A Review of Silent Substitution Devices for Melanopsin Stimulation in Humans
Abstract
1. Introduction
1.1. Silent Substitution Technique
1.2. Light Sources
1.3. Optical Setup for Silent Substitution
2. Materials and Methods
2.1. Publication Search
2.2. Device Description
3. Results
3.1. Publications Search
3.2. Devices Description
4. Discussion
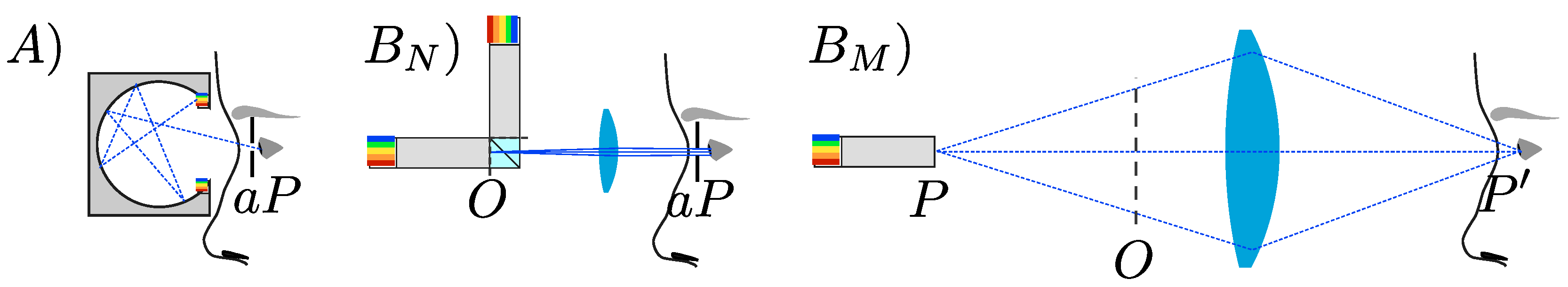
- A)
- Ganzfeld with clever light mixing where the eye of the subject is close to the light source, eventually with an artificial pupil to control the retinal illumination. Because of the lack of space, pupil diameter changes are measured on the contralateral eye.
- BN)
- One or more homogenized light sources with a field stop observed with a magnifier and eventually an artificial pupil to control the retinal illumination. Due to a lack of space, pupil diameter changes are also measured on the contralateral eye.
- BM)
- One homogeneous pupil light source illuminating an object plane with stops and projected into the eye of the subject, so that retinal illumination is controlled by the exit pupil of the instrument. In that case, more space is available and pupil diameter changes can be measured on the excited eye.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CIE | International Commission on Illumination |
| ERG | Electroretinogram |
| FOV | Field of view |
| IEEE | Institute of Electrical and Electronics Engineers |
| ipRGC | intrinsically photosensitive retinal ganglion cells |
| JOSA A | Journal of the Optical Society of America A |
| L | L-cone for long (red) |
| LED | Light emitting diode |
| M | M-cone for middle (green) |
| mRGC | melanopsin retinal ganglion cells |
| PWM | Pulse width modulation |
| S | S-cone for short (blue) |
| SPIE | International society for optics and photonics |
References
- Hattar, S. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Lok, C. Seeing without seeing. Nature 2011, 469, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M. Melanopsin contributions to non-visual and visual function. Curr. Opin. Behav. 2019, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; Martial, F.P.; Lucas, R.J. Form vision from melanopsin in humans. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.S.; Feigl, B.; Cao, D.; Zele, A.J. Temporal characteristics of melanopsin inputs to the human pupil light reflex. Vision Res. 2015, 107, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Dacey, D.M.; Liao, H.W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749–754. [Google Scholar] [CrossRef]
- CIE. System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light, CIE S 026 TOOLBOX. Available online: http://cie.co.at/news/launch-cie-s-026-toolbox-and-user-guide (accessed on 10 June 2020).
- Brown, T.M.; Tsujimura, S.I.; Allen, A.E.; Wynne, J.; Bedford, R.; Vickery, G.; Vugler, A.; Lucas, R.J. Melanopsin-based brightness discrimination in mice and humans. Curr. Biol. 2012, 22, 1134–1141. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. The circadian clock and human health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Douglas, R.H.; Foster, R.G. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 2001, 4, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.S.; Feigl, B.; Zele, A.J. The effects of short-term light adaptation on the human post-illumination pupil response. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.W.; Hannibal, J.; Hagiwara, G.; Colas, D.; Ruppert, E.; Ruby, N.F.; Heller, H.C.; Franken, P.; Bourgin, P. Melanopsin as a sleep modulator: Circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol. 2009, 7, e1000125. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, G.; Schwartz, S.; Grandjean, D.; Wuillaume, C.; Balteau, E.; Degueldre, C.; Schabus, M.; Phillips, C.; Luxen, A.; Dijk, D.J.; et al. Spectral quality of light modulates emotional brain responses in humans. Proc. Natl. Acad. Sci. USA 2010, 107, 19549–19554. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.P.; Rollag, M.D.; Greeson, J.; Byrne, B.; Glickman, G.; Gerner, E.; Sanford, B. Human melatonin regulation is not mediated by the three cone photopic visual system. J. Clin. Endocrinol. Metab. 2001, 86, 433–436. [Google Scholar] [CrossRef][Green Version]
- LeGates, T.A.; Altimus, C.M.; Wang, H.; Lee, H.K.; Yang, S.; Zhao, H.; Kirkwood, A.; Weber, E.T.; Hattar, S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012, 491, 594–598. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Blattner, P.; Oelhafen, P.; Götz, T.; Cajochen, C. Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert? PloS one 2011, 6, e16429. [Google Scholar] [CrossRef]
- Ostrin, L.A.; Abbott, K.S.; Queener, H.M. Attenuation of short wavelengths alters sleep and the ipRGC pupil response. Ophthalmic. Physiol. Opt. 2017, 37, 440–450. [Google Scholar] [CrossRef]
- Gracitelli, C.P.B.; Duque-Chica, G.L.; Roizenblatt, M.; Moura, A.L.d.A.; Nagy, B.V.; Ragot de Melo, G.; Borba, P.D.; Teixeira, S.H.; Tufik, S.; Ventura, D.F.; et al. Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology 2015, 122, 1139–1148. [Google Scholar] [CrossRef]
- Kelbsch, C.; Maeda, F.; Strasser, T.; Blumenstock, G.; Wilhelm, B.; Wilhelm, H.; Peters, T. Pupillary responses driven by ipRGCs and classical photoreceptors are impaired in glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Maynard, M.L.; Zele, A.; Kwan, A.; Feigl, B. Intrinsically photosensitive retinal ganglion cell function, sleep efficiency and depression in advanced age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Maynard, M.L.; Zele, A.J.; Feigl, B. Melanopsin-mediated post-illumination pupil response in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6906–6913. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, C.; Zhang, L.; Qi, L.; Yao, L.; Chen, J.; Yang, G.; Chen, T.; Zhang, Z. Dark adaptation-induced changes in rod, cone and intrinsically photosensitive retinal ganglion cell (ipRGC) sensitivity differentially affect the pupil light response (PLR). Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1997–2005. [Google Scholar] [CrossRef]
- Lovasik, J.V.; Kergoat, H.; Wajszilber, M.A. Blue flicker modifies the subfoveal choroidal blood flow in the human eye. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H683–H691. [Google Scholar] [CrossRef][Green Version]
- Estévez, O.; Spekreijse, H. The “silent substitution” method in visual research. Vision Res. 1982, 22, 681–691. [Google Scholar] [CrossRef]
- Spitschan, M.; Woelders, T. The method of silent substitution for examining melanopsin contributions to pupil control. Front. Neurol. 2018, 9, 941. [Google Scholar] [CrossRef]
- Google. Ngram Viewer. Available online: https://books.google.com/ngrams (accessed on 12 June 2020).
- Google. Trends. Available online: https://trends.google.com/ (accessed on 12 June 2020).
- Spitschan, M.; Aguirre, G.K.; Brainard, D.H. Selective stimulation of penumbral cones reveals perception in the shadow of retinal blood vessels. PloS One 2015, 10, e0124328. [Google Scholar] [CrossRef]
- Viénot, F.; Bailacq, S.; Rohellec, J.L. The effect of controlled photopigment excitations on pupil aperture. Ophthalmic Physiol. Opt. 2010, 30, 484–491. [Google Scholar] [CrossRef]
- Spitschan, M.; Jain, S.; Brainard, D.H.; Aguirre, G.K. Opponent melanopsin and S-cone signals in the human pupillary light response. Proc. Natl. Acad. Sci. USA 2014, 111, 15568–15572. [Google Scholar] [CrossRef]
- Horiguchi, H.; Winawer, J.; Dougherty, R.F.; Wandell, B.A. Human trichromacy revisited. Proc. Natl. Acad. Sci. USA 2013, 110, E260–E269. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.i.; Ukai, K.; Ohama, D.; Nuruki, A.; Yunokuchi, K. Contribution of human melanopsin retinal ganglion cells to steady-state pupil responses. Proc. Royal Soc. B 2010, 277, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Kuze, M.; Morita, T.; Fukuda, Y.; Kondo, M.; Tsubota, K.; Ayaki, M. Electrophysiological responses from intrinsically photosensitive retinal ganglion cells are diminished in glaucoma patients. J. Optom. 2017, 10, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Agrici, S.; Geiser, M.; Rovati, L. Device for silent substitution excitation of melanopsin for human eye. Ophthalmic Technol. XXIX 2019, 10858. [Google Scholar] [CrossRef]
- Pokorny, J.; Smithson, H.; Quinlan, J. Photostimulator allowing independent control of rods and the three cone types. Vis. Neurosci. 2004, 21, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Parry, N.R.A.; McKeefry, D.J.; Kremers, J.; Murray, I.J. A dim view of M-cone onsets. J. Opt. Soc. Am. A 2016, 33, A207–A213. [Google Scholar] [CrossRef]
- Cao, D.; Nicandro, N.; Barrionuevo, P.A. A five-primary photostimulator suitable for studying intrinsically photosensitive retinal ganglion cell functions in humans. J. Vis. 2015, 15, 27. [Google Scholar] [CrossRef]
- Allen, A.E.; Hazelhoff, E.M.; Martial, F.P.; Cajochen, C.; Lucas, R.J. Exploiting metamerism to regulate the impact of a visual display on alertness and melatonin suppression independent of visual appearance. Sleep 2018, 41, zsy100. [Google Scholar] [CrossRef]
- Hexley, A.C.; Yöntem, A.O.; Spitschan, M.; Smithson, H.E.; Mantiuk, R. Demonstrating a multi-primary high dynamic range display system for vision experiments. J. Opt. Soc. Am. A 2020, 37, A271–A284. [Google Scholar] [CrossRef]
- Luminus Devices, I. PT-39-L51 Product Datasheet. Available online: https://download.luminus.com/datasheets/Luminus_PT-39-L51_Datasheet.pdf (accessed on 1 June 2020).
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef]

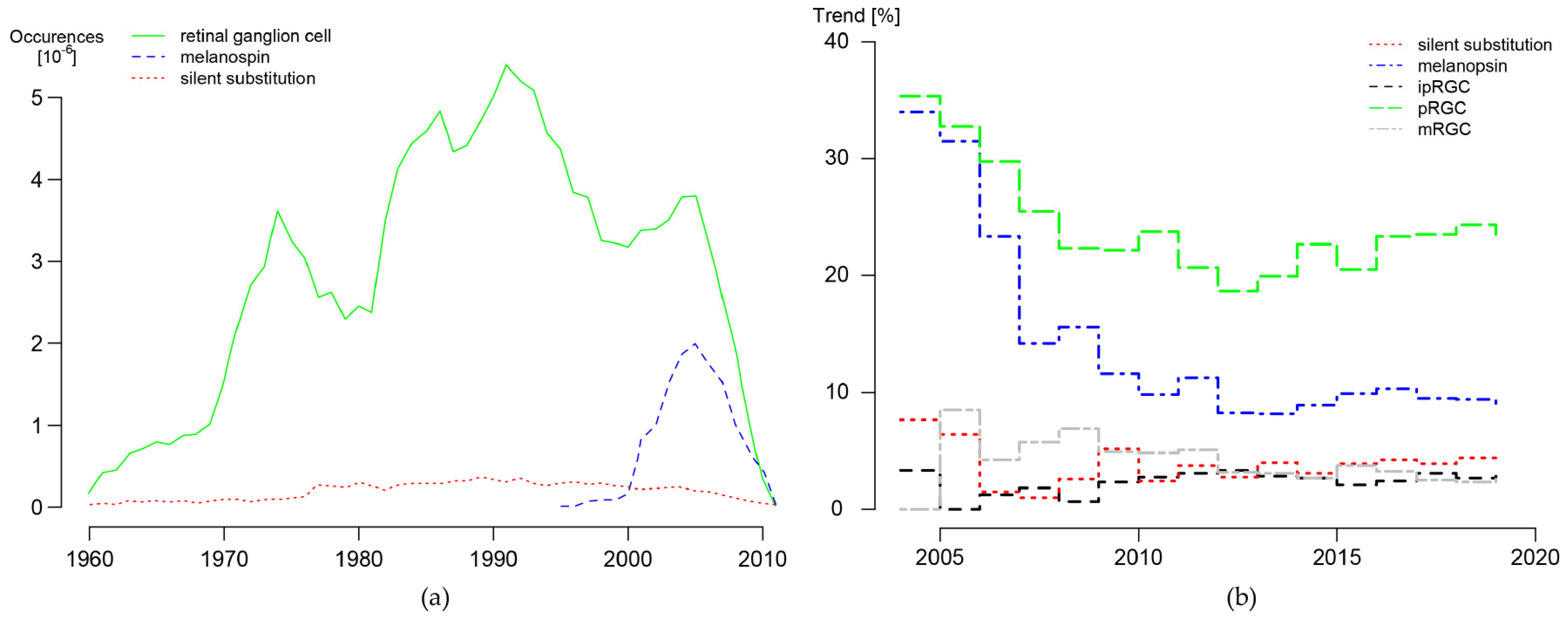
| silent substitution | melanopsin | ipRGC | pRGC | mRGC | circadian | Ngram (2019) | |
|---|---|---|---|---|---|---|---|
| silent substitution | 252 | 20 | 5 | 0 | 2 | 6 | 0.9 |
| melanopsin | 1018 | 151 | 10 | 21 | 527 | 72.1 | |
| ipRGC | 194 | 0 | 1 | 102 | 2.8 | ||
| pRGC | 25 | 0 | 9 | 1.8 | |||
| mRGC | 68 | 15 | 0.2 | ||||
| circadian | 90550 | 1665.0 |
| Reference | [42] | [41] | [32] | [35] | [36] | [39] | [33] | [40] | [38] | [34] | [37] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| View type | Newtonian | Maxwellian | |||||||||
| Pupil type | Normal | Artificial | Artificial | Normal | |||||||
| Nb primary n | 6 | 5 | 5 | 4 | 4 | 4 | 56 | 5 | 4 | 6 | 4 |
| [bit] | 8 | 16 | 8 | - | - | - | - | 12 | 8 | 12 | - |
| Light source | PRJ | PRJ | LED | LED | LED | LED | DSI | LED | LED | LED | LED |
| Type of field | IM | IM | HO | HO | HO | HO | HO | HO | 2 HO | HO | HO |
| C. obsc. [∘] | 0 | 0 | 0 | 0 | 0 | no | 5 | 10.5 | - | 0 | 10 |
| FOV [∘] | - | - | 120 | 20 | 26.5 | 180 | 27.5 | 30 | - | 20 | 52 |
| Light units | lux | lux | lux | lux * | lux | lux | lux | tr | tr | lux | lux |
| 117 | - | - | 53 | 45 | - | 300 | 50 | - | - | 0 | |
| - | - | 1.05 | - | 18 | - | 50 | 25.1 | - | - | 85 | |
| Biosignal | - | PU | PU | PU | ERG | PU | PU | PU | PU | PU | PU |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conus, V.; Geiser, M. A Review of Silent Substitution Devices for Melanopsin Stimulation in Humans. Photonics 2020, 7, 121. https://doi.org/10.3390/photonics7040121
Conus V, Geiser M. A Review of Silent Substitution Devices for Melanopsin Stimulation in Humans. Photonics. 2020; 7(4):121. https://doi.org/10.3390/photonics7040121
Chicago/Turabian StyleConus, Vincent, and Martial Geiser. 2020. "A Review of Silent Substitution Devices for Melanopsin Stimulation in Humans" Photonics 7, no. 4: 121. https://doi.org/10.3390/photonics7040121
APA StyleConus, V., & Geiser, M. (2020). A Review of Silent Substitution Devices for Melanopsin Stimulation in Humans. Photonics, 7(4), 121. https://doi.org/10.3390/photonics7040121






