Dual-Wavelength Confocal Laser Speckle Contrast Imaging Using a Deep Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. DW-LSCI Imaging Setup
2.2. Principle of Speckle Contrast Imaging
2.3. Principle of Blood Oxygenation Imaging
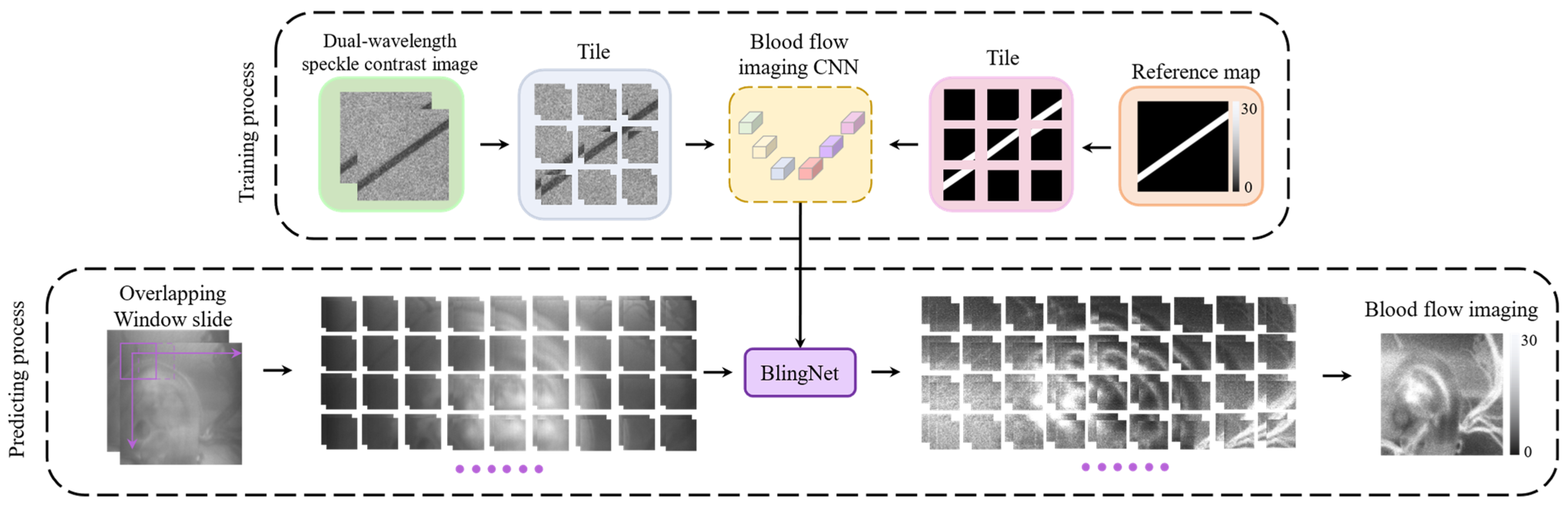
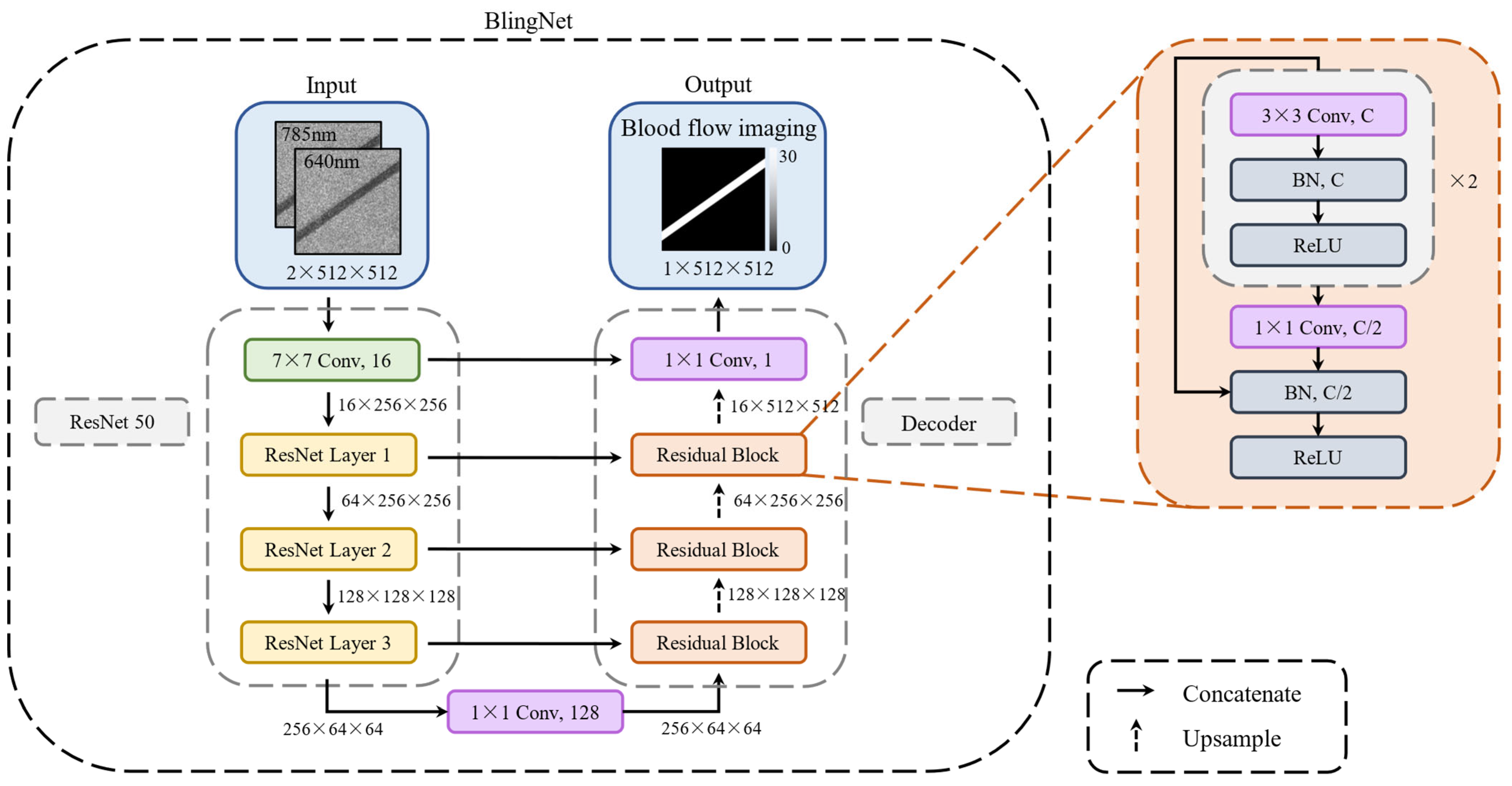
2.4. Deep Learning Framework of Blood Flow Imaging CNN (BlingNet)
2.5. Sample Preparation
3. Results
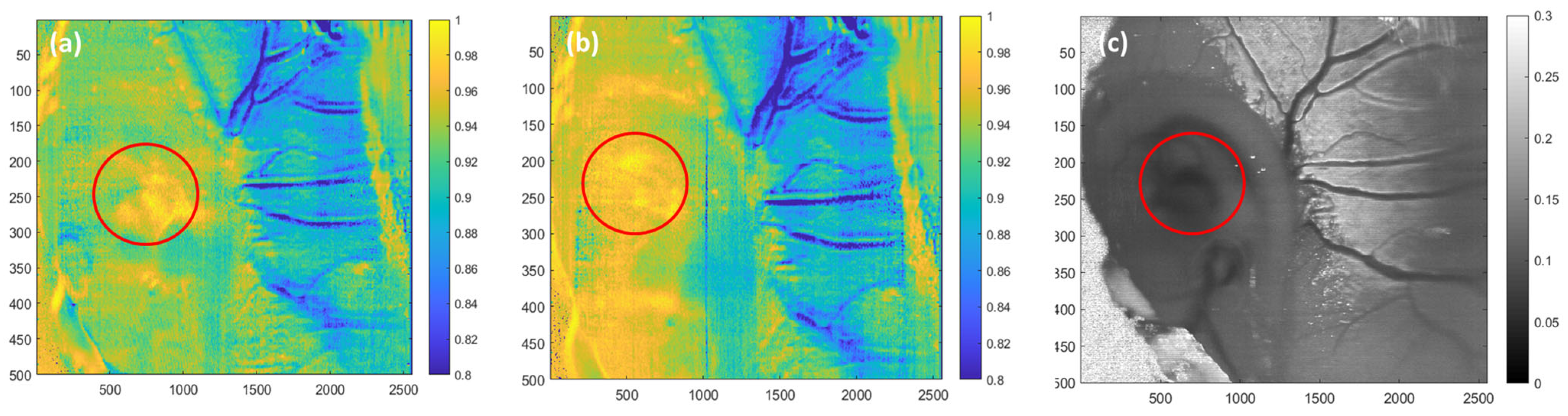
3.1. Flow Phantom Experiment
3.2. Blood Flow Imaging of the Deep Tissue
3.3. Hemoglobin Oxygen Saturation SO2 Imaging of the Deep Tissue
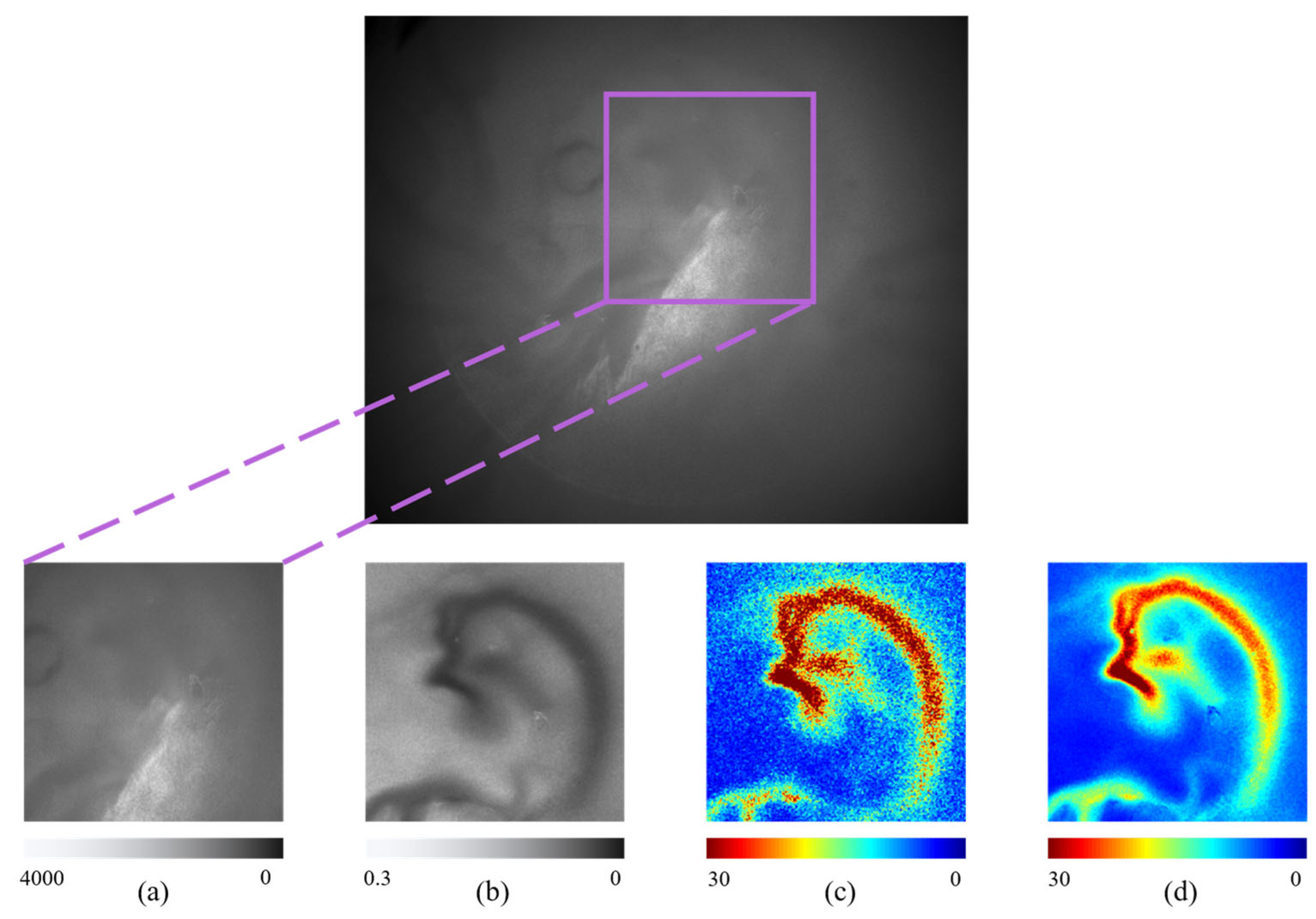
3.4. Speckle Contrast Imaging Combined with Deep Learning Approach
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pijls, N.H.; de Vos, A.M.; Keulards, D.C. Measurement ofabsolute coronary blood flow and microvascular resistance: A new window to coronary microcirculation. J. Am. Coll. Cardiol. 2021, 77, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Awuah, A.; Moore, J.S.; Nesbit, M.A.; Ruddock, M.W.; Brennan, P.F.; Mailey, J.A.; McNeil, A.J.; Jing, M.; Finlay, D.D.; Trucco, E.; et al. A novel algorithm for cardiovascular screening using conjunctival microcirculatory parameters and blood biomarkers. Sci. Rep. 2022, 12, 6545. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Upputuri, P.K.; Sivasubramanian, K.; Mark, C.S.; Pramanik, M. Recent developments in vascular imaging techniques in tissue engineering and regenerative medicine. BioMed Res. Int. 2015, 2015, 783983. [Google Scholar] [CrossRef] [PubMed]
- Senarathna, J.; Rege, A.; Li, N.; Thakor, N.V. Laser speckle contrast imaging: Theory, instrumentation and applications. IEEE Rev. Biomed. Eng. 2013, 6, 99–110. [Google Scholar] [CrossRef]
- Rajan, V.; Varghese, B.; Van Leeuwen, T.G.; Steenbergen, W. Review of methodological developments in laser Doppler flowmetry. Lasers Med. Sci. 2009, 24, 269–283. [Google Scholar] [CrossRef]
- Allam, N.; Zabel, W.J.; Demidov, V.; Jones, B.; Flueraru, C.; Taylor, E.; Vitkin, I.A. Longitudinal in-vivo quantification of tumour microvascular heterogeneity by optical coherence angiography in pre-clinical radiation therapy. Sci. Rep. 2022, 12, 6140. [Google Scholar] [CrossRef]
- Sakadžić, S.; Roussakis, E.; Yaseen, M.A.; Mandeville, E.T.; Srinivasan, V.J.; Arai, K.; Ruvinskaya, S.; Devor, A.; Lo, E.H.; Vinogradov, S.A.; et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat. Methods 2010, 7, 755–759. [Google Scholar] [CrossRef]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85, 51–63. [Google Scholar] [CrossRef]
- Qureshi, M.M.; Allam, N.; Im, J.; Kwon, H.S.; Chung, E.; Vitkin, I.A. Advances in laser speckle imaging: From qualitative to quantitative hemodynamic assessment. J. Biophotonics 2024, 17, e202300126. [Google Scholar] [CrossRef]
- Nitta, F.; Kunikata, H.; Aizawa, N.; Omodaka, K.; Shiga, Y.; Yasuda, M.; Nakazawa, T. The effect of intravitreal bevacizumab on ocular blood flow in diabetic retinopathy and branch retinal vein occlusion as measured by laser speckle flowgraphy. Clin. Ophthalmol. 2014, 8, 1119–1127. [Google Scholar]
- Molnár, E.; Molnár, B.; Lohinai, Z.; Tóth, Z.; Benyó, Z.; Hricisák, L.; Windisch, P.; Vág, J. Evaluation of laser speckle contrast imaging for the assessment of oral mucosal blood flow following periodontal plastic surgery: An exploratory study. BioMed Res. Int. 2017, 1, 4042902. [Google Scholar] [CrossRef] [PubMed]
- White, S.M.; Valdebran, M.; Kelly, K.M.; Choi, B. Simultaneous blood flow measurement and dermoscopy of skin lesions using Dual-Mode dermascope. Sci. Rep. 2018, 8, 16941. [Google Scholar] [CrossRef] [PubMed]
- Farraro, R.; Fathi, O.; Choi, B. Handheld, point-of-care laser speckle imaging. J. Biomed. Opt. 2016, 21, 094001. [Google Scholar] [CrossRef] [PubMed]
- Hecht, N.; Woitzik, J.; König, S.; Horn, P.; Vajkoczy, P. Laser speckle imaging allows real-time intraoperative blood flow assessment during neurosurgical procedures. J. Cereb. Blood Flow Metab. 2013, 33, 1000–1007. [Google Scholar] [CrossRef]
- Eriksson, S.; Nilsson, J.; Lindell, G.; Sturesson, C. Laser speckle contrast imaging for intraoperative assessment of liver microcirculation: A clinical pilot study. Med. Devices Evid. Res. 2014, 7, 257–261. [Google Scholar] [CrossRef]
- Ambrus, R.; Svendsen, L.B.; Secher, N.H.; Rünitz, K.; Frederiksen, H.J.; Svendsen, M.B.; Siemsen, M.; Kofoed, S.C.; Achiam, M.P. A reduced gastric corpus microvascular blood flow during Ivor-Lewis esophagectomy detected by laser speckle contrast imaging technique. Scand. J. Gastroenterol. 2017, 52, 455–461. [Google Scholar] [CrossRef]
- Bray, R.C.; Forrester, K.R.; Reed, J.; Leonard, C.; Tulip, J. Endoscopic laser speckle imaging of tissue blood flow: Applications in the human knee. J. Orthop. Res. 2006, 24, 1650–1659. [Google Scholar] [CrossRef]
- Du, E.; Shen, S.; Chong, S.P.; Chen, N. Multifunctional laser speckle imaging. Biomed. Opt. Express 2020, 11, 2007–2016. [Google Scholar] [CrossRef]
- Du, E.; Shen, S.; Qiu, A.; Chen, N. Line scan spatial speckle contrast imaging and its application in blood flow imaging. Appl. Sci. 2021, 11, 10969. [Google Scholar] [CrossRef]
- Du, E.; Shen, S.; Qiu, A.; Chen, N. Depth-dependent microscopic flow imaging with line scan laser speckle acquisition and analysis. In Proceedings of the Optics in Health Care and Biomedical Optics XI, Nantong, China, 10–20 October 2021; SPIE: Washington, WA, USA, 2021; Volume 11900, pp. 20–26. [Google Scholar]
- Du, E.; Shen, S.; Qiu, A.; Chen, N. Confocal laser speckle autocorrelation imaging of dynamic flow in microvasculature. Opto-Electron. Adv. 2022, 5, 210045-1. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, Z.; Pan, Y.; Du, C. Simultaneous imaging of cortical hemodynamics and blood oxygenation change during cerebral ischemia using dual-wavelength laser speckle contrast imaging. Opt. Lett. 2009, 34, 1480–1482. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Dosovitskiy, A. An image is worth 16x16 words: Transformers for image recognition at scale. arXiv 2020, arXiv:2010.11929. [Google Scholar]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- Gu, J.; Wang, Z.; Kuen, J.; Ma, L.; Shahroudy, A.; Shuai, B.; Liu, T.; Wang, X.; Wang, G.; Cai, J.; et al. Recent advances in convolutional neural networks. Pattern Recognit. 2018, 77, 354–377. [Google Scholar] [CrossRef]
- Stebakov, I.; Kornaeva, E.; Stavtsev, D.; Potapova, E.; Dremin, V. Laser speckle contrast imaging and machine learning in application to physiological fluids flow rate recognition. Vibroeng. Procedia 2021, 38, 50–55. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Chammas, M.; Gurden, H.; Lin, H.-H.; Pain, F. Design and validation of a convolutional neural network for fast, model-free blood flow imaging with multiple exposure speckle imaging. Biomed. Opt. Express 2023, 14, 4439–4454. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Ku, G.; Wang, L.V.; Stoica, G. Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography. J. Biomed. Opt. 2006, 11, 024015. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015, Proceedings of the 18th International Conference, Munich, Germany, 5–9 October 2015; Proceedings, Part III 18; Springer International Publishing: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Mao, A.; Mohri, M.; Zhong, Y. Cross-entropy loss functions: Theoretical analysis and applications. In Proceedings of the International Conference on Machine Learning, Honolulu, HI, USA, 23–29 July 2023; pp. 23803–23828. [Google Scholar]
- Zhao, R.; Qian, B.; Zhang, X.; Li, Y.; Wei, R.; Liu, Y.; Pan, Y. Rethinking dice loss for medical image segmentation. In Proceedings of the 2020 IEEE International Conference on Data Mining (ICDM), Sorrento, Italy, 17–20 November 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 851–860. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, E.; Zheng, H.; He, H.; Li, S.; Qiu, C.; Zhang, W.; Wang, G.; Li, X.; Ma, L.; Shen, S.; et al. Dual-Wavelength Confocal Laser Speckle Contrast Imaging Using a Deep Learning Approach. Photonics 2024, 11, 1085. https://doi.org/10.3390/photonics11111085
Du E, Zheng H, He H, Li S, Qiu C, Zhang W, Wang G, Li X, Ma L, Shen S, et al. Dual-Wavelength Confocal Laser Speckle Contrast Imaging Using a Deep Learning Approach. Photonics. 2024; 11(11):1085. https://doi.org/10.3390/photonics11111085
Chicago/Turabian StyleDu, E, Haohan Zheng, Honghui He, Shiguo Li, Cong Qiu, Weifeng Zhang, Guoqing Wang, Xingquan Li, Lan Ma, Shuhao Shen, and et al. 2024. "Dual-Wavelength Confocal Laser Speckle Contrast Imaging Using a Deep Learning Approach" Photonics 11, no. 11: 1085. https://doi.org/10.3390/photonics11111085
APA StyleDu, E., Zheng, H., He, H., Li, S., Qiu, C., Zhang, W., Wang, G., Li, X., Ma, L., Shen, S., & Zhou, Y. (2024). Dual-Wavelength Confocal Laser Speckle Contrast Imaging Using a Deep Learning Approach. Photonics, 11(11), 1085. https://doi.org/10.3390/photonics11111085




