Photobiomodulation in Complex Female Infertility Profile: A Case Report with 12-Month Follow-Up and Review of Current Mechanism in Reproductive Photomedicine
Abstract
1. Introduction
1.1. Pathophysiology of Female Infertility
1.1.1. Endometriosis and Infertility
1.1.2. Polycystic Ovarian Syndrome (PCOS) and Infertility
1.1.3. Low Ovarian Reserve in the Context of Endometriosis and PCOS
1.2. Current Fertility Treatments
1.2.1. Prescribed Medication
Metformin
Clomiphene Citrate (CC)
Letrozole
Gonadotrophins
1.2.2. Surgery
1.2.3. Anti-Inflammatory Diet
1.2.4. Dietary Supplements
1.2.5. Photobiomodulation Therapy
2. Materials and Methods
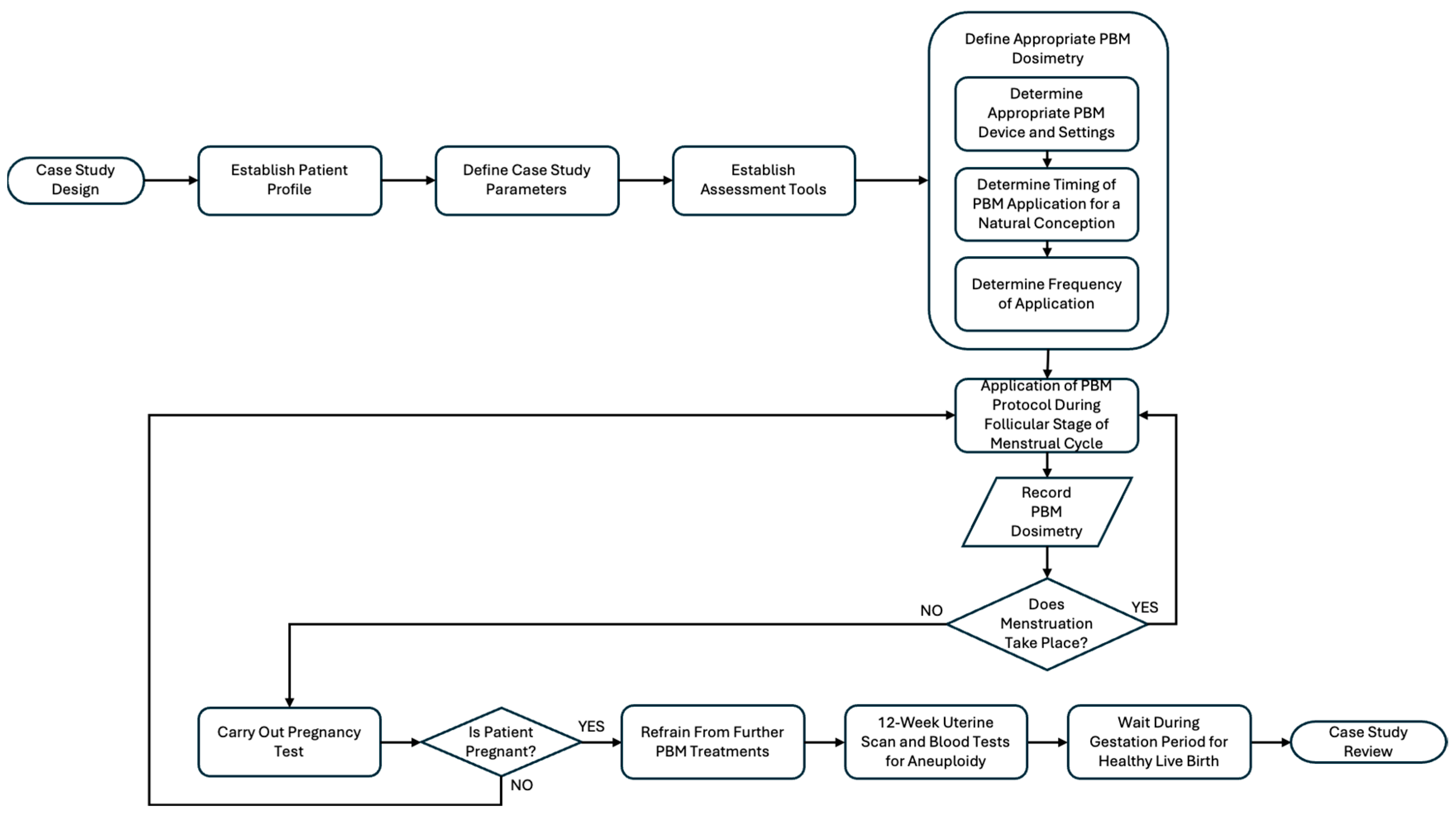
2.1. Study Design
2.2. Subject Clinical Profile
2.3. Research Focused Questions and PICO
2.4. Treatment Protocol and PBM Irradiation Points
- Lymph nodes: a low-dose short protocol of PBM was applied to four lymph nodes to prepare the system for PBM:
- ○
- Groin: Bilateral inguinal lymph nodes;
- ○
- Clavicle: Bilateral thymus lymph nodes.
- Inverted triangle in the lower abdominal area above the female reproductive system, primarily to target the ovaries and the uterus, approximately the size of a stretched open hand. To calculate dosimetry the irradiated area was assumed to be 10 cm2.
- The area around the naval and upper digestive tract to aid with digestion and waste. PBM improves gut microbiome and digestive function, which has a positive effect on genital tract microbiome to aid fertility outcomes [53]. To calculate dosimetry the irradiated area was assumed to be 10 cm2.
- Lower back, lumbar spine from L3 to the base of sacrum. PBM was applied to target the back of the uterus and lower abdominal area. Holes in the sacrum allow light wavelengths to reach the back of the uterus, and based on traditional Chinese medicine, the second holes on the sacrum are relative to the menstrual cycle. To calculate dosimetry the irradiated area was assumed to be 5 cm2.
- Cervical spine from C1 to T1, to cover the nerves connecting to the thyroid gland and the Vagus nerve, to positively impact the parasympathetic nervous system. To calculate dosimetry the irradiated area was assumed to be 5 cm2.
- The PBM dosimetry was determined based on previously published studies for PBM and female fertility [22,48,49,54]:
- ○
- 12,600 J per PBM session in 20 min 45 s;
- ○
- Infrared wavelengths 800 nm, 900 nm and 970 nm for deep absorption into the tissue, and 660 nm to include superficial absorption in the tissue.
2.5. Assessment Tools
2.5.1. Method of Conception
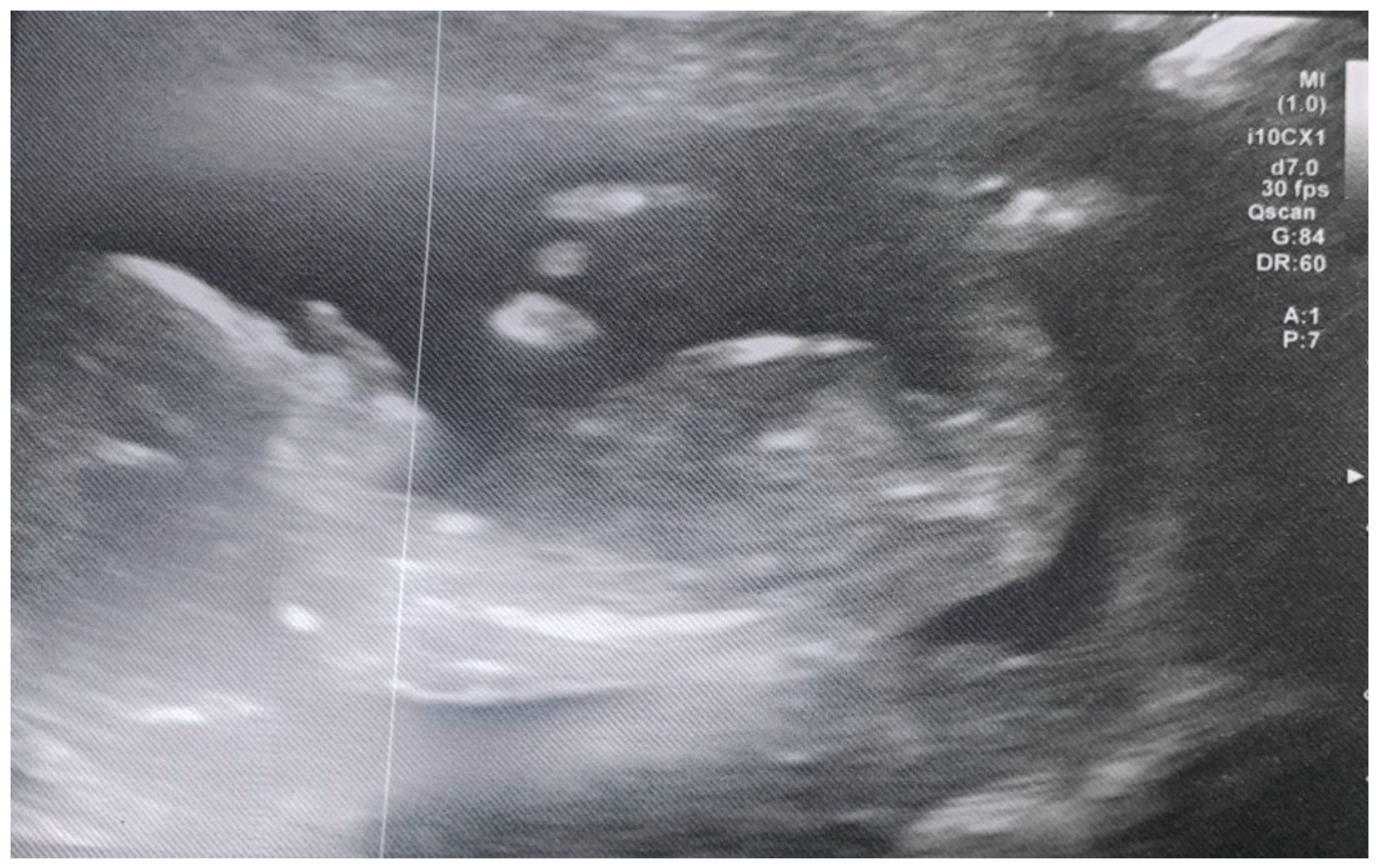
2.5.2. Uterine Scans
2.5.3. Biochemical Markers
- Pregnancy-associated plasma protein A (PAPP-A) is a biomarker included in the first-trimester combined screening, typically conducted between 11 and 14 weeks of gestation. Low levels of PAPP-A (≤0.4 MoM, multiples of the median) have been associated with an increased risk of adverse outcomes, including low birth weight, pre-term birth, preeclampsia (characterised by hypertension and proteinuria) and mid-trimester miscarriage [56].
- Free beta human chorionic gonadotropin (β-hCG) is also measured during early screening to assess the risk of chromosomal abnormalities such as Down syndrome. Abnormal levels of free β-hCG, specifically values < 0.5 MoM or >2.0 MoM have been linked to various pregnancy complications, including late foetal loss, gestational hypertension, preeclampsia, intrauterine growth restriction (IUGR), pre-term delivery and intrauterine foetal demise (IUFD) [57].
2.6. Endpoints
2.6.1. Primary Outcome
2.6.2. Secondary Outcomes
- We assessed and reported any adverse effects of PBM in the case of endometriosis, PCOS and low ovarian reserve.
- We defined effective PBM and treatment protocols for future adjunct treatments for complex infertility profiles.
3. Case Description and Results
3.1. Cohort Demographic Characteristics
3.2. Cohort Biochemical Markers
3.3. Case Subject
- 2018: Elevated progesterone levels indicated PCOS, and a clinical diagnosis.
- 2019–2020: Clomid prescribed to support ovulation for a natural conception, without success.
- 2021: Chemical pregnancy recorded in December (positive pregnancy test post-ovulation resulted in menstruation within the same cycle).
- July 2022: Failed IVF cycle.
- 2022: Surgery for the removal of a small mass/stage 1–2 endometriosis in December.
- 2023: PBM therapy given pre-ovulation for 4 consecutive months June to September.
- Metformin prescribed July-September.
- From June to September the patient made dietary changes in line with the anti-inflammatory Mediterranean diet [33].
- Patient progress assessed at each PBM treatment, with the patient reporting improved energy and a healthy weight loss. No side effects of the PBM treatment were observed.
- September 2023: Natural conception achieved.
3.4. PBM Treatment Protocol Leading to a Natural Conception
4. Discussion
4.1. Role of PBM in Ovarian Health
4.2. Role of PBM in Enhancing Endometrial Receptivity
- Improved microcirculation: PBM has been shown to enhance local blood flow by stimulating NO release and vasodilation, leading to better oxygenation and nutrient delivery to the endometrial tissue [59].
- Mitochondrial activation: PBM activates cytochrome c oxidase in the mitochondrial respiratory chain, increasing ATP production. This boost in cellular energy supports endometrial cell repair, angiogenesis and cellular proliferation [59].
- Stimulation of growth factors and cytokines: PBM may promote the release of vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and fibroblast growth factors (FGFs), which are critical for endometrial growth, vascularization and receptivity [60].
- Cytokine modulation: PBM appears to modulate the expression of pro-inflammatory and anti-inflammatory cytokines, creating an immune environment favourable for embryo implantation. This modulation is believed to be mediated through various mechanisms, including the activation of mitochondrial pathways and the regulation of ROS. These effects may contribute to improved endometrial receptivity and successful embryo implantation [61]. Hence, the evidence supports the role of PBM in modulating cytokine expression, which may contribute to creating an immune environment conducive to embryo implantation. However, further research is needed to fully understand the mechanisms involved and to establish standardised protocols for clinical application.
4.3. PBM Effects on Oocyte Quality and Maturation
4.4. Natural Conception as an Outcome
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chauhan, S.; More, A.; Chauhan, V.; Kathane, A. Endometriosis: A Review of Clinical Diagnosis, Treatment, and Pathogenesis. Cureus 2022, 14, e28864. [Google Scholar] [CrossRef]
- Strathy, J.H.; Molgaard, C.A.; Coulam, C.B.; Melton, L.J. Endometriosis and Infertility: A Laparoscopic Study of Endometriosis among Fertile and Infertile Women. Fertil. Steril. 1982, 38, 667–672. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and Treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Macer, M.L.; Taylor, H.S. Endometriosis and Infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, Z.; Li, M.; Zhang, Y.; Nan, F. Decreased Oocyte Quality in Patients with Endometriosis Is Closely Related to Abnormal Granulosa Cells. Front. Endocrinol. 2023, 14, 1226687. [Google Scholar] [CrossRef]
- Prescott, J.; Farland, L.V.; Tobias, D.K.; Gaskins, A.J.; Spiegelman, D.; Chavarro, J.E.; Rich-Edwards, J.W.; Barbieri, R.L.; Missmer, S.A. A Prospective Cohort Study of Endometriosis and Subsequent Risk of Infertility. Hum. Reprod. 2016, 31, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.K.; Kao, C.-N.; Quinn, M.; Lenhart, N.; Rosen, M.; Cedars, M.I.; Huddleston, H. Differential Rate in Decline in Ovarian Reserve Markers in Women with Polycystic Ovary Syndrome Compared with Control Subjects: Results of a Longitudinal Study. Fertil. Steril. 2018, 109, 526–531. [Google Scholar] [CrossRef]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Kousta, E.; White, D.M.; Cela, E.; McCarthy, M.I.; Franks, S. The Prevalence of Polycystic Ovaries in Women with Infertility. Hum. Reprod. 1999, 14, 2720–2723. [Google Scholar] [CrossRef][Green Version]
- Cunha, A.; Póvoa, A.M. Infertility Management in Women with Polycystic Ovary Syndrome: A Review. Porto Biomed. J. 2021, 6, e116. [Google Scholar] [CrossRef]
- Hart, R.; Doherty, D.A. The Potential Implications of a PCOS Diagnosis on a Woman’s Long-Term Health Using Data Linkage. J. Clin. Endocrinol. Metab. 2015, 100, 911–919. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.-D.; Amer, S. The Role of Chronic Inflammation in Polycystic Ovarian Syndrome—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Gupta, Y.; Khemani, M.; Aggarwal, S. Thyroid Disorders and Polycystic Ovary Syndrome: An Emerging Relationship. Indian J. Endocr. Metab. 2015, 19, 25. [Google Scholar] [CrossRef]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished Ovarian Reserve, Premature Ovarian Failure, Poor Ovarian Responder—A Plea for Universal Definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Gong, X.; Wang, C.C.; Zhang, T.; Huang, J. Diminished Ovarian Reserve in Endometriosis: Insights from In Vitro, In Vivo, and Human Studies—A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15967. [Google Scholar] [CrossRef] [PubMed]
- Lessans, N.; Gilan, A.; Dick, A.; Bibar, N.; Saar, T.D.; Porat, S.; Dior, U.P. Ovarian Reserve Markers of Women with Superficial Endometriosis. Int. J. Gynecol. Obstet. 2024, 165, 696–702. [Google Scholar] [CrossRef]
- Waghmare, S.V.; Shanoo, A. Polycystic Ovary Syndrome: A Literature Review With a Focus on Diagnosis, Pathophysiology, and Management. Cureus 2023, 15, e47408. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, C.; Yang, C.; Shan, X.; Meng, X.-Q.; Zhang, M. Unveiling the Role of Chronic Inflammation in Ovarian Aging: Insights into Mechanisms and Clinical Implications. Hum. Reprod. 2024, 39, 1599–1607. [Google Scholar] [CrossRef]
- Uri-Belapolsky, S.; Shaish, A.; Eliyahu, E.; Grossman, H.; Levi, M.; Chuderland, D.; Ninio-Many, L.; Hasky, N.; Shashar, D.; Almog, T.; et al. Interleukin-1 Deficiency Prolongs Ovarian Lifespan in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, 12492–12497. [Google Scholar] [CrossRef]
- Treasure, T. Fertility: Assessment and Treatment for People with Fertility Problems; National Institute for Health and Clinical Excellence: Manchester, UK, 2013; ISBN 978-1-4731-0029-9. [Google Scholar]
- Phypers, R.; Berisha-Muharremi, V.; Hanna, R. The Efficacy of Multiwavelength Red and Near-Infrared Transdermal Photobiomodulation Light Therapy in Enhancing Female Fertility Outcomes and Improving Reproductive Health: A Prospective Case Series with 9-Month Follow-Up. J. Clin. Med. 2024, 13, 7101. [Google Scholar] [CrossRef] [PubMed]
- Morley, L.C.; Tang, T.M.H.; Balen, A.H. Metformin Therapy for the Management of Infertility in Women with Polycystic Ovary Syndrome: Scientific Impact Paper No. 13. BJOG 2017, 124, e306–e313. [Google Scholar] [CrossRef]
- Tso, L.O.; Costello, M.F.; Albuquerque, L.E.T.; Andriolo, R.B.; Macedo, C.R. Metformin Treatment before and during IVF or ICSI in Women with Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. 2020, 2020, CD006105. [Google Scholar] [CrossRef]
- Romanski, P.A.; Bortoletto, P.; Malmsten, J.E.; Tan, K.S.; Spandorfer, S.D. Pregnancy Outcomes after Oral and Injectable Ovulation Induction in Women with Infertility with a Low Antimüllerian Hormone Level Compared with Those with a Normal Antimüllerian Hormone Level. Fertil. Steril. 2022, 118, 1048–1056. [Google Scholar] [CrossRef]
- Conforti, A.; Carbone, L.; Di Girolamo, R.; Iorio, G.G.; Guida, M.; Campitiello, M.R.; Ubaldi, F.M.; Rienzi, L.; Vaiarelli, A.; Cimadomo, D.; et al. Therapeutic Management in Women with a Diminished Ovarian Reserve: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Fertil. Steril. 2025, 123, 457–476. [Google Scholar] [CrossRef]
- Diamond, M.P.; Legro, R.S.; Coutifaris, C.; Alvero, R.; Robinson, R.D.; Casson, P.; Christman, G.M.; Ager, J.; Huang, H.; Hansen, K.R.; et al. Letrozole, Gonadotropin, or Clomiphene for Unexplained Infertility. N. Engl. J. Med. 2015, 373, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt-Hawes, E.M.; Campbell, N.; Maley, P.E.; Won, H.; Hooshmand, D.; Henry, A.; Ledger, W.; Abbott, J.A. The Surgical Treatment of Severe Endometriosis Positively Affects the Chance of Natural or Assisted Pregnancy Postoperatively. BioMed Res. Int. 2015, 2015, 438790. [Google Scholar] [CrossRef]
- La Marca, A.; Semprini, M.; Mastellari, E.; Donno, V.; Capuzzo, M.; Alboni, C.; Giulini, S. Fertility Preservation in Women with Endometriosis. Hum. Reprod. Open 2025, 2025, hoaf012. [Google Scholar] [CrossRef]
- Krämer, B.; Andress, J.; Neis, F.; Hoffmann, S.; Brucker, S.; Kommoss, S.; Höller, A. Adhesion Prevention after Endometriosis Surgery—Results of a Randomized, Controlled Clinical Trial with Second-Look Laparoscopy. Langenbeck’s Arch. Surg. 2021, 406, 2133–2143. [Google Scholar] [CrossRef]
- Alesi, S.; Villani, A.; Mantzioris, E.; Takele, W.W.; Cowan, S.; Moran, L.J.; Mousa, A. Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients 2022, 14, 3914. [Google Scholar] [CrossRef] [PubMed]
- Nirgianakis, K.; Egger, K.; Kalaitzopoulos, D.R.; Lanz, S.; Bally, L.; Mueller, M.D. Effectiveness of Dietary Interventions in the Treatment of Endometriosis: A Systematic Review. Reprod. Sci. 2022, 29, 26–42. [Google Scholar] [CrossRef]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 Restores Oocyte Mitochondrial Function and Fertility during Reproductive Aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef]
- Abodi, M.; De Cosmi, V.; Parazzini, F.; Agostoni, C. Omega-3 Fatty Acids Dietary Intake for Oocyte Quality in Women Undergoing Assisted Reproductive Techniques: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 275, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Trop-Steinberg, S.; Gal, M.; Azar, Y.; Kilav-Levin, R.; Heifetz, E.M. Effect of Omega-3 Supplements or Diets on Fertility in Women: A Meta-Analysis. Heliyon 2024, 10, e29324. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Demidova, T.N. Mechanisms of Low Level Light Therapy. In Proceedings of the Mechanisms for Low-Light Therapy, San Jose, CA, USA, 21–26 January 2006; Hamblin, M.R., Waynant, R.W., Anders, J., Eds.; SPIE: Bellingham, WA, USA, 2006; Volumn 6140, p. 614001. [Google Scholar] [CrossRef]
- Hanna, R.; Miron, I.C.; Benedicenti, S. A Novel Therapeutic Approach of 980 Nm Photobiomodulation Delivered with Flattop Beam Profile in Management of Recurrent Aphthous Stomatitis in Paediatrics and Adolescents—A Case Series with 3-Month Follow-Up. J. Clin. Med. 2024, 13, 2007. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, B.; Mohammadi, K.; Hodjat, M.; Hakimiha, N.; Sayar, F.; Kharazi Fard, M.J.; Sadatmansouri, S.; Hanna, R. An Evaluation of Photobiomodulation Effects on Human Gingival Fibroblast Cells under Hyperglycemic Condition: An in Vitro Study. Lasers Med. Sci. 2023, 39, 9. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A Comparative Study Between the Effectiveness of 980 Nm Photobiomodulation Delivered by Hand-Piece With Gaussian vs. Flat-Top Profiles on Osteoblasts Maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Raber-Durlacher, J.E.; Benedicenti, S. Role of Photobiomodulation Therapy in Neurological Primary Burning Mouth Syndrome. A Systematic Review and Meta-Analysis of Human Randomised Controlled Clinical Trials. Pharmaceutics 2021, 13, 1838. [Google Scholar] [CrossRef]
- Hanna, R.; Bensadoun, R.J.; Beken, S.V.; Burton, P.; Carroll, J.; Benedicenti, S. Outpatient Oral Neuropathic Pain Management with Photobiomodulation Therapy: A Prospective Analgesic Pharmacotherapy-Paralleled Feasibility Trial. Antioxidants 2022, 11, 533. [Google Scholar] [CrossRef]
- Oubiña, G.; Pascuali, N.; Scotti, L.; Di Pietro, M.; La Spina, F.A.; Buffone, M.G.; Higuera, J.; Abramovich, D.; Parborell, F. Low Level Laser Therapy (LLLT) Modulates Ovarian Function in Mature Female Mice. Prog. Biophys. Mol. Biol. 2019, 145, 10–18. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, R.; Peng, Y.; Pei, Q.; Wu, L.; Wang, C.; Ni, W.; Li, M.; Zhang, Y.; Yao, M. Photobiomodulation Ameliorates Ovarian Aging by Alleviating Oxidative Stress and Inflammation Damage and Improving Mitochondrial Function. J. Photochem. Photobiol. B Biol. 2024, 260, 113024. [Google Scholar] [CrossRef] [PubMed]
- Oubiña, G.; Pascuali, N.; Scotti, L.; Bianchi, S.; May, M.; Martínez, J.E.; Marchese Ragona, C.; Higuera, J.; Abramovich, D.; Parborell, F. Local Application of Low Level Laser Therapy in Mice Ameliorates Ovarian Damage Induced by Cyclophosphamide. Mol. Cell. Endocrinol. 2021, 531, 111318. [Google Scholar] [CrossRef]
- Hochler, H.; Lipschuetz, M.; Suissa-Cohen, Y.; Weiss, A.; Sela, H.Y.; Yagel, S.; Rosenbloom, J.I.; Grisaru-Granovsky, S.; Rottenstreich, M. The Impact of Advanced Maternal Age on Pregnancy Outcomes: A Retrospective Multicenter Study. J. Clin. Med. 2023, 12, 5696. [Google Scholar] [CrossRef]
- Grinsted, A.; Grinsted Hillegass, M. Photobiomodulation for Infertility. Available online: https://ecronicon.com/ecgy/photobiomodulation-for-infertility (accessed on 1 July 2025).
- Ohshiro, T. Personal overview of the application of lllt in severely infertile japanese females. Laser Ther. 2012, 21, 97–103. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute. Available online: www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm (accessed on 1 July 2025).
- Gupta, V.; Sharma, V.K. Skin Typing: Fitzpatrick Grading and Others. Clin. Dermatol. 2019, 37, 430–436. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Jahani-Sherafat, S.; Taghavi, H.; Asri, N.; Rezaei Tavirani, M.; Razzaghi, Z.; Rostami-Nejad, M. The Effectiveness of Photobiomodulation Therapy in Modulation the Gut Microbiome Dysbiosis Related Diseases. Gastroenterol. Hepatol. Bed Bench 2023, 16, 386–393. [Google Scholar] [CrossRef]
- Fujii, S.; Ohshiro, T.; Ohshiro, T.; Sasaki, K.; Taniguchi, Y. Proximal priority treatment using the neck irradiator for adjunctive treatment of female infertility. Laser Ther. 2007, 16, 133–136. [Google Scholar] [CrossRef][Green Version]
- Detti, L.; Francillon, L.; Christiansen, M.E.; Peregrin-Alvarez, I.; Goedecke, P.J.; Bursac, Z.; Roman, R.A. Early Pregnancy Ultrasound Measurements and Prediction of First Trimester Pregnancy Loss: A Logistic Model. Sci. Rep. 2020, 10, 1545. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Cividino, A.; Rossetti, E.; Maurigh, A.; Londero, A.P.; Driul, L. First Trimester PAPP-A Serum Levels and Long-Term Metabolic Outcome of Mothers and Their Offspring. Sci. Rep. 2020, 10, 5131. [Google Scholar] [CrossRef]
- Younesi, S.; Eslamian, L.; Khalafi, N.; Taheri Amin, M.M.; Saadati, P.; Jamali, S.; Balvayeh, P.; Modarressi, M.-H.; Savad, S.; Amidi, S.; et al. Extreme βHCG Levels in First Trimester Screening Are Risk Factors for Adverse Maternal and Fetal Outcomes. Sci. Rep. 2023, 13, 1228. [Google Scholar] [CrossRef]
- Alves, E.D.; Bonfá, A.L.O.; Pigatto, G.R.; Anselmo-Franci, J.A.; Achcar, J.A.; Parizotto, N.A.; Montrezor, L.H. Photobiomodulation Can Improve Ovarian Activity in Polycystic Ovary Syndrome-Induced Rats. J. Photochem. Photobiol. B Biol. 2019, 194, 6–13. [Google Scholar] [CrossRef]
- Jafarabadi, M.; Farbod, Y.; Shariat, M. Low-Level Laser Therapy for Improvement of In Vitro Fertilization Outcomes in Patients with Recurrent Implantation Failure: A Randomized Clinical Trial. J. Lasers Med. Sci. 2024, 15, e15. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, J.; Sun, Y. Platelet-Rich Plasma as a Potential New Strategy in the Endometrium Treatment in Assisted Reproductive Technology. Front. Endocrinol. 2021, 12, 707584. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, S.; Defensor, E.; Ciari, P.; Ogawa, G.; Vidano, L.; Lin, J.S.; Fortkort, J.A.; Shamloo, M.; Barron, A.E. The Anti-Inflammatory Effects of Photobiomodulation Are Mediated by Cytokines: Evidence from a Mouse Model of Inflammation. Front. Neurosci. 2023, 17, 1150156. [Google Scholar] [CrossRef] [PubMed]
- Sahraeian, S.; Abbaszadeh, H.A.; Taheripanah, R.; Edalatmanesh, M.A. Extracellular Vesicle-Derived Cord Blood Plasma and Photobiomodulation Therapy Down-Regulated Caspase 3, LC3 and Beclin 1 Markers in the PCOS Oocyte: An In Vitro Study. J. Lasers Med. Sci. 2023, 14, e23. [Google Scholar] [CrossRef] [PubMed]
| Age at Conception (Years) | Age at Birth (Years) | Weight (Pre-Conception) (kg) | Height (cm) | BMI | Skin Colour | |
|---|---|---|---|---|---|---|
| Subject | 27 | 28 | 72 | 158 | 28.8 | III |
| Manufacturer | K-Laser |
|---|---|
| Semiconductor materials (emitter type) | GaAIAs |
| Probe design | 4 wavelengths probe |
| Device classification | Type 4 Laser |
| Beam delivery system | Fibre |
| Laser-aiming beam | None |
| Wavelength (nm) | 660, 800, 905, 970 |
| Operating emission mode | A combination of CW and SP |
| Polarisation | Linear |
| Therapeutic power output for 800, 905, 970 (W) | ~15 |
| Ratio of power output divided equally between 800, 905, 970 nm | 1:1:1 |
| Therapeutic power output for 660 nm (mW) | ~120 |
| Total fluence (J/cm2) per point (irradiation area of 10 cm2) | 3150 |
| Total irradiation time over 10 cm2 | 5 mins and 15 s |
| Total number of irradiated points above the ovaries/uterus/abdomen | 3 |
| Total number of irradiated points at lower back/sacrum/cervical spine | 1 |
| Total of fluence (J/cm2) per session | 12,600 |
| Total irradiation time per session | 20 mins and 45 s |
| Time interval | Relatively every three weeks |
| Treatment frequency | 1 or 2 sessions per month |
| Total treatment sessions | 5 sessions |
| Treatment duration | 4 months |
| Scanning technique | Moveable application |
| Light-skin tissue distance (cm) | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phypers, R.; Hanna, R. Photobiomodulation in Complex Female Infertility Profile: A Case Report with 12-Month Follow-Up and Review of Current Mechanism in Reproductive Photomedicine. Photonics 2025, 12, 1021. https://doi.org/10.3390/photonics12101021
Phypers R, Hanna R. Photobiomodulation in Complex Female Infertility Profile: A Case Report with 12-Month Follow-Up and Review of Current Mechanism in Reproductive Photomedicine. Photonics. 2025; 12(10):1021. https://doi.org/10.3390/photonics12101021
Chicago/Turabian StylePhypers, Ruth, and Reem Hanna. 2025. "Photobiomodulation in Complex Female Infertility Profile: A Case Report with 12-Month Follow-Up and Review of Current Mechanism in Reproductive Photomedicine" Photonics 12, no. 10: 1021. https://doi.org/10.3390/photonics12101021
APA StylePhypers, R., & Hanna, R. (2025). Photobiomodulation in Complex Female Infertility Profile: A Case Report with 12-Month Follow-Up and Review of Current Mechanism in Reproductive Photomedicine. Photonics, 12(10), 1021. https://doi.org/10.3390/photonics12101021






