.; Fazeli, B.; Bartuś, S.; Sutkowska, E. The role of endothelium in physiological and pathological states-new data. BioMed Res. Int. 2018, 2018, 1098039. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, T.S.; Serra, A.J.; Manchini, M.T.; Bassaneze, V.; Krieger, J.E.; de Tarso Camillo de Carvalho, P.; Espindola Antunes, D.; Sales Bocalini, D.; Ferreira Tucci, P.J.; Silva, J.A., Jr. Effects of low level laser therapy on attachment, proliferation, and gene expression of VEGF and VEGF receptor 2 of adipocyte-derived mesenchymal stem cells cultivated under nutritional deficiency. Lasers Med. Sci. 2015, 30, 217–223. [Google Scholar] [CrossRef]
- Silveira, P.C.L.; Scheffer, D.D.L.; Glaser, V.; Remor, A.P.; Pinho, R.A.; Junior, A.S.A.; Latini, A. Low-level laser therapy attenuates the acute inflammatory response induced by muscle traumatic injury. Free. Radic. Res. 2016, 50, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Szymczyszyn, A.; Doroszko, A.; Szahidewicz-Krupska, E.; Rola, P.; Gutherc, R.; Jasiczek, J.; Mazur, G.; Derkacz, A. Effect of the transdermal low-level laser therapy on endothelial function. Lasers Med. Sci. 2016, 31, 1301–1307. [Google Scholar] [CrossRef]
- Ribatti, D.; Solimando, A.; Pezzella, F. The Anti-VEGF(R) Drug Discovery Legacy: Improving Attrition Rates by Breaking the Vicious Cycle of Angiogenesis in Cancer. Cancers 2021, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Umitsu, M.; de Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Argüelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Imamura, R.; Matsumoto, K. Hepatocyte growth factor in physiology and infectious diseases. Cytokine 2017, 98, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-S.; Wang, H.; Zhang, Q.-L.; Yang, Z.-J.; Kong, F.; Wu, C.-T. Hepatocyte Growth Factor Gene Therapy for Ischemic Diseases. Hum. Gene Ther. 2018, 29, 413–423. [Google Scholar] [CrossRef]
- Park, I.-S.; Chung, P.-S.; Ahn, J.C. Enhancement of ischemic wound healing by spheroid grafting of human adipose-derived stem cells treated with low-level light irradiation. PLoS ONE 2015, 10, e0122776. [Google Scholar] [CrossRef]
- Araújo, T.G.; de Oliveira, A.G.; Tobar, N.; Saad, M.J.A.; Moreira, L.R.; Reis, E.R.; Nicola, E.M.D.; de Jorge, G.L.; dos Tártaro, R.R.; Boin, I.F.S.F.; et al. Liver regeneration following partial hepatectomy is improved by enhancing the HGF/Met axis and Akt and Erk pathways after low-power laser irradiation in rats. Lasers Med. Sci. 2013, 28, 1511–1517. [Google Scholar] [CrossRef]
- Miyanaga, T.; Ueda, Y.; Miyanaga, A.; Yagishita, M.; Hama, N. Angiogenesis after administration of basic fibroblast growth factor induces proliferation and differentiation of mesenchymal stem cells in elastic perichondrium in an in vivo model: Mini review of three sequential republication-abridged reports. Cell. Mol. Biol. Lett. 2018, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kang, X.; Jin, L.; Bai, J.; Liu, W.; Wang, Z. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int. J. Nanomed. 2018, 13, 3897–3906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef]
- Chen, M.; Bao, L.; Zhao, M.; Cao, J.; Zheng, H. Progress in Research on the Role of FGF in the Formation and Treatment of Corneal Neovascularization. Front. Pharmacol. 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Akishima-Fukasawa, Y.; Honma, N.; Ogata, H.; Akasaka, Y.; Mikami, T. Angiogenesis in mammary Paget disease: Histopathological analyses of blood vessel density and angiogenic factors. Diagn. Pathol. 2020, 15, 75. [Google Scholar] [CrossRef]
- Yeung, C.H.C.; Schooling, C.M. Systemic inflammatory regulators and risk of Alzheimer’s disease: A bidirectional Mendelian-randomization study. Int. J. Epidemiol. 2021, 50, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, J.; Zhang, K.; Liu, J.; Li, Y.; Su, W.; Song, N. Advances of Fibroblast Growth Factor/Receptor Signaling Pathway in Hepatocellular Carcinoma and its Pharmacotherapeutic Targets. Front. Pharmacol. 2021, 12, 650388. [Google Scholar] [CrossRef]
- Li, R.; Li, D.-H.; Zhang, H.-Y.; Wang, J.; Li, X.-K.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef]
- Panés, J.; Rimola, J. Perianal fistulizing Crohn’s disease: Pathogenesis, diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 652–664. [Google Scholar] [CrossRef]
- Taradaj, J.; Shay, B.; Dymarek, R.; Sopel, M.; Walewicz, K.; Beeckman, D.; Schoonhoven, L.; Gefen, A.; Rosińczuk, J. Effect of laser therapy on expression of angio-and fibrogenic factors, and cytokine concentrations during the healing process of human pressure ulcers. Int. J. Med. Sci. 2018, 15, 1105. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Sun, Z.; Zhang, L.; Li, X.; Dong, Y.; Liu, T.C.-Y. Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-β1 in skeletal muscle during the repair process. Lasers Med. Sci. 2013, 28, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tozum, T. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed. Laser Surg. 2012, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Mayahara, K.; Kiyosaki, T.; Yamaguchi, A.; Ozawa, Y.; Abiko, Y. Low-intensity laser irradiation stimulates bone nodule formation via insulin-like growth factor-I expression in rat calvarial cells. Lasers Surg. Med. 2007, 39, 551–559. [Google Scholar] [CrossRef]
- Gaptulbarova, K.A.; Tsyganov, M.M.; Pevzner, A.M.; Ibragimova, M.K.; Litviakov, N.V. NF-kB as a potential prognostic marker and a candidate for targeted therapy of cancer. Exp. Oncol 2020, 42, 263–269. [Google Scholar] [CrossRef]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Muscle Atrophy 2018, 1088, 267–279. [Google Scholar] [CrossRef]
- Rizzi, C.F.; Mauriz, J.L.; Freitas Correa, D.S.; Moreira, A.J.; Zettler, C.G.; Filippin, L.I.; Marroni, N.P.; Gonzalez-Gallego, J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-κB signaling pathway in traumatized muscle. Lasers Surg. Med. 2006, 38, 704–713. [Google Scholar] [CrossRef]
- Tamura, A.; Matsunobu, T.; Tamura, R.; Kawauchi, S.; Sato, S.; Shiotani, A. Photobiomodulation rescues the cochlea from noise-induced hearing loss via upregulating nuclear factor κB expression in rats. Brain Res. 2016, 1646, 467–474. [Google Scholar] [CrossRef]
- Yin, K.; Zhu, R.; Wang, S.; Zhao, R.C. Low level laser (LLL) attenuate LPS-induced inflammatory responses in mesenchymal stem cells via the suppression of NF-κB signaling pathway in vitro. PLoS ONE 2017, 12, e0179175. [Google Scholar] [CrossRef]
- Gupta, A.; Keshri, G.K.; Yadav, A.; Gola, S.; Chauhan, S.; Salhan, A.K.; Singh, S.B. Superpulsed (Ga-As, 904 nm) low-level laser therapy (LLLT) attenuates inflammatory response and enhances healing of burn wounds. J. Biophotonics 2015, 8, 489–501. [Google Scholar] [CrossRef]
- Cury, V.; Moretti, A.I.S.; Assis, L.; Bossini, P.; Crusca, J.D.S.; Neto, C.B.; Fangel, R.; de Souza, H.P.; Hamblin, M.; Parizotto, N.A. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1α and MMP-2. J. Photochem. Photobiol. B Biol. 2013, 125, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, Q.; Shi, M.; Gan, P.; Huang, Q.; Wang, A.; Tan, G.; Fang, Y.; Liao, H. Low-level laser therapy induces human umbilical vascular endothelial cell proliferation, migration and tube formation through activating the PI3K/Akt signaling pathway. Microvasc. Res. 2020, 129, 103959. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.-H.; Moon, J.-H.; Choi, S.-H.; Ahn, J.-C. Low-level laser therapy promoted aggressive proliferation and angiogenesis through decreasing of transforming growth factor-β1 and Increasing of Akt/hypoxia inducible factor-1α in anaplastic thyroid cancer. Photomed. Laser Surg. 2016, 34, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zou, Z.; Li, J.; Shen, Q.; Liu, L.; An, X.; Yang, S.; Xing, D. Photoactivation of mitochondrial reactive oxygen species-mediated Src and protein kinase C pathway enhances MHC class II-restricted T cell immunity to tumours. Cancer Lett. 2021, 523, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Autophagy and apoptosis cascade: Which is more prominent in neuronal death? Cell. Mol. Life Sci. 2021, 78, 8001–8047. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, T.; Xing, D.; Wang, F.; Pei, Y.; Wei, X. Single cell analysis of PKC activation during proliferation and apoptosis induced by laser irradiation. J. Cell. Physiol. 2006, 206, 441–448. [Google Scholar] [CrossRef]
- Wang, F.; Chen, T.-S.; Xing, D.; Wang, J.-J.; Wu, Y.-X. Measuring dynamics of caspase-3 activity in living cells using FRET technique during apoptosis induced by high fluence low-power laser irradiation. Lasers Surg. Med. 2005, 36, 2–7. [Google Scholar] [CrossRef]
- Izadi, M.; Ali, T.A.; Pourkarimi, E. Over Fifty Years of Life, Death, and Cannibalism: A Historical Recollection of Apoptosis and Autophagy. Int. J. Mol. Sci. 2021, 22, 12466. [Google Scholar] [CrossRef]
- Martines-Arano, H.; Palacios-Barreto, S.; Castillo-Cruz, J.; Meda-Campaña, J.A.; García-Pérez, B.E.; Torres-Torres, C. Fractional photodamage triggered by chaotic attractors in human lung epithelial cancer cells. Int. J. Therm. Sci. 2022, 181, 107734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).