The Efficacy of Phototherapy for the Treatment of Onychomycosis: An Observational Study
Abstract
:1. Introduction
1.1. The Scoring Clinical Index for OM (SCIO)
1.2. Diagnosis
1.3. Current Treatment Modalities for OM
1.4. Phototherapy
2. Materials and Methods
Data and Analysis
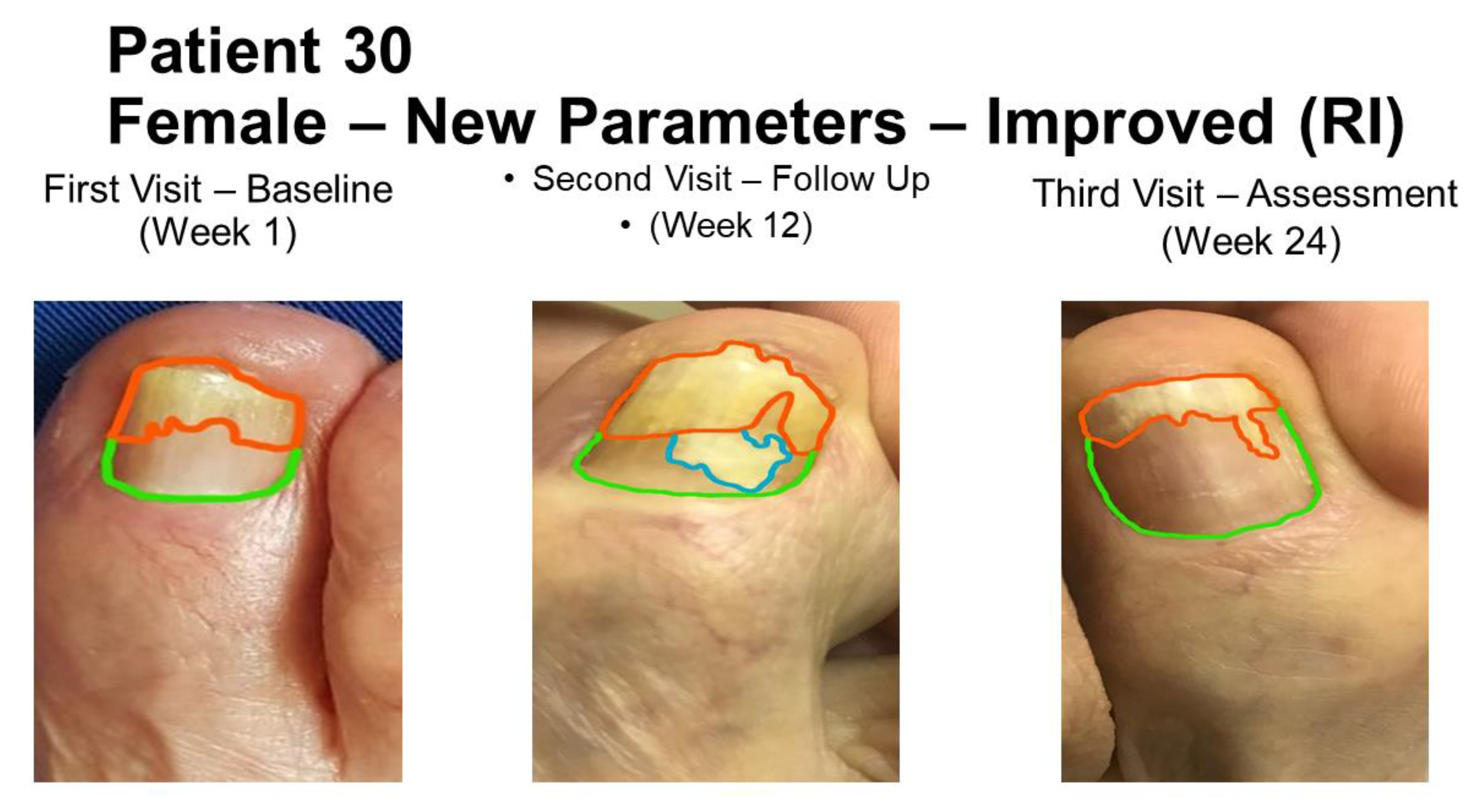
3. Results
3.1. PAS Stain and Fungal MC&S
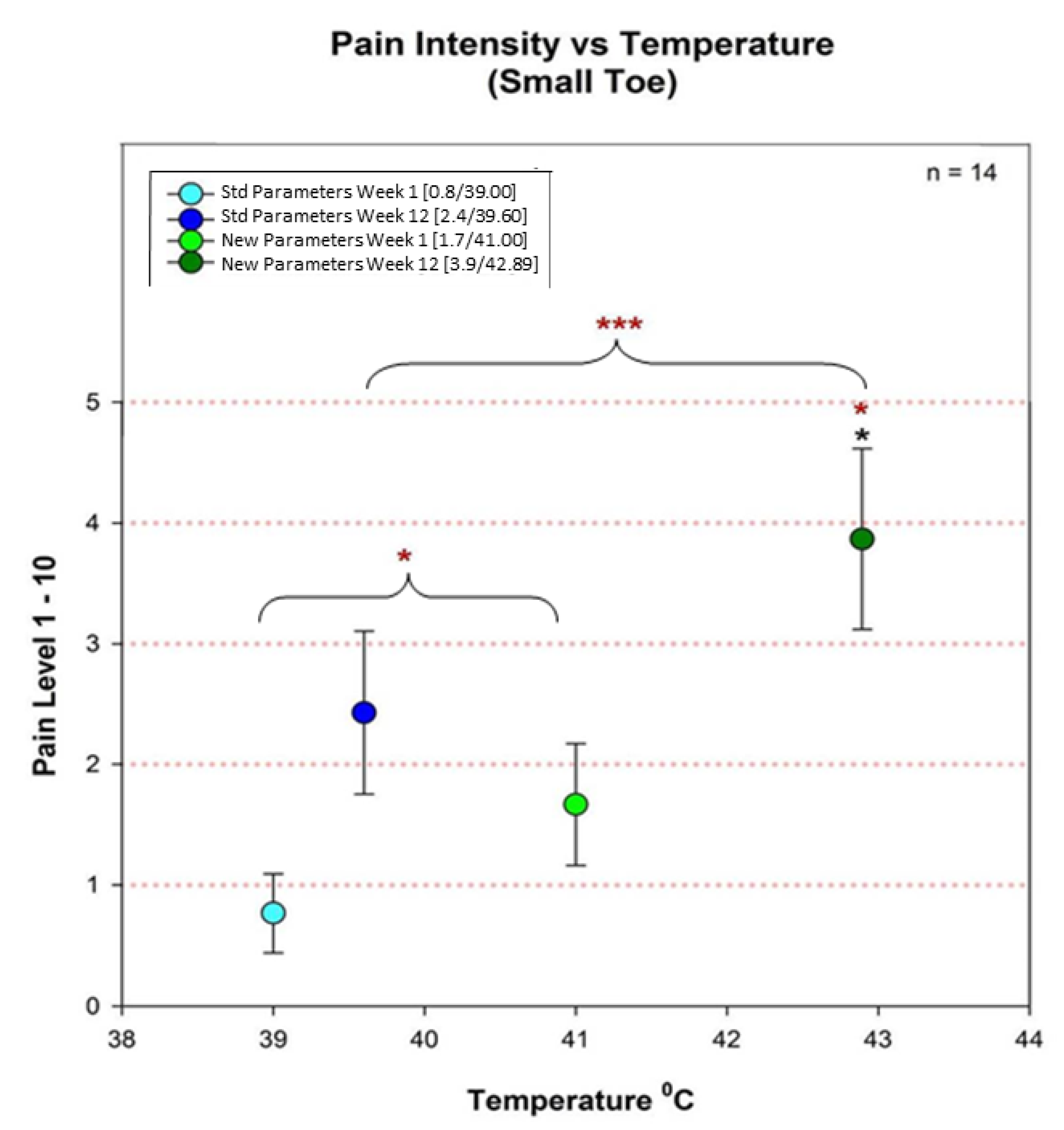
3.2. Overall Pain and Temperature Differences between Groups 2 and 3
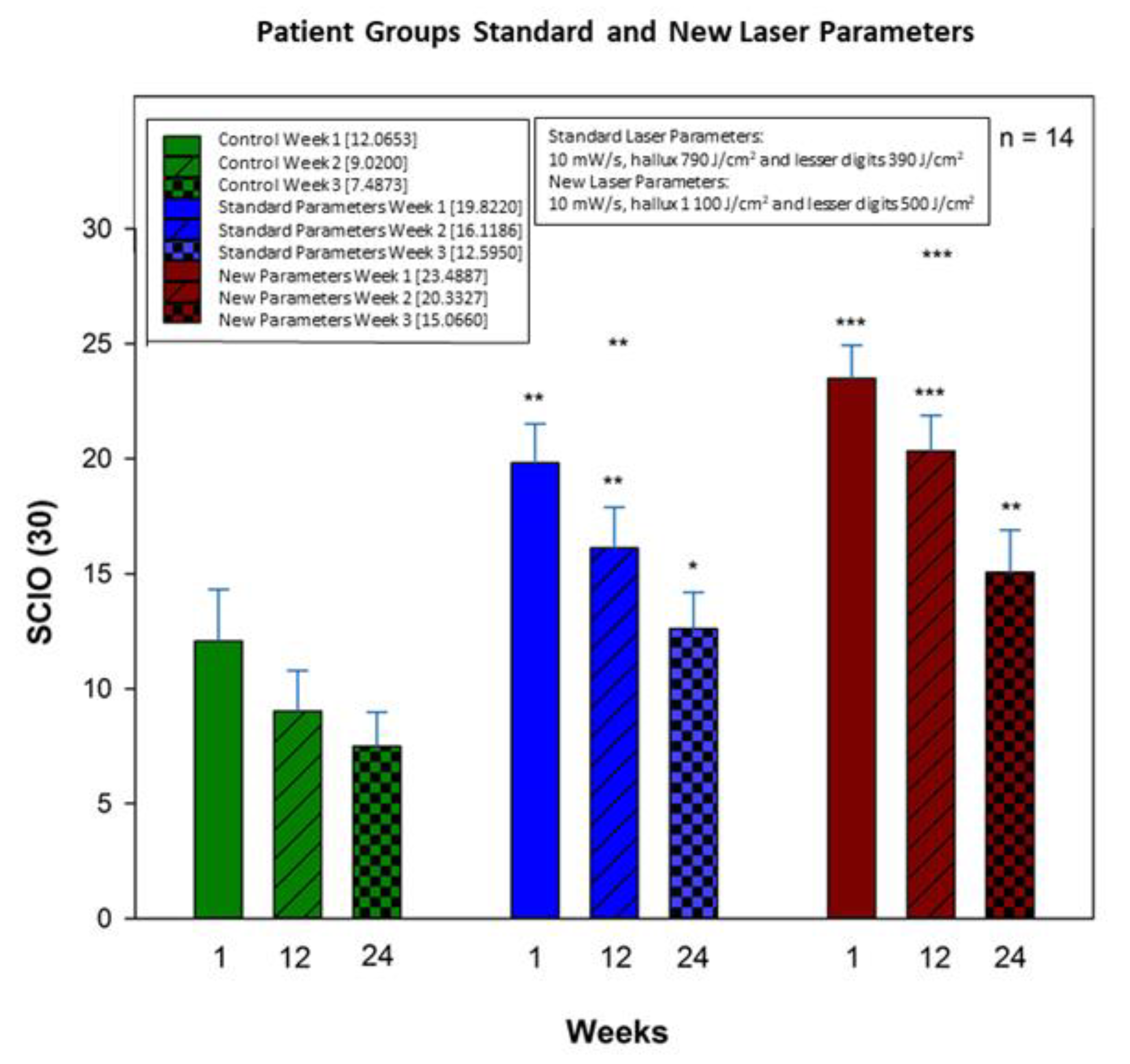
3.3. Overall Treatment Results
4. Discussion
4.1. Standard Podiatric Treatment Efficacy
4.2. Specimen Sampling and Analysis
4.3. Infective Organisms
4.4. SCIO Decrease
4.5. Temperature and Pain Correlation
4.6. Overall Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
Abbreviations
| OM | Onychomycosis |
| DSO | Distal Subungual Onychomycosis |
| DLSO | Distolateral Subungual Onychomycosis |
| PSO | Proximal Subungual Onychomycosis |
| PWSO | Proximal White Superficial Onychomycosis |
| TDO | Total Dystrophic Onychomycosis |
| WSO | White Superficial Onychomycosis |
| SCIO | Scoring Clinical Index for Onychomycosis |
| Nd: YAG | Neodymium Yttrium Aluminium Garnet |
| FDA | Food and Drug Administration |
| KOH | Potassium Hydroxide |
| PAS | Periodic Acid-Schiff |
| MC&S | Fungal microscopy and culture |
References
- Baran, R.; Kaoukhov, A. Topical antifungal drugs for the treatment of onychomycosis: An overview of current strategies for monotherapy and combination therapy. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 21–29. [Google Scholar] [CrossRef]
- Kimura, U.; Takeuchi, K.; Kinoshita, A.; Takamor, K.; Hirum, M.; Suga, Y. Treating onychomycosis of the toenail: Clinical efficacy of the sub-millisecond 1064 nm Nd. YAG laser using a 5 mm spot diameter. J. Drugs Dermatol. 2012, 11, 496–504. [Google Scholar] [PubMed]
- Ortiz, A.E.; Avram, M.M.; Wanner, M.A. A review of lasers and light for the treatment of onychomycosis. Lasers Surg. Med. 2014, 46, 117–124. [Google Scholar] [CrossRef]
- Suga, Y.; Kimura, U.; Hiruma, M. Can persistent toenail fungus be successfully treated with laser? J. Med. Mycol. 2014, 55, J65–J71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tcherney, G.; Penev, P.K.; Nenoff, P.; Zisova, L.G.; Cardoso, G.C.; Taneva, T.; Ginter-Hanselmayer, G.; Ananiev, J.; Gulubova, M.; Hristova, R.; et al. Onychomycosis: Modern diagnostic and treatment approaches. Wien. Med. Wochenschr. 2013, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A. Efinaconazole solution 10%: Topical antifungal therapy for toenail onychomycosis. Cutis 2013, 92, 203–208. [Google Scholar] [PubMed]
- Welsh, O.; Vera-Cabrera, L.; Welsh, E. Onychomycosis. Clin. Dermatol. 2010, 28, 151–159. [Google Scholar] [CrossRef]
- Nenoff, P.; Grunewald, S.; Paasch, U. Laser therapy of onychomycosis. J. Der Dtsch. Dermatol. Ges. 2014, 12, 33–38. [Google Scholar] [CrossRef]
- Waibel, J.; Wulkan, A.J.; Rudnick, A. Prospective efficacy and safety evaluation of laser treatments with real-time temperature feedback for fungal onychomycosis. J. Drugs Dermatol. 2013, 12, 1237–1242. [Google Scholar]
- Kaur, R.; Kashyap, B.; Bhalla, P. Onychomycosis—Epidemiology, diagnosis and management. Indian J. Med. Microbiol. 2008, 26, 108–116. [Google Scholar] [CrossRef]
- Evans, E.G. Causative pathogens in onychomycosis and the possibility of treatment resistance: A review. J. Am. Acad. Dermatol. 1988, 38, S32–S36. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paquet, M.; Simpson, F.C. Therapies for the treatment of onychomycosis. Clin. Dermatol. 2013, 31, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Miyata, K.; Sugita, T.; Hiruma, M.; Hiruma, M. Treatment of onychomycosis using a 1064 nm Nd. YAG laser. J. Med. Mycol. 2013, 54, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Krűger, C.; Ginter-Hanselmayer, G.; Tietz, H.J. Mycology—An update. Part 1: Dermatomycosis: Causative agents, epidemiology and pathogenesis. J. Ger. Soc. Dermatol. 2014, 1203, 188–210. [Google Scholar] [CrossRef]
- Nenoff, P.; Krűger, C.; Ginter-Hanselmayer, G.; Schulte-Beerbühl, R.; Tietz, H.-J. Mycology—An update. Part 2: Dermatomycosis: Clinical picture and diagnosis. J. Ger. Soc. Dermatol. 2014, 1209, 749–777. [Google Scholar]
- Thomas, J.; Jacobson, G.A.; Narkowicz, C.K.; Peterson, G.; Burnet, H.; Sharpe, C. Toenail onychomycosis: An important global disease burden. J. Clin. Pharm. Ther. 2010, 35, 497–519. [Google Scholar] [CrossRef]
- Baudraz-Rosselet, F.; Ruffieux, C.; Lurati, M.; Bontems, O.; Monod, M. Onychomycosis insensitive to systemic terbinafine and azole treatments reveals non-dermatophyte molds as infectious agents. Dermatology 2010, 220, 164–168. [Google Scholar] [CrossRef]
- Westerberg, D.P.; Voyack, M.J. Onychomycosis: Current trends in diagnosis and treatment. Indian J. Clin. Pract. 2014, 25, 309–319. [Google Scholar]
- Ghannoum, M.; Isham, N. Fungal nail infections (onychomycosis): A never-ending story? PLoS Pathog. 2014, 10, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.T.; Taylor, W.D.; Boyle, J. Guidelines for treatment of onychomycosis. Br. J. Dermatol. 2003, 148, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, A.Y.; Gupta, A.K.; Sergeev, Y.V. The scoring clinical index for onychomycosis (SCIO Index). Ski. Ther. Lett. 2002, 7, 6–7. [Google Scholar]
- Gupta, A.K.; Simpson, F.C. New therapeutic options for onychomycosis. Expert Opin. Pharmacother. 2012, 13, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Wilsmann-Theis, D.; Sareika, F.; Bieber, T.; Schmid-Wendtner, M.-H.; Wenzel, J. New reasons for histopathological nail clipping examination in the diagnosis of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 235–237. [Google Scholar] [CrossRef]
- Zaias, N. Onychomycosis. Arch. Dermatol. 1972, 105, 263–274. [Google Scholar] [CrossRef]
- Bristow, I.R. The effectiveness of lasers in the treatment of onychomycosis: A systemic review. Br. J. Foot Ankle Res. 2014, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, E.G. Resistance of Candida species to antifungal agents used in the treatment of onychomycosis: A review of current problems. Br. J. Dermatol. 1999, 141, 33–35. [Google Scholar] [CrossRef]
- Bunert, N.; Homey, B.; Gerber, P.A. Onychomycosis. Successful treatment with a 1064-nm Nd. YAG laser. Der Hautarzt 2013, 64, 716–718. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paquet, M. A retrospective chart review of the clinical efficacy of Nd. YAG 1064-nm laser for toenail onychomycosis. J. Dermatol. Treat. 2014, 5, 1–3. [Google Scholar]
- Heers, H.; Jäger, M.W.; Raulin, C. Treatment of onychomycosis using the 1064 nm Nd. YAG laser: A clinical pilot study. J. Der Dtsch. Dermatol. Ges. 2014, 12, 322–329. [Google Scholar]
- Wantiphakdeedecha, R.; Thanomkitti, K.; Bunyaratavej, S.; Manuskiatti, W. Efficacy and safety of 1064 nm Nd: YAG laser in the treatment of onychomycosis. J. Dermatol. Treat. 2015, 27, 75–79. [Google Scholar] [CrossRef]
- Dembskey, N.; Abrahamse, H. Laser Therapy for the Treatment of Onychomycosis: Best Evidence Based Practice or Not? Clin. Res. Foot Ankle 2016, 4, 3–7. [Google Scholar] [CrossRef]
- Malay, D.S. Efficacy of debridement alone versus debridement combined with topical antifungal nail lacquer for the treatment of pedal onychomycosis: A randomised, controlled trial. J. Foot Ankle Surg. 2009, 48, 294–308. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ryder, J.E.; Summerbell, R.C. The diagnosis of non-dermatophyte mold onychomycosis. Int. J. Dermatol. 2003, 24, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, B.; Olafsson, J.H.; Steinsson, J.T.; Kerrouche, N.; Sidou, F. Efficacy of amorolfine nail lacquer for the prophylaxis of onychomycosis over 3 years. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Aditya, K. Gupta, Maanasa Venkataraman, Emma M Quinlan. Efficacy of lasers for the management of dermatophyte toenail onychomycosis. J. Am. Podiatr. Med. Assoc. 2021, 20–236. [Google Scholar] [CrossRef]
- Ameen, M.; Lear, T.J.; Madan, V.; Mustapa, M.F.M.; Richardson, M. British Association of Dermatologists’ guidelines for the management of onychomycosis 2014. Br. J. Dermatol. 2014, 171, 937–958. [Google Scholar] [CrossRef]
- Gupta, A.K.; Jain, H.C.; Lynde, C.W.; Macdonald, P.; Cooper, E.A.; Summerbell, R.C. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: A multicenter Canadian survey of 15,000 patients. J. Am. Acad. Dermatol. 2000, 43 (Pt. 1), 244–248. [Google Scholar] [CrossRef]
- Fernández, J.; Del Valle Fernández, I.; Villar, C.J.; Lombó, F. Combined laser and ozone therapy for onychomycosis in an in vitro and ex vivo model. PLoS ONE 2021, 16, e0253979. [Google Scholar] [CrossRef]
- Kandpal, R.; Arora, S.; Arora, D. Study of Q-switched Nd: YAG Laser versus Itraconazole in Management of Onychomycosis. J. Cutan. Aesthet. Surg. 2021, 14, 93–100. [Google Scholar]
- Kosarev, J. Novel laser therapy in the treatment of onychomycosis. J. Laser Health Acad. 2010, 1, 1–8. [Google Scholar]
- Gupta, A.K.; Simpson, F.C. Device-based therapies for onychomycosis treatment. Skin Ther. Lett. 2012, 17, 57–61. [Google Scholar]
- Hollmig, T.; Rahman, Z.; Henderson, M.T.; Rotatori, R.M.; Gladstone, H.; Tang, J.Y. Lack of efficacy with 1064 nm Nd: YAG laser for the treatment of onychomycosis: A randomised, controlled trial. J. Am. Acad. Derm. 2014, 70, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.N.; Wang, D.K.; Zhuo, F.L.; Duan, X.-H.; Zhang, X.-Y.; Zhao, J.-Y. Long-pulse Nd: YAG 1064 nm laser treatment of onychomycosis. Chin. Med. J. 2012, 125, 3288–3291. [Google Scholar] [PubMed]
- Hochman, L.G. Laser treatment of onychomycosis using a novel 0.65-milisecond pulsed Nd:YAG 1064 nm laser. J. Cosmet. Laser Ther. 2011, 13, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, E.; Koussidou-Ermonti, T.; Chaidemenos, G.; Apalla, Z.; Ioannides, D. Photodynamic therapy for distal and lateral subungual toenail onychomycosis caused by Trichophyton rubrum: Preliminary results of a single-centre open trial. Acta Derm. Venerol. 2010, 90, 216–217. [Google Scholar] [CrossRef] [Green Version]
- Shemer, A.; Davidovici, B.; Grunwald, M.H.; Lyakhovitsky, A.; Amichai, B. Onychomycosis: A simpler in-office technique for sampling specimens. J. Fam. Pract. 2012, 61, 552–554. [Google Scholar]
- Gupta, A.K.; Ryder, J.E.; Baran, R. The use of topical therapeutics to treat onychomycosis. Derm. Clin. 2003, 21, 481–489. [Google Scholar] [CrossRef]
- Helou, J.; Maatouk, I.; Soutou, B. Big toenail onychomycosis features associated with response to 1064 nm Nd: YAG laser treatment. J. Cosmet. Derm. 2021. [Google Scholar] [CrossRef]

| Classification | Description | Causative Organism |
|---|---|---|
| Distal Subungual Onychomycosis (DSO) |
|
|
| Lateral and Distolateral Subungual Onychomycosis (DLSO) |
|
|
| Proximal Subungual Onychomycosis (PSO) |
|
|
| White Superficial Onychomycosis (WSO) |
|
|
| Proximal White Subungual Onychomycosis (PWSO) |
|
|
| Total Dystrophic Onychomycosis (TDO) |
|
|
| Scio | Treatment Approach |
|---|---|
| 1–3 |
|
| 3–6 |
|
| 6–9 |
|
| 9–12 |
|
| 12–16 |
|
| 16–20 |
|
| 20–30 |
|
| Group | Wavelength | Frequency | Pulse Length | Pulse Interval | Aiming Beam | Spot Size |
|---|---|---|---|---|---|---|
| 2 | 10 mW/s | Hallux; 790 J/cm2 | 0.1 ms | 0.1 ms | Green; 532 nm | 2 mm |
| Lesser Digits; 390 J/cm2 | 0.1 ms | 0.1 ms | Green; 532 nm | 2 mm | ||
| 3 | 10 mW/s | Hallux; 1 100 J/cm2 | 0.1 ms | 0.1 ms | Green; 532 nm | 2 mm |
| Lesser Digits; 500 J/cm2 | 0.1 ms | 0.1 ms | Green; 532 nm | 2 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembskey, N.; Abrahamse, H. The Efficacy of Phototherapy for the Treatment of Onychomycosis: An Observational Study. Photonics 2021, 8, 350. https://doi.org/10.3390/photonics8090350
Dembskey N, Abrahamse H. The Efficacy of Phototherapy for the Treatment of Onychomycosis: An Observational Study. Photonics. 2021; 8(9):350. https://doi.org/10.3390/photonics8090350
Chicago/Turabian StyleDembskey, Nadia, and Heidi Abrahamse. 2021. "The Efficacy of Phototherapy for the Treatment of Onychomycosis: An Observational Study" Photonics 8, no. 9: 350. https://doi.org/10.3390/photonics8090350
APA StyleDembskey, N., & Abrahamse, H. (2021). The Efficacy of Phototherapy for the Treatment of Onychomycosis: An Observational Study. Photonics, 8(9), 350. https://doi.org/10.3390/photonics8090350






