Raman Hyperspectroscopy and Chemometric Analysis of Blood Serum for Diagnosing Celiac Disease in Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Serum Samples
2.2. Sample Preparation and Raman Spectral Collection Method
2.3. Statistical Analysis
3. Results and Discussion
3.1. Visual Analysis of Blood Serum Samples of Healthy and Celiac Disease Donors
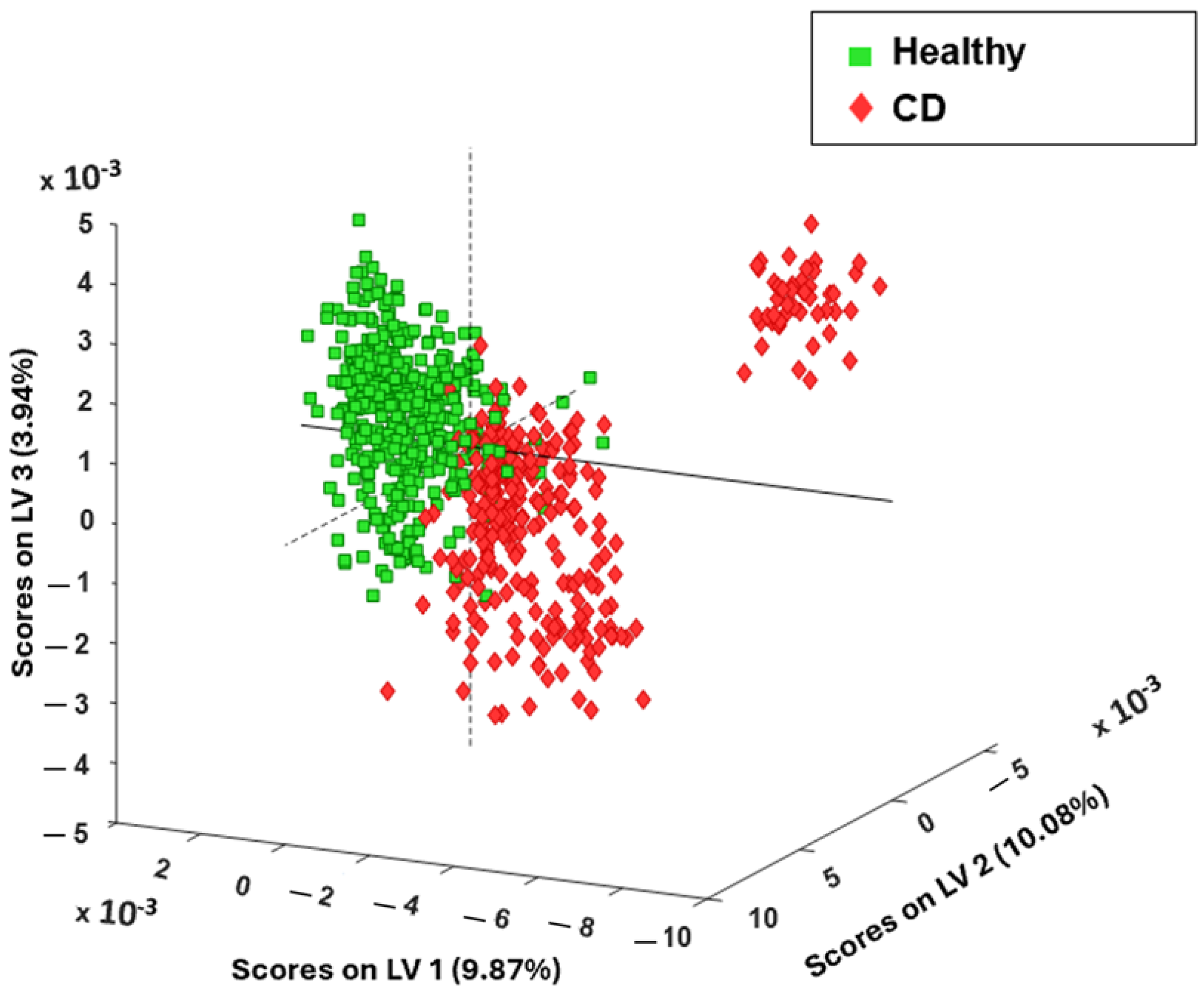
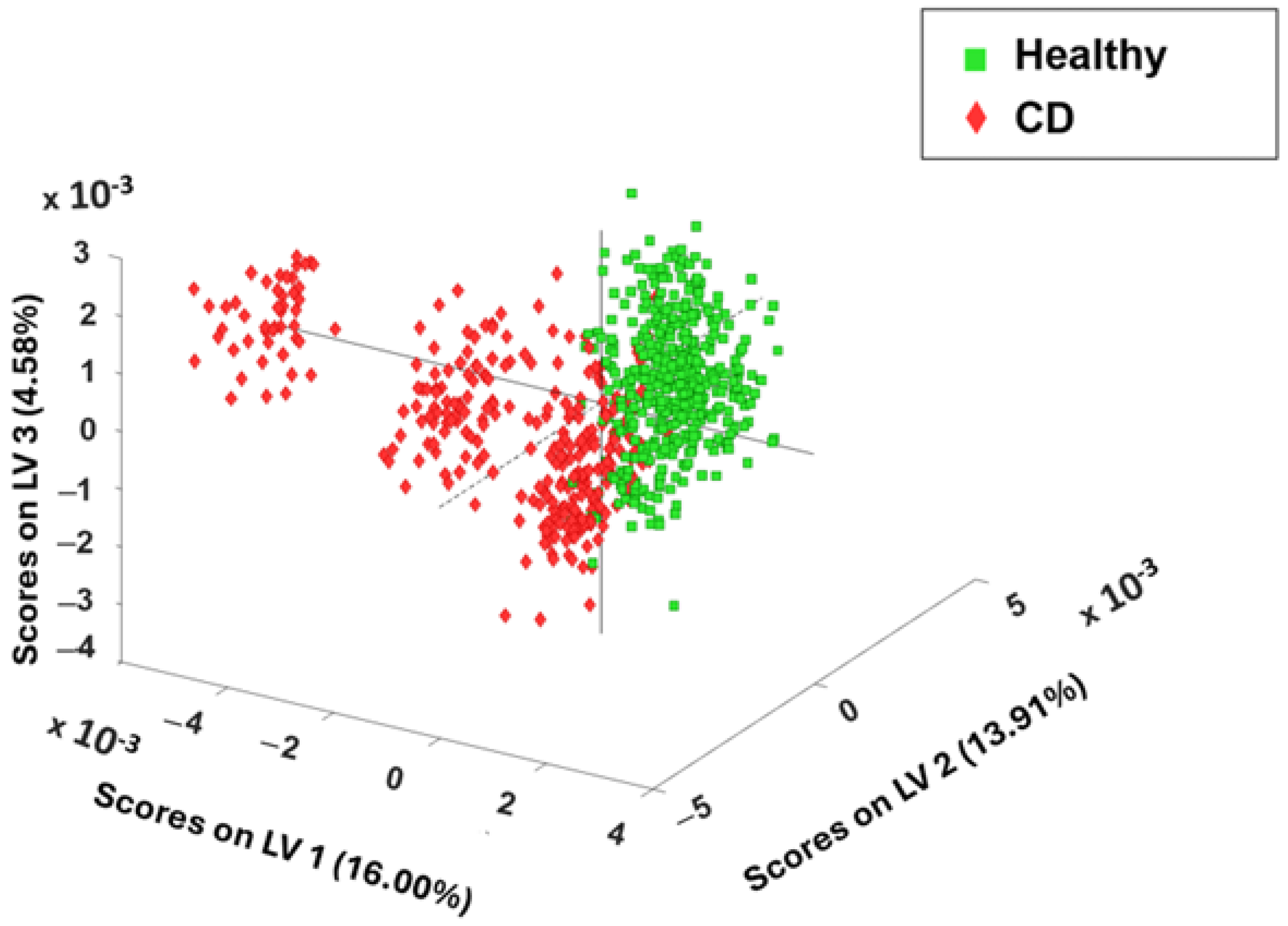
3.2. Chemometric Spectral Analysis of Blood Serum Samples Collected from Healthy and Celiac Disease Samples
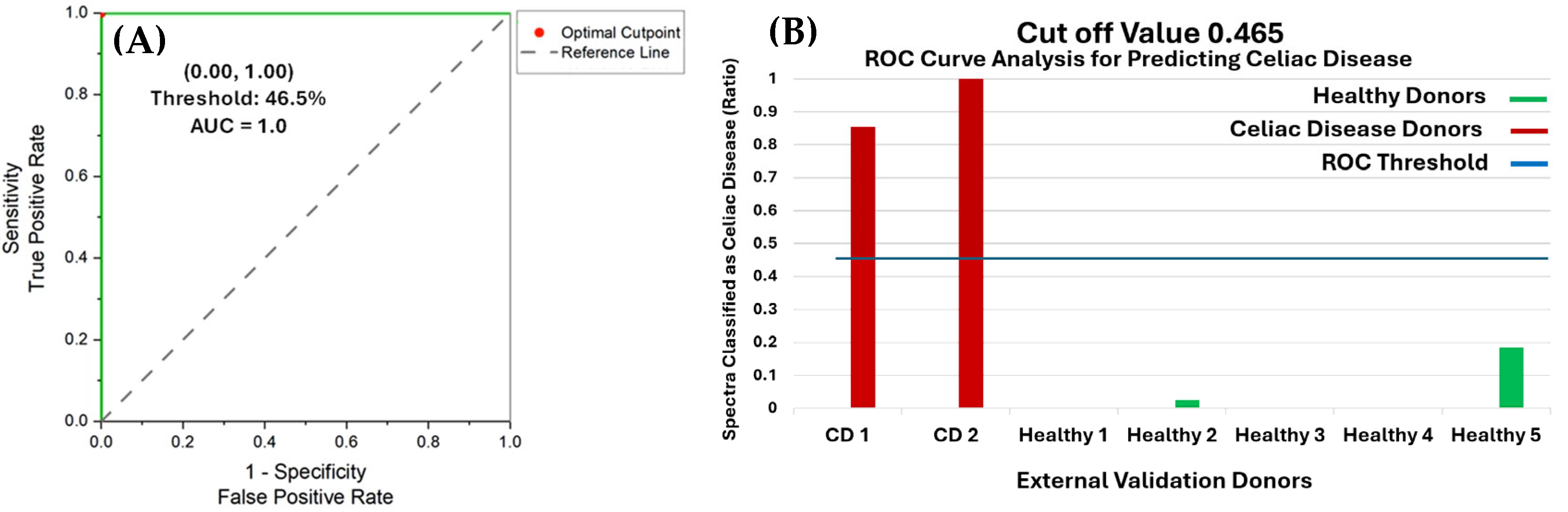
3.3. Receiver Operating Characteristic Curve Analysis of External Validation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van der Windt, D.A.W.M.; Jellema, P.; Mulder, C.J.; Kneepkens, C.M.F.; van der Horst, H.E. Diagnostic Testing for Celiac Disease Among Patients with Abdominal Symptoms: A Systematic Review. JAMA 2010, 303, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Akbari, M.; Vanga, R.; Kelly, C.P.; Hansen, J.; Theethira, T.; Tariq, S.; Dennis, M.; Leffler, D.A. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am. J. Gastroenterol. 2014, 109, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.S.; Ciclitira, P.J. Pathogenesis of coeliac disease: Implications for treatment. World J. Gastroenterol. 2001, 7, 772–776. [Google Scholar] [CrossRef]
- Kaswala, D.H.; Veeraraghavan, G.; Kelly, C.P.; Leffler, D.A. Celiac Disease: Diagnostic Standards and Dilemmas. Diseases 2015, 3, 86–101. [Google Scholar] [CrossRef]
- Vacca, M.; Porrelli, A.; Calabrese, F.M.; Lippolis, T.; Iacobellis, I.; Celano, G.; Pinto, D.; Russo, F.; Giannelli, G.; De Angelis, M. How Metabolomics Provides Novel Insights on Celiac Disease and Gluten-Free Diet: A Narrative Review. Front. Microbiol. 2022, 13, 859467. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Lee, J. Serologic testing in celiac disease: Practical guide for clinicians. Can. Fam. Physician 2016, 62, 38–43. [Google Scholar]
- Rostom, A.; Dubé, C.; Cranney, A.; Saloojee, N.; Sy, R.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V. The diagnostic accuracy of serologic tests for celiac disease: A systematic review. Gastroenterology 2005, 128, S38–S46. [Google Scholar] [CrossRef]
- Dieterich, W.; Ehnis, T.; Bauer, M.; Donner, P.; Volta, U.; Riecken, E.O.; Schuppan, D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 1997, 3, 797–801. [Google Scholar] [CrossRef]
- Lenhardt, A.; Plebani, A.; Marchetti, F.; Gerarduzzi, T.; Not, T.; Meini, A.; Villanacci, V.; Martelossi, S.; Ventura, A. Role of human-tissue transglutaminase IgG and anti-gliadin IgG antibodies in the diagnosis of coeliac disease in patients with selective immunoglobulin A deficiency. Dig. Liver Dis. 2004, 36, 730–734. [Google Scholar] [CrossRef]
- Raivio, T.; Kaukinen, K.; Nemes, E.; Laurila, K.; Collin, P.; Kovács, J.; Mäki, M.; Korponay-Szabó, I.R. Self transglutaminase-based rapid coeliac disease antibody detection by a lateral flow method. Aliment. Pharmacol. Ther. 2006, 24, 147–154. [Google Scholar] [CrossRef]
- Fasano, A.; Berti, I.; Gerarduzzi, T.; Not, T.; Colletti, R.B.; Drago, S.; Elitsur, Y.; Green, P.H.; Guandalini, S.; Hill, I.D. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: A large multicenter study. Arch. Intern. Med. 2003, 163, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Brar, P.; Holleran, S.; Ramakrishnan, R.; Green, P.H. Effect of gender on the manifestations of celiac disease: Evidence for greater malabsorption in men. Scand. J. Gastroenterol. 2005, 40, 183–187. [Google Scholar] [CrossRef]
- Green, P.H.R. The Role of Endoscopy in the Diagnosis of Celiac Disease. Gastroenterol. Hepatol. 2014, 10, 522–524. [Google Scholar]
- Bonamico, M.; Mariani, P.; Thanasi, E.; Ferri, M.; Nenna, R.; Tiberti, C.; Mora, B.; Mazzilli, M.C.; Magliocca, F.M. Patchy villous atrophy of the duodenum in childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 204–207. [Google Scholar] [CrossRef]
- Khristoforova, Y.; Bratchenko, L.; Bratchenko, I. Raman-Based Techniques in Medical Applications for Diagnostic Tasks: A Review. Int. J. Mol. Sci. 2023, 24, 15605. [Google Scholar] [CrossRef]
- Vyas, B.; Khatiashvili, A.; Galati, L.; Ngo, K.; Gildener-Leapman, N.; Larsen, M.; Lednev, I.K. Raman hyperspectroscopy of saliva and machine learning for Sjögren’s disease diagnostics. Sci. Rep. 2024, 14, 11135. [Google Scholar] [CrossRef]
- Hanna, K.; Krzoska, E.; Shaaban, A.M.; Muirhead, D.; Abu-Eid, R.; Speirs, V. Raman spectroscopy: Current applications in breast cancer diagnosis, challenges and future prospects. Br. J. Cancer 2022, 126, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Knorr, F.; Latka, I.; Vogt, M.; Hofmann, G.O.; Popp, J.; Schie, I.W. Real-time molecular imaging of near-surface tissue using Raman spectroscopy. Light Sci. Appl. 2022, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikova, E.; Ralbovsky, N.M.; Sikirzhytski, V.; Kazakov, O.; Halamkova, L.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Raman spectroscopy and machine learning for biomedical applications: Alzheimer’s disease diagnosis based on the analysis of cerebrospinal fluid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119188. [Google Scholar] [CrossRef]
- Ralbovsky, N.M.; Lednev, I.K. Towards development of a novel universal medical diagnostic method: Raman spectroscopy and machine learning. Chem. Soc. Rev. 2020, 49, 7428–7453. [Google Scholar] [CrossRef]
- Ralbovsky, N.M.; Dey, P.; Galfano, A.; Dey, B.K.; Lednev, I.K. Diagnosis of a model of Duchenne muscular dystrophy in blood serum of mdx mice using Raman hyperspectroscopy. Sci. Rep. 2020, 10, 11734. [Google Scholar] [CrossRef]
- Ryzhikova, E.; Ralbovsky, N.M.; Halámková, L.; Celmins, D.; Malone, P.; Molho, E.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Multivariate Statistical Analysis of Surface Enhanced Raman Spectra of Human Serum for Alzheimer’s Disease Diagnosis. Appl. Sci. 2019, 9, 3256. [Google Scholar] [CrossRef]
- Shi, T.; Li, J.; Li, N.; Chen, C.; Chen, C.; Chang, C.; Xue, S.; Liu, W.; Reyim, A.M.; Gao, F.; et al. Rapid diagnosis of celiac disease based on plasma Raman spectroscopy combined with deep learning. Sci. Rep. 2024, 14, 15056. [Google Scholar] [CrossRef] [PubMed]
- Acri, G.; Romano, C.; Costa, S.; Pellegrino, S.; Testagrossa, B. Raman Spectroscopy Technique: A Non-Invasive Tool in Celiac Disease Diagnosis. Diagnostics 2021, 11, 1277. [Google Scholar] [CrossRef] [PubMed]
- Ralbovsky, N.M.; Fitzgerald, G.S.; McNay, E.C.; Lednev, I.K. Towards development of a novel screening method for identifying Alzheimer’s disease risk: Raman spectroscopy of blood serum and machine learning. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119603. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikova, E.; Kazakov, O.; Halamkova, L.; Celmins, D.; Malone, P.; Molho, E.; Zimmerman, E.A.; Lednev, I.K. Raman spectroscopy of blood serum for Alzheimer’s disease diagnostics: Specificity relative to other types of dementia. J. Biophotonics 2015, 8, 584–596. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Serum metabolomics as a novel diagnostic approach for disease: A systematic review. Anal. Bioanal. Chem. 2012, 404, 1239–1245. [Google Scholar] [CrossRef]
- Vanmassenhove, J.; Vanholder, R.; Nagler, E.; Van Biesen, W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol. Dial. Transplant. 2013, 28, 254–273. [Google Scholar] [CrossRef]
- Schöler, N.; Langer, C.; Döhner, H.; Buske, C.; Kuchenbauer, F. Serum microRNAs as a novel class of biomarkers: A comprehensive review of the literature. Exp. Hematol. 2010, 38, 1126–1130. [Google Scholar] [CrossRef]
- Ogruc Ildiz, G.; Bayari, S.; Aksoy, U.M.; Yorguner, N.; Bulut, H.; Yilmaz, S.S.; Halimoglu, G.; Kabuk, H.N.; Yavuz, G.; Fausto, R. Auxiliary differential diagnosis of schizophrenia and phases of bipolar disorder based on the blood serum Raman spectra. J. Raman Spectrosc. 2020, 51, 2233–2244. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, A.; Rong, Z.; Wang, C.; Jia, X.; Zhang, K.; Xiao, R.; Wang, S. Fast and non-invasive serum detection technology based on surface-enhanced Raman spectroscopy and multivariate statistical analysis for liver disease. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.G.; Buckley, K.; Blades, M.W.; Turner, R.F.B. Raman Spectroscopy of Blood and Blood Components. Appl. Spectrosc. 2017, 71, 767–793. [Google Scholar] [CrossRef]
- Nargis, H.F.; Nawaz, H.; Ditta, A.; Mahmood, T.; Majeed, M.I.; Rashid, N.; Muddassar, M.; Bhatti, H.N.; Saleem, M.; Jilani, K.; et al. Raman spectroscopy of blood plasma samples from breast cancer patients at different stages. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117210. [Google Scholar] [CrossRef]
- Saade, J.; Silva, J.d.N.; de Farias, P.M.A.; Lopes, D.F.; Santos, C.T.d.; Farias, B.d.A.; Rodrigues, K.C.; Martin, A.A. Glicemical Analysis of Human Blood Serum Using FT-Raman: A New Approach. Photomed. Laser Surg. 2012, 30, 388–392. [Google Scholar] [CrossRef]
- Udensi, J.; Loughman, J.; Loskutova, E.; Byrne, H.J. Raman Spectroscopy of Carotenoid Compounds for Clinical Applications—A Review. Molecules 2022, 27, 9017. [Google Scholar] [CrossRef]
- Mędza, A.; Szlagatys-Sidorkiewicz, A. Nutritional Status and Metabolism in Celiac Disease: Narrative Review. J. Clin. Med. 2023, 12, 5107. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Singh, A.; Mehtab, W.; Gupta, V.; Chauhan, A.; Rajput, M.S.; Singh, N.; Ahuja, V.; Makharia, G.K. Patients with celiac disease are at high risk of developing metabolic syndrome and fatty liver. Intest. Res. 2021, 19, 106–114. [Google Scholar] [CrossRef]
- Kårhus, L.L.; Kriegbaum, M.; Grand, M.K.; Lind, B.S.; Møllehave, L.T.; Rumessen, J.J.; Andersen, C.L.; Linneberg, A. Biochemical abnormalities among patients referred for celiac disease antibody blood testing in a primary health care setting. Sci. Rep. 2022, 12, 6407. [Google Scholar] [CrossRef] [PubMed]
- Mediene, S.; Hakem, S.; Bard, J.; Medjaoui, I.; Benhamamouch, S.; Lebel, P.; Fruchart, J.; Clavey, V. Serum lipoprotein profile in Algerian patients with celiac disease. Clin. Chim. Acta 1995, 235, 189–196. [Google Scholar] [CrossRef]
- Leffler, D.A.; Edwards-George, J.; Dennis, M.; Schuppan, D.; Cook, F.; Franko, D.L.; Blom-Hoffman, J.; Kelly, C.P. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig. Dis. Sci. 2008, 53, 1573–1581. [Google Scholar] [CrossRef]
- Uribe, B.; González, O.; Blanco, M.E.; Albóniga, O.E.; Alonso, M.L.; Alonso, R.M. Analysis of the Heterogeneous Distribution of Amiloride and Propranolol in Dried Blood Spot by UHPLC-FLD and MALDI-IMS. Molecules 2019, 24, 4320. [Google Scholar] [CrossRef] [PubMed]
- Ralbovsky, N.M.; Lednev, I.K. Analysis of individual red blood cells for Celiac disease diagnosis. Talanta 2021, 221, 121642. [Google Scholar] [CrossRef] [PubMed]
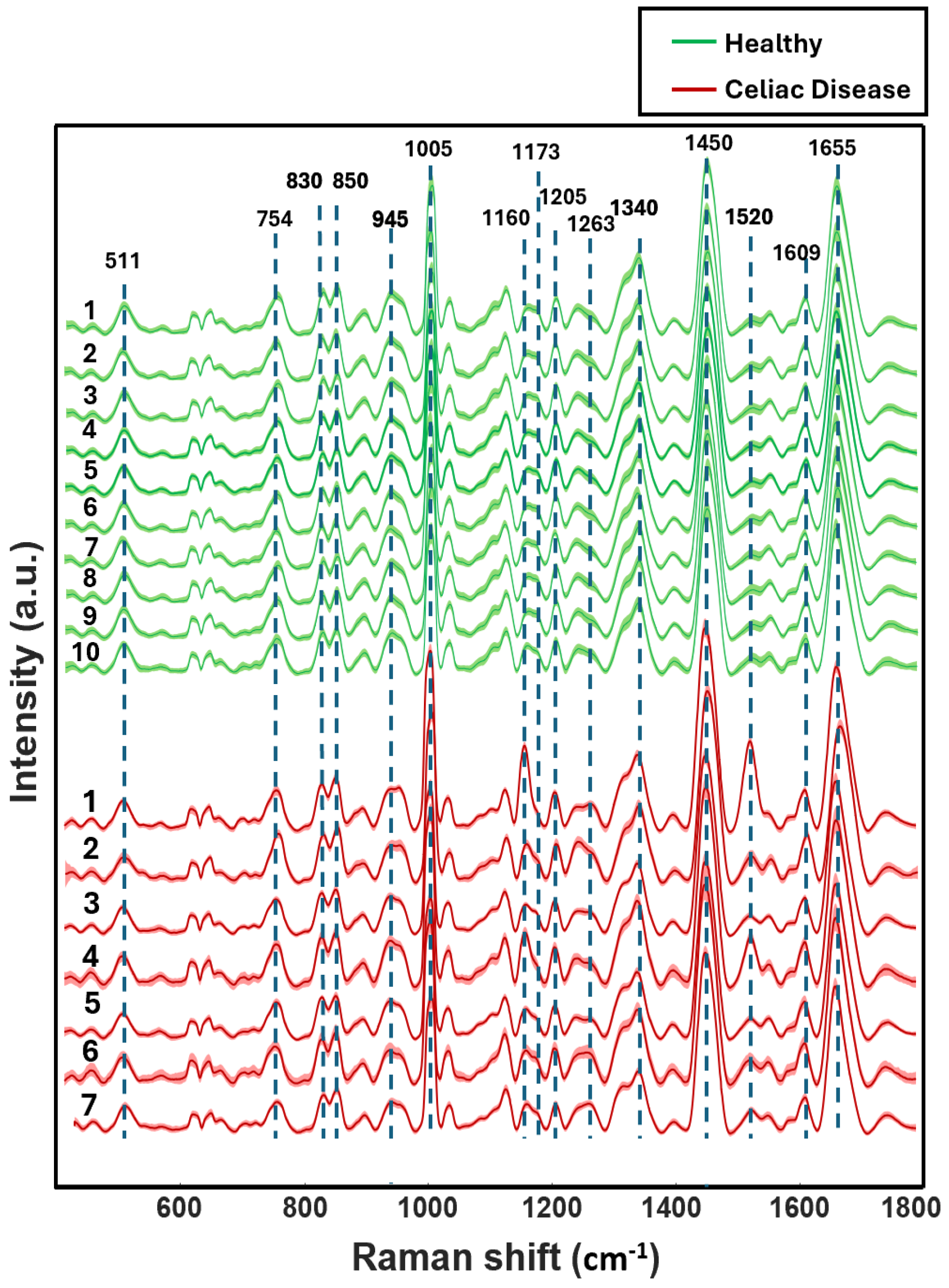
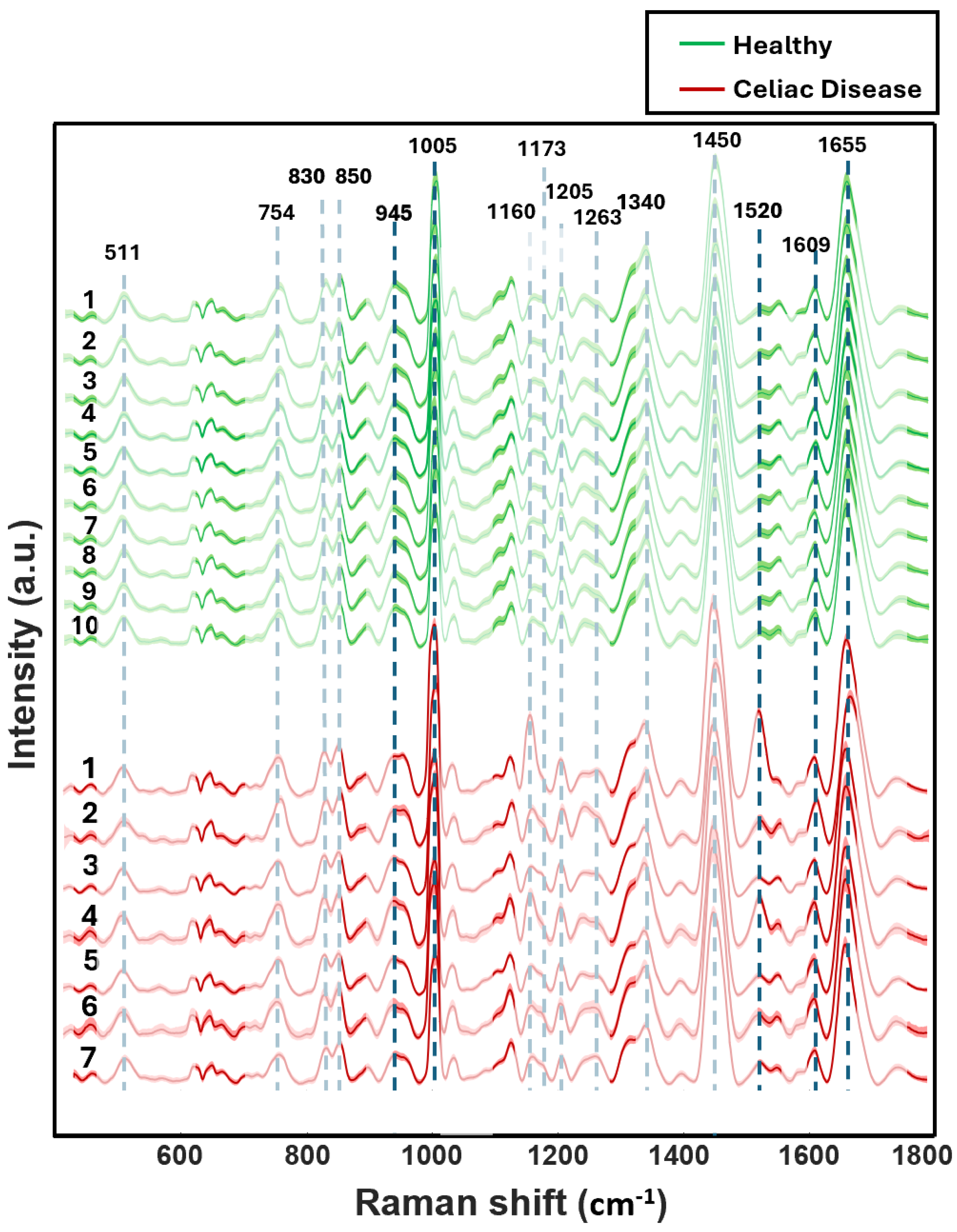
| Raman Bands (cm−1) | Tentative Assignment |
|---|---|
| 1655 | Protein (Amide I a-helix) phospholipids |
| 1609 | Phenylalanine ν(C=C)/Carotenoids |
| 1520 | Carotenoids |
| 1450 | Lipoproteins, phospholipids, δ(CH2), δ(CH3) |
| 1340 | Proteins (tryptophan) |
| 1263 | Phospholipids δ(CH) |
| 1205 | Amino acids ν(C=C) |
| 1173 | Cytosine, guanine |
| 1160 | Carotenoids |
| 1005 | Phenylalanine ν(C–H) |
| 945 | Phenylalanine ν(C–C) |
| 850 | Tyrosine |
| 830 | Tyrosine |
| 754 | Guanine, thymine |
| 511 | Cystine ν(S–S) |
| Internal Validation (PLS-DA) Based on 6 LVs | ||
| Prediction: Most Probable | Actual Class CD | Actual Class Healthy |
| Predicted as CD | 212 | 11 |
| Predicted as Healthy | 11 | 210 |
| Predicted as Unassigned | 0 | 0 |
| CD Prediction Sensitivity % 95 | CD Prediction Specificity % 95 | |
| External Validation (PLS-DA) | ||
| Prediction: Most Probable | Actual Class CD | Actual Class Healthy |
| Predicted as CD | 62 | 13 |
| Predicted as Healthy | 17 | 190 |
| Predicted as Unassigned | 0 | 0 |
| CD Prediction Sensitivity % 79 | CD Prediction Specificity % 94 | |
| External Validation (PLS-DA) | ||
| External Validation (PLS-DA) | External Validation (PLS-DA) | External Validation (PLS-DA) |
| Prediction: % 50 Threshold | Prediction: % 50 Threshold | Prediction: % 50 Threshold |
| Actual Class CD | Actual Class CD | Actual Class CD |
| Actual Class Healthy | Actual Class Healthy | Actual Class Healthy |
| CD Prediction Sensitivity % 79 | CD Prediction Specificity % 94 | |
| Internal Validation (GA-PLS-DA) Based on 2 LVs | ||
| Prediction: Most Probable | Actual Class CD | Actual Class Healthy |
| Predicted as CD | 208 | 15 |
| Predicted as Healthy | 15 | 206 |
| Predicted as Unassigned | 0 | 0 |
| CD Prediction Sensitivity % 93 | CD Prediction Specificity % 93 | |
| External Validation (GA-PLS-DA) | ||
| Prediction: Most Probable | Actual Class CD | Actual Class Healthy |
| Predicted as CD | 73 | 9 |
| Predicted as Healthy | 6 | 194 |
| Predicted as Unassigned | 0 | 0 |
| CD Prediction Sensitivity % 92 | CD Prediction Specificity % 96 | |
| External Validation (GA-PLS-DA) | ||
| Prediction: % 50 Threshold | Actual Class CD | Actual Class Healthy |
| Predicted as CD | 73 | 9 |
| Predicted as Healthy | 6 | 194 |
| Predicted as Unassigned | 0 | 0 |
| CD Prediction Sensitivity % 92 | CD Prediction Specificity % 96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hetlani, E.; Almehmadi, L.M.; Lednev, I.K. Raman Hyperspectroscopy and Chemometric Analysis of Blood Serum for Diagnosing Celiac Disease in Adults. Photonics 2025, 12, 553. https://doi.org/10.3390/photonics12060553
Al-Hetlani E, Almehmadi LM, Lednev IK. Raman Hyperspectroscopy and Chemometric Analysis of Blood Serum for Diagnosing Celiac Disease in Adults. Photonics. 2025; 12(6):553. https://doi.org/10.3390/photonics12060553
Chicago/Turabian StyleAl-Hetlani, Entesar, Lamyaa M. Almehmadi, and Igor K. Lednev. 2025. "Raman Hyperspectroscopy and Chemometric Analysis of Blood Serum for Diagnosing Celiac Disease in Adults" Photonics 12, no. 6: 553. https://doi.org/10.3390/photonics12060553
APA StyleAl-Hetlani, E., Almehmadi, L. M., & Lednev, I. K. (2025). Raman Hyperspectroscopy and Chemometric Analysis of Blood Serum for Diagnosing Celiac Disease in Adults. Photonics, 12(6), 553. https://doi.org/10.3390/photonics12060553






