Abstract
Stimulated Raman scattering (SRS) microscopy is a high-speed imaging modality based on intrinsic molecular vibrations, producing chemical maps in living systems. Such capability, allowing for direct visualization without the perturbation of biological processes, has enabled a plethora of biological and medical applications. In this review, after introducing the basic theory and competitive effects of SRS, some crucial features for SRS microscopy implementations, such as noise, spectral bandwidth, speed, chemical sensitivity, spatial resolution, and quantum enhancement, are discussed. Finally, some SRS applications in biological and medical imaging are described. Even if certainly not exhaustive, we aimed to offer a broad overview, providing guidance for newcomers and hinting at a more detailed investigation to interested researchers in this rapidly growing field.
1. Introduction
Today, fluorescence microscopy is used worldwide, even if it suffers from drawbacks such as photobleaching, phototoxicity, and challenging quantification. Most small biomolecules, such as nucleosides, amino acids, fatty acids, choline, glucose, and cholesterol, are intrinsically nonfluorescent, so fluorescent tags are mandatory. Unluckily, the latter, such as organic dyes, fluorescent proteins, or quantum dots, are all relatively larger than small biomolecules; consequently, they can severely falsify their native biochemical or biophysical properties. Therefore, label-free imaging, with high sensitivity and high chemical selectivity of unlabeled living cells, is preferable [1,2,3].
In this framework, vibrational microscopy techniques have emerged as a powerful approach based on a chemically label-free selective contrast due to intrinsic molecule vibrations. Among them, stimulated Raman scattering (SRS) microscopy, which was adapted to microscopy only in the last two decades, is one of the most promising techniques. SRS microscopy is sensitive to the same molecular vibrations probed in spontaneous Raman spectroscopy but exhibits a nonlinear dependence on the incoming light fields, producing coherent radiation. Because of nonlinear excitation, taking advantage of SRS microscopy, it is possible to achieve images faster than conventional Raman microscopes, with typical acquisition times of a few seconds. Other advantages of SRS microscopy are high sensitivity, spatial and spectral resolution, and 3D sectioning capability. Since the invention of SRS microscopy, it has been extensively employed to study composition, structure, chemical distribution, molecular transport, metabolic conversion in living cells, and disease in biological systems in a label-free manner. Applications of SRS have generated new insights in many fields, including lipid droplet biology, cell metabolism, drug delivery, neurobiology, tumor biology, and developmental biology [4,5,6,7,8,9,10].
In Raman amplification, two laser pulses, i.e., a pump and a probe (Stokes) beams, at frequencies and (with > ) are incident on a material sample. If their difference frequency matches a particular molecular vibrational frequency, the energy from the intense pump beam is transferred to the weaker signal beam. The SRS effect occurs in the form of a gain of the Stokes beam (stimulated Raman gain, SRG) and a loss of the pump beam (stimulated Raman loss, SRL). Because of its coherent nature, the molecular bonds oscillate in phases and interfere constructively inside the focus area of the laser beam so that the SRS signal can be orders of magnitude greater than spontaneous Raman scattering. Although it is impossible to detect a single vibrational mode at room temperature, detecting a macromolecule with thousands of identical vibrational modes that interfere coherently is feasible [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26].
This review article is organized as follows. In the next paragraph, some crucial features of SRS implementations, such as noise, spectral bandwidth, speed, chemical sensitivity, spatial resolution, and quantum enhancement, are discussed. These features are important for at least two reasons. First, there are significant efforts to improve them in many directions. Second, these features allow for the matching of the potentials of SRS microscopes with the requirements imposed by applications. Finally, in the last section, some life science applications of SRS are reported. Of course, covering all the application fields would be impossible, so only some recent results have been reported.
2. Features of SRS Microscopes
Almost all SRS microscopes reported in the literature are home-built with highly different laser sources, optics, and detection mechanisms. They present a large variety in terms of main features, such as noise, spectral bandwidth, speed, chemical sensitivity, and spatial resolution. As a consequence, there is a lack of standard procedures or reference materials for SRS, which has important implications for reliability, reproducibility, and consistency [27]. This section analyzes and discusses the main features of SRS microscopes.
2.1. Noise and Competitive Effects
We note that the SRS signal is generated at the frequency of incident beams acting as local oscillators. Such heterodyne detection boosts the signal level and removes the non-resonant background, which limits CARS microscopy. Therefore, initially, it was suggested that SRS microscopy was free of spurious background signals. However, the SRS signal is generally overlaid with parasitic signals stemming from various linear (scattering and absorption) and nonlinear optical effects whose sources are ubiquitous [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26].
Due to other nonlinear optical processes, unwanted signals, which can overlay with SRS, can be classified into two groups: nonlinear transient absorption [28,29] and nonlinear transient scattering processes [30,31] (see Figure 1). Both heterodyne modalities produce a spectrally overlapped background with the SRS signal. Nonlinear transient absorption (TA) is a pump–probe process involving electronic-energy-level excitations, which may be classified into excited-state absorption, induced fluorescence, and ground-state photobleaching processes [28]. The resulting attenuation of the probe beam can be misinterpreted as an SRS signal [29] but has no chemical specificity. Nonlinear transient scattering includes two main effects: cross-phase modulation (XPM) and thermal lensing (TL). In cross-phase modulation (XPM), the pump beam (ωp) changes the nonlinear refractive index at the focus through the optical Kerr effect, thus affecting the probe beam (ωpr) propagation and frequency. [30]. TL is induced by local heating due to the absorption of pump photons, which changes the refractive index at the focus, thus affecting the propagation of the probe beam [31]. Both effects can be reduced by using collection optics with a large numerical aperture, i.e., employing an objective collecting lens with a numerical aperture that exceeds that of the excitations.
We note that all these nonlinear optical (NLO) techniques, SRS, nonlinear transient absorption, and nonlinear transient scattering processes, are based on the detection of small intensity changes (of the order of 10−5–10−4) of large signals, in which the signal wavelengths do not depend on the nonlinear processes. The signal exists at the same wavelengths as the excitation pulses, and the spectral separation of the relatively weak signal from the excitation beams is impossible. In these techniques, the signal, i.e., a small alternating voltage (AC) at the sub-microvolt level, must be discriminated from the laser 1/f noise. For this reason, most of the investigations relied on high-frequency optical modulation schemes and phase-sensitive detection [32,33,34].
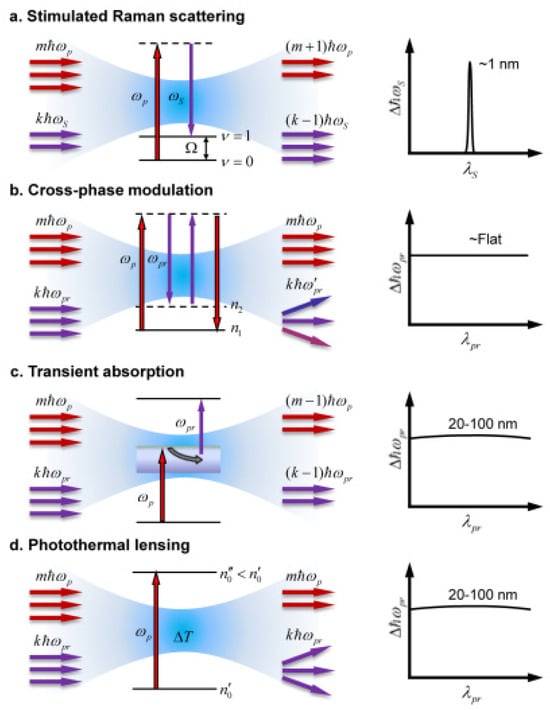
Figure 1.
Basic principles of pump–probe modalities and their spectral characteristics: (a), stimulated Raman scattering (SRS); (b), cross-phase modulation (XPM); (c), transient absorption (TA); and (d), thermal lensing. Adapted from [35] Copyright 2013 Optica Publishing Group.
We note that XPM involves only energy states, so the XPM signal can be considered wavelength-independent for a small spectral range. Transient absorption and photothermal effects are based on electronic transitions and, therefore, have weak wavelength dependence. In contrast, the SRS signal originates from vibrational transitions, which have very sharp spectral features. As a consequence, one may selectively detect the SRS process by modulating a laser beam’s wavelength within a few nm. Based on this concept, frequency-modulated SRS is one of the most successful methods proposed to reduce the background signal [35]. In this method, either the pump or Stokes input of the laser pulse is frequency-modulated so that their difference is modulated on and off the vibrational resonance. This results in a signal at the Stokes frequency that is modulated between two values, one corresponding to purely non-resonant interaction and the other to both resonant interactions. The difference between these two signals effectively removes the average background contribution and gives much-improved image contrast.
In [35], a simple but robust FM-SRS (Frequency Modulation-SRS) microscopy strategy was based on a commercial laser system and standard optics. Effective suppression of backgrounds, including non-resonant cross-phase modulation and an electronic background from two-photon absorption or the pump–probe process, was demonstrated on a variety of Raman modes.
It is worth noting that due to the weak Raman cross-section of biomolecules, SRS microscopy often suffers from a low signal-to-noise ratio (SNR), and SRS applications in biological/biomedical imaging could be compromised. In the literature on SRS, noise investigations are often overlooked, even if the signal-to-noise ratio (SNR) is crucial for spectroscopy [36,37] and biological imaging [38,39,40,41,42,43,44,45]. A safer way would be to evaluate both the stimulated Raman loss and the stimulated Raman gain, as these two contributions should be equal in the absence of artifacts. In ref [45], an SRS scheme, SRGAL (stimulated Raman gain and loss), was presented. The SRGAL concept modulates the pump at frequency f1 and the Stokes at frequency f2 while simultaneously demodulating the pump at f2 to access SRL and the Stokes at f1 to access SRG. In the SRGAL scheme, the SRL and SRG images are collected simultaneously but within separate signal channels. As a result, the SRGAL scheme allows for (1) faster imaging for a given signal-to-noise ratio (SNR), as the SRG and SRL channels can be added to double the SRS signal, and (2) the semi-quantitative evaluation of the SRS artifacts by directly comparing the magnitude of the SRG and SRL channels at each pixel level.
One key SRGAL advantage is to improve the SNR ratio by summating the SRL and SRG images. In Figure 2, the SNR improvement is demonstrated for an onion surface thick slice (about 150 microns). The SNR was measured within the delineated region of interest (ROI) (loss: dashed green ROI, gain: dashed red ROI). By summing the SRL and SRG images of the onion cell, a 2-fold improvement of the SNRSRGAL (black curve) compared to SNRSRL (green curve) and SNRSRG (red curve) was obtained. The 2-fold improvement is further shown in Figure 2 through the ratio 2 SNRSRGAL/(SNRSRL+SNRSRGº that is constant along the scan direction and equal to 2 [45].
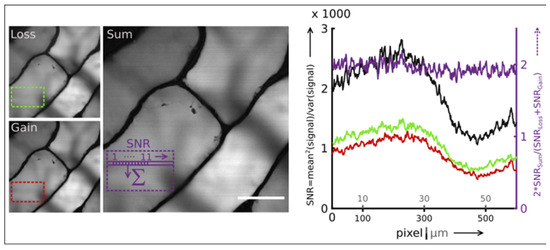
Figure 2.
SRGAL signal-to-noise (SNRSRGAL) improvement. Scale bar: 50 µm. Pixel integration time: 20 ms. Adapted from [45] Copyright 2020 Optica Publishing Group.
Visualizing heterogeneous morphology, segmentation, and quantifying image features is crucial for nonlinear optics microscopy applications. The identification and quantification of the structure and the spatial distributions of chemicals and organelles are necessary in order to accurately compare images and make objective conclusions about an experiment. On this line of argument, recently, there has been a sudden increase in applications of quantitative biological imaging, a field that has been advanced by developments in optical imaging technology, as well as in computational image analysis techniques [46,47]. However, many pitfalls are associated with quantification due to complications such as sample scattering and a non-Raman background. A useful and detailed discussion of SRS-based quantitative analysis is reported in reference [47].
2.2. Spectral Bandwidth and Speed
Label-free stimulated Raman scattering microscopy allows for the imaging of a variety of molecular species, targeting their intrinsic chemical bonds in the C-H region (2800–3100 cm−1), the silent region (1800–2800 cm−1), and the fingerprint region (<1800 cm−1). Almost all biomolecules contain carbon and hydrogen, so the CH region is the most used in SRS microscopy. The two Raman bands, CH2, near 2845 cm−1, and CH3, near 2930 cm−1, corresponding to lipids and proteins, respectively, are typically investigated. Due to the difference between peaks (about 95 cm−1) and large spectral shapes (about 100 cm−1), the CH2 and CH3 Raman bands partially overlap. In the C-H region, because the density of CH bonds is high, the SRS signal level is high, while the SRS molecular specificity is low [48,49,50,51].
SRS imaging can be demonstrated in three modalities: single-frequency, hyperspectral, and multiplex.
Single-frequency SRS microscopy is implemented by using a pair of Fourier transform-limited (FTL) picosecond laser sources. This approach ensures a high spectral resolution (*10 cm−1) that is very useful in the fingerprint region (~800 and 1800 cm−1), where Raman peaks are narrow, closely spaced, and may be in abundance for a particular chemical [48,49]. Utilizing a picosecond (ps) pump and Stokes lasers, single-color SRS has reached video-rate speeds, opening doors for a plethora of biological and medical applications [52,53]. The drawback is that ps pulses show a low peak intensity, thus needing high laser power for imaging.
Two-color stimulated Raman scattering (SRS) microscopy has succeeded in label-free digital histology, with diagnostic results similar to the hematoxylin and eosin stain. In ref. [54], the pulse profiles of Stokes beams were precisely engineered to detect two Raman frequencies simultaneously through a dual-phase lock-in amplifier in-phase and quadrature output channels. Such a method could reach the maximum speed as in single-color SRS.
High-throughput-stimulated Raman scattering (SRS) microscopy is highly desired for large tissue imaging. However, in most cases, SRS microscopy was limited to a very small field of view (FOV) of about 300 μm or to mapping a relatively large tissue in a very long time [55,56]. In [57], an inertia-free acousto-optic deflector (AOD)-based high-speed large-field stimulated Raman scattering microscopy was presented. The immune system of the mechanical response time ensures both speed and integration time. An SRS imaging of a 12 × 8 mm2 mouse brain slice in only 8 min at an image resolution of approximately 1 μm and 32 slices from a whole brain in 12 h were achieved.
We note that while single-frequency with ps coherent Raman scattering guarantees the best spectral resolution, the optimal image contrast and signal intensity ratio are obtained by matching the spectral resolution with the width of the Raman lines under consideration (5–100 cm−1) [58]. Because picosecond pulse excitation can only match the linewidths in the fingerprint region (5–20 cm−1), the question is raised as to whether broader bandwidth femtosecond (fs) pulses can be suited to the optimal excitation of CH stretching vibrations [59,60] In addition, we note that when femtosecond (fs) pulses are used, the SRS signal is improved by one order of magnitude [61]. On the other hand, low spectral selectivity is obtained using fs sources, and multi-band excitation can occur, which does not allow for, in principle, the separation of lipids and proteins.
Hyperspectral SRS (hsSRS) imaging can be obtained by changing the wavelength of one or both lasers. Different Raman bands, corresponding to different chemical components of the sample, can be imaged by tuning the frequency either of the pump or the Stokes beams in sequential scans, allowing for the detection of one chemical contrast for each scanning. Thus, CH2 (2845 cm−1) and CH3 (2940 cm−1) stretching signals can be collected at one Raman shift at a time, leading, in principle, to mapping the distributions of the lipid and protein contents. Nevertheless, such improvement comes at the price of reduced imaging speed. When fs pulses are used, SRS images acquired at 2845 cm−1 can be attributed to the lipids, while in order to obtain protein content, SRS images acquired at 2940 cm−1 contain both lipids and protein signals. Therefore, the calibration of individual components is mandatory [59,60,62].
Hyperspectral SRS (hsSRS) imaging, i.e., the spectral scanning of a narrowband laser pulse and collection of images at a series of Raman shifts, has reached the speed of a few seconds per stack [63,64,65,66,67]. Hyperspectral SRS (hsSRS) imaging can also be achieved by chirping pump and Stokes lasers and tuning the time delay between them. In this technique, called spectral focusing [64], each temporal delay between the chirped pulses corresponds to a Raman shift. SRS spectroscopic imaging can be obtained by scanning the temporal delay of one of the pulses and recording a series of images. A spectral-focusing scheme has been demonstrated with a total acquisition time of several tens of seconds [64,68,69,70], where the spectral acquisition speed was limited by the waiting time for the stabilization and communication of a motorized translational stage used for delay tuning. In [71], microsecond-scale SRS spectroscopic imaging was presented by temporally tuning two spectrally focused pulses through a resonant delay line. The platform was able to form a spectral image of 40,000 pixels within 3.3 s. The SRS spectrum at each pixel was acquired within 83 μs, covering the 200 cm−1 spectral window with 25 cm−1 spectral resolution.
Another possibility is given by femtosecond pulse-shaping technology [72], which is able to obtain a spectral resolution of about 10 cm−1. In [73], the development of a unique spatial light-modulated stimulated Raman scattering (SLM-SRS) microscopy, tailoring the broadband excitation beam for multiplexed vibrational imaging with higher sensitivity, is reported. Pulse-shaping is achieved using a sparse-sampling mask designed to allow for the collective excitation of the predominant spectral windows against the overlapping spectra. SLM-SRS microscopy permits rapid multiplexed SRS imaging of polystyrene and polymethyl methacrylate beads in Brownian motion in dimethyl sulfoxide (DMSO) at 70 ms intervals without motion artifacts.
In multiplex SRS imaging, the SRS spectrum at each pixel is captured simultaneously over a large spectral window by using broadband excitation at the cost of more pixel integration time [74,75]. In multiplex SRS imaging, by replacing the lock-in amplifier with a gate array, lock-in-free single-color-line-scan SRS imaging that acquired 20 frames per second was obtained [76]. Using a 32-channel tuned amplifier array, Zhang et al. [77] obtained an acquisition speed of 5 μs per spectrum (~200 cm−1).
Several computational methods have been proposed to break the speed limit of Raman imaging bounded by signal integration time. In [78], the sparsity of natural images was used to record a Raman image. This work reported three-dimensional sparsely sampled spectroscopic SRS imaging that measures ~20% of pixels throughout the stack. An acquisition speed of 0.8 s per image stack, with 50 frames in the spectral domain and 40,000 pixels in the spatial domain, was achieved.
2.3. Improvement in Chemical Sensitivity
Strategies aimed at enhancing the visualization and quantitation of biomolecules by SRS have focused on the introduction of small Raman-active tags, which present their Raman band in the cellular silent region of the Raman spectrum, where the background interference from intrinsic cellular components is minimal, and the signal contrast is maximized. Two main strategies are employed; the former concerns using Raman-active groups, which are either inherent to the molecule under investigation or selectively introduced for imaging purposes. The tiny size and good biocompatibility of vibrational tags enable the nonperturbative tracing of small molecules [79], which is difficult to achieve with fluorescence because of the bulky fluorophores.
Furthermore, by virtue of much narrower vibrational peaks (≈10 cm–1) compared to fluorescence peaks (≈500 cm–1), Raman imaging offers scalable multiplexity and breaks the “palette number barrier” of fluorescence, which can typically only image 4–5 colors simultaneously. By combining electronic pre-resonance spectroscopy with stimulated Raman scattering (SRS) microscopy (i.e., epr- SRS), the Raman cross-sections of electronically coupled vibrational modes in light-absorbing dyes can be enhanced by 1013-fold [80,81,82,83]. This drastic enhancement achieved the nanomolar sensitivity of Raman-active dyes.
2.4. Super-Resolution
Because SRS is an optical technique using near-infrared or visible light, its spatial resolution is governed by the diffraction limit (≈300 nm).
In order to break the diffraction limit, the approach is focused on reducing the point spread function (PSD) or increasing the cutoff frequency of the optical transfer function (OFT). It includes the following three different directions: (a) the effective PSF reduction based on nonlinear optical processes such as stimulated emission depletion (STED) microscopy [84] and saturation excitation (SAX) microscopy [85]; (b) OTF expansion by Moire effects, such as structured illumination microscopy (SIM) [86]; and (c) PSF, which is suppressed by focal volume engineering, such as superoscillation [87].
For direction (b), we note that the Moiré fringe is a wide-field technique, while CRS is a nonlinear optical process requiring a tightly focused laser beam. Although SIM-based super-resolution CARS microscopy was theoretically proposed [88,89], the related experiment has not yet been demonstrated. Therefore, the feasible approaches for super-resolution CRS microscopy should be approach (a) [90,91,92,93,94,95,96,97,98] and approach (c) [99,100,101]. However, approach (c) can only improve the resolution up to 2-fold, while approach (a) provides, in principle, unlimited resolution, so, in recent years, it has attracted much more attention.
Note that the CRS process is the 3rd-order nonlinear process; for super-resolution CRS imaging, the nonlinear optical process of even higher orders (n > 3) should be involved [102]. The key to achieving super-resolution CRS imaging is to properly detect the higher-order nonlinear process with a relatively high excitation power without damaging the sample. In approach (a), several theoretical schemes are proposed based on either the saturation [98] or suppression [93,96,98] of the CRS signal. To apply super-resolution CRS techniques in biological samples, saturated SRS (SSRS) microscopy and higher-order CARS (HO-CARS) are proposed and demonstrated experimentally [90,91].
In reference [91], saturated stimulated-Raman-scattering (SSRS) microscopy was presented to break the diffraction limit for far-field super-resolution imaging in biological systems. An experimental implementation of SSRS microscopy was developed to enhance the spatial resolution of SRS imaging in samples (e.g., plant cells, live HeLa cells) with a spectral-focusing hyperspectral SRS system. With saturation occurring in coherent Raman processes under a certain level of excitation powers, it was demonstrated that the SSRS technique has the ability to become an appealing tool for label-free far-field super-resolution SRS imaging in biological and biomedical systems (see Figure 3).
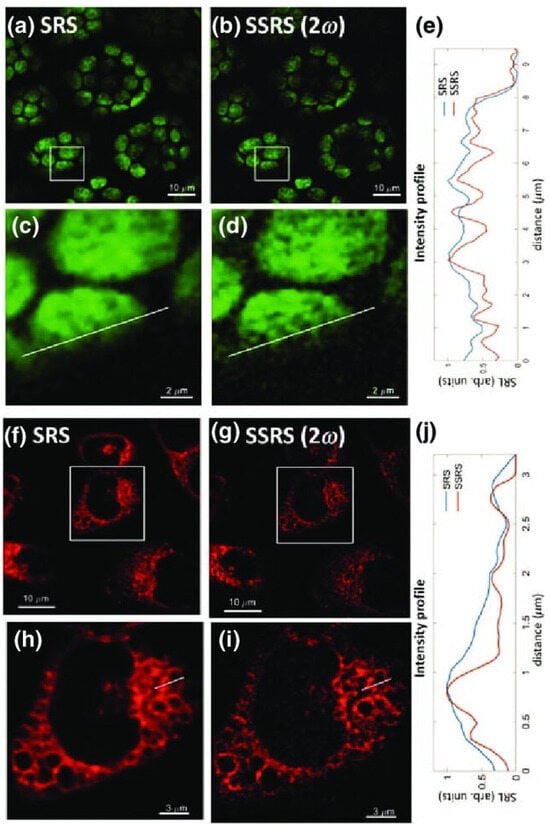
Figure 3.
Comparison of the conventional SRS and super-resolution SSRS images of chloroplast and Raman-tagged mitochondria in live HeLa cells. (a,b) SRS image and super-resolution SSRS image of chloroplasts in leaf sheath cells of rice. The Raman shift is 1530 cm−1. (c,d) Enlarged SRS and SSRS images of the selected areas. (e) Intensity profiles across the lines are indicated in (c,d). (f,g) SRS image and second-order super-resolution SSRS image of mitochondria in Hela cells. The Raman shift is 2216 cm−1. (h,i) Enlarged SRS and SSRS images of the selected areas. (j), Intensity profiles across the lines indicated in (h,i). Adapted from [91] with the permission number RNP/24/MAY/078995 of the American Physical Society.
Over the last half-decade, a sample-oriented nanoscale imaging technique known as expansion microscopy (ExM) has emerged as a powerful technique for nanoscale optical imaging through the physical magnification of the tissue samples embedded in a water-swellable polymer gel [103,104,105].
By combining SRS imaging with this framework, a new technique called molecule anchorable gel-enabled nanoscale imaging of fluorescence and stimulated Raman scattering microscopy (MAGNIFIERS), integrating SRS microscopy with expansion microscopy (ExM), has been described in ref [105]. MAGNIFIERS offers chemical-specific nanoscale imaging with sub-50 nm resolution and has scalable multiplexity when combined with multiplex Raman probes and fluorescent labels. MAGNIFIERS has been used to visualize nanoscale features in a label-free manner with the C–H vibration of proteins, lipids, and DNA in a broad range of biological specimens [105].
2.5. Quantum Enhancement
It is known that quantum correlations can be used to extract more information per photon used in an optical measurement [106,107,108,109]. This breaks the trade-off between the signal-to-noise ratio and intensity [110]. For this reason, quantum correlations are now used routinely to improve many optical measurements in proof-of-principle experiments [111]. While several different quantum states of light can, in principle, be used to provide such a quantum advantage, so far, it is only the ubiquitous squeezed states of light that have demonstrably been shown to provide a real practical advantage [112] due to their generation simplicity and robustness to loss. Squeezed light, first observed in the 1980s [113], is a unique type of quantum state of light in which the amplitude or the phase has a lower quantum uncertainty than that of a coherent state, but the uncertainty principle is not violated. Squeezed vacuum states can be generated by means of parametric down-conversion (PDC), which converts a pump photon of frequency o0 into a signal photon of frequency o1 and an idler photon of frequency o2. The generated photons are quantum-mechanically correlated in their photon number and quadratures. As a result of the quadrature correlations, the joint measurement of the photons yields quadrature noise reduction, that is, squeezing. Because squeezed vacuum states have an electric field with zero mean value, they are not directly suitable for SRS, but its mean value must be coherently amplified to empower the squeezed vacuum state.
In SRS, the sensitivity and imaging speed depend on the background noise of the probe beam. This background noise is fundamentally limited by shot noise when the probe beam is in a coherent state, produced by a conventional laser [114]. Still, in principle, it can be arbitrarily improved by increasing the input beams’ power. However, in biological systems, especially in living systems, the power must be kept low to avoid changing the biological dynamics of the specimens and, in particular, to avoid damage due to excessive heating. For SRS, photodamage places acute constraints on sensitivity and imaging speed [115], presenting a roadblock for powerful prospective applications such as label-free spectrally multiplexed imaging. State-of-the-art coherent Raman microscopes are already limited by shot noise. Consequently, the roadblock cannot be overcome through improvements in instrumentation.
Although quantum-enhanced stimulated Raman scattering (QE-SRS) helps to improve sensitivity by reducing the shot noise, it is still challenging to exceed the sensitivity of classical SRS microscopy. This is mainly because of the difficulty in applying high-power squeezed light pulses (SRS pump or Stokes) in QE-SRS. The typical average power of laser pulses for SRS imaging is several tens of milliwatts. Therefore, to exceed the sensitivity of classical SRS, developing a QE-SRS system that can operate at a high-power regime is crucial.
To improve the signal-to-noise ratio (SNR), the squeezed light-enhanced SRS (SQ-SRS) has been demonstrated in both SRS spectroscopy [116] and SRS microscopy [117]. In [116], SRS spectroscopy was demonstrated with quantum-enhanced balanced detection (QE-BD). In Figure 4, the QE-SRS spectra are measured by observing the output signal of the LIA on an oscilloscope (OSC), which is shown [116]. The noise became stronger or weaker depending on anti-squeezing (ASQ) or squeezing (SQ). The histogram of the voltage signals in Figure 4b is plotted to estimate the noise level quantitatively. Applying Gaussian fitting, the standard deviation σ was derived as 0.378, 0.139, and 0.109 V for ASQ, shot noise limited (SNL), and SQ-SRS traces, respectively. This corresponds to the quantum enhancement of 2.11 dB.
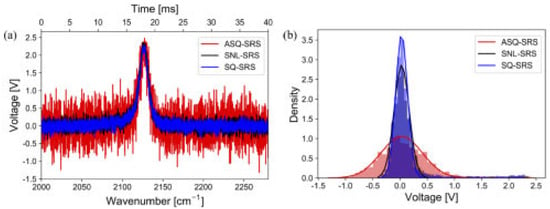
Figure 4.
The QE-SRS spectra were acquired with a lock-in amplifier and an oscilloscope. (a) The measured SRS spectra with and without SQV. (b) Noise distribution with Gaussian fitting (σASQ = 0.40 V, σSNL = 0.152 V, σSQ = 0.116 V). Adapted from [116] Copyright 2022 Optica Publishing Group.
In [118], the quantum enhancement of continuous-wave (CW) SRS spectroscopy was demonstrated by spectroscopically measuring the carbon–hydrogen (C-H) vibrations of polymethylmethacrylate (PMMA) and polydimethylsiloxane (PDMS) with a sensitivity improvement of approximately 56% relative to shot-noise-limited Raman spectroscopy.
In [119], it was experimentally shown that quantum correlations allow for a signal-to-noise ratio beyond the photodamage limit of conventional microscopy. The correlations allow for the imaging of molecular bonds within a cell with a 35-percent improved signal-to-noise ratio compared with conventional microscopy, corresponding to a 14-percent improvement in concentration sensitivity.
In [120], the deconvoluted SQ-SRS (Dcv-SQ-SRS) super-resolution SRS imaging technique, in which the resolution of the deconvoluted image is further enhanced by using quantum light, was presented. Firstly, SQ-SRS was adopted to improve the SNR of conventional SRS beyond the shot noise limit. Then, the deconvolution method was applied to retrieve Dcv-SQ-SRS images beyond the diffraction limit. Thanks to a higher SNR, with the Dcv-SQ-SRS technique, ∼a 2.2-fold improvement in spatial resolution compared to conventional SRS microscopy was proved.
Several super-resolution methods have been proposed to break the diffraction limit in coherent Raman scattering microscopy. Typically, these methods require a higher excitation power than conventional SRS imaging, leading to a high risk of photodamage to the tissue samples. On the other hand, the deconvolution methods could extract information beyond the diffraction limit without increasing the laser power. Still, its performance is limited by SNR [121].
3. Life Science Applications
Tremendous work has been conducted on cellular imaging, and the developments and applications of SRS in this area have been thoroughly discussed and reviewed [4,5,6,7,8,9,10,122]. Mammalian cells store excess lipid molecules in specialized intracellular organelles called lipid droplets (LDs). The sizes of LDs vary from tens of nm to tens of μm in diameter. LDs are ubiquitously conserved from yeast to mammals and are involved in maintaining lipid homeostasis through lipid synthesis, metabolism, and transportation. Based on controlling these important cellular functions, LDs are closely associated with many human diseases but also with membrane trafficking, cell signaling, proliferation, and apoptosis [123]. This is why great attention has been reserved for LD biology in the last decade.
Although intracellular lipid accumulation has been observed in human cancer tissues and cells, it has not been widely used as a prognostic factor or therapeutic target due to a limited understanding of lipid metabolism in cancer. In particular, the role of lipid accumulation in cancer progression remains elusive, partly because of the lack of tools for mapping lipid species at a single-cell level. Quantitative analysis of lipid content at a single-cell level in human patient cancerous tissue by coupling confocal Raman microscopy with SRS microscopy enabled the identification of the metabolic signature of aggressive human prostate cancer [124].
One advantage of label-free imaging is the capability of quantitative long-duration imaging. This is especially important when studying biomolecule dynamics and tracking changes during ongoing processes, such as embryonic development and injury. Dou et al. performed time-lapse SRS imaging of a developing Drosophila embryo to track single-lipid droplet motion within large populations of droplets (Figure 5). By analyzing the velocity and turning frequency of each droplet, the mathematical model for the LD movement was developed to show the key regulatory point of LD dynamics in the developmental process. This work offers the potential of using the SRS imaging technique to study lipid trafficking in living cells and organisms [125].
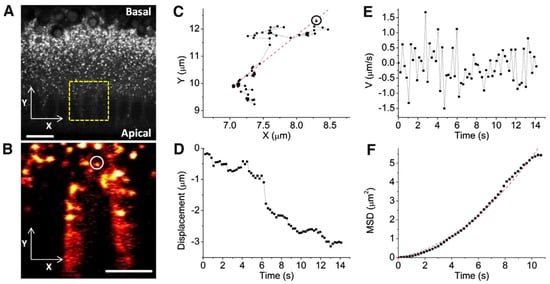
Figure 5.
Droplet bidirectional motion is characterized by single-droplet tracking. (A) A snapshot of the embryo cortex. (Yellow box) Imaged region for droplet tracking. Acquisition time, 2 μs per pixel. Bar, 10 μm. (B) A typical trafficking droplet tracked over 10 s. Acquisition time, 2 μs per pixel (4∼5 frames/s). Bar, 5 μm. (C) The trajectory of the droplet is marked in phase B (dashed line) least-squares linear fitting that characterizes the major axis of displacement. (D) Droplet displacement (along the dashed line in phase C, where segments of continuous runs were extracted). Decreasing ordinate values indicates apically directed motion. (E,F) The instantaneous velocity and MSD curve of the tracked droplet. An overall quadratic shape of the MSD curve corresponds to directed transport. The fitting of this MSD curve led to a diffusion coefficient (D) of 0.032 ± 0.003 μm2/s and an average velocity (V) of 0.201 ± 0.041 μm/s. It is reproduced by [125] under license number 5711300529639 with permission under the Elsevier agreement.
SRS is severely limited in imaging depth due to the turbidity and heterogeneity of tissue in both transmissive and epi-mode imaging. The studies, which explicitly report the achieved imaging depths of coherent Raman microscopy in tissue, suggest the limit to be around 20–100 μm, with varying tissue types being the cause of the large variance. Hill et al. [126] investigated maximum signal sizes, scattering lengths, and achievable imaging depths as a function of tissue type and sample thickness using a simultaneous SRS epi/transmissive imaging approach in four different ex vivo murine tissues: brain, lung, kidney, and liver. As one would expect, their findings demonstrated that epi (transmissive) SRS signal size and imaging depth increase (decrease) as tissue thickness grows, reflecting an increase (reduction) in backscattered (transmitted) photons. When tissue thicknesses reach 2 mm, epi and transmissive signal sizes are roughly equivalent, and epi images provide slightly higher penetration depths than transmissive images.
Hematoxylin and eosin (H&E) staining has been the “gold standard” for pathologies like cancer, but it requires extensive time and labor, making it not ideal in the operation room where quick decisions must be made. Fast and accurate pathological diagnoses require real-time assessment of excised tissue. Two-color SRS microscopy has been used for virtual histology by imaging at 2845 cm−1 and 2930 cm−1, respectively, for lipid and protein and has achieved excellent results comparable to H&E staining [127].
Lu et al. [128] compared the large-scale SRS imaging of brain tumor tissue resection from patients with H&E staining and found that SRS microscopy could capture not only fundamental diagnostic hallmarks (such as cell nuclei and cell density) for tumor classification but also features not detectable by H&E approaches, including abundant lipid droplets within glioma cells, collagen deposition in gliosarcoma, and the disruption of myelinated fibers.
In addition to tumors, SRS imaging has been applied to detect a variety of brain diseases, such as Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS). Ji et al. [129] applied multicolor narrowband SRS microscopy to detect Aβ plaques in the APP/PS1 AD mouse model ex vivo brain tissue. They differentiated misfolded Aβ plaques from normal proteins and lipids in frozen and fresh brain tissues based on the spectral shift (~10 cm−1) of the amide-I band of SRS spectra.
Studying amyotrophic lateral sclerosis (ALS) and exploring potential interventions would be easier if we could more effectively visualize motor axon degeneration. Tian et al. [130] demonstrated in their research that SRS microscopy could be employed to monitor peripheral nerve degeneration sensitively in ALS mouse models (Figure 6). Their findings showed that significant degeneration of peripheral nerves could be detected concurrently with the earliest observable signs of muscle denervation, occurring before any measurable decline in motor function. Moreover, through serial imaging, they could observe the disease’s long-term progression and the effects of drugs in living animals.
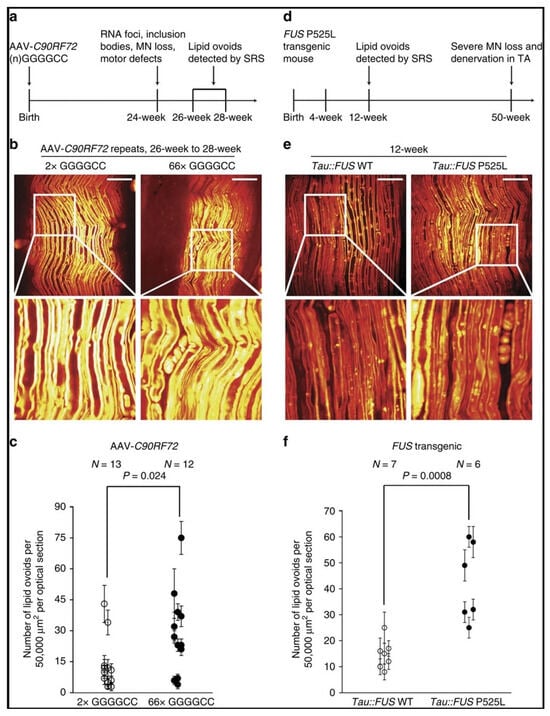
Figure 6.
SRS imaging of non-SOD1 mouse models has demonstrated its broad applicability in the study of ALS. The information below is adapted from [130] and is used under a Creative Commons CC-BY license. (a) The progression of the disease in the AAV-C9ORF72 repeat expansion over-expression mouse model of ALS is illustrated. (b) Representative SRS images of the AAV-C9ORF72 mouse model showcase the healthy nerve fiber morphology for 2 repeats (2Rs) and the deposition of lipid ovoids for 66 repeats (66Rs). The scale bar is set at 50 μm. (c) The quantification of lipid ovoids in the AAV-C9ORF72 mouse model of ALS is presented. The data are expressed as mean±s.e.m., with error bars indicating the standard error of the mean. (d) The progression of disease-related phenotypes in the FUSP525L mouse ALS model is depicted. (e) Ex vivo SRS images of FUSP525L versus FUS WT at 12 weeks of age are provided, with a scale bar of 50 μm. (f) The quantification of lipid ovoids and the corresponding statistical significance analysis for the FUSP525L mouse model of ALS are included.
Acetylcholine (ACh) is a neurotransmitter that plays a crucial role in the central nervous system. Fu et al. [131] reported the first label-free imaging of ACh in frog muscle ex vivo by employing frequency-modulated spectral-focusing SRS microscopy and using the vibrational signature of ACh at 720 cm−1 (intrinsic C-N bonds stretching).
The miniaturization of the SRS microscopy system is desired for in vivo and in situ imaging of live animals and in the clinic. A fiber-delivered handheld SRS imaging system was reported in [132]. Background-free in situ and in vivo imaging was achieved on normal and tumorigenic dog brain tissues and live human skin. However, achieving real-time SRS imaging for intraoperative assessment is still a challenge.
The high attrition rates of candidate drugs during clinical development remain a common feature within the pharmaceutical industry. Incorporating imaging into complex drug-screening models has the potential to improve the robustness of preclinical studies of drug uptake, retention, and metabolism.
However, imaging the uptake of drugs and drug carriers presents a significant challenge. Thus, spectroscopically bio-orthogonal Raman-active tags have been adopted, which improve detection sensitivity. The use of isotopes has recently been demonstrated as an alternative Raman-tagging strategy. Deuterium and carbon-13 are useful isotopic substitutions for Raman spectroscopy. The principal benefit of this strategy is that isotopes have almost the identical size and electronic characteristics as the parent compounds. Still, they may display favorable Raman spectral characteristics, particularly as C–D bands fall into the cellular silent region (~2100 cm−1). Replacing the hydrogen atoms in a molecule with deuterium may even improve metabolic stability, and the resultant deuterated molecules are typically safe to handle and dose to patients. When coupled with biorthogonal-labeling strategies, live-cell Raman imaging is a promising imaging modality applicable to drug discovery and medicine. However, the low sensitivity of the Raman scattering process remains a significant limitation of the approach, where, in many cases, typical detection limits are above physiologically relevant drug concentrations. Several studies using Raman imaging to probe intracellular drug distribution have been reviewed by Tipping et al. [133].
Antimicrobial resistance threatens the effective treatment of infectious diseases caused by bacteria and fungi. Rapid antimicrobial susceptibility testing (AST) plays a significant role in suppressing the emergence of antimicrobial resistance to combat this crisis. Current gold standard AST methods are quite time-consuming and lead to the emergence and spread of antimicrobial resistance. The rapid AST of infectious pathogens is urgently needed to guide appropriate antibiotic prescriptions in a timely manner before clinical treatment.
Hong et al. [134] reviewed the utility of SRS for rapidly determining the antimicrobial susceptibility of bacteria and fungi by single-cell metabolic imaging. Rapid AST was achieved in functional analysis by single-cell SRS imaging of the deuterated glucose metabolic activity, D2O metabolic incorporation, and de novo lipogenesis. Unlike fluorescent labels, the deuterium substitution does not affect glucose metabolism inside the cell. By mapping the signal arising from the unnatural C-D bond, one is able to determine the concentration and the speed at which glucose or D2O is consumed by a bacterial or fungal cell under normal or antimicrobial-laden conditions. SRS microscopy was further substantiated as a feasible platform for the rapid detection of antifungal susceptibility by the direct imaging of lipogenesis activity. The transformative aspect of SRS metabolic imaging lies in its real-time analysis capability, enabling the determination of antimicrobial susceptibility within a single cell cycle. This functionality facilitates the timely identification of suitable antimicrobial agents for precise treatment.
4. Conclusions
In the last two decades, significant biological and medical insights have been made possible by the chemical specificity and nonperturbative nature of SRS microscopy. However, improvements in SRS detection sensitivity and spatial resolution are always desired.
Super-resolution and quantum enhancement have recently opened the door to new possibilities for SRS spectroscopy and microscopy. Super-resolution could allow for the resolution of many crucial nanoscale organizations in cells and tissues. On the other hand, using squeezed light to enhance the sensitivity of the stimulated Raman signal enables studies of biological samples with a lower risk of damage and biophysical effects that may not be visible using the standard classical approaches. With further development of advanced quantum light generation and detection schemes in the near future, the QE-SRS technique with much-reduced shot noise can benefit many aspects of bioimaging in terms of its sensitivity, biomolecular specificity, imaging speed, and spatial resolution beyond the limit of classical light [10,118,119].
Author Contributions
The authors participated equally in this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Streets, A.M.; Li, A.; Chen, T.; Huang, Y. Imaging without fluorescence: Nonlinear optical microscopy for quantitative cellular imaging. Anal. Chem. 2014, 86, 8506–8513. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Freudiger, C.W.; Lu, S.; Xie, X.S. Coherent nonlinear optical imaging: Beyond fluorescence microscopy. Annu. Rev. Phys. Chem. 2011, 62, 501–530. [Google Scholar] [CrossRef]
- Popp, J.; Kiefer, W. Raman scattering, fundamentals. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 13104–13142. [Google Scholar]
- Ferrara, M.A.; Ranjan, R.; Righini, G.C.; Sirleto, L. Stimulated Raman scattering: Towards applications in nano and biophotonics. In Woodhead Publishing Series in Electronic and Optical Materials, Advances in Nonlinear Photonics, Woodhead Publishing; Righini, G.C., Sirleto, L., Eds.; Woodhead Publishing: Thorston, UK, 2023; pp. 489–515. ISBN 9780323983846. [Google Scholar] [CrossRef]
- Zumbusch, A.; Langbein, A.; Borri, P. Nonlinear vibrational microscopy applied to lipid biology. Prog. Lipid Res. 2013, 52, 615–632. [Google Scholar] [CrossRef]
- Alfonso-García, A.; Mittal, R.; Lee, E.S.; Potma, E.O. Biological imaging with coherent Raman scattering microscopy: A tutorial. J. Biomed. Opt. 2014, 19, 071407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.X.; Xie, X.S. Vibrational spectroscopic imaging of living systems: An emerging platform for biology and medicine. Science 2015, 350, aaa10670. [Google Scholar]
- Lee, H.J.; Cheng, J.-X. Imaging chemistry inside living cells by stimulated Raman scattering microscopy. Methods 2017, 128, 119–128. [Google Scholar] [CrossRef]
- Hill, A.H.; Fu, D. Cellular Imaging Using Stimulated Raman Scattering Microscopy. Anal. Chem. 2019, 91, 9333–9342. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fung, A.A.; Zhou, A. Advances in stimulated Raman scattering imaging for tissues and animals. Quant. Imaging Med. Surg. 2021, 11, 1078–1101. [Google Scholar] [CrossRef]
- McClung, F.J.; Hellwarth, R.W. Giant Optical Pulsations from ruby. J. Appl. Phys. 1962, 33, 828–829. [Google Scholar] [CrossRef]
- Eckhardt, G.; Hellwarth, R.W.; McClung, F.J.; Schwarz, S.E.; Weiner, D.; Woodbury, E.J. Stimulated Raman scattering from organic liquids. Phys. Rev. Lett. 1962, 9, 455–457. [Google Scholar] [CrossRef]
- Eckhardt, G.; Bortfeld, D.P.; Geller, M. Stimulated emission of stokes and anti-stokes raman lines from diamond, calcite, and α-sulfur single crystals. Appl. Phys. Lett. 1963, 3, 137–138. [Google Scholar] [CrossRef]
- Lallemand, P.; Simova, P.; Bret, G. Pressure-induced line shift and collisional narrowing in hydrogen gas determined by stimulated Raman emission. Phys. Rev. Lett. 1966, 17, 1239–1241. [Google Scholar] [CrossRef]
- Boyd, R.W. Nonlinear Optics, 2nd ed.; Academic Press: Boston, MA, USA, 2003. [Google Scholar]
- Shen, Y.R. The Principles of Nonlinear Optics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Yariv, A. Quantum Electronics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1967. [Google Scholar]
- Armstrong, J.A.; Bloembergen, N.; Ducuing, J.; Pershan, P.S. Interactions between light waves in a nonlinear dielectric. Phys. Rev. 1962, 127, 1918–1939. [Google Scholar] [CrossRef]
- Bloembergen, N.; Shen, Y.R. Coupling between vibrations and light waves in Raman Laser Media. Phys. Rev. Lett. 1964, 12, 504–507. [Google Scholar] [CrossRef]
- Bloembergen, N.; Shen, Y.R. Multimode effects in stimulated Raman emission. Phys. Rev. Lett. 1964, 13, 720–724. [Google Scholar] [CrossRef]
- Shen, Y.R.; Bloembergen, N. Theory of stimulated Brillouin and Raman scattering. Phys. Rev. 1965, 137, 6A. [Google Scholar] [CrossRef]
- Bloembergen, N. The Stimulated Raman Effect. Am. J. Phys. 1967, 35, 989–1022. [Google Scholar] [CrossRef]
- Wang, C.S. Theory of Stimulated Raman Scattering. Phys. Rev. 1969, 182, 484–494. [Google Scholar] [CrossRef]
- Hellwarth, R.W. Theory of Stimulated Raman Scattering. Phys. Rev. 1963, 130, 1850–1852. [Google Scholar] [CrossRef]
- Raymer, M.G.; Mostowski, J. Stimulated raman scattering: Unified treatment of spontaneous initiation and spatial propagation. Phys. Rev. A 1981, 24, 1980–1993. [Google Scholar] [CrossRef]
- Raymer, M.G.; Walmsley, I.A.; Mostowski, J.; Sobolewska, B. Quantum theory of spatial and temporal coherence properties of stimulated Raman scattering. Phys. Rev. A 1985, 32, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Tsikritsis, D.; Legge, E.J.; Belsey, N.A. Practical considerations for quantitative and reproducible measurements with stimulated Raman scattering microscopy. Analyst 2022, 147, 4642–4656. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Ye, T.; Matthews, T.E.; Yurtsever, G.; Warren, W.S. Two-color, two-photon, and excited-state absorption microscopy. J. Biomed. Opt. 2007, 12, 054004. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Kawano, H.; Suda, A.; Kumagai, A.; Miyawaki, A.; Midorikawa, K. Simultaneous imaging of two-photon absorption and stimulated Raman scattering by spatial overlap modulation nonlinear optical microscopy. Biomed. Opt. Express 2013, 4, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Samineni, P.; Warren, W.S.; Fischer, M.C. Cross-phase modulation spectral shifting: Nonlinear phase contrast in a pump-probe microscope. Biomed. Opt. Express 2012, 3, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Hibara, A.; Kimura, H.; Sawada, T.; Kitamori, T. Thermal lens microscope. Jpn. J. Appl. Phys. 2000, 39, 5316–5322. [Google Scholar] [CrossRef]
- D’Arco, A.; Ferrara, M.A.; Indolfi, M.; Tufano, V.; Sirleto, L. Label-free imaging of small lipid droplets by femtosecond-stimulated Raman scattering microscopy. J. Nonlinear Opt. Phys. Mater. 2017, 26, 1750052. [Google Scholar] [CrossRef]
- Ranjan, R.; Indolfi, M.; Ferrara, M.A.; Sirleto, L. Implementation of a nonlinear microscope based on stimulated Raman scattering. J. Vis. Exp. 2019, 149, e59614. [Google Scholar]
- Ranjan, R.; Ferrara, M.A.; Filograna, A.; Valente, C.; Sirleto, L. Femtosecond stimulated Raman microscopy: Home-built realization and a case study of biological imaging. J. Instrum. 2019, 14, P09008. [Google Scholar] [CrossRef]
- Zhang, D.; Slipchenko, M.N.; Leaird, D.E.; Weiner, A.M.; Cheng, J.-X. Spectrally modulated stimulated Raman scattering imaging with an angle-to-wavelength pulse shaper. Opt. Express 2013, 21, 13864–13874. [Google Scholar] [CrossRef]
- Ozeki, Y.; Dake, F.; Kajiyama, S.; Fukui, K.; Itoh, K. Analysis and experimental assessment of the sensitivity of stimulated Raman scattering microscopy. Opt. Express 2009, 17, 3651. [Google Scholar] [CrossRef] [PubMed]
- Dietze, D.R.; Mathies, R.A. Femtosecond stimulated Raman spectroscopy. ChemPhysChem 2016, 17, 1224–1251. [Google Scholar] [CrossRef] [PubMed]
- Audier, X.; Heuke, S.; Volz, P.; Rimk, I.; Rigneault, H. Noise in stimulated Raman scattering measurement: From basics to practice. APL Photonics 2020, 5, 011101. [Google Scholar] [CrossRef]
- Nose, K.; Ozeki, Y.; Kishi, T.; Sumimura, K.; Nishizawa, N.; Fukui, K.; Kanematsu, Y.; Itoh, K. Sensitivity enhancement of fiber-laser-based stimulated Raman scattering microscopy by collinear balanced detection technique. Opt. Express 2012, 20, 13958. [Google Scholar] [CrossRef] [PubMed]
- Zada, L.; Fokker, B.; Leslie, H.A.; Vethaak, A.D.; de Boer, J.F.; Ariese, F. Stimulated Raman scattering simulation for imaging optimization. J. Eur. Opt. Soc. Rapid Publ. 2017, 17, 1. [Google Scholar]
- Ranjan, R.; Costa, G.; Ferrara, M.A.; Sansone, M.; Sirleto, L. Noises investigations and image denoising in femtosecond stimulated Raman scattering microscopy. J. Biophotonics 2022, 15, e202100379. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Costa, G.; Ferrara, M.A.; Sansone, M.; Sirleto, L. Noise Measurements and Noise Statistical Properties Investigations in a Stimulated Raman Scattering Microscope Based on Three Femtoseconds Laser Sources. Photonics 2022, 9, 910. [Google Scholar] [CrossRef]
- Berto, P.; Andresen, E.R.; Rigneault, H. Background-free stimulated Raman spectroscopy and microscopy. Phys. Rev. Lett. 2014, 112, 053905. [Google Scholar] [CrossRef]
- Ranjan, R.; D’arco, A.; Ferrara, M.A.; Indolfi, M.; Larobina, M.; Sirleto, L. Integration of stimulated Raman gain and stimulated Raman losses detection modes in a single nonlinear microscope. Opt. Express 2018, 26, 26317. [Google Scholar] [CrossRef]
- Heuke, S.; Lombardini, A.; Büttner, E.; Rigneault, H. Simultaneous stimulated Raman gain and loss detection (SRGAL). Opt. Express 2020, 28, 29619–29630. [Google Scholar] [CrossRef]
- D’Arco, A.; Brancati, N.; Ferrara, M.A.; Indolfi, M.; Frucci, M.; Sirleto, L. Subcellular chemical and morphological analysis by stimulated Raman scattering microscopy and image analysis techniques. Biomed. Opt. Express 2016, 7, 1853. [Google Scholar] [CrossRef] [PubMed]
- Manifold, B.; Fu, D. Quantitative stimulated Raman scattering microscopy: Promises and pitfalls. Annu. Rev. Anal. Chem. 2022, 15, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Saar, B.G.; Freudiger, C.W.; Reichman, J.; Stanley, C.M.; Holtom, G.R.; Xie, X.S. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 2010, 330, 1369–1370. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-Free Biomedical Imaging with High Sensitivity by Stimulated Raman Scattering Microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef]
- Ranjan, R.; Antonietta Ferrara, M.; Sirleto, L. Femtosecond Stimulated Raman Microscopy in C-H Region of Raman Spectra of Biomolecules and Its Extension to Silent and Fingerprint Regions [Internet]. Novel Imaging and Spectroscopy; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ferrara, M.A.; Filograna, A.; Ranjan, R.; Corda, D.; Valente, C.; Sirleto, L. Threedimensional label-free imaging throughout adipocyte differentiation by stimulated Raman microscopy. PLoS ONE 2019, 14, e0216811. [Google Scholar] [CrossRef]
- Fu, D.; Zhou, J.; Zhu, W.S.; Manley, P.W.; Wang, Y.K.; Hood, T.; Wylie, A.; Xie, X.S. Imaging the intracellular distribution of tyrosine kinase inhibitors in living cells with quantitative hyperspectral stimulated Raman scattering. Nat. Chem. 2014, 6, 614–622. [Google Scholar] [CrossRef]
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.; Maher, C.O.; et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 2017, 1, 0027. [Google Scholar] [CrossRef]
- He, R.; Xu, Y.; Zhang, L.; Ma, S.; Wang, X.; Ye, D.; Ji, M. Dual-phase stimulated Raman scattering microscopy for real-time two-color imaging. Optica 2017, 4, 44–47. [Google Scholar] [CrossRef]
- Galli, R.; Uckermann, O.; Temme, A.; Leipnitz, E.; Meinhardt, M.; Koch, E.; Schackert, G.; Steiner, G.; Kirsch, M. Assessing the Efficacy of Coherent Anti-Stokes Raman Scattering Microscopy for the Detection of Infiltrating Glioblastoma in Fresh Brain Samples. J. Biophotonics 2017, 10, 404–414. [Google Scholar] [CrossRef]
- Pekmezci, M.; Morshed, R.A.; Chunduru, P.; Pandian, B.; Young, J.; Villanueva-Meyer, J.E.; Tihan, T.; Sloan, E.A.; Aghi, M.K.; Molinaro, A.M.; et al. Detection of Glioma Infiltration at the Tumor Margin Using Quantitative Stimulated Raman Scattering Histology. Sci. Rep. 2021, 11, 12162. [Google Scholar] [CrossRef]
- Yan, S.; Li, Y.; Huang, Z.; Yuan, X.; Wang, P. High-Speed Stimulated Raman Scattering Microscopy Using Inertia-Free AOD Scanning. J. Phys. Chem. B 2023, 127, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, A.F.; Ridsdale, A.; Moffatt, D.J.; Jia, Y.W.; Pezacki, J.P.; Stolow, A. Optimally chirped multimodal CARS microscopy based on a single Ti:sapphire oscillator. Opt. Express 2009, 17, 2984–2996. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Berry, K.; Chen, Y.; Figueroa, B.; Fu, D. Label-free pathology by spectrally sliced femtosecond stimulated Raman scattering (SRS) microscopy. PLoS ONE 2017, 12, e0178750. [Google Scholar] [CrossRef] [PubMed]
- Sirleto, L.; Ranjan, R.; Ferrara, M.A. Analysis of pulses bandwidth and spectral resolution in femtosecond stimulated Raman scattering microscopy. Appl. Sci. 2019, 11, 3903. [Google Scholar] [CrossRef]
- Zhang, D.; Slipchenko, M.N.; Cheng, J.X. Highly Sensitive Vibrational Imaging by Femtosecond Pulse Stimulated Raman Loss. J. Phys. Chem. Microsc. Lett. 2011, 2, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Lu, F.K.; Zhang, X.; Freudiger, C.; Pernik, D.R.; Holtom, G.; Xie, X.S. Quantitative Chemical Imaging with Multiplex Stimulated Raman Scattering. J. Am. Chem. Soc. 2012, 134, 3623–3626. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.J.; Ji, M.B.; Holtom, G.R.; Fu, D.; Freudiger, C.W.; Xie, X.S. Multicolor stimulated Raman scattering microscopy with a rapidly tunable optical parametric oscillator. Opt. Lett. 2013, 38, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Holtom, G.; Freudiger, C.; Zhang, X.; Xie, X.S. Hyperspectral imaging with stimulated Raman scattering by chirped femtosecond lasers. J. Phys. Chem. B 2013, 117, 4634–4640. [Google Scholar] [CrossRef]
- Ozeki, Y.; Umemura, W.; Otsuka, Y.; Satoh, S.; Hashimoto, H.; Sumimura, K.; Nishizawa, N.; Fukui, K.; Itoh, K. High-speed molecular spectral imaging of tissue with stimulated Raman scattering. Nat. Photonics 2012, 6, 845–851. [Google Scholar] [CrossRef]
- Ozeki, Y.; Umemura, W.; Sumimura, K.; Nishizawa, N.; Fukui, K.; Itoh, K. Stimulated Raman hyperspectral imaging based on spectral filtering of broadband fiber laser pulses. Opt. Lett. 2012, 37, 431–433. [Google Scholar] [CrossRef]
- Karpf, S.; Eibl, M.; Wieser, W.; Klein, T.; Huber, R. A time-encoded technique for fibre-based hyperspectral broadband stimulated 711 Raman microscopy. Nat. Commun. 2015, 6, 6784. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Polzer, C.; Hellerer, T. Resolution of spectral focusing in coherent Raman imaging. Opt. Express 2018, 26, 10230–10241. [Google Scholar] [CrossRef] [PubMed]
- Hellerer, T.; Enejder, A.M.K.; Zumbusch, A. Spectral focusing: High spectral resolution spectroscopy with broad-bandwidth laser pulses. Appl. Phys. Lett. 2004, 85, 25–27. [Google Scholar] [CrossRef]
- Liu, B.; Lee, H.J.; Zhang, D.L.; Liao, C.-S.; Ji, N.; Xia, Y.Q.; Cheng, J.-X. Label-free spectroscopic detection of membrane potential using stimulated Raman scattering. Appl. Phys. Lett. 2015, 106, 173704. [Google Scholar] [CrossRef]
- Liao, C.-S.; Huang, K.-C.; Hong, W.; Chen, A.J.; Karanja, C.; Wang, P.; Eakins, G.; Cheng, J.-X. Stimulated Raman spectroscopic imaging by microsecond delay-line tuning. Optica 2016, 3, 1377–1380. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, P.; Slipchenko, M.N.; Ben-Amotz, D.; Weiner, A.M.; Cheng, J.X. Quantitative vibrational imaging by hyperspectral stimulated Raman scattering microscopy and multivariate curve resolution analysis. Anal. Chem. 2013, 85, 98–106. [Google Scholar] [CrossRef]
- Bae, K.; Zheng, W.; Huang, Z. Spatial light-modulated stimulated Raman scattering (SLM-SRS) microscopy for rapid multiplexed vibrational imaging. Theranostics 2020, 10, 312–322. [Google Scholar] [CrossRef]
- Camp, C.H., Jr.; Lee, Y.J.; Heddleston, J.M.; Hartshorn, C.M.; Walker, A.R.H.; Rich, J.N.; Lathia, J.D.; Cicerone, M.T. High-speed coherent Raman fingerprint imaging of biological tissues. Nat. Photonics 2014, 8, 627–634. [Google Scholar] [CrossRef]
- Hashimoto, K.; Takahashi, M.; Ideguchi, T.; Goda, K. Broadband coherent Raman spectroscopy running at 24,000 spectra per second. Sci. Rep. 2016, 6, 21036. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, W.; Huang, Z. Lock-in-detection-free linescan stimulated Raman scattering microscopy for near video-rate Raman imaging. Opt. Lett. 2016, 41, 3960–3963. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, K.C.; Rajwa, B.; Li, J.; Yang, S.; Lin, H.; Liao, C.S.; Eakins, G.; Kuang, S.; Patsekin, V.; et al. Stimulated Raman scattering flow cytometry for labelfree single-particle analysis. Optica 2017, 4, 103–109. [Google Scholar] [CrossRef]
- Lin, H.; Liao, C.S.; Wang, P.; Kong, N.; Cheng, J.X. Spectroscopic stimulated Raman scattering imaging of highly dynamic specimens through matrix completion. Light Sci. Appl. 2018, 7, 17179. [Google Scholar] [CrossRef]
- Wei, L.; Hu, F.; Chen, Z.; Shen, Y.; Zhang, L.; Min, W. Live- Cell Bioorthogonal Chemical Imaging: Stimulated Raman Scattering Microscopy of Vibrational Probes. Acc. Chem. Res. 2016, 49, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, Z.; Shi, L.; Long, R.; Anzalone, A.V.; Zhang, L.; Hu, F.; Yuste, R.; Cornish, V.W.; Min, W. Super-multiplex vibrational imaging. Nature 2017, 544, 465–470. [Google Scholar] [CrossRef]
- Hu, F.; Zeng, C.; Long, R.; Miao, Y.; Wei, L.; Xu, Q.; Min, W. Supermultiplexed optical imaging and barcoding with engineered polyynes. Nat. Methods 2018, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Qian, N.; Shi, L.; Hu, F.; Min, W. 9-Cyanopyronin probe palette for super-multiplexed vibrational imaging. Nat. Commun. 2021, 2, 4518. [Google Scholar] [CrossRef]
- Shi, L.; Wei, M.; Miao, Y.; Qian, N.; Shi, L.; Singer, R.A.; Benninger, R.K.; Min, W. Highly-multiplexed volumetric mapping with Raman dye imaging and tissue clearing. Nat. Biotechnol. 2022, 40, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780. [Google Scholar] [CrossRef]
- Fujita, K.; Kobayashi, M.; Kawano, S.; Yamanaka, M.; Kawata, S. High-resolution confocal microscopy by saturated excitation of fluorescence. Phys. Rev. Lett. 2007, 99, 228105. [Google Scholar] [CrossRef]
- Gustafsson, M.G.L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82. [Google Scholar] [CrossRef]
- Huang, F.M.; Zheludev, N.I. Super-resolution without evanescent waves. Nano Lett. 2009, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Hajek, K.M.; Littleton, B.; Turk, D.; McIntyre, T.J.; Rubinsztein-Dunlop, H. A method for achieving super-resolved widefield CARS microscopy. Opt. Express 2010, 18, 19263. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.-W.; Lee, E.S.; Lee, J.Y. A method for super-resolved CARS microscopy with structured illumination in two dimensions. Opt. Express 2014, 22, 9854. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zheng, W.; Ma, Y.; Huang, Z. Higher-order coherent anti-stokes Raman scattering microscopy realizes label-free super-resolution vibrational imaging. Nat. Photonics 2020, 14, 115. [Google Scholar] [CrossRef]
- Gong, L.; Zheng, W.; Ma, Y.; Huang, Z. Saturated stimulated-Raman-scattering microscopy for far-field superresolution vibrational imaging. Phys. Rev. Appl. 2019, 11, 034041. [Google Scholar] [CrossRef]
- Gong, L.; Wang, H. Breaking the diffraction limit by saturation in stimulated-Raman-scattering microscopy: A theoretical study. Phys. Rev. A 2014, 90, 013818. [Google Scholar] [CrossRef]
- Gong, L.; Wang, H. Suppression of stimulated Raman scattering by an electromagnetically-induced-transparency-like scheme and its application for super-resolution microscopy. Phys. Rev. A 2015, 92, 023828. [Google Scholar] [CrossRef]
- Silva, W.R.; Graefe, C.T.; Frontiera, R.R. Toward label-free super-resolution microscopy. ACS Photonics 2016, 3, 79. [Google Scholar] [CrossRef]
- Yonemaru, Y.; Palonpon, A.F.; Kawano, S.; Smith, N.I.; Kawata, S.; Fujita, K. Super-spatial- and -spectral-resolution in vibrational imaging via saturated coherent anti-stokes Raman scattering. Phys. Rev. Appl. 2015, 4, 014010. [Google Scholar] [CrossRef]
- Beeker, W.P.; Groß, P.; Lee, C.J.; Cleff, C.; Offerhaus, H.L.; Fallnich, C.; Herek, J.L.; Boller, K.-J. A route to sub-diffraction-limited CARS microscopy. Opt. Express 2019, 17, 22632. [Google Scholar] [CrossRef]
- Beeker, W.P.; Lee, C.J.; Boller, K.-J.; Groß, P.; Cleff, C.; Fallnich, C.; Offerhaus, H.L.; Herek, J.L. Spatially dependent Rabi oscillations: An approach to sub-diffraction-limited coherent anti-stokes Raman-scattering microscopy. Phys. Rev. A 2010, 81, 012507. [Google Scholar] [CrossRef]
- Cleff, C.; Groß, P.; Fallnich, C.; Offerhaus, H.L.; Herek, J.L.; Kruse, K.; Beeker, W.P.; Lee, C.J.; Boller, K.-J. Ground-state depletion for subdiffractionlimited spatial resolution in coherent anti-stokes Raman scattering microscopy. Phys. Rev. A 2012, 86, 023825. [Google Scholar] [CrossRef]
- Gong, L.; Lin, J.; Hao, C.; Zheng, W.; Wu, S.Q.Y.; Teng, J.; Qiu, C.-W.; Huang, Z. Supercritical focusing coherent anti-stokes Raman scattering microscopy for high-resolution vibrational imaging. Opt. Lett. 2018, 43, 5615. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bryant, G.W.; Stranick, S.J. Superresolution four-wave mixing microscopy. Opt. Express 2012, 20, 6042. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, V.; Potma, E.O. Multiplicative and subtractive focal volume engineering in coherent Raman microscopy. J. Opt. Soc. Am. A 2010, 27, 2365. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.C.; Potma, E.O. Going visible: High-resolution coherent Raman imaging of cells and tissues. Light Sci. Appl. 2019, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Wassie, A.T.; Zhao, Y.; Boyden, E.S. Expansion microscopy: Principles and uses in biological research. Nat. Methods 2016, 16, 33–41. [Google Scholar] [CrossRef]
- Klimas, A.; Gallagher, B.; Wijesekara, P.; Fekir, S.; Stolz, D.; Cambi, F.; Watkins, S.; Barth, A.; Moore, C.; Ren, X.; et al. Magnify is a universal molecular anchoring strategy for expansion microscopy. Nat. Biotechnol. 2023, 41, 858–869. [Google Scholar] [CrossRef]
- Shi, L.; Klimas, A.; Gallagher, B.; Cheng, Z.; Fu, F.; Wijesekara, P.; Miao, Y.; Ren, X.; Zhao, Y.; Min, W. Super-Resolution Vibrational Imaging Using Expansion Stimulated Raman Scattering Microscopy. Adv. Sci. 2022, 9, 2200315. [Google Scholar] [CrossRef]
- Lawrie, B.J.; Lett, P.D.; Marino, A.M.; Pooser, R.C. Quantum sensing with squeezed light. ACS Photonics 2019, 6, 1307–1318. [Google Scholar] [CrossRef]
- Pirandola, S.; Bardhan, B.R.; Gehring, T.; Weedbrook, C.; Lloyd, S. Advances in photonic quantum sensing. Nat. Photonics 2018, 12, 724–733. [Google Scholar] [CrossRef]
- Taylor, M.A.; Bowen, W.P. Quantum metrology and its application in biology. Phys. Rep. 2016, 615, 1–59. [Google Scholar] [CrossRef]
- Slusher, R.E. Quantum optics in the ’80s. Opt. Photonics News 1990, 1, 27–30. [Google Scholar] [CrossRef]
- Sewell, R.J.; Napolitano, M.; Behbood, N.; Colangelo, G.; Mitchell, M.W. Certified quantum non-demolition measurement of a macroscopic material system. Nat. Photonics 2013, 7, 517–520. [Google Scholar] [CrossRef]
- Giovannetti, V.; Lloyd, S.; Maccone, L. Advances in quantum metrology. Nat. Photonics 2011, 5, 222–229. [Google Scholar] [CrossRef]
- Andersen, U.L.; Gehring, T.; Marquardt, C.; Leuchs, G. 30 Years of squeezed light generation. Phys. Scr. 2016, 91, 053001. [Google Scholar] [CrossRef]
- Slusher, R.E.; Hollberg, L.W.; Yurke, B.; Mertz, J.C.; Valley, J.F. Observation of squeezed states generated by four-wave mixing in an optical cavity. Phys. Rev. Lett. 1985, 55, 2409–2412. [Google Scholar] [CrossRef]
- Moreau, P.-A.; Toninelli, E.; Gregory, T.; Padgett, M.J. Imaging with quantum states of light. Nat. Rev. Phys. 2019, 1, 367–380. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, H.; Shi, R.; Cheng, J.-X. Characterization of photodamage in coherent anti-Stokes Raman scattering microscopy. Opt. Express 2006, 14, 3942–3951. [Google Scholar] [CrossRef]
- Xu, Z.; Oguchi, K.; Taguchi, Y.; Sano, Y.; Miyawaki, Y.; Cheon, D.; Katoh, K.; Ozeki, Y. Stimulated Raman scattering spectroscopy with quantum-enhanced balanced detection. Opt. Express 2022, 30, 18589. [Google Scholar] [CrossRef]
- Xu, Z.; Oguchi, K.; Taguchi, Y.; Takahashi, S.; Sano, Y.; Mizuguchi, T.; Katoh, K.; Ozeki, Y. Quantum-enhanced stimulated Raman scattering microscopy in a high-power regime. Opt. Lett. 2022, 47, 5829. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, R.B.; Kerdoncuff, H.; Berg-Sørensen, K.; Gehring, T.; Lassen, M.; Andersen, U.L. Quantum-enhanced continuous-wave stimulated Raman scattering spectroscopy. Optica 2020, 7, 470–475. [Google Scholar] [CrossRef]
- Casacio, C.A.; Madsen, L.S.; Terrasson, A.; Waleed, M.; Barnscheidt, K.; Hage, B.; Taylor, M.A.; Bowen, W.P. Quantum-enhanced nonlinear microscopy. Nature 2021, 594, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Lin, S.; Huang, Z. Super-resolution stimulated Raman scattering microscopy enhanced by quantum light and deconvolution. Opt. Lett. 2023, 48, 6516–6519. [Google Scholar] [CrossRef] [PubMed]
- Beskrovnyy, V.; Kolobov, M. Quantum limits of super-resolution in reconstruction of optical objects. Phys. Rev. A 2005, 71, 043802. [Google Scholar] [CrossRef]
- Yue, S.; Cheng, J.X. Deciphering single cell metabolism by coherent Raman scattering microscopy. Curr. Opin. Chem. Biol. 2016, 33, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Tirinato, L.; Pagliari, F.; Limongi, T.; Marini, M.; Falqui, A.; Seco, J.; Candeloro, P.; Liberale, C.; Di Fabrizio, E. An Overview of Lipid Droplets in Cancer and Cancer Stem Cells. Stem Cells Int. 2017, 2017, 1656053. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell. Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, D.; Jung, Y.; Cheng, J.X.; Umulis, D.M. Label-free imaging of lipid droplet intracellular motion in early Drosophila embryos using femtosecond stimulated Raman loss microscopy. Biophys. J. 2012, 102, 1666–1675. [Google Scholar] [CrossRef]
- AH, H.; Manifold, B.; Fu, D. Tissue imaging depth limit of stimulated Raman scattering microscopy. Biomed. Opt. Express 2020, 11, 762–774. [Google Scholar]
- Ji, M.; Orringer, D.A.; Freudiger, C.W.; Ramkissoon, S.; Liu, X.; Lau, D.; Golby, A.J.; Norton, I.; Hayashi, M.; Agar, N.Y.; et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci. Transl. Med. 2013, 5, 201ra119. [Google Scholar] [CrossRef]
- Lu, F.K.; Calligaris, D.; Olubiyi, O.I.; Norton, I.; Yang, W.; Santagata, S.; Xie, X.S.; Golby, A.J.; Agar, N.Y. Label-Free Neurosurgical Pathology with Stimulated Raman Imaging. Cancer Res. 2016, 76, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Arbel, M.; Zhang, L.; Freudiger, C.W.; Hou, S.S.; Lin, D.; Yang, X.; Bacskai, B.J.; Xie, X.S. Label-free imaging of amyloid plaques in Alzheimer’s disease with stimulated Raman scattering microscopy. Sci. Adv. 2018, 4, eaat7715. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Yang, W.; Mordes, D.A.; Wang, J.Y.; Salameh, J.S.; Mok, J.; Chew, J.; Sharma, A.; Leno-Duran, E.; Suzuki-Uemats Suzuki, N.; et al. Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging. Nat. Commun. 2016, 7, 13283. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Yang, W.; Xie, X.S. Label-free Imaging of Neurotransmitter Acetylcholine at Neuromuscular Junctions with Stimulated Raman Scattering. J. Am. Chem. Soc. 2017, 139, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.S.; Wang, P.; Huang, C.Y.; Lin, P.; Eakins, G.; Bentley, R.T.; Liang, R.; Cheng, J.X. In Vivo and in Situ Spectroscopic Imaging by a Handheld Stimulated Raman Scattering Microscope. ACS Photonics 2018, 5, 947–954. [Google Scholar] [CrossRef]
- Tipping, W.J.; Lee, M.; Serrels, A.; Brunton, V.G.; Hulme, A.N. Stimulated Raman scattering microscopy: An emerging tool for drug discovery. Chem. Soc. Rev. 2016, 45, 2075–2089. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, M.; Cheng, J.-X. Rapid determination of antimicrobial susceptibility by SRS single-cell metabolic imaging. In Chapter 29—Title: Stimulated Raman Scattering Microscopy; Cheng, J.-X., Min, W., Ozeki, Y., Polli, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 445–461. ISBN 9780323851589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).