Advances in Photoacoustic Endoscopic Imaging Technology for Prostate Cancer Detection
Abstract
1. Introduction
2. Photoacoustic Endoscope Imaging Technique
3. Research on Photoacoustic Endoscopic Imaging Technology for Prostate Cancer Detection
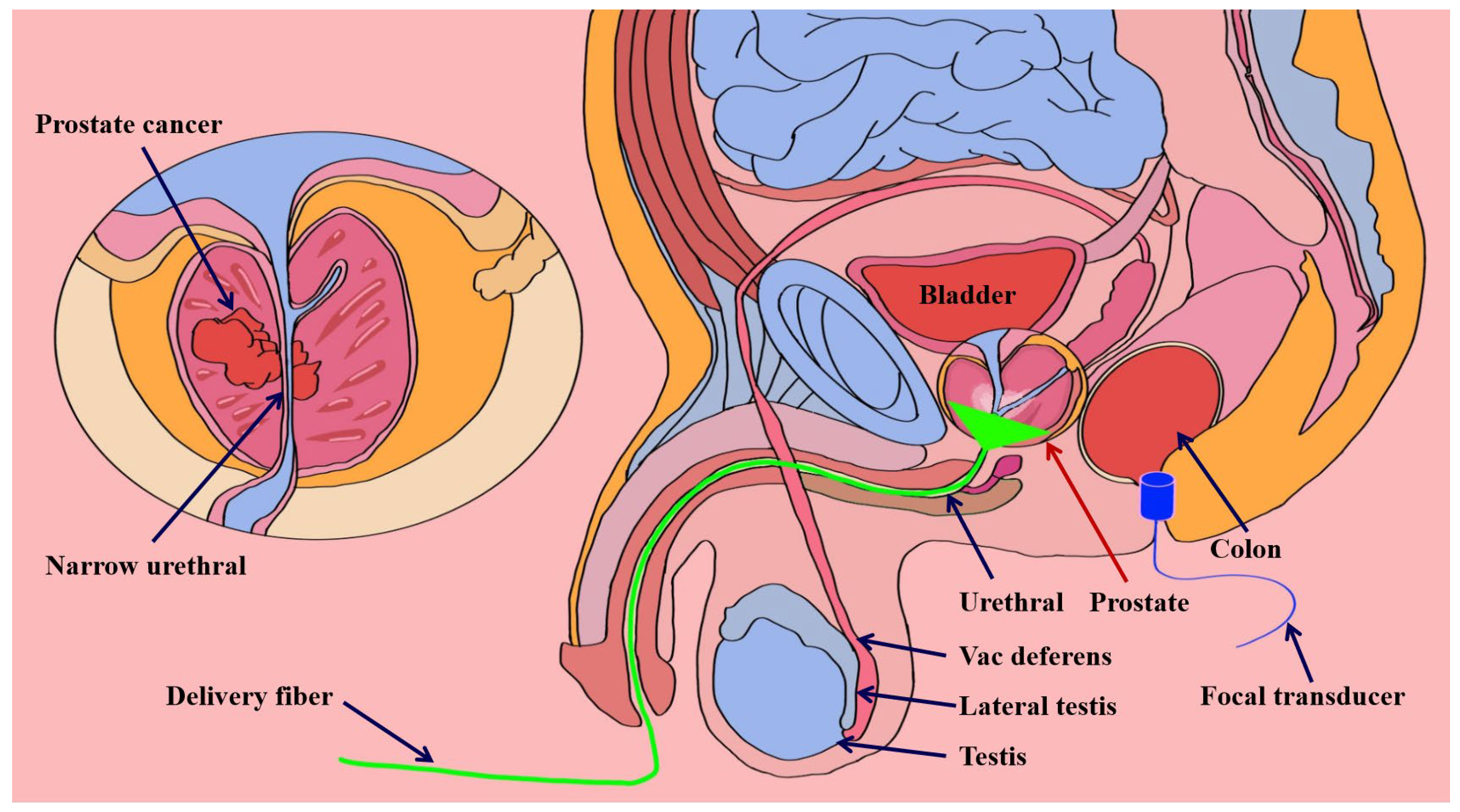
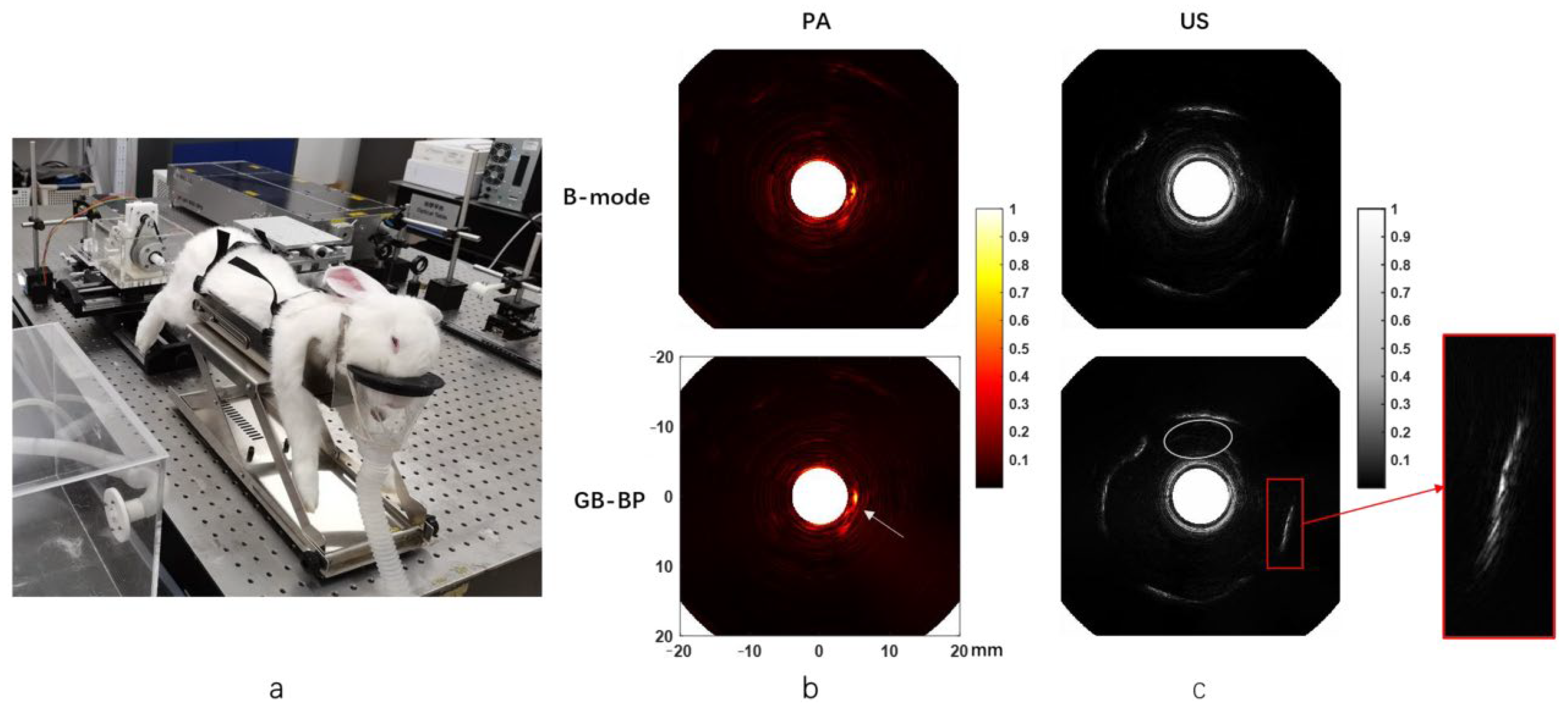
3.1. Prostate Detection System Utilizing Photoacoustic Endoscopic Imaging Technology
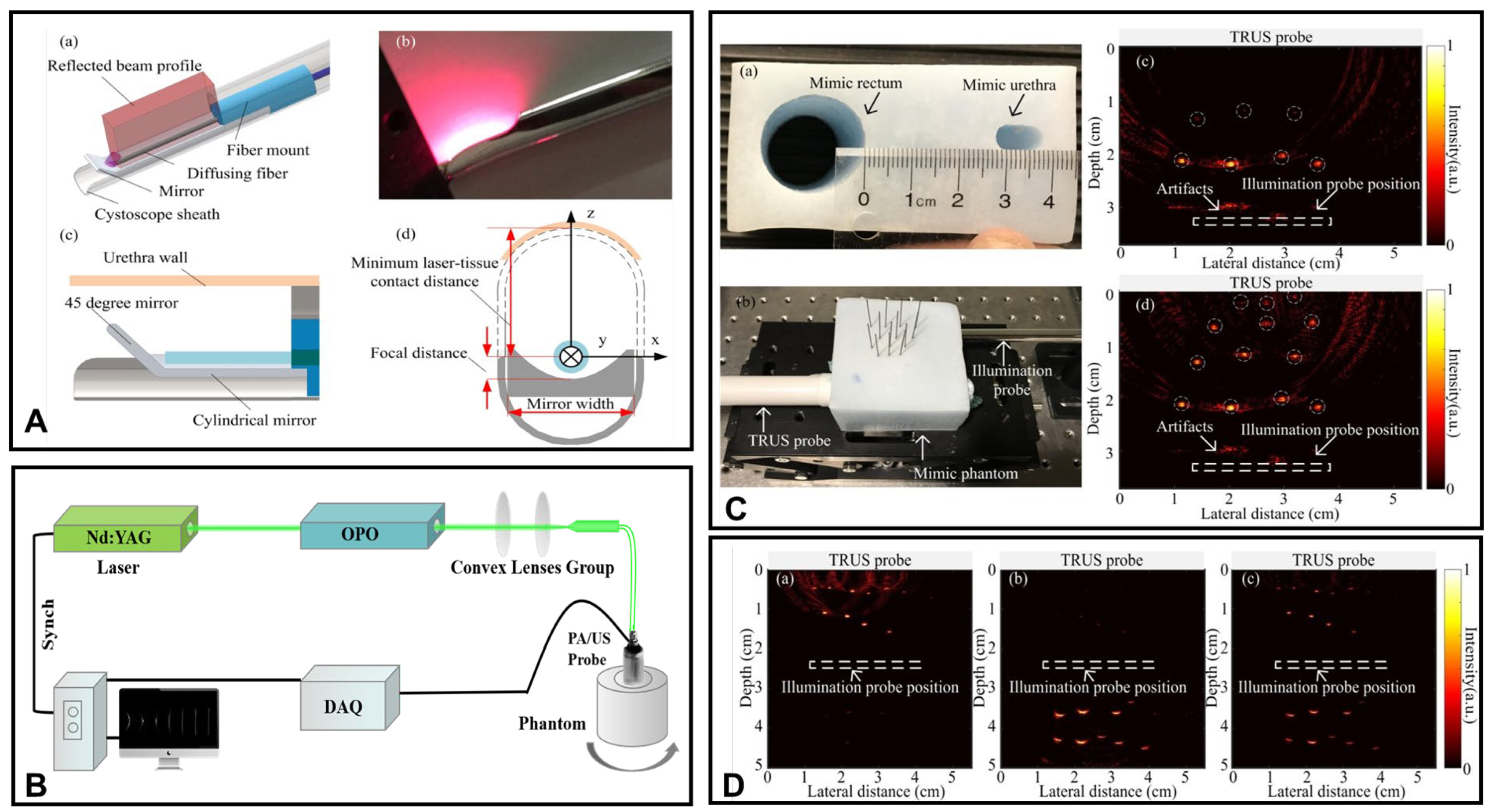
3.1.1. Transrectal Photodelivery
3.1.2. Transurethral Light Delivery
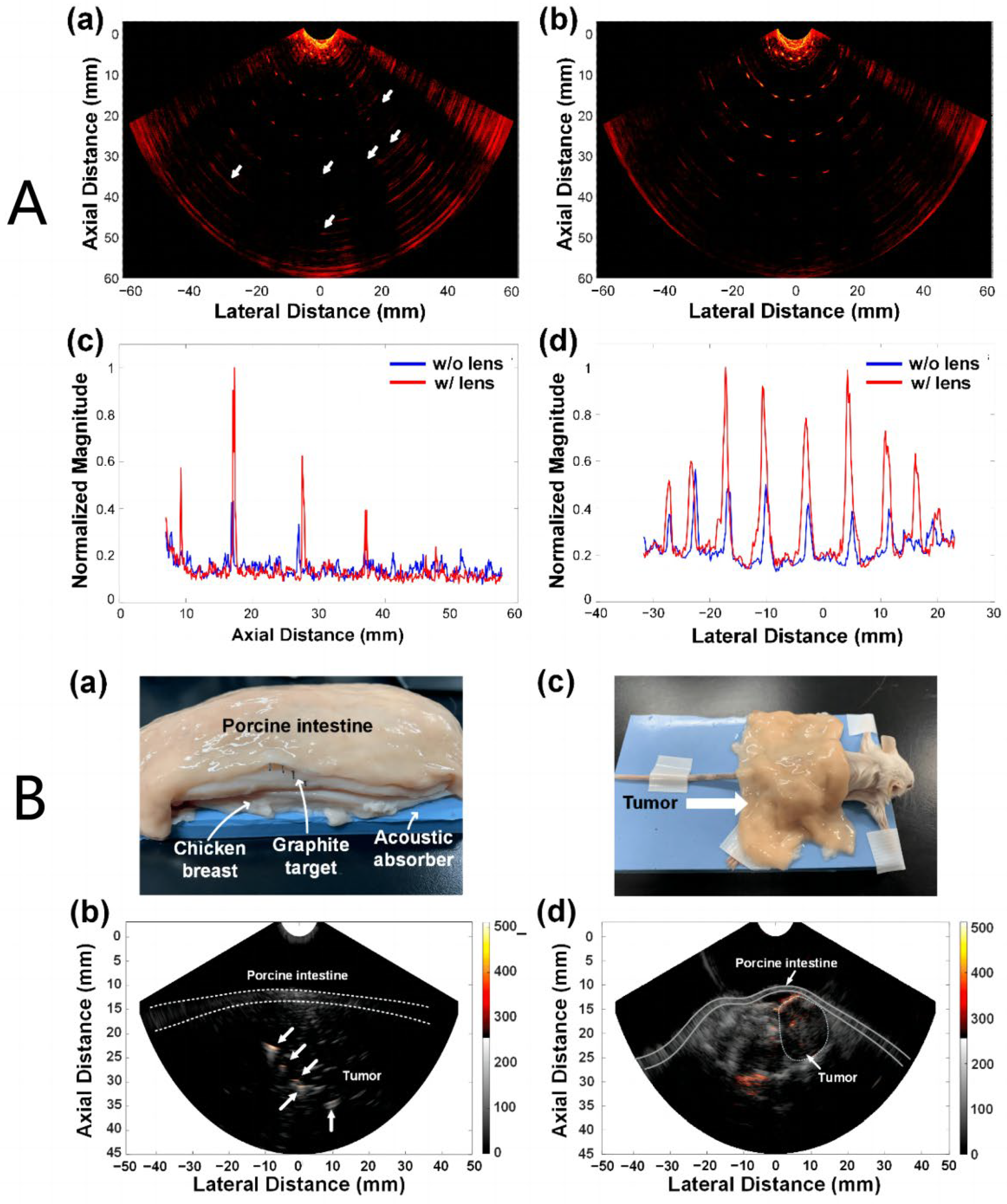
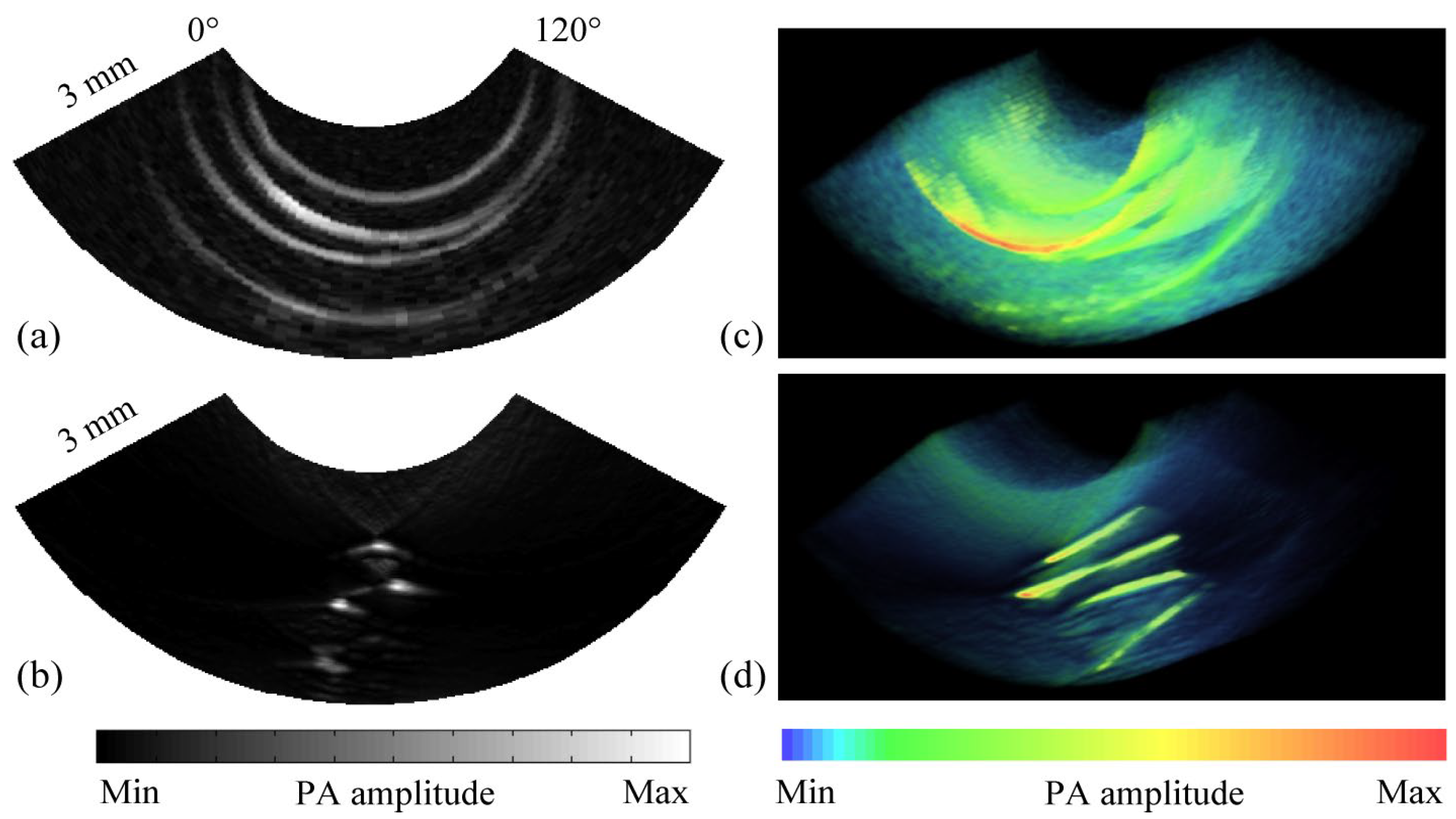
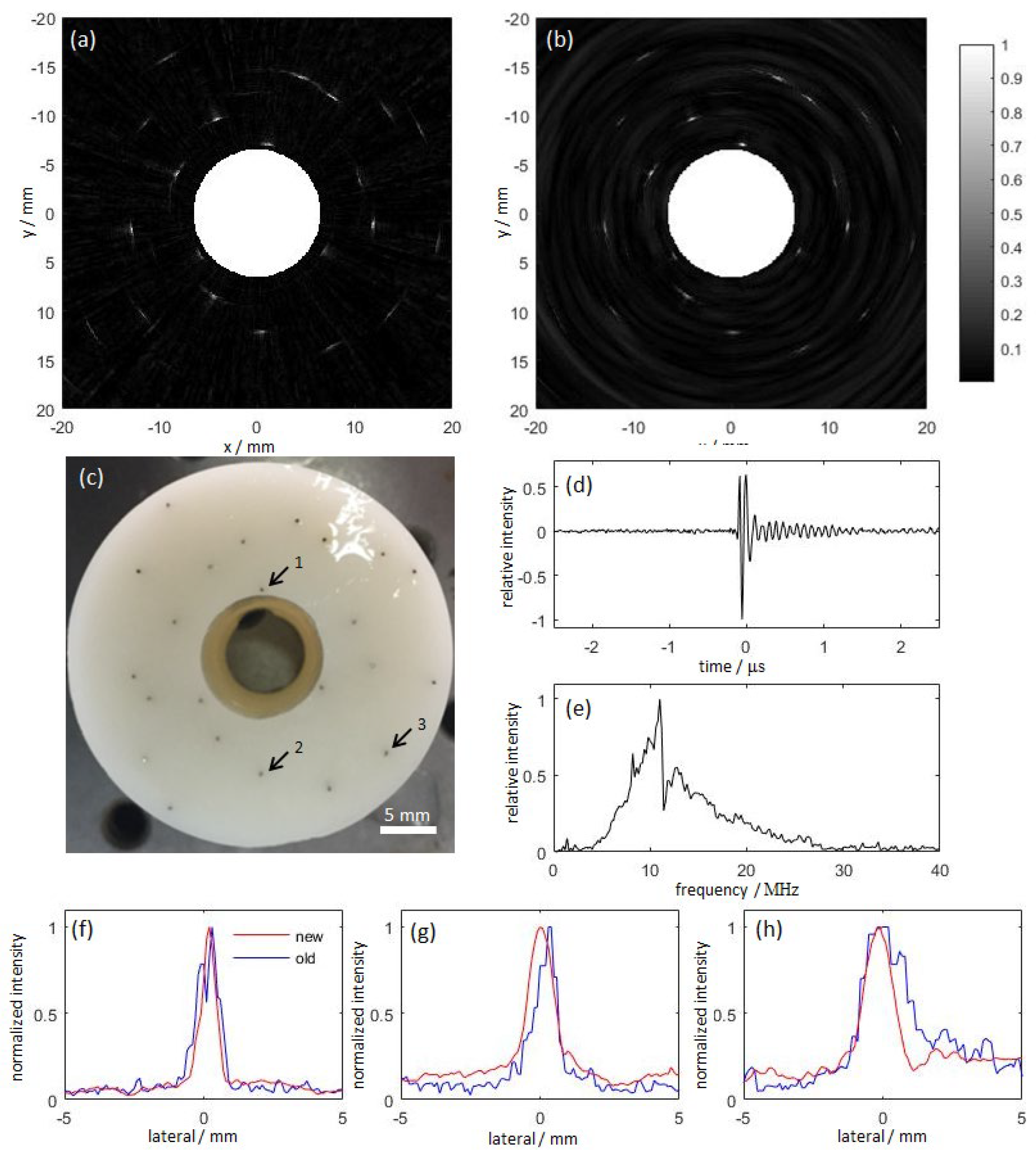
3.2. Research Progress on Photoacoustic Endoscopic Imaging Reconstruction Algorithms
3.2.1. General PAE Algorithm
3.2.2. Improved PAE Imaging Algorithm
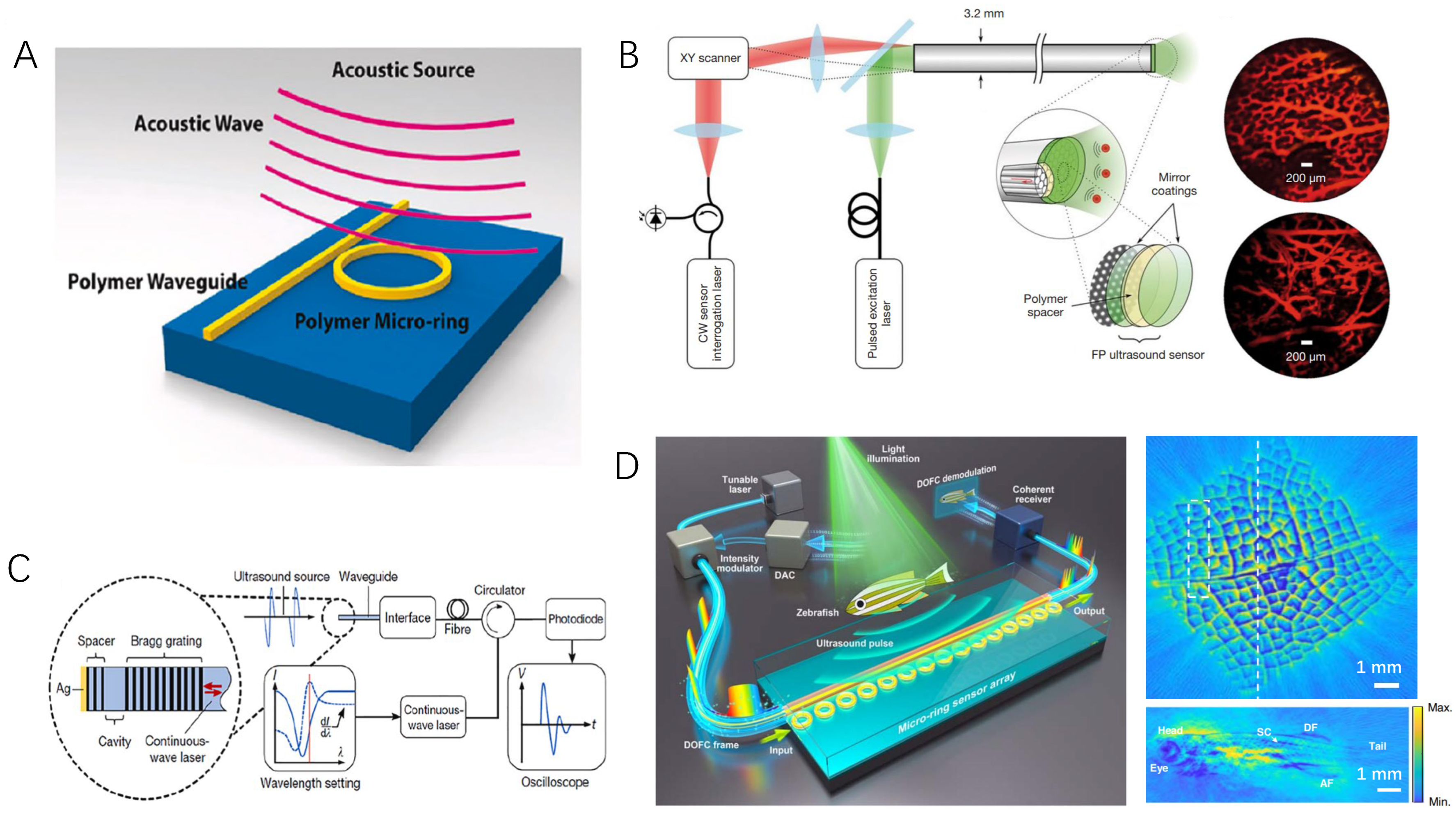
3.3. Optical Ultrasound Sensing Technologies and Their Applications in PAE
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Borno, H.; George, D.J.; Schnipper, L.E.; Cavalli, F.; Cerny, T.; Gillessen, S. All men are created equal: Addressing disparities in prostate cancercare. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.L.; Zhang, Q.C. Research progress of Chinese traditional medicine on the treatment of prostate cancer. Glob. Tradit. Chin. Med. CNKI China 2015, 8, 253–256. [Google Scholar]
- Miao, Y.S.U. New progress of traditional Chinese medicine and pharmacy in the treatment of prostate cancer. Glob. Tradit. Chin. Med. CNKI China 2012, 5, 152–156. [Google Scholar]
- Descotes, J.L. Diagnosis of prostate cancer. Asian J. Urol. 2019, 6, 129–136. [Google Scholar] [CrossRef]
- Andersson, J.; Palsdottir, T.; Lantz, A.; Aly, M.; Grönberg, H.; Egevad, L.; Eklund, M.; Nordström, T. Digital Rectal Examination in Stockholm3 Biomarker-based Prostate Cancer Screening. Eur. Urol. Open Sci. 2022, 44, 69–75. [Google Scholar] [CrossRef]
- Roobol, M.J.; Vugt, H.A.V.; Loeb, S.; Zhu, X.; Bul, M.; Bangma, C.H.; VanLeenders, A.G.L.J.H.; Steyerberg, E.W.; Schröder, F.H. Prediction of prostate cancer risk: The role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur. Urol. 2012, 61, 577–583. [Google Scholar] [CrossRef]
- Ankerst, D.P.; Straubinger, J.; Selig, K.; Guerrios, L.; De Hoedt, A.; Hernandez, J.; Liss, M.A.; Leach, R.J.; Freedland, S.J.; Kattan, M.W.; et al. A contemporary prostate biopsy risk calculator based on multiple heterogeneous cohorts. Eur. Urol. 2018, 74, 197–203. [Google Scholar] [CrossRef]
- Ferraro, S.; Bussetti, M.; Panteghini, M. Serum prostate-specific antigen testing for early detection of prostate cancer: Managing the gap between clinical and laboratory practice. Clin. Chem. 2021, 67, 602–609. [Google Scholar] [CrossRef]
- Halpern, J.A.; Oromendia, C.; Shoag, J.E.; Mittal, S.; Cosiano, M.F.; Ballman, K.V.; Vickers, A.J.; Hu, J.C. Use of digital rectal examination as an adjunct to prostate specific antigen in the detection of clinically significant prostate cancer. J. Urol. 2018, 199, 947–953. [Google Scholar] [CrossRef]
- Beemsterboer, P.M.M.; Kranse, R.; Koning, H.J.D.; Habbema, J.D.F.; Schröder, F.H. Changing role of 3 screening modalities in the European randomized study of screening for prostate cancer (Rotterdam). Int. J. Cancer 1999, 84, 437–441. [Google Scholar] [CrossRef]
- Schröder, F.H.; Roobol-Bouts, M.; Vis, A.N.; Kwast, T.V.D.; Kranse, R. Prostate-specific antigen-based early detection of prostate cancer—Validation of screening without rectal examination. Urology 2001, 57, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Merriel, S.W.D.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.M.; Spencer, A.; Hamilton, W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.N.; Berglund, R.K.; Jones, J.S. A practical guide to prostate cancer diagnosis and management. Cleve. Clin. J. Med. 2011, 78, 321–331. [Google Scholar] [CrossRef][Green Version]
- Klotz, L.; Chin, J.; Black, P.C.; Finelli, A.; Anidjar, M.; Bladou, F.; Machado, A.; Levental, M.; Ghai, S.; Chang, S. Comparison of multiparametric magnetic resonance imaging–targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021, 7, 534–542. [Google Scholar] [CrossRef]
- Bass, E.J.; Pantovic, A.; Connor, M.J.; Loeb, S.; Rastinehad, A.R.; Winkler, M.; Gabe, R.; Ahmed, H.U. Diagnostic accuracy of magnetic resonance imaging targeted biopsy techniques compared to transrectal ultrasound guided biopsy of the prostate: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022, 25, 174–179. [Google Scholar] [CrossRef]
- Loeb, S.; Gonzalez, C.M.; Roehl, K.A.; Han, M.; Antenor, J.A.V.; Yap, R.L.; Catalona, W.J. Pathological characteristics of prostate cancer detected through prostate specific antigen based screening. Urology 2006, 175, 902–906. [Google Scholar] [CrossRef]
- Mottet, N.; Bergh, R.C.N.V.D.; Briers, E.; Broeck, T.V.D.; Catto, J. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Jonathan Bloom, M.D.; Gurram, S.; Siddiqui, M.; Pinsky, P.; et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef]
- de Rooij, M.; Hamoen, E.H.J.; Fütterer, J.J.; Barentsz, J.O.; Rovers, M.M. Accuracy of multiparametric MRI for prostate cancer detection: A meta-analysis. Ajr Am. J. Roentgenol. 2014, 202, 343–351. [Google Scholar] [CrossRef]
- Liu, L.; Tian, Z.; Zhang, Z.; Fei, B. Computer-aided detection of prostate cancer with MRI: Technology and applications. Acad. Radiol. 2016, 23, 1024–1046. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect. Agents Cancer 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, J.; Wang, L.V. Tutorial on photoacoustic tomography. Biomed. Opt. 2016, 21, 061007. [Google Scholar] [CrossRef]
- Maslov, K.I.; Wang, L.V. Photoacoustic imaging of biological tissue with intensity-modulated continuous-wave laser. J. Biomed. Opt. 2008, 13, 024006–024011. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, S.; Liang, Z.; Wang, Y.; Ge, J.; Chen, W.; Qi, L. Advanced image post-processing methods for photoacoustic tomography: A review. Photonics 2023, 10, 707–730. [Google Scholar] [CrossRef]
- Le, T.D.; Kwon, S.Y.; Lee, C. Segmentation and quantitative analysis of photoacoustic imaging: A review. Photonics 2022, 9, 176. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Qi, W.; Xi, L. Photoacoustic endoscopy: A progress review. Biophotonics 2020, 13, e202000217. [Google Scholar] [CrossRef]
- Ji, X.; Xiong, K.; Yang, S.; Xing, D. Intravascular confocal photoacoustic endoscope with dual-element ultrasonic transducer. Opt. Express 2015, 23, 9130–9136. [Google Scholar] [CrossRef]
- Chen, B.Z.; Hang, Y.; Yang, J.G.; Chi, Z.H.; Jian, R.; Bing, H.; Jiang, H.B. The human colorectal cancer tissue in vitro experimental study based on photoacoustic endoscopic system. Acta Phys. Sin. 2014, 63, 84204. [Google Scholar] [CrossRef]
- Aguirre, A.; Ardeshirpour, Y.; Sanders, M.M.; Brewer, M.; Zhu, Q. Potential role of coregistered photoacoustic and ultrasound imaging in ovarian cancer detection and characterization. Transl. Oncol. 2011, 4, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, H.; Klippel, P.; Zhang, B.; Huang, H.Y.S.; Jing, Y.; Jiang, X.; Yao, J. Deep thrombosis characterization using photoacoustic imaging with intravascular light delivery. Biomed. Eng. Lett. 2022, 12, 135–145. [Google Scholar] [CrossRef]
- Yang, J.M.; Favazza, C.; Yao, J.; Chen, R.; Zhou, Q.; Shung, K.K.; Wang, L.V. Three-dimensional photoacoustic endoscopic imaging of the rabbit esophagus. PLoS ONE 2015, 10, e0120269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, G.; Zhou, Q.; Chen, Z. Advances in endoscopic photoacoustic imaging. Photonics 2021, 8, 281. [Google Scholar] [CrossRef]
- Gao, W.; Mo, H.; Wu, G.; Yang, D.; Yin, L. Compressive endoscopic imaging with complementary light modulation. Appl. Opt. 2021, 60, 8221–8225. [Google Scholar] [CrossRef]
- Yang, D.W.; Zeng, L.M.; Ji, X.R.; Huang, Z.; Chen, X.H.; Tan, Z. Fast photoacoustic imaging of blood vessels based on an annular transducer array. Chin. Phys. Lett. 2012, 29, 1–5. [Google Scholar] [CrossRef]
- Yang, D.W.; Zhou, Z.B.; Zeng, L.M.; Xin, Z.; Chen, X.H. Photoacoustic Imaging of Animals with an Annular Transducer Array. Chin. Phys. Lett. 2014, 31, 1–4. [Google Scholar] [CrossRef]
- Bauer-Marschallinger, J.; Höllinger, A.; Jakoby, B.; Burgholzer, P.; Berer, T. Fiber-optic annular detector array for large depth of field photoacoustic macroscopy. Photoacoustics 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, T.; Peng, K.; Zhang, X.; Wang, B. Lithography of Aluminum Coated PVDF Annular Array for Photoacoustic Endoscopy. Commun. Comput. Phys. 2018, 23, 561–571. [Google Scholar] [CrossRef]
- Passler, K.; Nuster, R.; Gratt, S.; Burgholzer, P.; Paltauf, G. Piezoelectric annular array for large depth of field photoacoustic imaging. Biomed. Opt. Express 2011, 2, 2655–2664. [Google Scholar] [CrossRef]
- Wang, H.; Xing, D.; Xiang, L. Photoacoustic imaging using an ultrasonic Fresnel zone plate transducer. J. Phys. D Appl. Phys. 2009, 41, 95111–95118. [Google Scholar] [CrossRef]
- Song, C.; Xi, L.; Jiang, H. Acoustic lens with variable focal length for photoacoustic microscopy. Appl. Phys. 2013, 114, 194703–194708. [Google Scholar] [CrossRef]
- Song, C.; Xi, L.; Jiang, H. Liquid acoustic lens for photoacoustic tomography. Opt. Lett. 2013, 38, 2930–2933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Ji, X.; Zhou, Q.; Xing, D. Characterization of lipid-rich aortic plaques by intravascular photoacoustic tomography: Ex vivo and in vivo validation in a rabbit atherosclerosis model with histologic correlation. Am. Coll. Cardiol. 2014, 64, 385–390. [Google Scholar] [CrossRef]
- Dai, X.; Yang, H.; Shan, T.; Xie, H.K.; Berceli, S.A.; Jiang, H. Miniature endoscope for multimodal imaging. ACS Photonics 2017, 4, 174–180. [Google Scholar] [CrossRef]
- Zhu, Q.C. A review of co-registered transvaginal Photoacoustic and ultrasound Imaging for Ovarian Cancer Diagnosis. Curr. Opin. Biomed. Eng. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Lei, P.; Wen, X.; Zhang, P.; Yang, S. Tapered fiber-based intravascular photoacoustic endoscopy for high-resolution and deep-penetration imaging of lipid-rich plaque. Opt. Express 2019, 27, 12832–12840. [Google Scholar] [CrossRef]
- Kothapalli, S.R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Chullee, B.; et al. Simultaneous transrectal ultrasound and photoacoustic human prostate imaging. Sci. Transl. Med. 2019, 11, eaav2169. [Google Scholar] [CrossRef]
- Agrawal, S.; Johnstonbaugh, K.; Clark, J.Y.; Raman, J.D.; Wang, X.; Kothapalli, S.R. Design, development, and multi-characterization of an integrated clinical transrectal ultrasound and photoacoustic device for human prostate imaging. Diagnostics 2020, 10, 566–582. [Google Scholar] [CrossRef]
- Ma, X.; Peng, C.; Yuan, J.; Cheng, Q.; Xu, G.; Wang, X.; Carson, P.L. Multiple delay and sum with enveloping beamforming algorithm for photoacoustic imaging. IEEE Trans. Med. Imaging 2019, 39, 1812–1821. [Google Scholar] [CrossRef]
- Sheu, Y.L.; Chou, C.Y.; Hsieh, B.Y.; Li, P.C. Image reconstruction in intravascular photoacoustic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, J.; Maurudis, A.; Aguirre, A.; Huang, F.; Guo, P.; Wang, L.V.; Zhu, Q. A real-time photoacoustic tomography system for small animals. Opt. Express 2009, 17, 10489–10498. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chatni, M.R.; Maslov, K.; Guo, Z.; Wang, K.; Anastasio, M.; Wang, L.V. Whole-body ring-shaped confocal photoacoustic computed tomography of small animals in vivo. J. Biomed. Opt. 2012, 17, 50506–50509. [Google Scholar] [CrossRef]
- Cai, D.; Li, G.; Xia, D.; Li, Z.; Guo, Z.; Chen, S.L. Synthetic aperture focusing technique for photoacoustic endoscopy. Opt. Express 2017, 25, 20162–20171. [Google Scholar] [CrossRef]
- El-Gohary, S.H.; Metwally, M.K.; Eom, S.; Jeon, S.H.; Byun, K.M.; Kim, T.S. Design study on photoacoustic probe to detect prostate cancer using 3D Monte Carlo simulation and finite element method. Biomed. Eng. Lett. 2014, 4, 250–257. [Google Scholar] [CrossRef]
- Lee, C.H.; Akin-Olugbade, O.; Kirschenbaum, A. Overview of prostate anatomy, histology, and pathology. Endocrinol. Metab. Clin. 2011, 40, 565–575. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Ermilov, S.A.; Brecht, H.P.; Su, R.; Oraevsky, A.A. Optoacoustic imaging of the prostate: Development toward image-guided biopsy. J. Biomed. Opt. 2010, 15, 021310. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Roberts, W.W.; Carson, P.L.; Wood, D.P.; Fowlkes, J.B. Photoacoustic tomography: A potential new tool for prostate cancer. Biomed. Opt. Express 2010, 1, 1117–1126. [Google Scholar] [CrossRef]
- Peng, D.Q.; Peng, Y.Y.; Guo, J.; Li, H. Laser illumination modality of photoacoustic imaging technique for prostate cancer. J. Phys. Conf. Ser. 2016, 679, 012026. [Google Scholar] [CrossRef]
- Tang, S.; Chen, J.; Samant, P.; Stratton, K.; Xiang, L. Transurethral photoacoustic endoscopy for prostate cancer: A simulation study. IEEE Trans. Med. Imaging 2016, 35, 1780–1787. [Google Scholar] [CrossRef]
- Singh, N.; Cherin, E.; Soenjaya, Y.; Matharoo, M.; Brisbane, B.; Papernick, S.; Roa, C.F.; Zheng, G.; Wodlinger, B.; Foster, F.S.; et al. A new photoacoustic imaging platform for potential applications in prostate cancer. In Proceedings of the 2020 IEEE International Ultrasonics Symposium (IUS), Las Vegas, NV, USA, 17 November 2020; pp. 1–4. [Google Scholar]
- Bauer, D.R.; Olafsson, R.; Montilla, L.G.; Witte, R.S. 3-D photoacoustic and pulse echo imaging of prostate tumor progression in the mouse window chamber. Biomed. Opt. 2011, 16, 026012. [Google Scholar] [CrossRef] [PubMed]
- Dogra, V.S.; Chinni, B.K.; Valluru, K.S.; Joseph, J.V.; Ghazi, A.; Yao, J.L.; Evans, K.; Messing, E.M.; Rao, N.A. Multispectral photoacoustic imaging of prostate cancer: Preliminary ex-vivo results. Clin. Imaging Sci. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, J.; Lee, H.J.; Chang, J.H. Transrectal ultrasound and photoacoustic imaging probe for diagnosis of prostate cancer. Sensors 2021, 21, 1217. [Google Scholar] [CrossRef]
- Liu, C.; Xing, M.; Cong, B.; Qiu, C.; He, D.; Wang, C.; Xiao, Y.; Yin, T.H.; Shao, M.; Qiu, W.B.; et al. In vivo transrectal imaging of canine prostate with a sensitive and compact handheld transrectal array photoacoustic probe for early diagnosis of prostate cancer. Biomed. Opt. Express 2019, 10, 1707–1717. [Google Scholar] [CrossRef]
- Horiguchi, A.; Tsujita, K.; Irisawa, K.; Kasamatsu, T.; Hirota, K.; Kawaguchi, M.; Shinchi, M.; Ito, K.; Asano, T.; Shinmoto, H.; et al. A pilot study of photoacoustic imaging system for improved real-time visualization of neurovascular bundle during radical prostatectomy. Prostate 2016, 76, 307–315. [Google Scholar] [CrossRef]
- IIshihara, M.; Shinchi, M.; Horiguchi, A.; Shinmoto, H.; Tsuda, H.; Irisawa, K.; Wada, T.; Asano, T. Possibility of transrectal photoacoustic imaging-guided biopsy for detection of prostate cancer. In Proceedings of the Photons Plus Ultrasound Imaging Sensing. SPIE 2017, BiOS, Medicine, Engineerin, San Francisco, CA, USA, 3 March 2017; Volume 10064, pp. 388–392. [Google Scholar]
- Agrba, P.D.; Kirillin, M.Y.; Abelevich, A.I.; Kamensky, V.A. Mechanical compression for biotissue image enhancement in optical coherence tomography. In Proceedings of the Saratov Fall Meeting 2009: International School for Junior Scientists and Students on Optics, Laser Physics, and Biophotonics. SPIE, Saratov Fall Meeting 2009, Saratov, Russia, 11 February 2010; Volume 7547, pp. 21–27. [Google Scholar]
- Mobley, J.; Vo-Dinh, T.; Tuchin, V.V. Optical properties of tissue. Biomed. Photonics Handb. 2003, 2, 1–2. [Google Scholar]
- Ai, M.; Youn, J.I.; Salcudean, S.E.; Rohling, R.; Abolmaesumi, P.; Tang, S. Photoacoustic tomography for imaging the prostate: A transurethral illumination probe design and application. Biomed. Opt. Express 2019, 10, 2588–2605. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Chen, Y.; Xie, W.; Zhang, M.; Pan, J.; Sato, N.; Wang, X.D.; Wu, D.L.; Cheng, Q. Examining the technical feasibility of prostate cancer molecular imaging by transrectal photoacoustic tomography with transurethral illumination. Exp. Biol. Med. 2020, 245, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Lediju Bell, M.A.; Kuo, N.P.; Song, D.Y.; Kang, J.U.; Boctor, E.M. In vivo visualization of prostate brachytherapy seeds with photoacoustic imaging. Biomed. Opt. 2014, 19, 126011. [Google Scholar] [CrossRef]
- Lediju Bell, M.A.; Guo, X.; Song, D.Y.; Boctor, E.M. Transurethral light delivery for prostate photoacoustic imaging. Biomed. Opt. 2015, 20, 036002. [Google Scholar] [CrossRef]
- Liao, C.K.; Li, M.L.; Li, P.C. Optoacoustic imaging with synthetic aperture focusing and coherence weighting. Opt. Lett. 2004, 29, 2506–2508. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yang, X.; Gong, H.; Luo, Q. Two-dimensional synthetic-aperture focusing technique in photoacoustic microscopy. J. Appl. Phys. 2011, 109, 104701. [Google Scholar] [CrossRef]
- Turner, J.; Estrada, H.; Kneipp, M.; Razansky, D. Improved optoacoustic microscopy through three-dimensional spatial impulse response synthetic aperture focusing technique. Opt. Lett. 2014, 39, 3390–3393. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeon, S.; Meng, J.; Song, L.; Lee, J.S.; Kim, C. Delay-multiply-and-sum-based synthetic aperture focusing in photoacoustic microscopy. Biomed. Opt. 2016, 21, 036010. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Park, J.; Managuli, R.; Kim, C. A novel 2-D synthetic aperture focusing technique for acoustic-resolution photoacoustic microscopy. IEEE Trans. Med. Imaging 2018, 38, 250–260. [Google Scholar] [CrossRef]
- Cai, D.; Li, Z.; Li, Y.; Guo, Z.; Chen, S.L. Photoacoustic microscopy in vivo using synthetic-aperture focusing technique combined with three-dimensional deconvolution. Opt. Express 2017, 25, 1421–1434. [Google Scholar] [CrossRef]
- Cai, D.; Li, Z.; Chen, S.L. Photoacoustic microscopy by scanning mirror-based synthetic aperture focusing technique. Chin. Opt. Lett. 2015, 13, 101101. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, R.; Zhang, X.; Li, Z.; Li, H. Image enhancement of photoacoustic imaging for early endometrial cancer detection by employing a filtered delay multiply and sum beamforming algorithm. AIP Adv. 2019, 9, 125303. [Google Scholar] [CrossRef]
- Paul, S.; Mulani, S.; Daimary, N.; Singh, M.S. Simplified-delay-multiply-and-sum-based promising beamformer for real-time photoacoustic imaging. IEEE Trans. Instrum. Meas. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Prakash, R.; Manwar, R.; Avanaki, K. Evaluation of 10 current image reconstruction algorithms for linear array photoacoustic imaging. Biophotonics 2024, 17, e202300117. [Google Scholar] [CrossRef]
- Wang, Z.X.; Wang, H.Q.; Ren, S.L. Research on ADMM Reconstruction algorithm of Photoacoustic tomography with limited sampling data. IEEE Access 2021, 9, 113631–113641. [Google Scholar] [CrossRef]
- Kong, Q.; Gong, R.; Liu, J.T.; Shao, X. Investigation on reconstruction for frequency domain photoacoustic imaging via TVAL3 regularization algorithm. IEEE Photonics J. 2018, 10, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zhang, Y.; Qiao, L. A photoacoustic imaging algorithm based on regularized smoothed L0 norm minimization. Mol. Imaging 2021, 2021, 6689194. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wei, N.N.; Peng, K.; Xiao, J.Y. Modified back-projection method in acoustic resolution based photoacoustic endoscopy for improved lateral resolution. Med. Phys 2018, 45, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, C.; Zhong, F.; Pang, W.; Guo, L.; Peng, K.; Xiao, J. 3D acoustic resolution-based photoacoustic endoscopy with dynamic focusing. Quant. Imaging Med. Surg. 2021, 11, 685–696. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Jiang, J.; Peng, K.; Wang, B. Photoacoustic/Ultrasound Endoscopic Imaging Reconstruction Algorithm Based on the Approximate Gaussian Acoustic Field. Biosensors 2022, 12, 463–477. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, D.; Zeng, Y.; Chen, Q. Photoacoustic imaging with deconvolution algorithm. Phys. Med. Biol. 2004, 49, 3117. [Google Scholar] [CrossRef]
- Ding, T.; Ren, K.; Vallélian, S. A one-step reconstruction algorithm for quantitative photoacoustic imaging. Inverse Probl. 2015, 31, 095005. [Google Scholar] [CrossRef]
- Mozaffarzadeh, M.; Mahloojifar, A.; Orooji, M.; Kratkiewicz, K.; Adabi, S.; Nasiriavanaki, M. Linear-array photoacoustic imaging using minimum variance-based delay multiply and sum adaptive beamforming algorithm. Biomed. Opt. 2018, 23, 026002. [Google Scholar] [CrossRef]
- Hakakzadeh, S.; Amjadian, M.; Zhang, Y.; Mostafavi, S.M.; Kavehvash, Z.; Wang, L. Signal restoration algorithm for photoacoustic imaging systems. Biomed. Opt. Express 2023, 14, 651–666. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Yang, H.; Jiang, H.; Ding, Y.; Xie, H. MEMS ultrasound transducers for endoscopic photoacoustic imaging applications. Micromachines 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Duoduo, H.; Yuan, Y. 2-D image reconstruction of photoacoustic endoscopic imaging based on time-reversal. Comput. Biol. Med. 2016, 76, 60–68. [Google Scholar] [CrossRef]
- Liu, X.; Peng, D.; Guo, W.; Ma, X.; Yang, X.; Tian, J. Compressed sensing photoacoustic imaging based on fast alternating direction algorithm. Int. J. Biomed. Imaging 2012, 2012, 206214. [Google Scholar] [CrossRef]
- Gulenko, O.; Yang, H.; Kim, K.; Youm, J.Y.; Kim, M.; Kim, Y.; Yang, J.M. Deep-learning-based algorithm for the removal of electromagnetic interference noise in photoacoustic endoscopic image processing. Sensors 2022, 22, 3961. [Google Scholar] [CrossRef]
- Gröhl, J.; Schellenberg, M.; Dreher, K.; Maier-Hein, L. Deep learning for biomedical photoacoustic imaging: A review. Photoacoustics 2021, 22, 100241. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Wang, Y.; Guo, L.; Wang, B.; Lai, P.; Xiao, J. Two-dimensional photoacoustic/ultrasonic endoscopic imaging based on a line-focused transducer. Front. Bioeng. Biotechnol. 2022, 9, 807633. [Google Scholar] [CrossRef]
- Li, B.B.; Ou, L.; Lei, Y.; Liu, Y.C. Cavity optomechanical sensing. Nanophotonics 2021, 10, 2799–2832. [Google Scholar] [CrossRef]
- Fu, B.; Cheng, Y.; Shang, C.; Li, J.; Wang, G.; Zhang, C.; Sun, J.; Ma, J.; Ji, X.; He, B. Optical ultrasound sensors for photoacoustic imaging: A narrative review. Quant. Imaging Med. Surg. 2022, 12, 1608. [Google Scholar] [CrossRef]
- Westerveld, W.J.; Mahmud-Ul-Hasan, M.; Shnaiderman, R.; Ntziachristos, V.; Rottenberg, X.; Severi, S.; Rochus, V. Sensitive, small, broadband and scalable optomechanical ultrasound sensor in silicon photonics. Nat. Photonics 2021, 15, 341–345. [Google Scholar] [CrossRef]
- Shnaiderman, R.; Wissmeyer, G.; Ülgen, O.; Mustafa, Q.; Chmyrov, A.; Ntziachristos, V. A submicrometre silicon-on-insulator resonator for ultrasound detection. Nature 2020, 585, 372–378. [Google Scholar] [CrossRef]
- Ansari, R.; Zhang, E.Z.; Desjardins, A.E.; Beard, P.C. All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy. Light Sci. Appl. 2018, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Chen, S.; Zhang, Z.; Sun, C.; Zhang, H.F. Photoacoustic probe using a microring resonator ultrasonic sensor for endoscopic applications. Opt. Lett. 2014, 39, 4372–4375. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Fu, W.; Li, Q.; Chen, X.; Sun, H.; Wang, L.; Jin, L.; Huang, W.; Guan, B.O. Optical-resolution functional gastrointestinal photoacoustic endoscopy based on optical heterodyne detection of ultrasound. Nat. Commun. 2022, 13, 7604. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, Q.; Feng, Y.; Zhong, R.; Fu, Z.; Yang, S.; Sun, W.; Zhang, B.; Sui, Q.; Chen, J.; et al. Parallel interrogation of the chalcogenide-based micro-ring sensor array for photoacoustic tomography. Nat. Commun. 2023, 14, 3250. [Google Scholar] [CrossRef]


| Method | Specificity | Sensitivity | Price | Damage |
|---|---|---|---|---|
| DRE | lower | lower | generally | no |
| TRUS | lower | lower | generally | no |
| PSA | lower | lower | generally | yes |
| MRI | higher | higher | higher | no |
| PAI | higher | higher | generally | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, N.; Chen, H.; Li, B.; Dong, X.; Wang, B. Advances in Photoacoustic Endoscopic Imaging Technology for Prostate Cancer Detection. Photonics 2024, 11, 872. https://doi.org/10.3390/photonics11090872
Wei N, Chen H, Li B, Dong X, Wang B. Advances in Photoacoustic Endoscopic Imaging Technology for Prostate Cancer Detection. Photonics. 2024; 11(9):872. https://doi.org/10.3390/photonics11090872
Chicago/Turabian StyleWei, Ningning, Huiting Chen, Bin Li, Xiaojun Dong, and Bo Wang. 2024. "Advances in Photoacoustic Endoscopic Imaging Technology for Prostate Cancer Detection" Photonics 11, no. 9: 872. https://doi.org/10.3390/photonics11090872
APA StyleWei, N., Chen, H., Li, B., Dong, X., & Wang, B. (2024). Advances in Photoacoustic Endoscopic Imaging Technology for Prostate Cancer Detection. Photonics, 11(9), 872. https://doi.org/10.3390/photonics11090872




