CB1 and CB2 Receptor Expression in Type 1 Diabetic Neuropathic Rats Is Enhanced by Photobiomodulation Therapy
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Streptozotocin (STZ)-Induced Type 1 Diabetes Mellitus
2.3. Photobiomodulation Therapy (PBMT) by Low-Level Laser Irradiation
2.4. Intrathecal (i.t.) Injection of CB1 and CB2 Antagonists
2.5. Electronic Von Frey Test (Mechanical Hyperalgesia)
2.6. Euthanasia, DRG and Thalamus Collection, and Homogenization
2.7. Western Blotting (WB) Assay
2.8. Statistical Analyses
3. Results
3.1. Efficacy of Type 1 Diabetes Induction by STZ and the Effect of PBMT on Metabolic Parameters
3.2. Effect of CB1 and CB2 Antagonists on Mechanical Hyperalgesia After PBMT Treatment
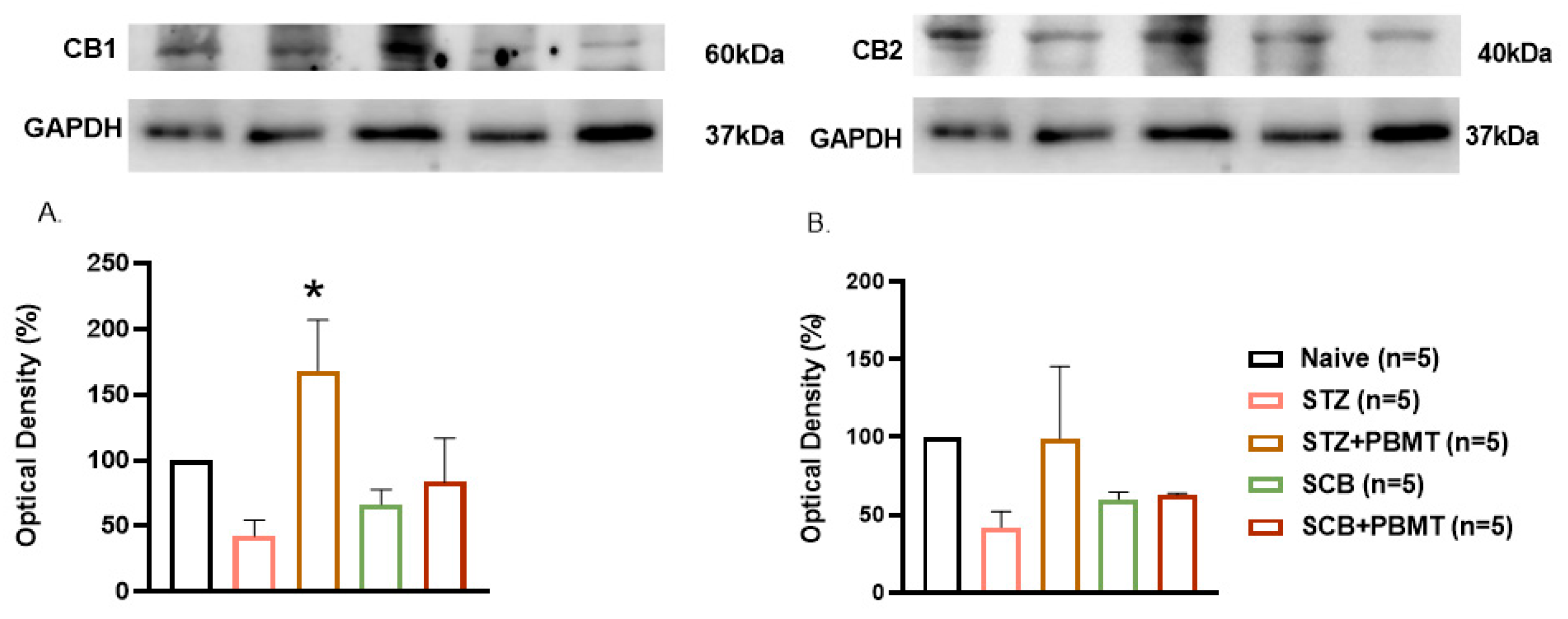
3.3. Effect of PBMT on CB1 and CB2 Receptors Expression in the DRG (L4 and L5)
3.4. Effect of PBMT on CB1 and CB2 Receptor Expression in the Thalamus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J.; Committee IDFD Atlas. Idf Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers. Clin. Diabetes 2020, 38, 10–38. [Google Scholar] [CrossRef]
- Pham, V.M.; Thakor, N. Insulin enhances neurite extension and myelination of diabetic neuropathy neurons. Korean J. Pain 2022, 35, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Boland, C.; Meade, L.; Battise, D. Adjunctive therapy for glucose control in patients with type 1 diabetes. Diabetes, Metabolic Syndrome and Obesity. 2018, 11, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.E.; Harris, N.D.; Rajbhandari, S.M.; Greenwood, P.; Wilkinson, I.D.; Ward, J.D.; Griffiths, P.D.; Tesfaye, S. Spinal-cord involvement in diabetic peripheral neuropathy. Lancet 2001, 358, 35–36. [Google Scholar] [CrossRef]
- Selvarajah, D.; Wilkinson, I.D.; Maxwell, M.; Davies, J.; Sankar, A.; Boland, E.; Gandhi, R.; Tracey, I.; Tesfaye, S. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care 2014, 37, 1681–1688. [Google Scholar] [CrossRef]
- Correia Rocha, I.R.; Chacur, M. Modulatory effects of photobiomodulation in the anterior cingulate cortex of diabetic rats. Photochem. Photobiol. Sci. 2021, 20, 781–790. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Yu, T.; Zhu, J.; Semyachkina-Glushkovskaya, O.; Zhu, D. Transcranial photobiomodulation improves insulin therapy in diabetic microglial reactivity and the brain drainage system. Commun. Biol. 2023, 6, 1239. [Google Scholar] [CrossRef]
- de la Torre, J.C. Treating cognitive impairment with transcranial low level laser therapy. J. Photochem. Photobiology B: Biology. 2017, 168, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Wen, J.; Sackett, S.; Tanaka, M.; Zhang, Y. Therapeutic Effects of Combined Treatment with the AEA Hydrolysis Inhibitor PF04457845 and the Substrate Selective COX-2 Inhibitor LM4131 in the Mouse Model of Neuropathic Pain. Cells 2023, 12, 1275. [Google Scholar] [CrossRef]
- Liang, Y.C.; Huang, C.C.; Hsu, K.S. Therapeutic potential of cannabinoids in trigeminal neuralgia. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Binda, K.H.; Chacur, M.; Martins, D.O. Exercise Improves Orofacial Pain and Modifies Neuropeptide Expression in a Rat Model of Parkinson’s Disease. Neurotox. Res. 2023, 41, 459–470. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Saito, V.M.; Wotjak, C.T.; Moreira, F.A. Pharmacological exploitation of the endocannabinoid system: New perspectives for the treatment of depression and anxiety disorders? Braz. J. Psychiatry 2010, 32 (Suppl. S1), 57–514. [Google Scholar] [CrossRef]
- Schreiber, A.K.; Neufeld, M.; Jesus, C.H.; Cunha, J.M. Peripheral antinociceptive effect of anandamide and drugs that affect the endocannabinoid system on the formalin test in normal and streptozotocin-diabetic rats. Neuropharmacology 2012, 63, 1286–1297. [Google Scholar] [CrossRef]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the endocannabinoid system in diabetes and diabetic complications. Br. J. Pharmacol. 2016, 173, 1116–1127. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhou, S.J.; Wang, X.Y.; Liu, J.F.; Xue, Y.X.; Jiang, W.; Lu, L. Effects of cannabinoid CB1 receptor antagonist rimonabant on acquisition and reinstatement of psychostimulant reward memory in mice. Behav. Brain Res. 2011, 217, 111–116. [Google Scholar] [CrossRef]
- Toth, C.C.; Jedrzejewski, N.M.; Ellis, C.L.; Frey, W.H. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol. Pain 2010, 6, 16. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.E.; Da Silva, J.T.; Brioschi, M.L.; Chacur, M. Effects of photobiomodulation therapy on neuropathic pain in rats: Evaluation of nociceptive mediators and infrared thermography. Lasers Med. Sci. 2021, 36, 1461–1467. [Google Scholar] [CrossRef]
- Rocha, I.R.C.; Ciena, A.P.; Rosa, A.S.; Martins, D.O.; Chacur, M. Photobiostimulation reverses allodynia and peripheral nerve damage in streptozotocin-induced type 1 diabetes. Lasers Med. Sci. 2017, 32, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.F.; de Magalhães, S.F.; Farias, F.H.; de Thomaz, A.A.; Parada, C.A. Raman spectroscopy of dorsal root ganglia from streptozotocin-induced diabetic neuropathic rats submitted to photobiomodulation therapy. J. Biophotonics 2019, 12, e201900135. [Google Scholar] [CrossRef] [PubMed]
- Mazuqueli Pereira, E.S.B.; Basting, R.T.; Abdalla, H.B.; Garcez, A.S.; Napimoga, M.H.; Clemente-Napimoga, J.T. Photobiomodulation inhibits inflammation in the temporomandibular joint of rats. J. Photochem. Photobiology B Biology 2021, 222, 112281. [Google Scholar] [CrossRef]
- Yamada, E.F.; Bobinski, F.; Martins, D.F.; Palandi, J.; Folmer, V.; da Silva, M.D. Photobiomodulation therapy in knee osteoarthritis reduces oxidative stress and inflammatory cytokines in rats. J. Biophotonics 2020, 13, e201900204. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Frigo, L.; Dos Reis Ferreira, T.C.; Casalechi, H.L.; Teixeira, S.; de Almeida, P.; Muscara, M.N.; Marcos, R.L.; Serra, A.J.; de Carvalho, P.T.C.; et al. Effects of photobiomodulation therapy and topical non-steroidal anti-inflammatory drug on skeletal muscle injury induced by contusion in rats-part 2: Biochemical aspects. Lasers Med. Sci. 2017, 32, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.M.S.; Gonçalves, E.C.D.; Cavalli, J.; Vieira, G.; Laurindo, L.R.; Simões, R.R.; Coelho, I.S.; Santos, A.R.S.; Marcolino, A.M.; Cola, M.; et al. Photobiomodulation Therapy Improves Acute Inflammatory Response in Mice: The Role of Cannabinoid Receptors/ATP-Sensitive K+ Channel/p38-MAPK Signalling Pathway. Mol. Neurobiol. 2018, 55, 5580–5593. [Google Scholar] [CrossRef]
- Moheghi, A.; Noori Mougehi, S.M.H.; Amini, A.; Mostafavinia, A.; Rezaei, F.; Bagheri Tadi, F.; Chien, S.; Bayat, M. Anti-inflammatory, Antioxidant, and Wound-Healing Effects of Photobiomodulation on Type-2 Diabetic Rats. J. Lasers Med. Sci. 2023, 14, e45. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Ma, K.; Li, H.; Fu, X.; Zhang, C. Photobiomodulation promotes hair regeneration in injured skin by enhancing migration and exosome secretion of dermal papilla cells. Wound Repair Regen. 2022, 30, 245–257. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wang, X.Y.; Chu, Y.X.; Wang, Y.Q. Light-emitting diode phototherapy: Pain relief and underlying mechanisms. Lasers Med. Sci. 2022, 37, 2343–2352. [Google Scholar] [CrossRef]
- Scarcella, G.; Tardugno, R.; Crupi, P.; Muraglia, M.; Clodoveo, M.L.; Corbo, F. Fluorescent Light Energy (FLE) Generated through Red LED Light and a Natural Photoconverter Gel as a New, Non-Invasive Approach for Facial Age Control: A Pilot Study. Cosmetics 2023, 10, 74. [Google Scholar] [CrossRef]
- Bettleyon, J.; Kaminski, T.W. Does Low-Level Laser Therapy Decrease Muscle-Damaging Mediators After Performance in Soccer Athletes Versus Sham Laser Treatment? A Critically Appraised Topic. J. Sport Rehabil. 2020, 29, 1210–1213. [Google Scholar] [CrossRef]
- Vieira, W.F.; Kenzo-Kagawa, B.; Britto, M.H.M.; Ceragioli, H.J.; Sakane, K.K.; Baranauskas, V.; da Cruz-Höfling, M.A. Vibrational spectroscopy of muscular tissue intoxicated by snake venom and exposed to photobiomodulation therapy. Lasers Med. Sci. 2018, 33, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.F.; Kenzo-Kagawa, B.; Alvares, L.E.; Cogo, J.C.; Baranauskas, V.; da Cruz-Höfling, M.A. Exploring the ability of low-level laser irradiation to reduce myonecrosis and increase Myogenin transcription after Bothrops jararacussu envenomation. Photochem. Photobiol. Sci. 2021, 20, 571–583. [Google Scholar] [CrossRef]
- Vieira, W.F.; Malange, K.F.; de Magalhães, S.F.; Lemes, J.B.P.; Dos Santos, G.G.; Nishijima, C.M.; de Oliveira, A.L.R.; da Cruz-Höfling, M.A.; Tambeli, C.H.; Parada, C.A. Anti-hyperalgesic effects of photobiomodulation therapy (904 nm) on streptozotocin-induced diabetic neuropathy imply MAPK pathway and calcium dynamics modulation. Sci. Rep. 2022, 12, 16730. [Google Scholar] [CrossRef] [PubMed]
- Athie, M.C.P.; Vieira, A.S.; Teixeira, J.M.; Dos Santos, G.G.; Dias, E.V.; Tambeli, C.H.; Sartori, C.R.; Parada, C.A. Transcriptome analysis of dorsal root ganglia’s diabetic neuropathy reveals mechanisms involved in pain and regeneration. Life Sci. 2018, 205, 54–62. [Google Scholar] [CrossRef]
- Teixeira, J.M.; Dos Santos, G.G.; Neves, A.F.; Athie, M.C.P.; Bonet, I.J.M.; Nishijima, C.M.; Farias, F.H.; Figueiredo, J.G.; Hernandez-Olmos, V.; Alshaibani, S.; et al. Diabetes-induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia. Neuroscience 2019, 398, 158–170. [Google Scholar] [CrossRef]
- Vieira, W.F.; Malange, K.F.; de Magalhães, S.F.; Dos Santos, G.G.; de Oliveira, A.L.R.; da Cruz-Höfling, M.A.; Parada, C.A. Gait analysis correlates mechanical hyperalgesia in a model of streptozotocin-induced diabetic neuropathy: A CatWalk dynamic motor function study. Neurosci. Lett. 2020, 736, 135253. [Google Scholar] [CrossRef]
- Rocha, I.R.C.; Perez-Reyes, E.; Chacur, M. Effect of photobiomodulation on mitochondrial dynamics in peripheral nervous system in streptozotocin-induced type 1 diabetes in rats. Photochem. Photobiol. Sci. 2021, 20, 293–301. [Google Scholar] [CrossRef]
- Ferreira, N.L.; Rocha, I.R.C.; Chacur, M. Unraveling the RAGE-NF-κB pathway: Implications for modulating inflammation in diabetic neuropathy through photobiomodulation therapy. Lasers Med. Sci. 2024, 39, 222. [Google Scholar] [CrossRef]
- Mestre, C.; Pélissier, T.; Fialip, J.; Wilcox, G.; Eschalier, A. A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods 1994, 32, 197–200. [Google Scholar] [CrossRef]
- Rosa, A.S.; Freitas, M.F.; Rocha, I.R.; Chacur, M. Gabapentin decreases microglial cells and reverses bilateral hyperalgesia and allodynia in rats with chronic myositis. Eur. J. Pharmacol. 2017, 799, 111–117. [Google Scholar] [CrossRef]
- Binda, K.H.; Lillethorup, T.P.; Real, C.C.; Bærentzen, S.L.; Nielsen, M.N.; Orlowski, D.; Brooks, D.J.; Chacur, M.; Landau, A.M. Exercise protects synaptic density in a rat model of Parkinson’s disease. Exp. Neurol. 2021, 342, 113741. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Machado, C.; De Marchi, T.; Casalechi, H.L.; Bjordal, J.M.; de Carvalho, P.T.C.; Leal-Junior, E.C.P. Infrared Low-Level Laser Therapy (Photobiomodulation Therapy) before Intense Progressive Running Test of High-Level Soccer Players: Effects on Functional, Muscle Damage, Inflammatory, and Oxidative Stress Markers-A Randomized Controlled Trial. Oxidative Med. Cell. Longev. 2019, 2019, 6239058. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, F.; Neshasteh-Riz, A.; Ghadaksaz, A.; Fazeli, S.M.; Janzadeh, A.; Hamblin, M.R. Mechanistic aspects of photobiomodulation therapy in the nervous system. Lasers Med. Sci. 2022, 37, 11–18. [Google Scholar] [CrossRef]
- Vieira, W.F.; Gersten, M.; Caldieraro, M.A.K.; Cassano, P. Photobiomodulation for Major Depressive Disorder: Linking Transcranial Infrared Light, Biophotons and Oxidative Stress. Harv. Rev. Psychiatry 2023, 31, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.R.A.; Salvi, J.D.; Vieira, W.F.; Cassano, P. Inflammation in obsessive-compulsive disorder: A literature review and hypothesis-based potential of transcranial photobiomodulation. J. Neurosci. Res. 2024, 102, e25317. [Google Scholar] [CrossRef]
- Coelho, D.R.A.; Renet, C.; López-Rodríguez, S.; Cassano, P.; Vieira, W.F. Transcranial photobiomodulation for neurodevelopmental disorders: A narrative review. Photochem. Photobiol. Sci. 2024, 23, 1609–1623. [Google Scholar] [CrossRef]
- Vieira, W.F. Beyond Digital Therapy: Augmenting Internet-Based Cognitive Behavioral Therapy With Brain Stimulation. Biol. Psychiatry Glob. Open Sci. 2025, 5, 100514. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]


| Laser | λ | Operation Mode | Frequency | Time |
|---|---|---|---|---|
| GaAs | 904 nm | Pulsed | 9500 Hz | 18″ per point |
| Total Energy | Nº of points | Nº of sessions | Treated area | Contact |
| 0.81 J | 2/animal/day | 8 sessions; 1/day | 1.17 cm2 | Direct |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.P.F.B.; Vendrame, N.; Vieira, W.F.; Chacur, M. CB1 and CB2 Receptor Expression in Type 1 Diabetic Neuropathic Rats Is Enhanced by Photobiomodulation Therapy. Photonics 2025, 12, 1060. https://doi.org/10.3390/photonics12111060
Silva DPFB, Vendrame N, Vieira WF, Chacur M. CB1 and CB2 Receptor Expression in Type 1 Diabetic Neuropathic Rats Is Enhanced by Photobiomodulation Therapy. Photonics. 2025; 12(11):1060. https://doi.org/10.3390/photonics12111060
Chicago/Turabian StyleSilva, Danielle Paula Freitas Bataus, Natalia Vendrame, Willians Fernando Vieira, and Marucia Chacur. 2025. "CB1 and CB2 Receptor Expression in Type 1 Diabetic Neuropathic Rats Is Enhanced by Photobiomodulation Therapy" Photonics 12, no. 11: 1060. https://doi.org/10.3390/photonics12111060
APA StyleSilva, D. P. F. B., Vendrame, N., Vieira, W. F., & Chacur, M. (2025). CB1 and CB2 Receptor Expression in Type 1 Diabetic Neuropathic Rats Is Enhanced by Photobiomodulation Therapy. Photonics, 12(11), 1060. https://doi.org/10.3390/photonics12111060







