Investigating the Coherence Between Motor Cortex During Rhythmic Finger Tapping Using OPM-MEG
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Subjects
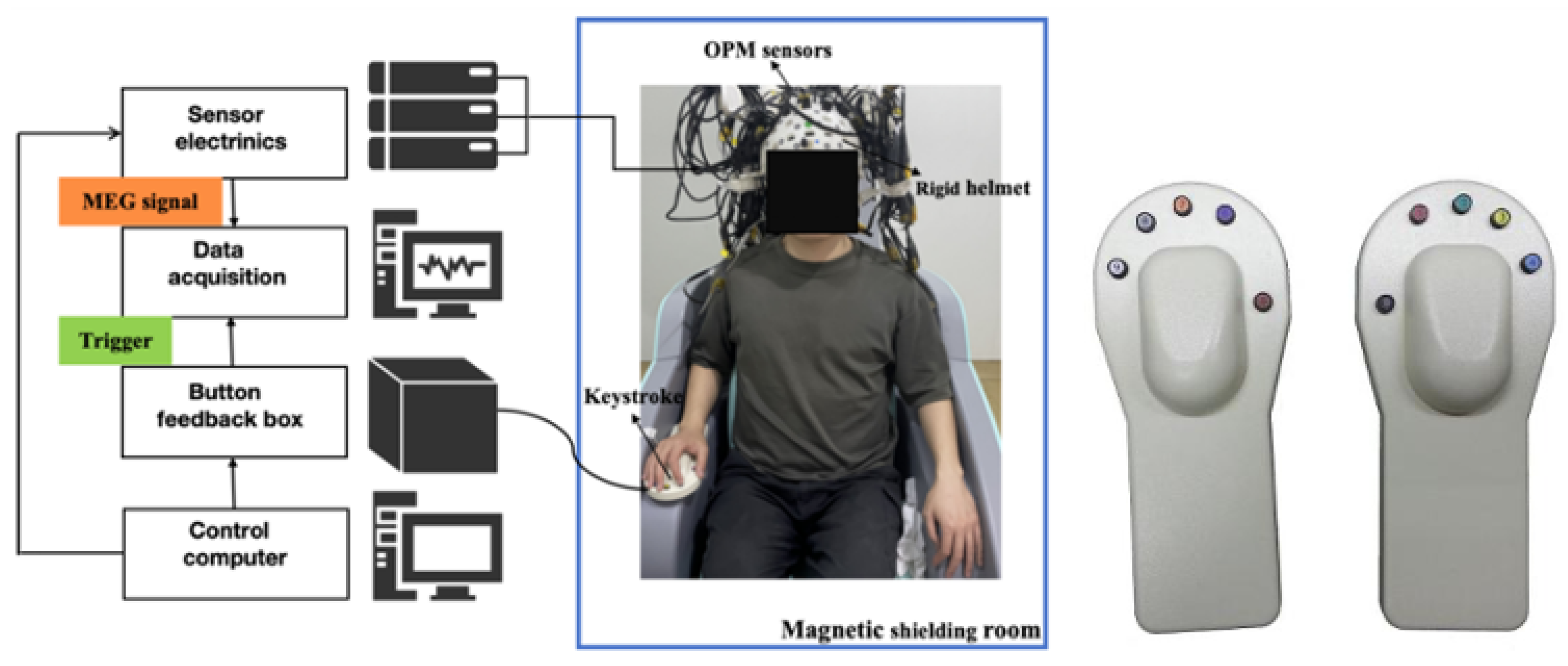
2.2. OPM-MEG System
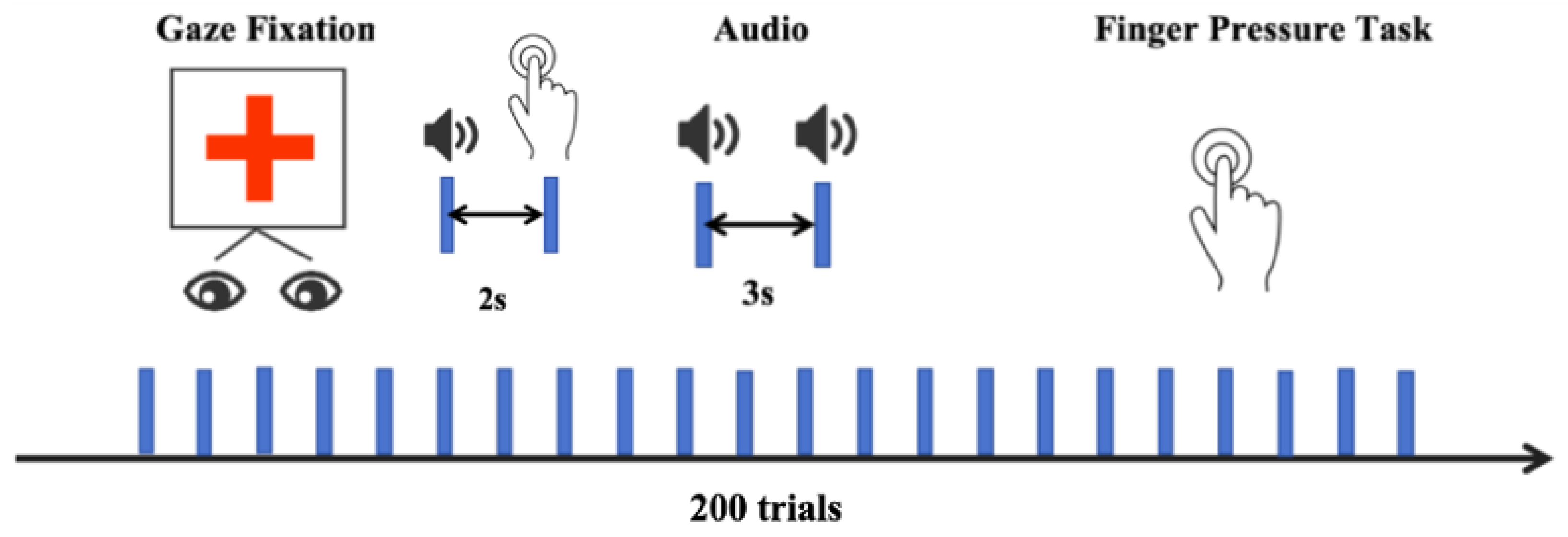
2.3. Experimental Design and Preprocessing
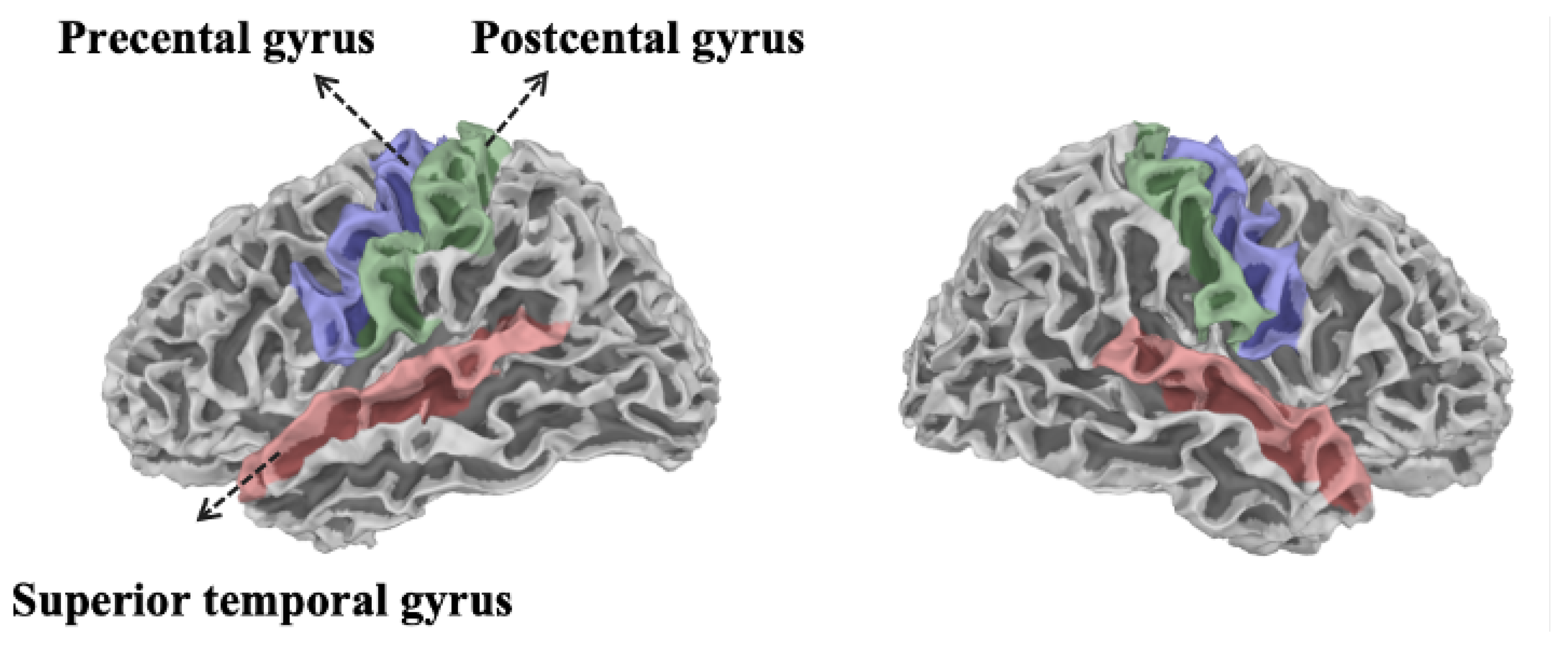
2.4. Registration and Source Localization
2.5. Registration and Source Localization
2.6. Coherence
2.7. Statistical Analysis
3. Results
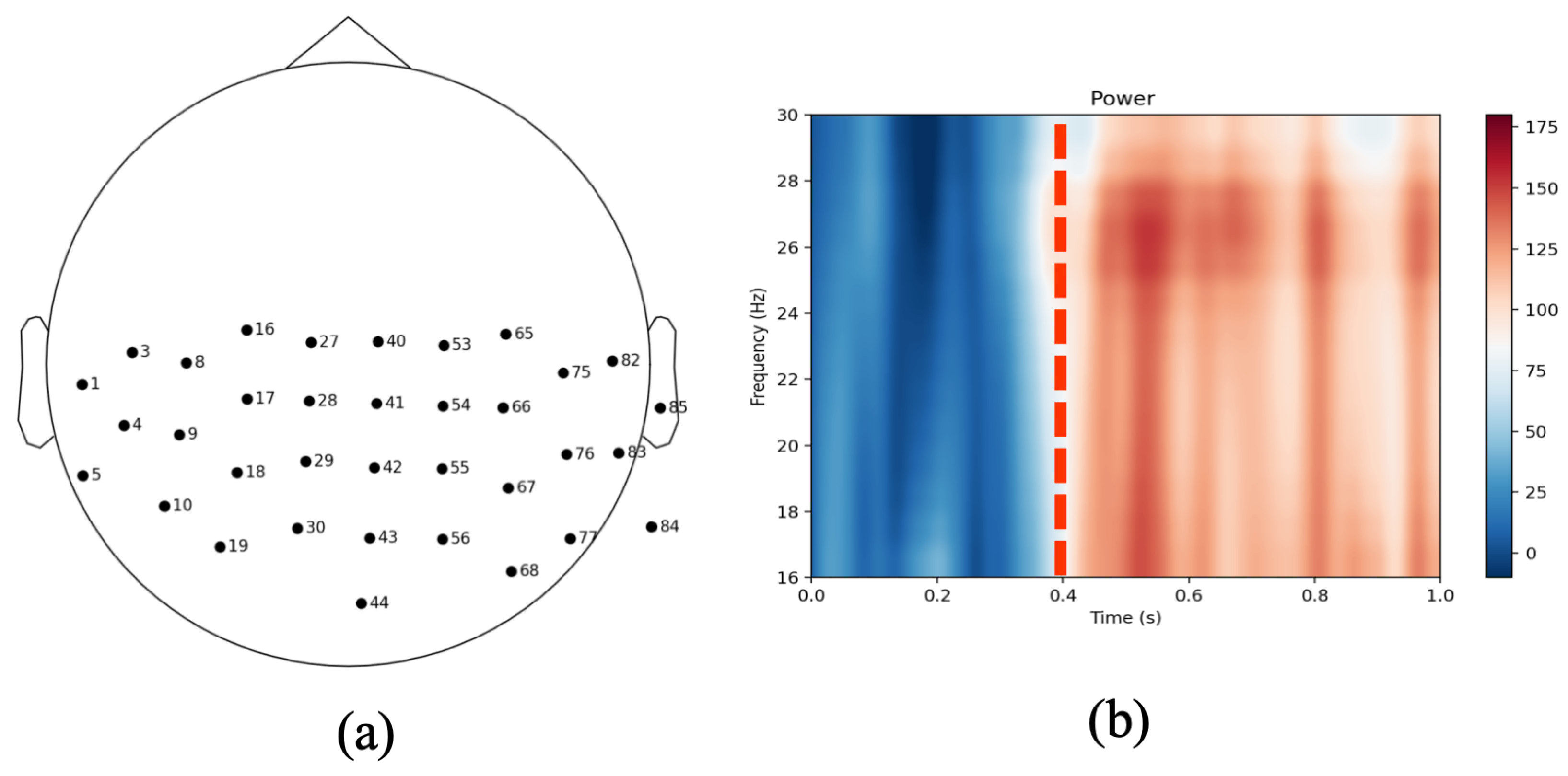
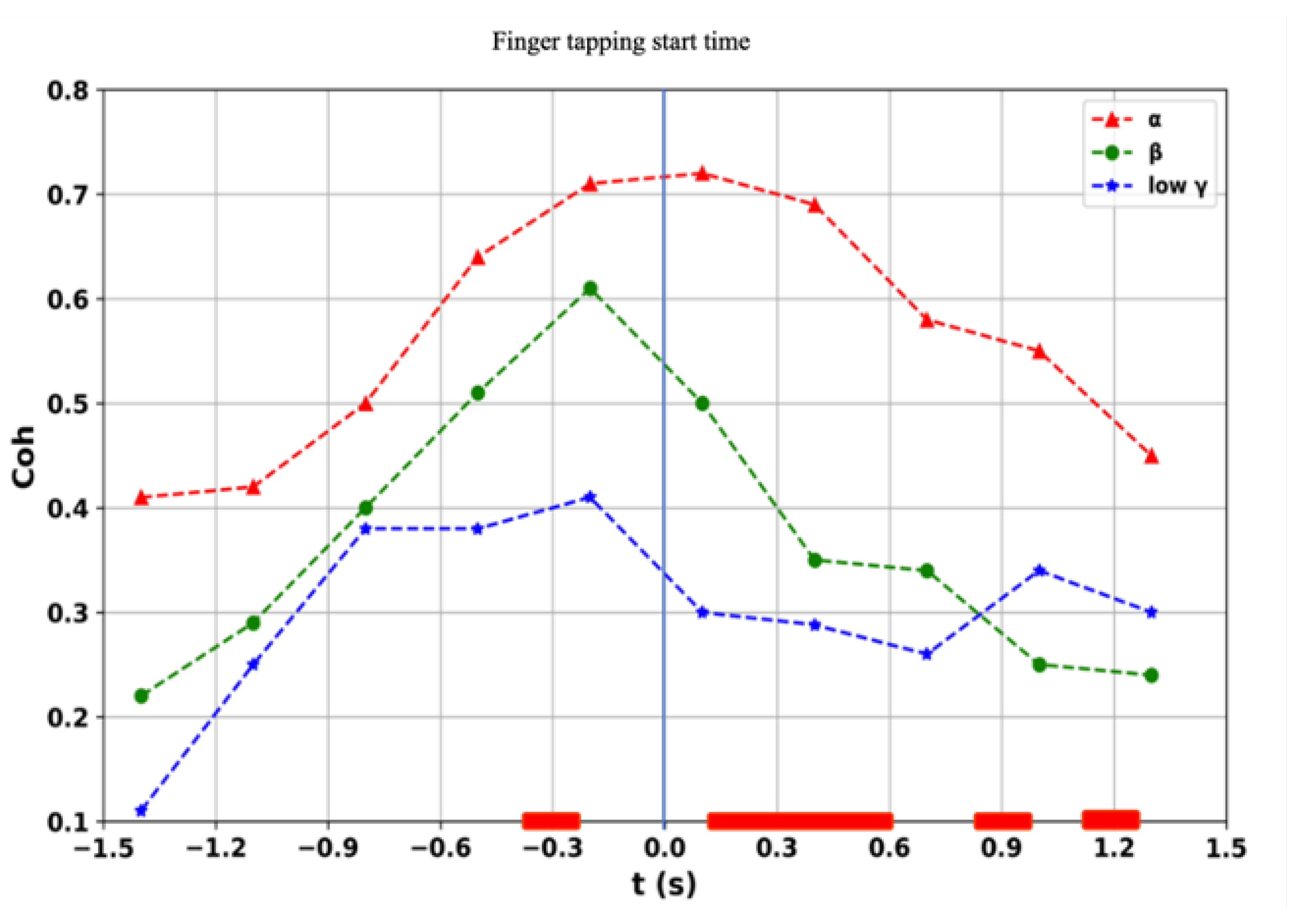
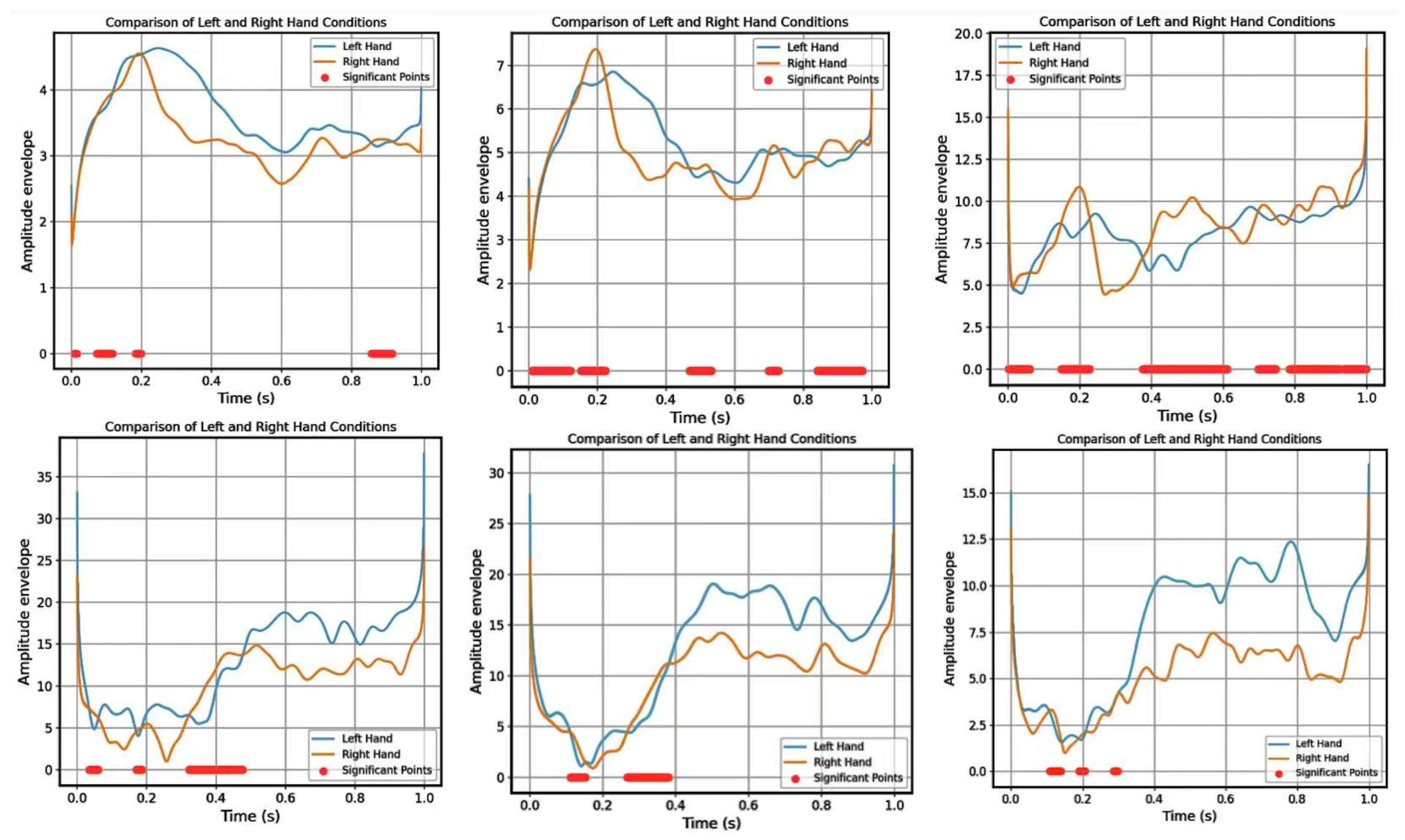
3.1. Sensor-Level Coherence Analysis
3.2. Source-Level Coherence Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.; Li, J.; Li, R.; Zhai, Y. Flow Rate Estimation Based on Magnetic Particle Detection Using a Miniatured High-Sensitivity OPM. IEEE Trans. Instrum. Meas. 2025, 74, 9518010. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, K. Instrumentation for measuring MEG signals. In Magnetoencephalography: From Signals to Dynamic Cortical Networks; Springer: Berlin/Heidelberg, Germany, 2019; pp. 41–71. [Google Scholar]
- Seymour, R.A.; Alexander, N.; Mellor, S.; O’Neill, G.C.; Tierney, T.M.; Barnes, G.R.; Maguire, E.A. Interference suppression techniques for OPM-based MEG: Opportunities and challenges. NeuroImage 2022, 247, 118834. [Google Scholar] [CrossRef]
- Gerloff, C.; Richard, J.; Hadley, J.; Schulman, A.E.; Honda, M.; Hallett, M. Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain J. Neurol. 1998, 121, 1513–1531. [Google Scholar] [CrossRef]
- Bourguignon, M.; Piitulainen, H.; De Tiège, X.; Jousmäki, V.; Hari, R. Corticokinematic coherence mainly reflects movement-induced proprioceptive feedback. Neuroimage 2015, 106, 382–390. [Google Scholar] [CrossRef]
- Andres, F.G.; Mima, T.; Schulman, A.E.; Dichgans, J.; Hallett, M.; Gerloff, C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 1999, 122, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, M.; De Tiège, X.; de Beeck, M.O.; Pirotte, B.; Van Bogaert, P.; Goldman, S.; Hari, R.; Jousmäki, V. Functional motor-cortex mapping using corticokinematic coherence. Neuroimage 2011, 55, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Sacchet, M.D.; Mellinger, J.; Sitaram, R.; Braun, C.; Birbaumer, N.; Fetz, E. Volitional control of neuromagnetic coherence. Front. Neurosci. 2012, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, M.; Jousmäki, V.; de Beeck, M.O.; Van Bogaert, P.; Goldman, S.; De Tiège, X. Neuronal network coherent with hand kinematics during fast repetitive hand movements. Neuroimage 2012, 59, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Daffertshofer, A.; Peper, C.; Frank, T.; Beek, P. Spatio-temporal patterns of encephalographic signals during polyrhythmic tapping. Hum. Mov. Sci. 2000, 19, 475–498. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Yuan, X.; Zheng, W. Attentional focus modulates automatic finger-tapping movements. Sci. Rep. 2021, 11, 698. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Song, X.; Ning, X.; Yang, J.; Qi, S.; Long, T.; Wu, Z.; Wang, R. Hardware-Based Interference Suppression Techniques for OPM-MEG: A Review. IEEE Sens. J. 2024, 25, 2090–2102. [Google Scholar] [CrossRef]
- Jia, L.; Song, X.; Suo, Y.; Li, J.; Long, T.; Ning, X. Magnetic field interference suppression for minimized SERF atomic magnetometer. Sens. Actuators A Phys. 2023, 351, 114188. [Google Scholar] [CrossRef]
- Yuan, S.; Cui, P.; Shi, M.; Zhang, X.; Yang, J.; Zhang, L.; Ma, Y. Biplanar coils design for spatial nonlinear magnetic fields using an enhanced target field method. J. Phys. D Appl. Phys. 2024, 57, 405002. [Google Scholar] [CrossRef]
- Yuan, S.; Shi, M.; Zhang, L.; Yang, J.; Li, T.; Ma, Y. A Design Method of Biplanar Uniform Field Coils Based on Magnetic Shielding Rooms. IEEE Trans. Magn. 2024, 60, 7201904. [Google Scholar] [CrossRef]
- Stavrinou, M.L.; Moraru, L.; Cimponeriu, L.; Della Penna, S.; Bezerianos, A. Evaluation of cortical connectivity during real and imagined rhythmic finger tapping. Brain Topogr. 2007, 19, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xiang, J.; Wang, Y.; O’Brien, H.; Kabbouche, M.; Horn, P.; Powers, S.W.; Hershey, A.D. Aberrant neuromagnetic activation in the motor cortex in children with acute migraine: A magnetoencephalography study. PLoS ONE 2012, 7, e50095. [Google Scholar] [CrossRef]
- Tierney, T.M.; Alexander, N.; Mellor, S.; Holmes, N.; Seymour, R.; O’Neill, G.C.; Maguire, E.A.; Barnes, G.R. Modelling optically pumped magnetometer interference in MEG as a spatially homogeneous magnetic field. NeuroImage 2021, 244, 118484. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Witzel, T.; Ahlfors, S.P.; Stufflebeam, S.M.; Belliveau, J.W.; Hämäläinen, M.S. Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. Neuroimage 2006, 31, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Sereno, M.I.; Tootell, R.B.; Dale, A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999, 8, 272–284. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE software for processing MEG and EEG data. Neuroimage 2014, 86, 446–460. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neuroinform. 2013, 7, 267. [Google Scholar] [CrossRef]
- An, N.; Gao, Z.; Li, W.; Cao, F.; Wang, W.; Xu, W.; Wang, C.; Xiang, M.; Gao, Y.; Wang, D.; et al. Source localization comparison and combination of OPM-MEG and fMRI to detect sensorimotor cortex responses. Comput. Methods Programs Biomed. 2024, 254, 108292. [Google Scholar] [CrossRef]
- Gross, J.; Kujala, J.; Hämäläinen, M.; Timmermann, L.; Schnitzler, A.; Salmelin, R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc. Natl. Acad. Sci. USA 2001, 98, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Pollok, B.; Gross, J.; Müller, K.; Aschersleben, G.; Schnitzler, A. The cerebral oscillatory network associated with auditorily paced finger movements. Neuroimage 2005, 24, 646–655. [Google Scholar] [CrossRef]
- Peng, J.; Zikereya, T.; Shao, Z.; Shi, K. The neuromechanical of Beta-band corticomuscular coupling within the human motor system. Front. Neurosci. 2024, 18, 1441002. [Google Scholar] [CrossRef]
- Akdogan, I.; Ogmen, H.; Kafaligonul, H. The phase coherence of cortical oscillations predicts dynamic changes in perceived visibility. Cereb. Cortex 2024, 34, bhae380. [Google Scholar] [CrossRef] [PubMed]
- Lukas, R.; Rhodes, N.; Boto, E.; Holmes, N.; Hill, R.M.; Gonzalo, R.R.; Shah, V.; Doyle, C.; Osborne, J.; Bowtell, R.W.; et al. Tracking the neurodevelopmental trajectory of beta band oscillations with optically pumped magnetometer-based magnetoencephalography. eLife 2024, 13, RP94561. [Google Scholar] [PubMed]
- Balasubramanian, P.; De Leon, R.P.; Snyder, D.B.; Beardsley, S.A.; Hyngstrom, A.S.; Schmit, B.D. Altered Cortical Activity during a Finger Tap in People with Stroke. Brain Topogr. 2024, 37, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; DeGrauw, X.; Korman, A.M.; Allen, J.R.; O’Brien, H.L.; Kabbouche, M.A.; Powers, S.W.; Hershey, A.D. Neuromagnetic abnormality of motor cortical activation and phases of headache attacks in childhood migraine. PLoS ONE 2013, 8, e83669. [Google Scholar] [CrossRef] [PubMed]
- Rier, L.; Zamyadi, R.; Zhang, J.; Emami, Z.; Seedat, Z.A.; Mocanu, S.; Gascoyne, L.E.; Allen, C.M.; Scadding, J.W.; Furlong, P.L.; et al. Mild traumatic brain injury impairs the coordination of intrinsic and motor-related neural dynamics. NeuroImage Clin. 2021, 32, 102841. [Google Scholar] [CrossRef]
- Mashat, M.E.M.; Lin, C.T.; Zhang, D. Effects of task complexity on motor imagery-based brain–computer interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.; Salleh, S.H.; Noor, A.M. Electroencephalographic motor imagery brain connectivity analysis for BCI: A review. Neural Comput. 2016, 28, 999–1041. [Google Scholar] [CrossRef]
- Meador, K.J.; Ray, P.G.; Echauz, J.R.; Loring, D.W.; Vachtsevanos, G.J. Gamma coherence and conscious perception. Neurology 2002, 59, 847–854. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Zalaudek, K.; Neuper, C. Event-related beta synchronization after wrist, finger and thumb movement. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1998, 109, 154–160. [Google Scholar] [CrossRef]
- Thaut, M.H.; Abiru, M. Rhythmic auditory stimulation in rehabilitation of movement disorders: A review of current research. Music Percept. 2010, 27, 263–269. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhang, Y.; Yan, R.; Liu, H.; Qiu, M. Conditional granger causality analysis of effective connectivity during motor imagery and motor execution in stroke patients. BioMed Res. Int. 2016, 2016, 3870863. [Google Scholar] [CrossRef]
- Benz, H.L.; Zhang, H.; Bezerianos, A.; Acharya, S.; Crone, N.E.; Zheng, X.; Thakor, N.V. Connectivity analysis as a novel approach to motor decoding for prosthesis control. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 20, 143–152. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Li, Y.; Gao, Y.; Liu, Y.; Ning, X. Investigating the Coherence Between Motor Cortex During Rhythmic Finger Tapping Using OPM-MEG. Photonics 2025, 12, 766. https://doi.org/10.3390/photonics12080766
Lu H, Li Y, Gao Y, Liu Y, Ning X. Investigating the Coherence Between Motor Cortex During Rhythmic Finger Tapping Using OPM-MEG. Photonics. 2025; 12(8):766. https://doi.org/10.3390/photonics12080766
Chicago/Turabian StyleLu, Hao, Yong Li, Yang Gao, Ying Liu, and Xiaolin Ning. 2025. "Investigating the Coherence Between Motor Cortex During Rhythmic Finger Tapping Using OPM-MEG" Photonics 12, no. 8: 766. https://doi.org/10.3390/photonics12080766
APA StyleLu, H., Li, Y., Gao, Y., Liu, Y., & Ning, X. (2025). Investigating the Coherence Between Motor Cortex During Rhythmic Finger Tapping Using OPM-MEG. Photonics, 12(8), 766. https://doi.org/10.3390/photonics12080766




