Design and Optimization of Surface Plasmon Resonance Spectroscopy for Optical Constant Characterization and Potential Sensing Application: Theoretical and Experimental Approaches
Abstract
:1. Introduction
2. Theory
3. Materials and Methods
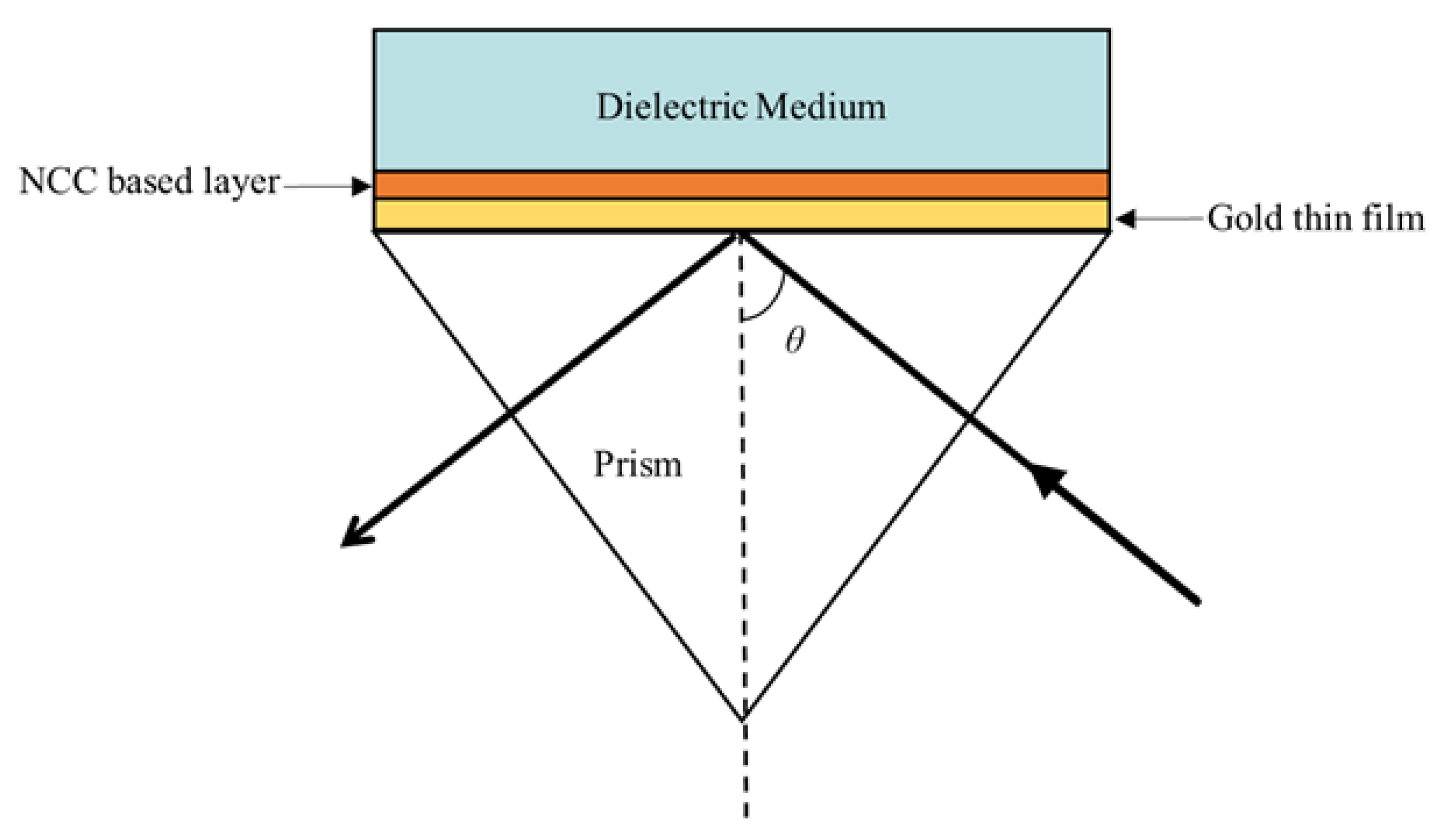
3.1. Surface Plasmon Resonance (SPR) Optimization
3.2. Preparation of Thin Film
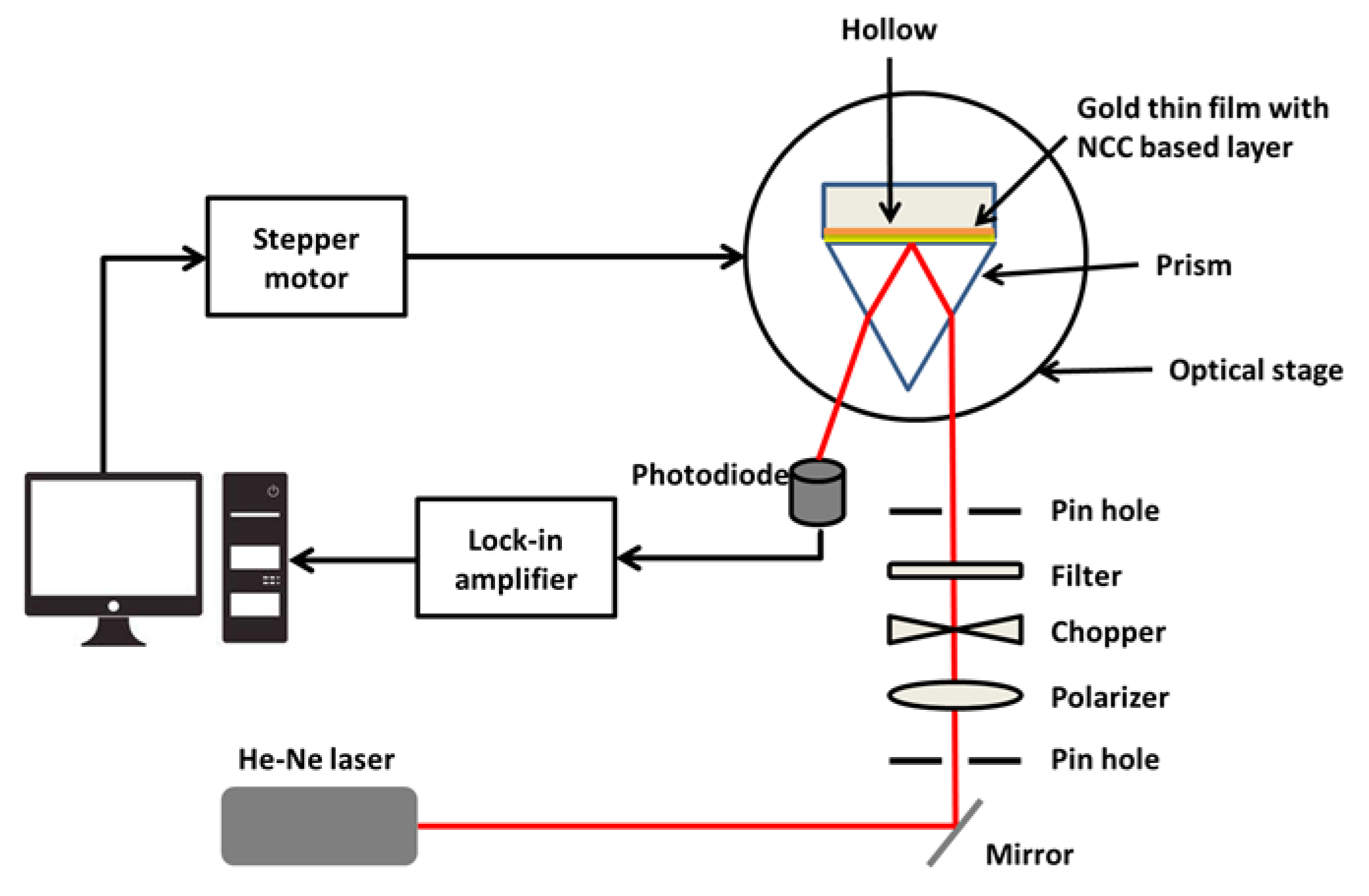
3.3. Surface Plasmon Resonance
3.4. Fitting Experimental to Theoretical
4. Results and Discussion
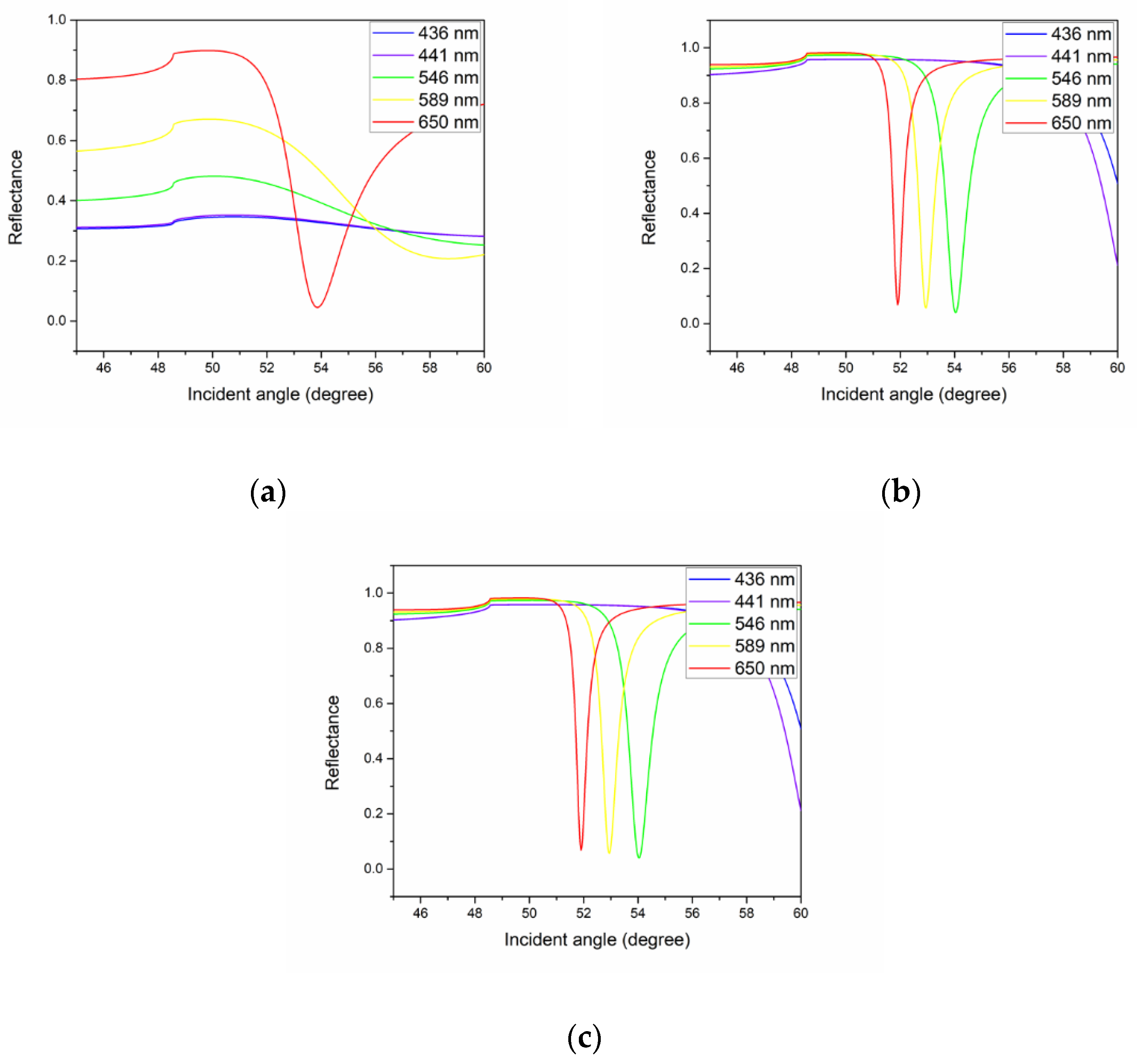
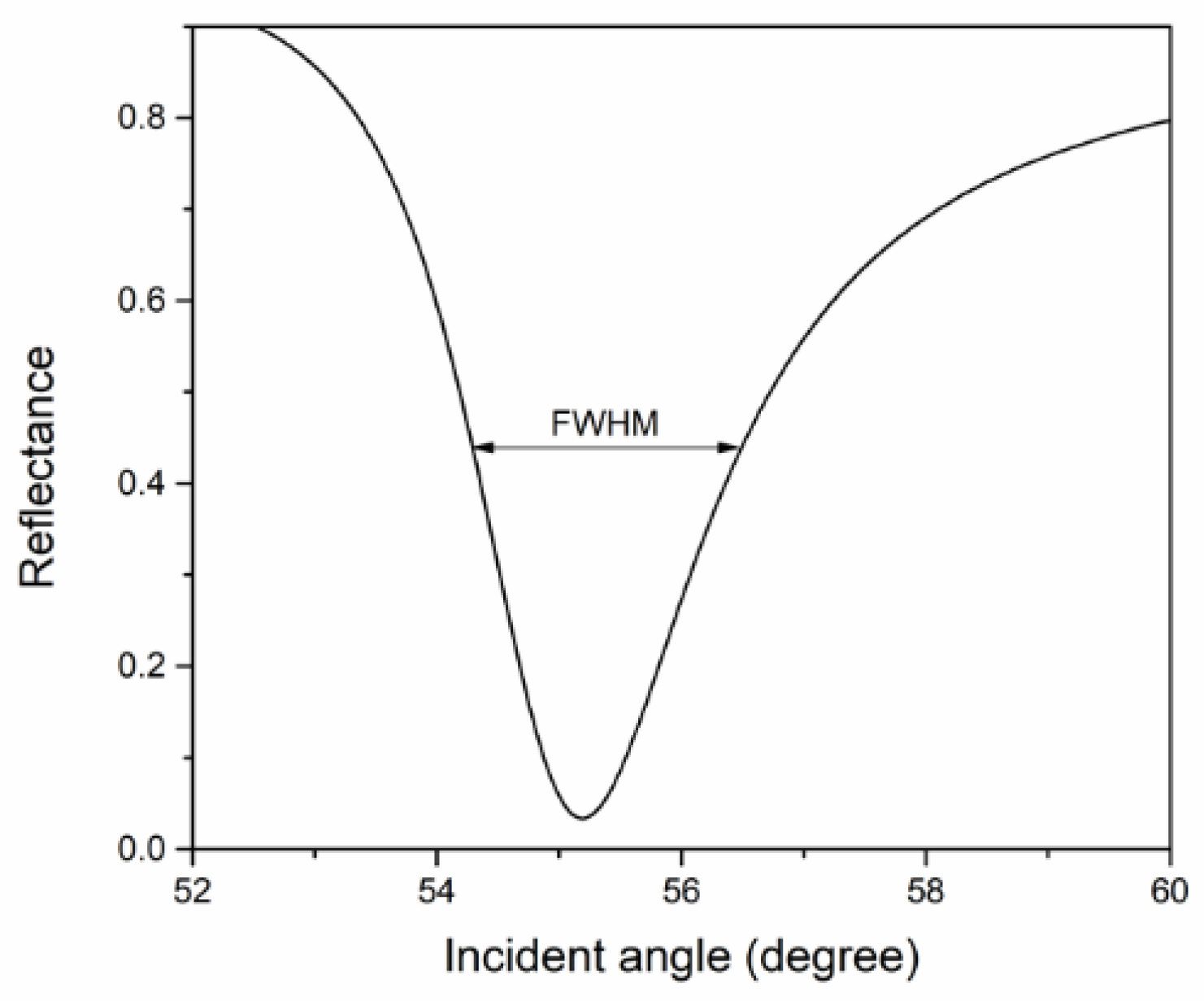
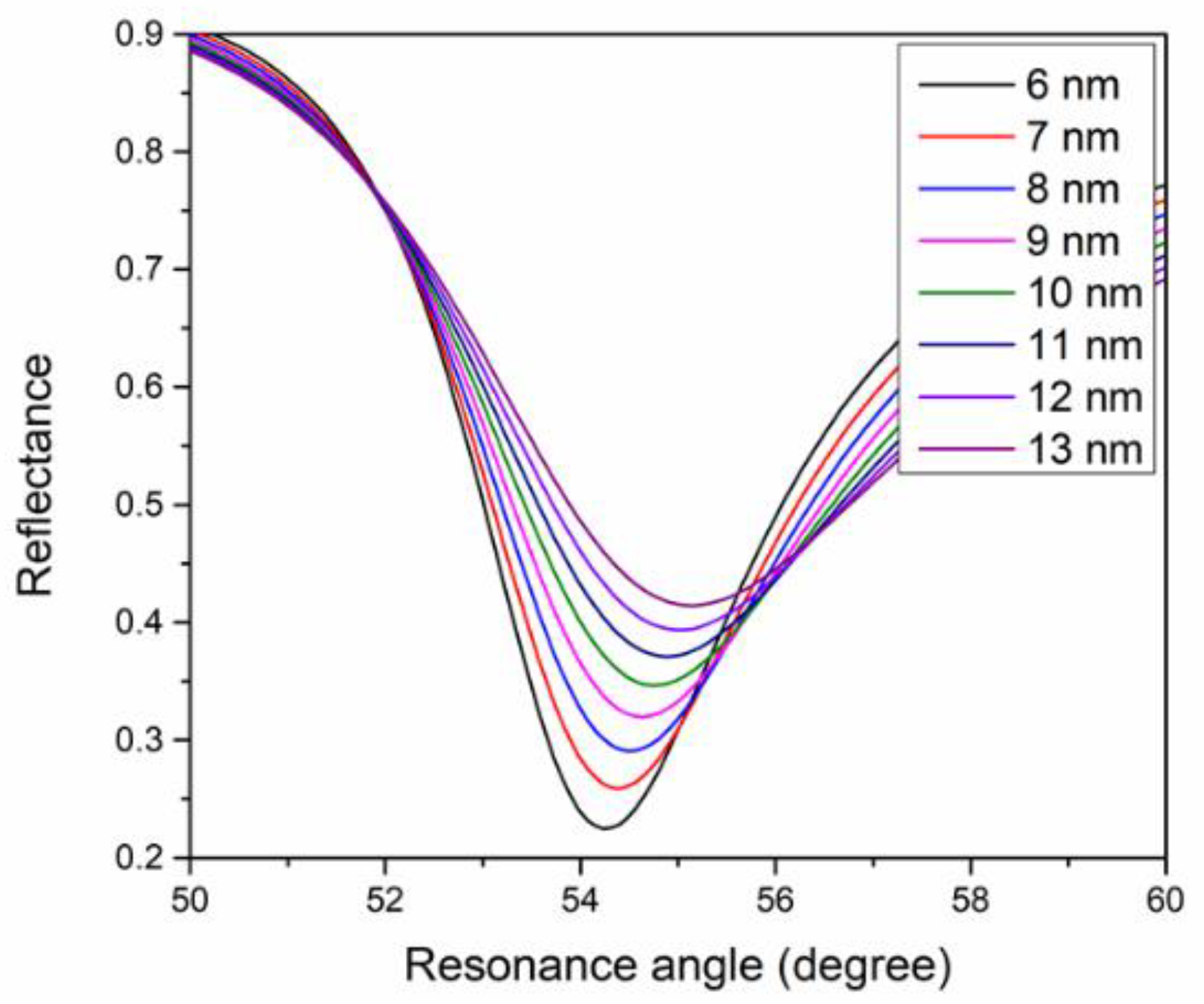
4.1. Simulation of SPR Signal
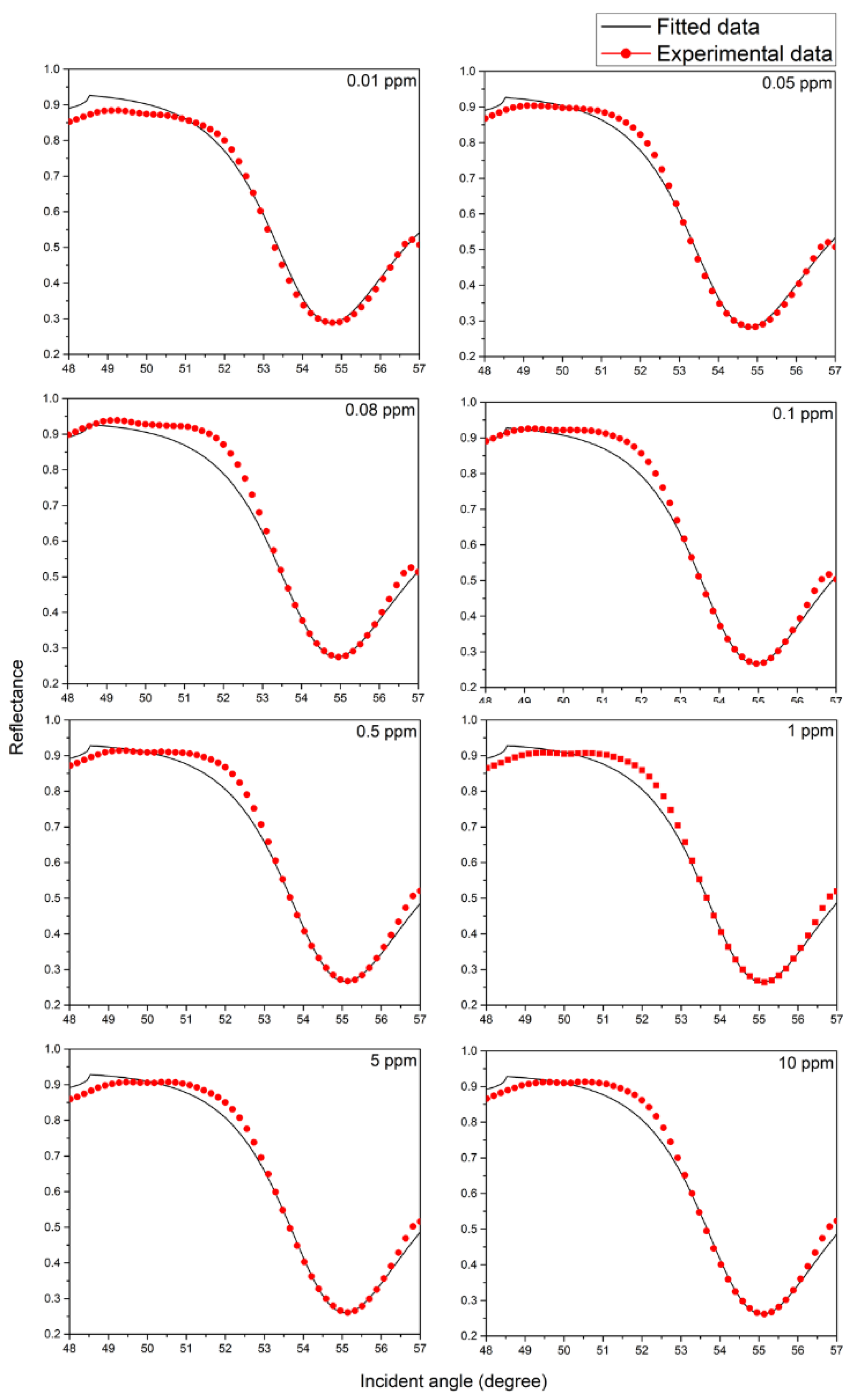
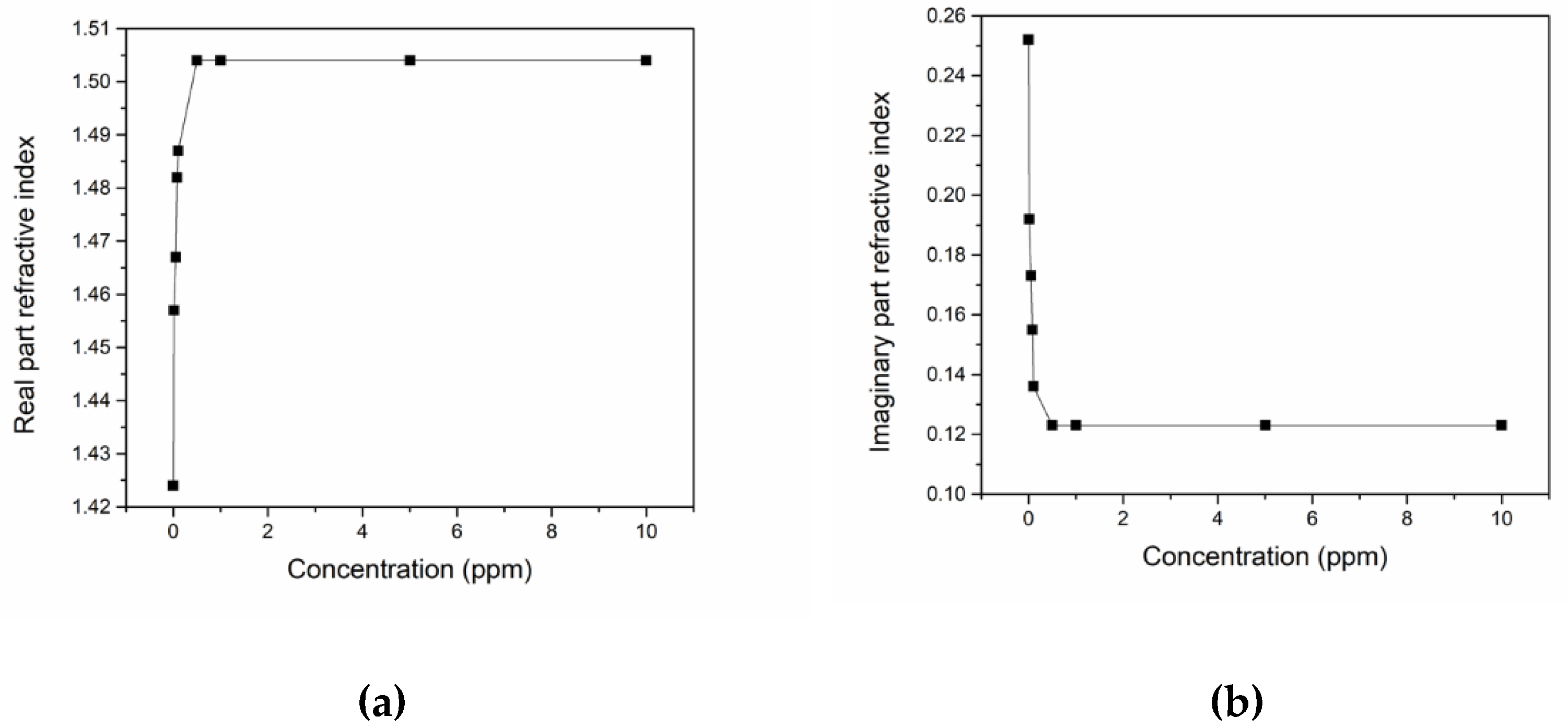
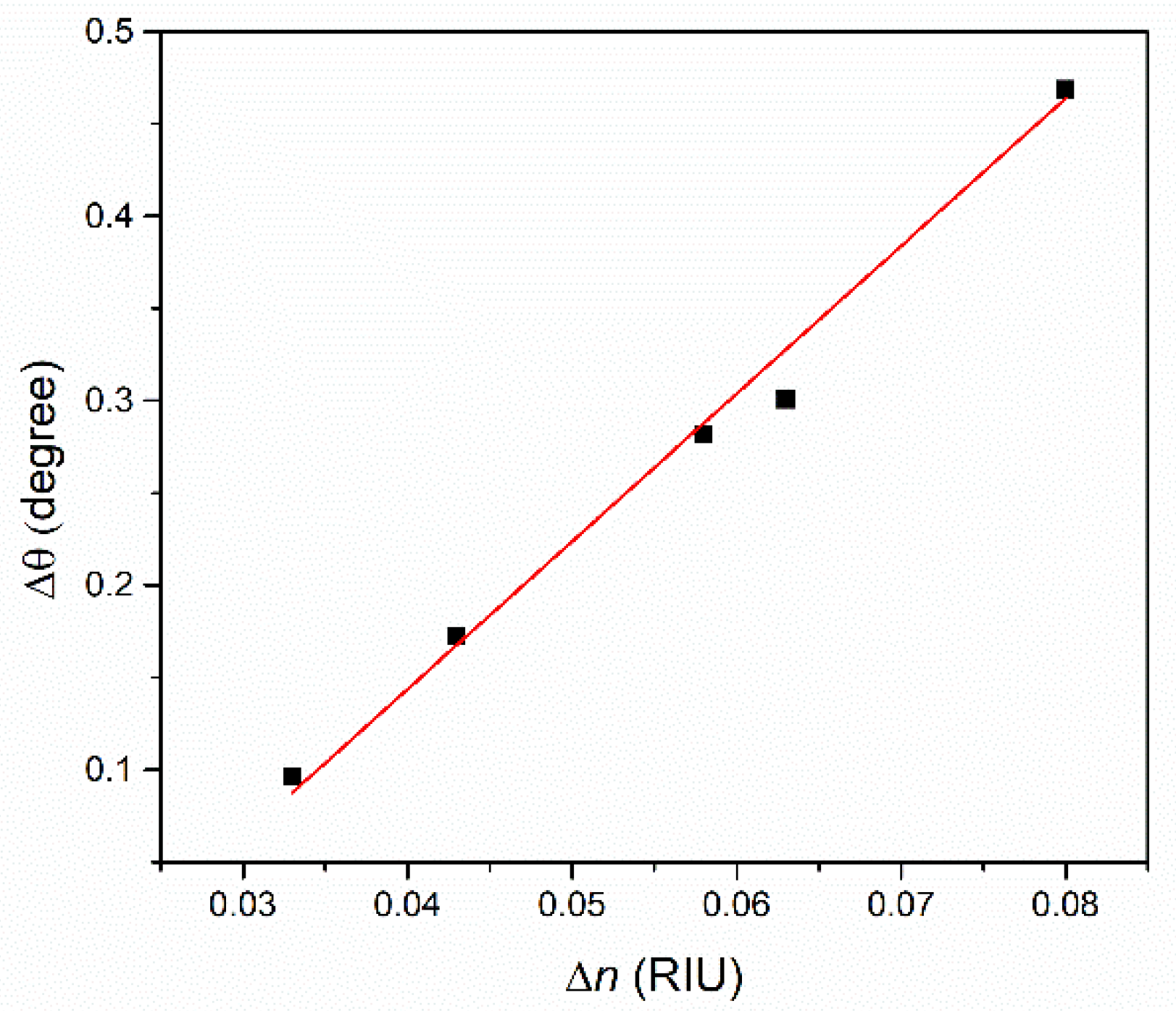
4.2. Analysis of Thin Films Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kravets, V.G.; Kabashin, A.V.; Barnes, W.L.; Grigorenko, A.N. Plasmonic surface lattice resonances: A review of properties and applications. Chem. Rev. 2018, 118, 5912–5951. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, W.M.; Susthitha Menon, P.; Shaari, S.; Malek, M.Z.A.; Abdullah, A.M. Angle shifting in surface plasmon resonance: Experimental and theoretical verification. J. Phys. Conf. Ser. 2013, 431, 012028. [Google Scholar] [CrossRef]
- Usman, F.; Ojur, J.; Yousif, A.; Cheng, K. Structural characterization and optical constants of p-toluene sulfonic acid doped polyaniline and its composites of chitosan and reduced graphene-oxide. Integr. Med. Res. 2019, 9, 1468–1476. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a graphene-based surface plasmon resonance optical sensor chip for potential biomedical application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerislioglu, B.; Dong, L.; Ahmadivand, A.; Hu, H.; Nordlander, P.; Halas, N.J. Monolithic metal dimer-on-film structure: New plasmonic properties introduced by the underlying metal. Nano Lett. 2020, 20, 2087–2093. [Google Scholar] [CrossRef]
- Mansouri, M.; Fathi, F.; Jalili, R.; Shoeibie, S.; Dastmalchi, S.; Khataee, A.; Rashidi, M.R. SPR enhanced DNA biosensor for sensitive detection of donkey meat adulteration. Food Chem. 2020, 331, 127163–127172. [Google Scholar] [CrossRef]
- Firdous, S.; Anwar, S.; Rafya, R. Development of surface plasmon resonance (SPR) biosensors for use in the diagnostics of malignant and infectious diseases. Laser Phys. Lett. 2018, 15, 18–23. [Google Scholar] [CrossRef]
- Rosddi, N.N.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Cationically modified nanocrystalline cellulose/carboxyl-functionalized graphene quantum dots nanocomposite thin film: Characterization and potential sensing application. Crystals 2020, 10, 875. [Google Scholar] [CrossRef]
- Kamaruddin, N.H.; Bakar, A.A.A.; Yaacob, M.H.; Mahdi, M.A.; Zan, M.S.D.; Shaari, S. Enhancement of chitosan-graphene oxide SPR sensor with a multi-metallic layers of Au-Ag-Au nanostructure for lead(II) ion detection. Appl. Surf. Sci. 2016, 361, 177–184. [Google Scholar] [CrossRef]
- Moon, J.; Kang, T.; Oh, S.; Hong, S.; Yi, J. In situ sensing of metal ion adsorption to a thiolated surface using surface plasmon resonance spectroscopy. J. Colloid Interface Sci. 2006, 298, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Eddin, F.B.K.; Fen, Y.W.; Omar, N.A.S.; Liew, J.Y.C.; Daniyal, W.M.E.M.M. Femtomolar detection of dopamine using surface plasmon resonance sensor based on chitosan/graphene quantum dots thin film. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 263, 120202. [Google Scholar] [CrossRef]
- Ock, K.; Jang, G.; Roh, Y.; Kim, S.; Kim, J.; Koh, K. Optical detection of Cu2+ ion using a SQ-dye containing polymeric thin-film on Au surface. Microchem. J. 2001, 70, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, T.; Abdalla, S.; Negm, S.; Talaat, H. Surface plasmons resonance technique for the detection of nicotine in cigarette smoke. Sens. Actuators A Phys. 2003, 102, 234–239. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Biosens. Bioelectron. 2020, 167, 112511–112523. [Google Scholar] [CrossRef] [PubMed]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciminelli, C.; Campanella, C.M.; Olio, F.D.; Campanella, C.E.; Armenise, M.N. Label-free optical resonant sensors for biochemical applications. Prog. Quantum Electron. 2013, 37, 51–107. [Google Scholar] [CrossRef]
- Sun, R.J.; Huang, H.J.; Hsiao, C.N.; Lin, Y.W.; Liao, B.H.; Chou Chau, Y.F.; Chiang, H.P. Reusable TiN substrate for surface plasmon resonance heterodyne phase interrogation sensor. Nanomaterials 2020, 10, 1325. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Du, Y.; Chen, C.; Li, B.; Zhou, M.; Dong, S. Au NPs-enhanced surface plasmon resonance for sensitive detection of mercury(II) ions. Biosens. Bioelectron. 2010, 25, 2622–2626. [Google Scholar] [CrossRef]
- Lee, K.S.; Son, J.M.; Jeong, D.Y.; Lee, T.S.; Kim, W.M. Resolution enhancement in surface plasmon resonance sensor based on waveguide coupled mode by combining a bimetallic approach. Sensors 2010, 10, 11390–11399. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Lin, S.; Wei, S.C.; Chen, C.Y.; Lin, C.W. An amplified surface plasmon resonance “turn-on” sensor for mercury ion using gold nanoparticles. Biosens. Bioelectron. 2011, 30, 235–240. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Thomas, P.A.; Kravets, V.G.; Arola, H.O.; Soikkeli, M.; Iljin, K.; Kim, G.; Kim, M.; Shin, H.S.; Andreeva, D.V.; et al. Layered material platform for surface plasmon resonance biosensing. Sci. Rep. 2019, 9, 20286. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Chen, Y.P.; Wang, W.; Shen, Y.; Guo, J.S. Surface plasmon resonance for water pollutant detection and water process analysis. Trends Anal. Chem. 2016, 85, 153–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Wang, Y.; Toledo, F.; Zhou, F. Studies of metal ion binding by apo-metallothioneins attached onto preformed self-assembled monolayers using a highly sensitive surface plasmon resonance spectrometer. Sens. Actuators B Chem. 2007, 123, 784–792. [Google Scholar] [CrossRef] [Green Version]
- Shaban, M.; Hady, A.G.A.; Serry, M. A new sensor for heavy metals detection in aqueous media. IEEE Sens. J. 2014, 14, 436–441. [Google Scholar] [CrossRef]
- Zijlstra, P.; Paulo, P.M.R.; Yu, K.; Xu, Q.H.; Orrit, M. Chemical interface damping in single gold nanorods and its near elimination by tip-specific functionalization. Angew. Chemie Int. Ed. 2012, 51, 8352–8355. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Han, S.-K.; Jang, G.-S.; Koh, K.-N.; Kang, S.-W.; Keum, S.-R.; Yoon, C.-M. Surface plasmon resonance study on the interaction of a dithiosquarylium dye with metal ions. Dyes Pigm. 2000, 44, 169–173. [Google Scholar] [CrossRef]
- Panta, Y.M.; Liu, J.; Cheney, M.A.; Joo, S.W.; Qian, S. Ultrasensitive detection of mercury (II) ions using electrochemical surface plasmon resonance with magnetohydrodynamic convection. J. Colloid Interface Sci. 2009, 333, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Saleviter, S.; Omar, N.A.S. Preparation and characterization of hexadecyltrimethylammonium bromide modified nanocrystalline cellulose/graphene oxide composite thin film and its potential in sensing copper ion using surface plasmon resonance technique. Optik 2018, 173, 71–77. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Zhao, Y.; Yan, X.; Cao, P. Poisoning Effect of SO2 on honeycomb cordierite-based Mn–Ce/Al2O3 catalysts for NO reduction with NH3 at low temperature. Appl. Sci. 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A. Analysis of Pb(II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy. Optik 2013, 124, 126–133. [Google Scholar] [CrossRef]
- Hong, S.; Kang, T.; Moon, J.; Oh, S.; Yi, J. Surface plasmon resonance analysis of aqueous copper ions with amino-terminated self-assembled monolayers. Colloid Surf. A Physicochem. Eng. Asp. 2007, 292, 264–270. [Google Scholar] [CrossRef]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt. Commun. 2016, 367, 102–107. [Google Scholar]
- Eddin, F.B.K.; Fen, Y.W. The principle of nanomaterials based surface plasmon resonance biosensors and its potential for dopamine detection. Molecules 2020, 25, 2769. [Google Scholar] [CrossRef] [PubMed]
- Chah, S.; Yi, J.; Zare, R.N. Surface plasmon resonance analysis of aqueous mercuric ions. Sensors Actuators B Chem. 2004, 99, 216–222. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Lai, E.P.C.; Sadeghi, S. Surface plasmon resonance sensor for Hg(II) detection by binding interactions with polypyrrole and 2-mercaptobenzothiazole. Sens. Actuators B Chem. 2004, 101, 236–241. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, S.W.; Kim, D.U.; Cui, J.Z.; Kim, S.H. Effect of metal ions on the absorption spectra and surface plasmon resonance of an azacrown indoaniline dye. Dyes Pigm. 2001, 49, 109–115. [Google Scholar] [CrossRef]
- Hur, Y.; Ock, K.; Kim, K.; Jin, S.; Gal, Y.; Kim, J.; Kim, S.; Koh, K. Surface plasmon resonance study on enhanced refractive index change of an Ag+ ion-sensing membrane containing dithiosquarylium dye. Anal. Chim. Acta 2002, 460, 133–139. [Google Scholar] [CrossRef]
- Forzani, E.S.; Zhang, H.; Chen, W.; Tao, N. Detection of heavy metal ions in drinking water using a high-resolution differential surface plasmon resonance sensor. Environ. Sci. Technol. 2005, 39, 1257–1262. [Google Scholar] [CrossRef]
- Chau, Y.C.; Wang, C.; Shen, L.; Lim, C.M.; Chao, C.C.; Huang, H.J.; Lin, C.; Kumara, N.T.R.N. Simultaneous realization of high sensing sensitivity and tunability in plasmonic nanostructures arrays. Sci. Rep. 2017, 7, 16817. [Google Scholar] [CrossRef] [Green Version]
- Pelossof, G.; Tel-Vered, R.; Willner, I. Amplified surface plasmon resonance and electrochemical detection of Pb2+ ions using the Pb2+-dependent DNAzyme and hemin/G-quadruplex as a label. Anal. Chem. 2012, 84, 3703–3709. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Chung, B.H.; Lee, H.J. Parts per trillion detection of Ni(II) ions by nanoparticle-enhanced surface plasmon resonance. Anal. Chem. 2012, 84, 10091–10096. [Google Scholar] [CrossRef]
- May, L.M.; Russell, D.A. Novel determination of cadmium ions using an enzyme self-assembled monolayer with surface plasmon resonance. Anal. Chim. Acta 2003, 500, 119–125. [Google Scholar] [CrossRef]
- Lin, T.J.; Chung, M.F. Detection of cadmium by a fiber-optic biosensor based on localized surface plasmon resonance. Biosens. Bioelectron. 2009, 24, 1213–1218. [Google Scholar] [CrossRef]
- Wang, S.; Forzani, E.S.; Tao, N. Detection of heavy metal ions in water by high-resolution surface plasmon resonance spectroscopy combined with anodic stripping voltammetry. Anal. Chem. 2007, 79, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Gupta, B.D. Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chem. 2015, 166, 568–575. [Google Scholar] [CrossRef]
- Eum, N.-S.; Lee, S.-H.; Lee, D.-R.; Kwon, D.-K.; Shin, J.-K.; Kim, J.-H.; Kang, S.-W. K+-ion sensing using surface plasmon resonance by NIR light source. Sens. Actuators B Chem. 2003, 96, 446–450. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Fen, Y.W.; Alwahib, A.A.; Yaacob, M.H.; Bidin, N.; Omar, N.A.S.; Mahdi, M.A. Detection of adulterated honey by surface plasmon resonance optical sensor. Optik 2018, 168, 134–139. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Moksin, M.M.; Talib, Z.A.; Yusof, N.A. Surface plasmon resonance optical sensor for mercury ion detection by crosslinked chitosan thin film. J. Optoelectron. Adv. Mater. 2011, 13, 279–285. [Google Scholar]
- Kumar, P.; Kim, K.-H.; Bansal, V.; Lazarides, T.; Kumar, N. Progress in the sensing techniques for heavy metal ions using nanomaterials. J. Ind. Eng. Chem. 2017, 54, 30–43. [Google Scholar] [CrossRef]
- Dhayal, M.; Ratner, D.M. XPS and SPR analysis of glycoarray surface density. Langmuir 2009, 25, 2181–2187. [Google Scholar] [CrossRef] [Green Version]
- Martinis, E.M.; Wuilloud, R.G. Enhanced spectrophotometric detection of Hg in water samples by surface plasmon resonance of Au nanoparticles after preconcentration with vortex-assisted liquid-liquid microextraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 167, 111–115. [Google Scholar] [CrossRef]
- Wu, C.M.; Lin, L.Y. Utilization of albumin-based sensor chips for the detection of metal content and characterization of metal-protein interaction by surface plasmon resonance. Sens. Actuators B Chem. 2005, 110, 231–238. [Google Scholar] [CrossRef]
- Yao, F.; He, J.; Li, X.; Zou, H.; Yuan, Z. Studies of interaction of copper and zinc ions with Alzheimer’s Aβ(1-16) using surface plasmon resonance spectrometer. Sens. Actuators B Chem. 2012, 161, 886–891. [Google Scholar] [CrossRef]
- Fahnestock, K.J.; Manesse, M.; McIlwee, H.A.; Schauer, C.L.; Boukherroub, R.; Szunerits, S. Selective detection of hexachromium ions by localized surface plasmon resonance measurements using gold nanoparticles/chitosan composite interfaces. Analyst 2009, 134, 881–886. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Zulholinda, M. Structural and optical properties of chitosan–poly(amidoamine) dendrimer composite thin film for potential sensing Pb2+ using an optical spectroscopy. Optik 2019, 185, 351–358. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Galatus, R.; Conte, L.; Zeni, L. Performance Comparison of Two Sensors Based on Surface Plasmon Resonance in a Plastic Optical Fiber. Sensors 2013, 13, 721–735. [Google Scholar] [CrossRef]
- Qiu, G.Y.; Law, A.H.L.; Ng, S.P.; Wu, C.M.L. Label-free detection of lead(II) ion using differential phase modulated localized surface plasmon resonance sensors. Procedia Eng. 2016, 168, 533–536. [Google Scholar] [CrossRef]
- Castillo, J.; Chirinos, J.; Gutiérrez, H.; La Cruz, M. Surface plasmon resonance sensor based on golden nanoparticles and cold vapour generation technique for the detection of mercury in aqueous samples. Opt. Laser Technol. 2017, 94, 34–39. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Hashim, H.S.; Fauzi, N.I.M.; Chanlek, N.; Mahdi, M.A. X-ray photoelectron study on gold/nanocrystalline cellulose-graphene oxide thin film as surface plasmon resonance active layer for metal ion detection. Thin Solid Films 2020, 713, 138340–138350. [Google Scholar] [CrossRef]
- Amjadi, M.; Shokri, R.; Hallaj, T. A new turn-off fluorescence probe based on graphene quantum dots for detection of Au(III) ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 619–624. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Abdullah, J.; Mahdi, M.A. Experimental evaluation on surface plasmon resonance sensor performance based on sensitive hyperbranched polymer nanocomposite thin films. Sensors Actuators A Phys. 2020, 303, 111830–111840. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Day, B.W. Theory and applications of surface plasmon resonance, resonant mirror, resonant waveguide grating, and dual polarization interferometry biosensors. Sensors 2010, 10, 9630–9646. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Q.; Hu, X. SPR phase detection for measuring the thickness of thin metal films. Opt. Express 2014, 22, 7574–7580. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Utilization of chitosan-based sensor thin films for the detection of lead ion by surface plasmon resonance optical sensor. IEEE Sens. J. 2013, 13, 1413–1418. [Google Scholar] [CrossRef]
- Shushama, K.N.; Rana, M.M.; Inum, R.; Hossain, M.B. Sensitivity enhancement of graphene coated surface plasmon resonance biosensor. Opt. Quantum Electron. 2017, 49, 381. [Google Scholar] [CrossRef]
- Yue, C.; Qin, Z.; Lang, Y.; Liu, Q. Determination of thin metal film’s thickness and optical constants based on SPR phase detection by simulated annealing particle swarm optimization. Opt. Commun. 2019, 430, 238–245. [Google Scholar] [CrossRef]
- Hasib, M.H.H.; Nur, J.N.; Rizal, C.; Shushama, K.N. Improved transition metal dichalcogenides-based surface plasmon resonance biosensors. Condens. Matter 2019, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Abitbol, T.; Marway, H.; Cranston, E.D. Surface modification of cellulose nanocrystals with cetyltrimethylammonium bromide. Nord. Pulp Pap. Res. J. 2014, 29, 46–57. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 48–52. [Google Scholar] [CrossRef]
- Babar, S.; Weaver, J.H. Optical constants of Cu, Ag, and Au revisited. Appl. Opt. 2015, 54, 477–481. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical constant of the nobel metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Based Sensors; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4, pp. 3–44. [Google Scholar]
- Kedenburg, S.; Vieweg, M.; Gissibl, T.; Giessen, H. Linear refractive index and absorption measurements of nonlinear optical liquids in the visible and near-infrared spectral region. Opt. Mat. Express 2012, 2, 1588–1611. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Seong, K.C.; Ahmed, A.Y.; Ferrell, T.L.; Fen, Y.W.; Sadrolhosseini, A.R.; Ayodele, O.B.; Meriaudeau, F.; Saidu, A. Enhanced sensitivity of surface plasmon resonance biosensor functionalized with doped polyaniline composites for the detection of low-concentration acetone vapour. J. Sensors 2019, 2019, 5786105. [Google Scholar] [CrossRef] [Green Version]
- Zynio, S.A.; Samoylov, A.V.; Surovtseva, E.R.; Mirsky, V.M.; Shirshov, Y.M. Bimetallic layers increase sensitivity of affinity sensors based on surface plasmon resonance. Sensors 2002, 2, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Landry, V.; Alemdar, A.; Blanchet, P. Nanocrystalline cellulose: Morphological, physical, and mechanical properties. For. Prod. J. 2011, 61, 104–112. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M. Highly sensitive surface plasmon resonance optical detection of ferric ion using CTAB/hydroxylated graphene quantum dots thin film. J. Appl. Phys. 2020, 128, 083105. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active layer. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef]
- Fouad, S.; Sabri, N.; Jamal, Z.A.Z.; Poopalan, P. Surface plasmon resonance sensor sensitivity enhancement using gold-dielectric material. Int. J. Nanoelectron. Mater. 2017, 10, 147–156. [Google Scholar]
- Mudgal, N.; Saharia, A.; Agarwal, A.; Singh, G. ZnO and Bi-metallic (Ag–Au) layers based surface plasmon resonance (SPR) biosensor with BaTiO3 and graphene for biosensing applications. IETE J. Res. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Agarwal, S.; Giri, P.; Prajapati, Y.K.; Chakrabarti, P. Effect of surface roughness on the performance of optical spr sensor for sucrose detection: Fabrication, characterization, and simulation study. IEEE Sens. J. 2016, 16, 8865–8873. [Google Scholar] [CrossRef]
- Hoseinian, M.S.; Bolorizadeh, M.A. Design and simulation of a highly sensitive SPR optical fiber sensor. Photonic Sens. 2019, 9, 33–42. [Google Scholar] [CrossRef] [Green Version]


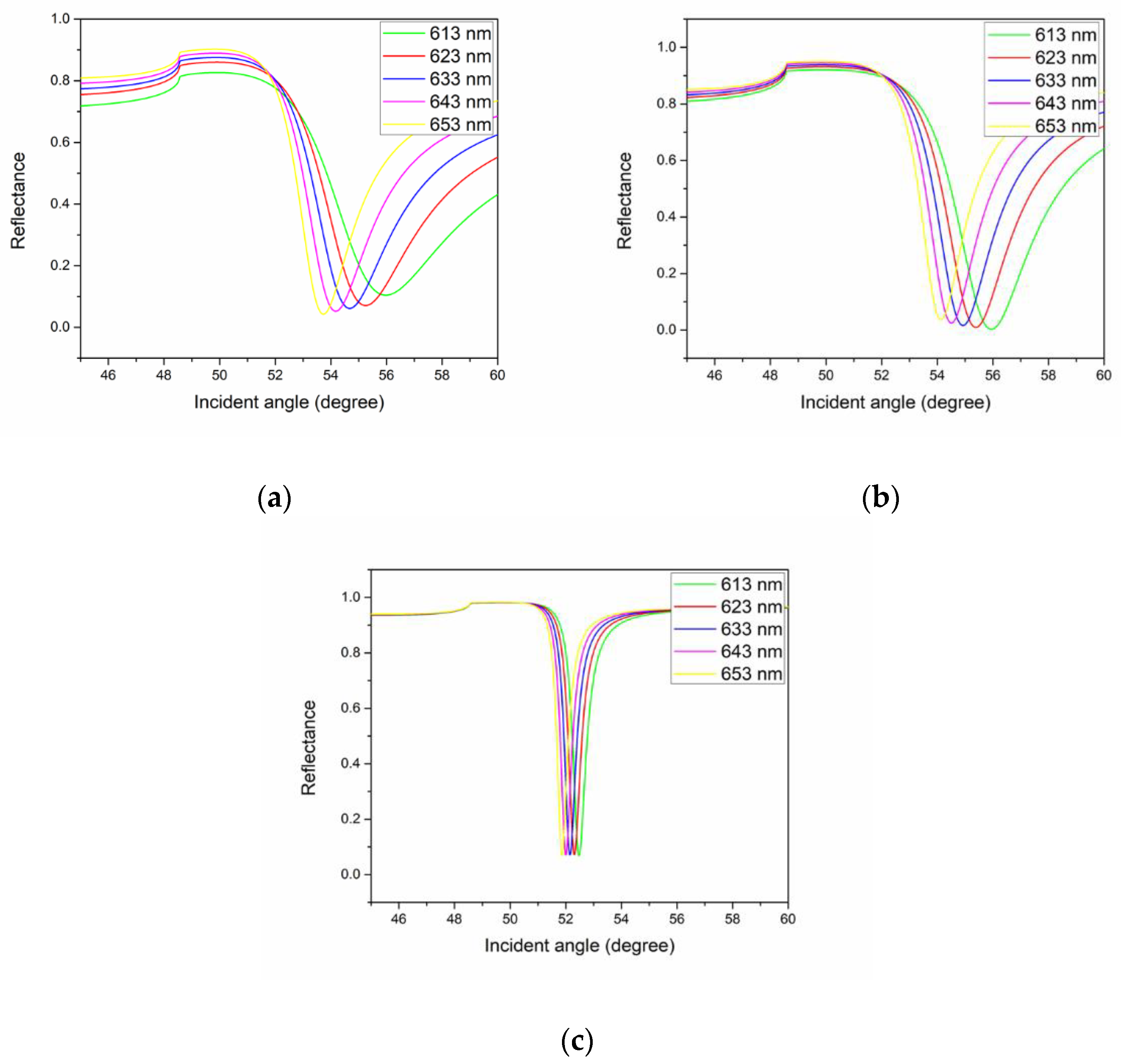
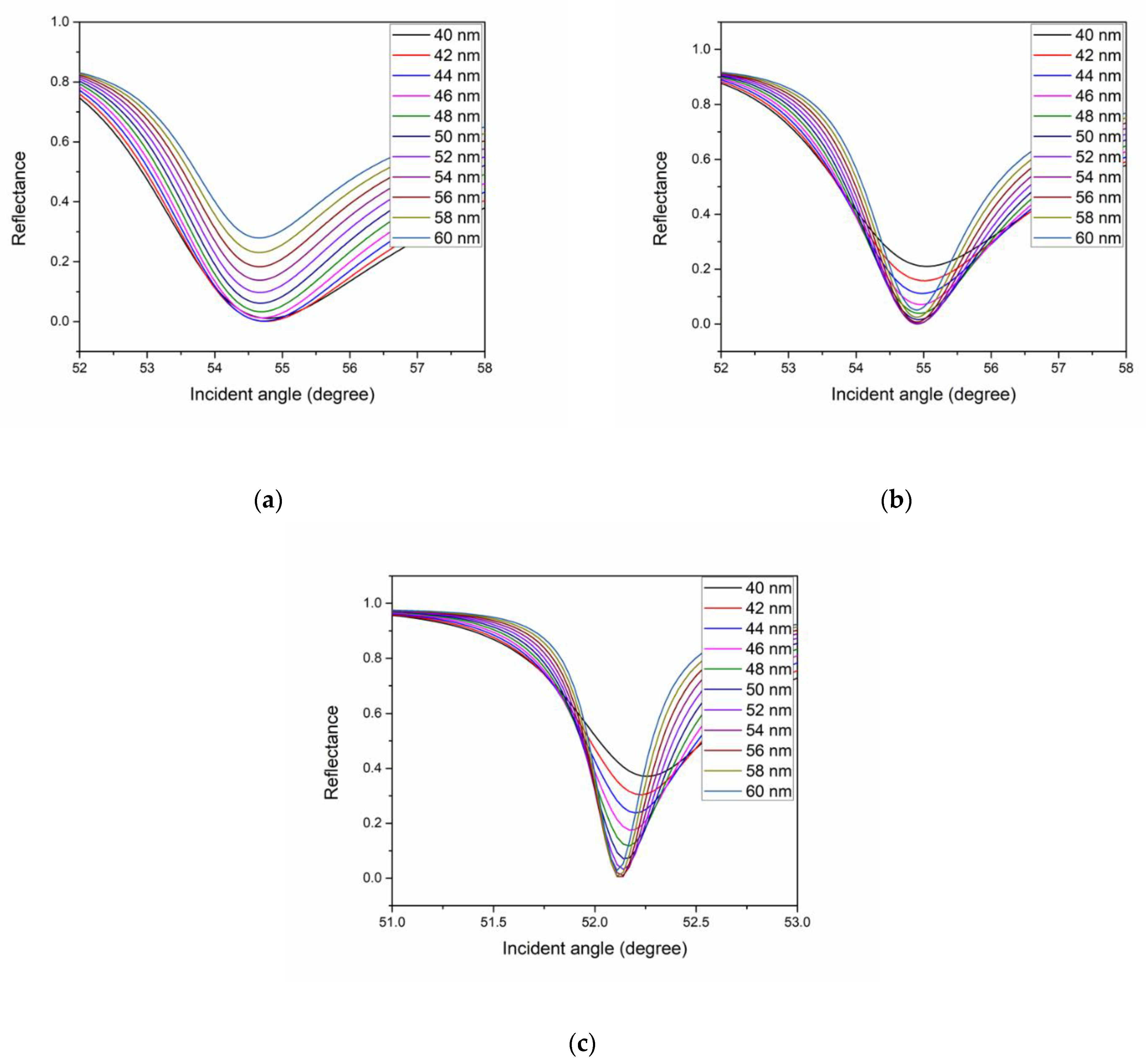
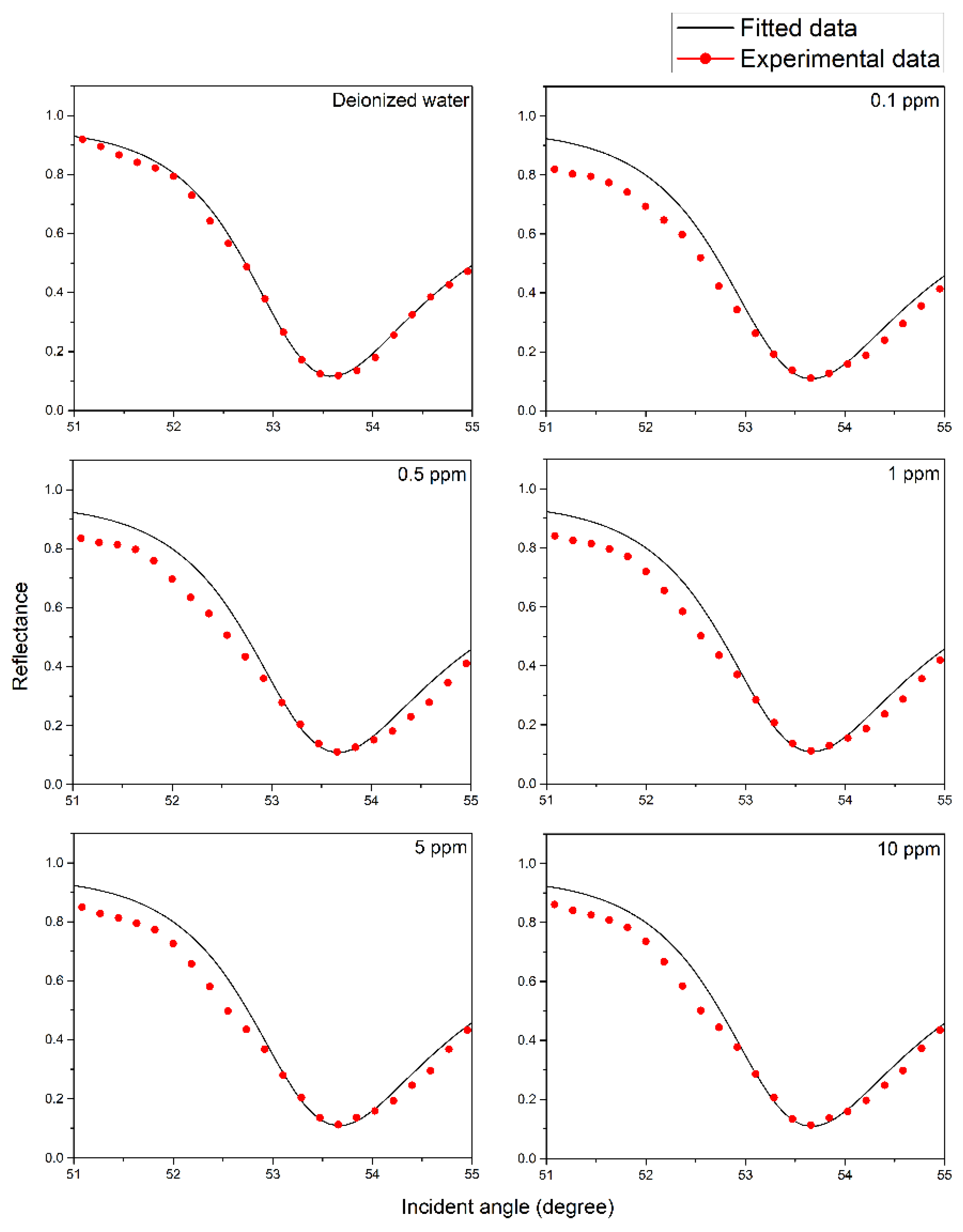
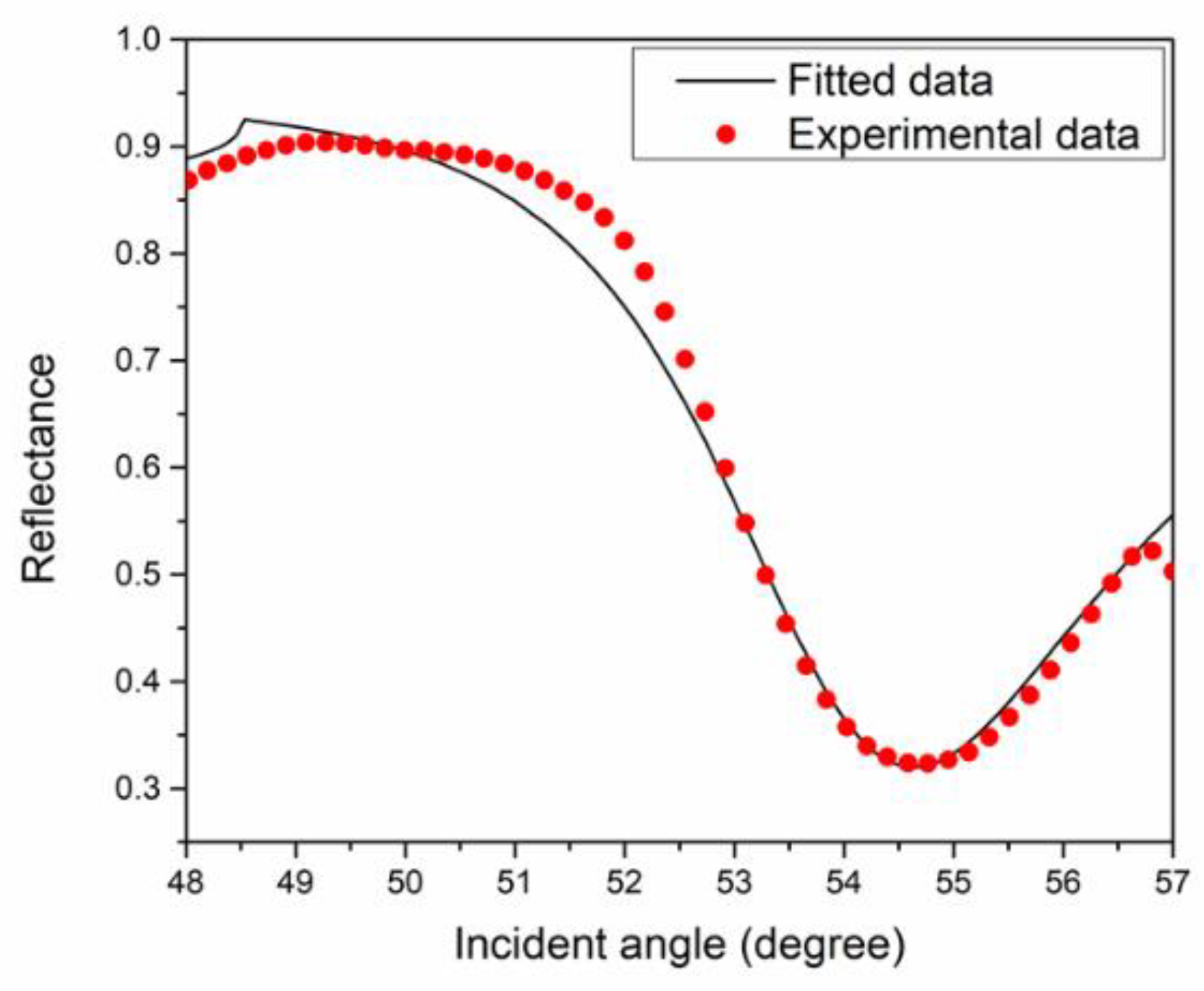
| Metal | Wavelength (nm) | FWHM (Degree) | Accuracy (Degree−1) |
|---|---|---|---|
| Copper | 613 | 3.2 | 0.31 |
| 623 | 3.0 | 0.33 | |
| 633 | 2.8 | 0.36 | |
| 643 | 2.5 | 0.40 | |
| 653 | 2.2 | 0.45 | |
| Gold | 613 | 2.5 | 0.40 |
| 623 | 2.3 | 0.43 | |
| 633 | 2.2 | 0.45 | |
| 643 | 2.2 | 0.45 | |
| 653 | 2.2 | 0.45 | |
| Silver | 613 | 0.5 | 2.0 |
| 623 | 0.5 | 2.0 | |
| 633 | 0.5 | 2.0 | |
| 643 | 0.4 | 2.5 | |
| 653 | 0.4 | 2.5 |
| Thickness (nm) | FWHM (Degree) | Accuracy (Degree−1) |
|---|---|---|
| 6 | 3.0 | 0.333 |
| 7 | 3.2 | 0.313 |
| 8 | 3.4 | 0.294 |
| 9 | 3.5 | 0.286 |
| 10 | 3.6 | 0.277 |
| 11 | 3.7 | 0.270 |
| 12 | 3.8 | 0.263 |
| 13 | 3.9 | 0.256 |
| Concentration (ppm) | Refractive Index of Sensing Layer After in Contact with Copper Ions | Thickness of NCC Based Layer (nm) | |
|---|---|---|---|
| Real Part, n | Imaginary Part, k | ||
| 0 | 1.424 | 0.252 | 9.5 |
| 0.01 | 1.457 | 0.192 | 9.7 |
| 0.05 | 1.467 | 0.173 | 9.9 |
| 0.08 | 1.482 | 0.155 | 10.3 |
| 0.1 | 1.487 | 0.136 | 10.5 |
| 0.5 | 1.504 | 0.123 | 11.0 |
| 1 | 1.504 | 0.123 | 11.0 |
| 5 | 1.504 | 0.123 | 11.0 |
| 10 | 1.504 | 0.123 | 11.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Mahdi, M.A. Design and Optimization of Surface Plasmon Resonance Spectroscopy for Optical Constant Characterization and Potential Sensing Application: Theoretical and Experimental Approaches. Photonics 2021, 8, 361. https://doi.org/10.3390/photonics8090361
Daniyal WMEMM, Fen YW, Abdullah J, Sadrolhosseini AR, Mahdi MA. Design and Optimization of Surface Plasmon Resonance Spectroscopy for Optical Constant Characterization and Potential Sensing Application: Theoretical and Experimental Approaches. Photonics. 2021; 8(9):361. https://doi.org/10.3390/photonics8090361
Chicago/Turabian StyleDaniyal, Wan Mohd Ebtisyam Mustaqim Mohd, Yap Wing Fen, Jaafar Abdullah, Amir Reza Sadrolhosseini, and Mohd Adzir Mahdi. 2021. "Design and Optimization of Surface Plasmon Resonance Spectroscopy for Optical Constant Characterization and Potential Sensing Application: Theoretical and Experimental Approaches" Photonics 8, no. 9: 361. https://doi.org/10.3390/photonics8090361
APA StyleDaniyal, W. M. E. M. M., Fen, Y. W., Abdullah, J., Sadrolhosseini, A. R., & Mahdi, M. A. (2021). Design and Optimization of Surface Plasmon Resonance Spectroscopy for Optical Constant Characterization and Potential Sensing Application: Theoretical and Experimental Approaches. Photonics, 8(9), 361. https://doi.org/10.3390/photonics8090361






