Investigating the Thermometric Performance of Inorganic Materials Doped with Nd3+ under Infrared LED Excitation: An Alternative for Deep Tissue Luminescent Thermometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Structural Synthesis and Characterization
2.2. Optical Characterization
2.3. Luminescence Intensity Ratio (LIR)
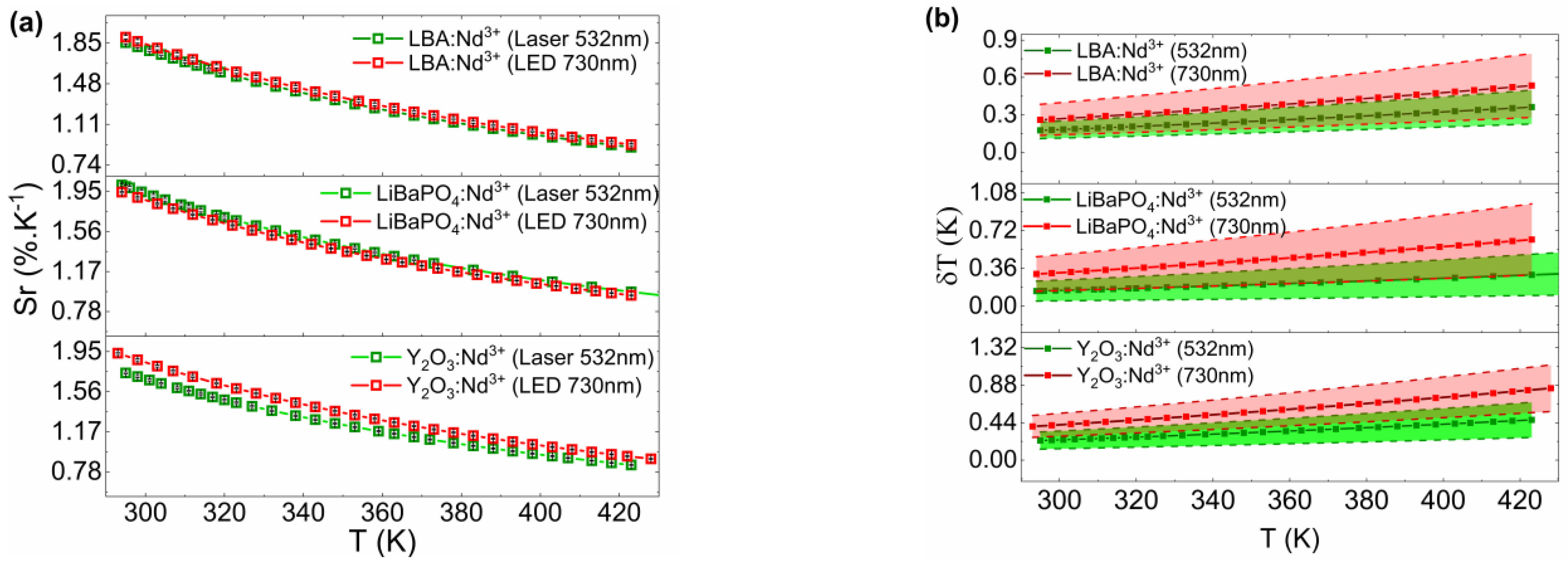
2.4. Sensitivity
3. Results
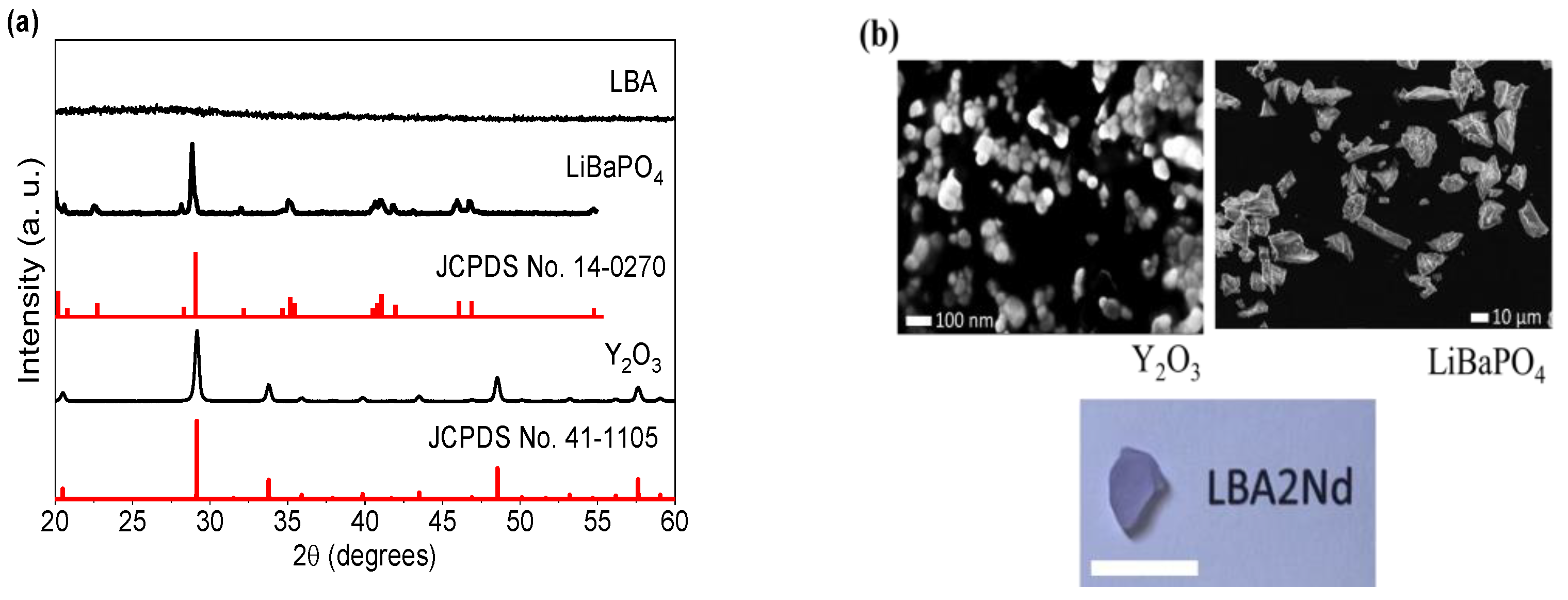
3.1. Structural and Morphological Properties
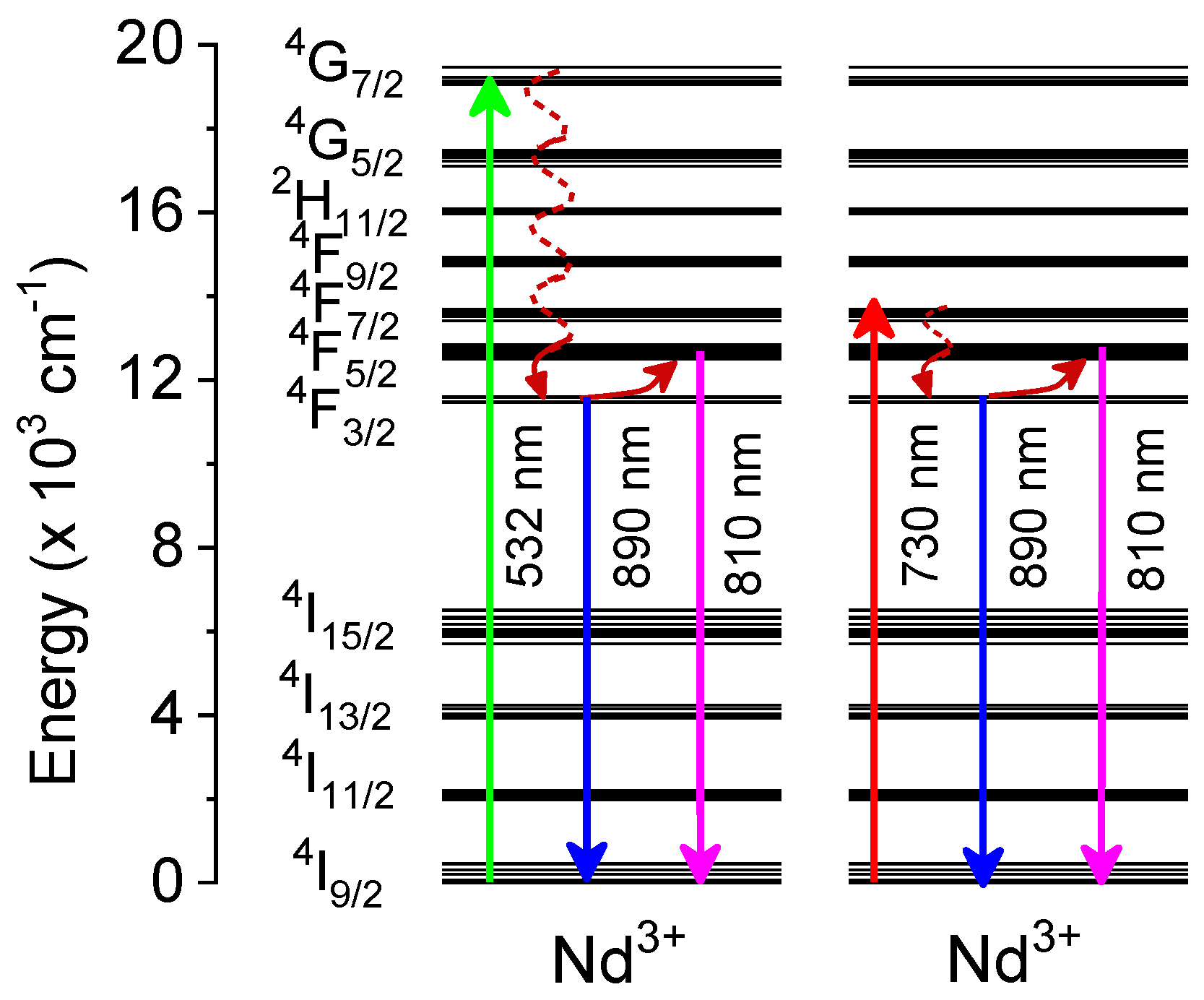
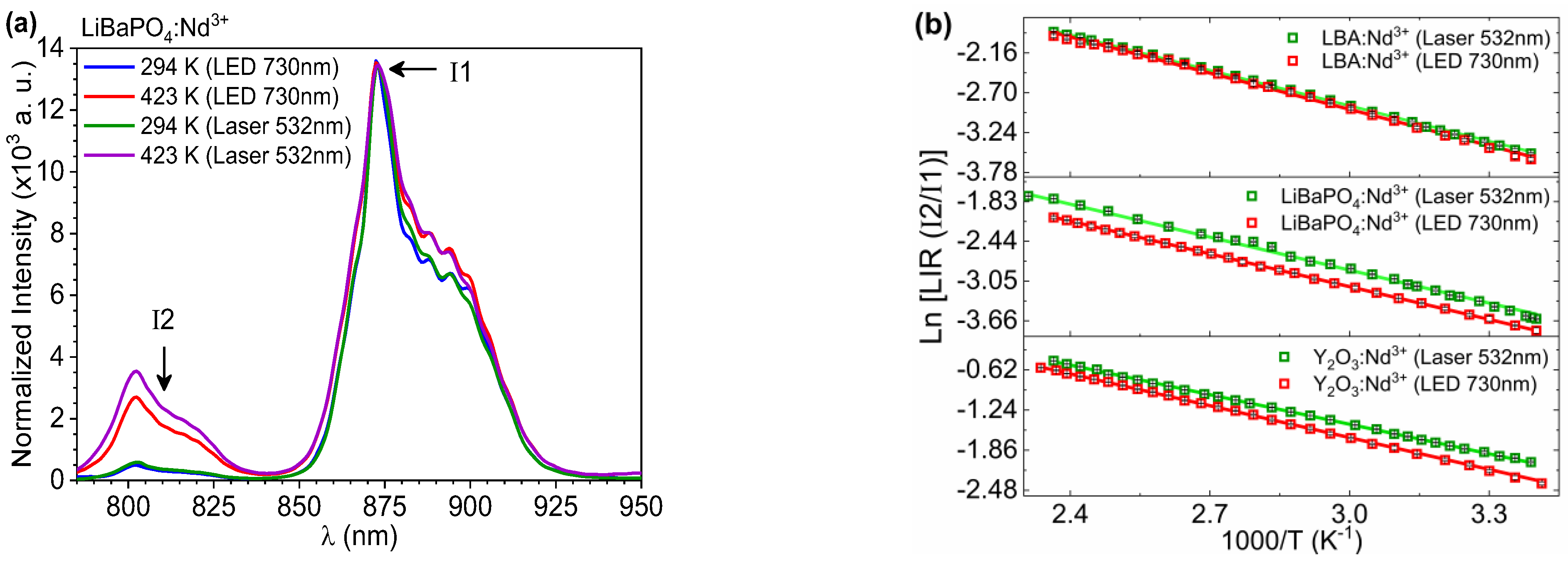
3.2. Optical Properties
3.3. Temperature Sensing Properties
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, X.; Zhou, Q.; Zhu, X.; Wu, Z.; Feng, W.; Li, F. Ratiometric Upconversion Nanothermometry with Dual Emission at the Same Wavelength Decoded via a Time-Resolved Technique. Nat. Commun. 2020, 11, 4. [Google Scholar] [CrossRef]
- Bradac, C.; Lim, S.F.; Chang, H.C.; Aharonovich, I. Optical Nanoscale Thermometry: From Fundamental Mechanisms to Emerging Practical Applications. Adv. Opt. Mater. 2020, 8, 2000183. [Google Scholar] [CrossRef]
- Del Rosal, B.; Jaque, D. Upconversion Nanoparticles for In Vivo Applications: Limitations and Future Perspectives. Methods Appl. Fluoresc. 2019, 7, 22001–22023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, X.; Wei, Z.; Feng, W.; Li, L.; Ma, L.; Li, F.; Zhou, J. Customized Photothermal Therapy of Subcutaneous Orthotopic Cancer by Multichannel Luminescent Nanocomposites. Adv. Mater. 2021, 33, 2008615. [Google Scholar] [CrossRef]
- Hemmer, E.; Vetrone, F.; Soga, K. Lanthanide-Based Nanostructures for Optical Bioimaging: Small Particles with Large Promise. MRS Bull. 2014, 39, 960–964. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Abdel-Wahab, B.A.; Alam, A.; Zafar, S.; Ahmad, J.; Ahmad, F.J.; Midoux, P.; Pichon, C.; Akhter, S. Toxicity of Inorganic Nanoparticles Used in Targeted Drug Delivery and Other Biomedical Application: An Updated Account on Concern of Biomedical Nanotoxicology. J. Nanosci. Nanotechnol. 2016, 16, 7873–7897. [Google Scholar] [CrossRef]
- Hora, D.A.; Silva, A.J.S.; Nascimento, P.A.M.; Sampaio, D.V.; Moulton, B.J.A.; Silva, R.S.; Rezende, M.V.d.S. Effect of the Amounts of Li+ Additive on the Luminescence Properties of LiBaPO4:Eu Phosphor. Opt. Mater. 2019, 89, 329–333. [Google Scholar] [CrossRef]
- Dramićanin, M.D. Sensing Temperature via Downshifting Emissions of Lanthanide-Doped Metal Oxides and Salts. A Review. Methods Appl. Fluoresc. 2016, 4, 042001–0420024. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Lu, H.; Yang, J.; Huang, D.; Zou, Q.; Yang, M.; Zhang, X.; Wang, Y.; Zhu, H. Ultranarrow NIR Bandwidth and Temperature Sensing of YOF:Yb3+/Tm3+ Phosphor in Low Temperature Range. J. Lumin. 2019, 206, 613–617. [Google Scholar] [CrossRef]
- Peng, H.; Song, H.; Chen, B.; Wang, J.; Lu, S.; Kong, X.; Zhang, J. Temperature Dependence of Luminescent Spectra and Dynamics in Nanocrystalline Y2O3: Eu3+. J. Chem. Phys. 2003, 118, 3277–3282. [Google Scholar] [CrossRef]
- Rocha, U.; Jacinto da Silva, C.; Ferreira Silva, W.; Guedes, I.; Benayas, A.; Martinez Maestro, L.; Acosta Elias, M.; Bovero, E.; van Veggel, F.C.; Garcia Solé, J.A.; et al. Subtissue Thermal Sensing Based on Neodymium-Doped LaF3 Nanoparticles. ACS Nano 2013, 7, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Schneider, C.; Maier, S.; Igusa, R.; Iwamoto, S.; Forchel, A.; Höfling, S.; Arakawa, Y.; Kamp, M. Temperature Dependency of the Emission Properties from Positioned In(Ga)As/GaAs Quantum Dots. AIP Adv. 2014, 4, 097128. [Google Scholar] [CrossRef]
- Lojpur, V.; Antić, Ž.; Dramićanin, M.D. Temperature Sensing from the Emission Rise Times of Eu3+ in SrY2O4. Phys. Chem. Chem. Phys. 2014, 16, 25636–25641. [Google Scholar] [CrossRef]
- Allison, S.; Goedeke, S.; Cates, M.; Bonsett, T.; Smith, D.; Brewington, A.; Bencic, T. Phosphor Thermometry in an Operating Turbine Engine; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2012; pp. 1–7. [Google Scholar] [CrossRef]
- Brandão-Silva, A.C.; Gomes, M.A.; Macedo, Z.S.; Avila, J.F.M.; Rodrigues, J.J.; Alencar, M.A.R.C. Multiwavelength Fluorescence Intensity Ratio Nanothermometry: High Sensitivity over a Broad Temperature Range. J. Phys. Chem. C 2018, 122, 20459–20468. [Google Scholar] [CrossRef]
- Balabhadra, S.; Debasu, M.L.; Brites, C.D.S.; Nunes, L.A.O.; Malta, O.L.; Rocha, J.; Bettinelli, M.; Carlos, L.D. Boosting the Sensitivity of Nd3+ -Based Luminescent Nanothermometers. Nanoscale 2015, 7, 17261–17267. [Google Scholar] [CrossRef] [PubMed]
- Benayas, A.; Del Rosal, B.; Pérez-Delgado, A.; Santacruz-Gómez, K.; Jaque, D.; Hirata, G.A.; Vetrone, F. Nd:YAG Near-Infrared Luminescent Nanothermometers. Adv. Opt. Mater. 2015, 3, 687–694. [Google Scholar] [CrossRef]
- Wawrzynczyk, D.; Bednarkiewicz, A.; Nyk, M.; Strek, W.; Samoc, M. Neodymium(Iii) Doped Fluoride Nanoparticles as Non-Contact Optical Temperature Sensors. Nanoscale 2012, 4, 6959–6961. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Golyeva, E.V.; Kurochkin, M.A.; Lähderanta, E.; Mikhailov, M.D. Nd3+-Doped YVO4 Nanoparticles for Luminescence Nanothermometry in the First and Second Biological Windows. Sensors Actuators B Chem. 2016, 235, 287–293. [Google Scholar] [CrossRef]
- Zairov, R.R.; Dovzhenko, A.P.; Podyachev, S.N.; Sudakova, S.N.; Kornev, T.A.; Shvedova, A.E.; Masliy, A.N.; Syakaev, V.V.; Alekseev, I.S.; Vatsouro, I.M.; et al. Role of PSS-Based Assemblies in Stabilization of Eu and Sm Luminescent Complexes and Their Thermoresponsive Luminescence. Colloids Surf. B Biointerfaces 2022, 217, 112664. [Google Scholar] [CrossRef]
- González-Béjar, M.; Pérez-Prieto, J. Upconversion Luminescent Nanoparticles in Physical Sensing and in Monitoring Physical Processes in Biological Samples. Methods Appl. Fluoresc. 2015, 3, 042002. [Google Scholar] [CrossRef]
- Wade, S.A.; Collins, S.F.; Baxter, G.W. Fluorescence Intensity Ratio Technique for Optical Fiber Point Temperature Sensing. J. Appl. Phys. 2003, 94, 4743–4756. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent Probes and Sensors for Temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Acosta-Mora, P.; Méndez-Ramos, J.; Fischer, S. Optical Nanoprobes for Biomedical Applications: Shining a Light on Upconverting and near-Infrared Emitting Nanoparticles for Imaging, Thermal Sensing, and Photodynamic Therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the Biological Windows: Current Perspectives on Fluorescent Bioprobes Emitting above 1000 Nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the Nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef]
- Nadort, A.; Zhao, J.; Goldys, E.M. Lanthanide Upconversion Luminescence at the Nanoscale: Fundamentals and Optical Properties. Nanoscale 2016, 8, 13099–13130. [Google Scholar] [CrossRef]
- Liu, G. Advances in Theoretical Understanding of Photon Upconversion in Rare—Earth Activated Nanophopshors. Chem. Soc. Rev. 2015, 44, 1635–1652. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Zhou, L.; Zhang, F. Epitaxial Seeded Growth of Rare-Earth Nanocrystals with Efficient 800 Nm Near-Infrared to 1525 Nm Short-Wavelength Infrared Downconversion Photoluminescence for In Vivo Bioimaging ** Angewandte. Angew. Chem. Int. Ed. 2014, 53, 12086–12090. [Google Scholar] [CrossRef]
- Marciniak, L.; Pilch, A.; Arabasz, S.; Jin, D.; Bednarkiewicz, A. Heterogeneously Nd3+ Doped Single Nanoparticles for NIR-Induced Heat Conversion, Luminescence, and Thermometry. Nanoscale 2017, 9, 8288–8297. [Google Scholar] [CrossRef]
- Quintanilla, M.; Zhang, Y.; Liz-Marzán, L.M. Subtissue Plasmonic Heating Monitored with CaF2:Nd3+,Y3+ Nanothermometers in the Second Biological Window. Chem. Mater. 2018, 30, 2819–2828. [Google Scholar] [CrossRef]
- Xu, W.; Qi, H.; Zheng, L.; Zhang, Z.; Cao, W. Multifunctional Nanoparticles Based on the Nd3+ /Yb3+ Codoped NaYF4. Opt. Lett. 2015, 40, 5678–5681. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Koughia, K.; Fujimoto, K.; Fujihashi, C.; Decorby, R.; Haugen, C.; Hewak, D.W.; Tonchev, D.; Kasap, S. Observation of 4F3/2→4I15/2 Radiative Current Topics in Solid State Physics Transition in Nd3+ Ions in GaLaS Glass Using Frequency-Resolved PL Spectroscopy. Phys. Status Solidi 2009, 6, 54–58. [Google Scholar] [CrossRef]
- Rakov, N.; Maciel, G.S. Near-Infrared Emission and Optical Temperature Sensing Performance of Nd3+:SrF2 Crystal Powder Prepared by Combustion Synthesis. J. Appl. Phys. 2017, 121, 113103. [Google Scholar] [CrossRef]
- Dantelle, G.; Matulionyte, M.; Testemale, D.; Cantarano, A.; Ibanez, A.; Vetrone, F. Nd3+ Doped Gd3Sc2Al3O12 Nanoparticles: Towards Efficient Nanoprobes for Temperature Sensing. Phys. Chem. Chem. Phys. 2019, 21, 11132–11141. [Google Scholar] [CrossRef]
- Tian, X.; Wei, X.; Chen, Y.; Duan, C.; Yin, M. Temperature Sensor Based on Ladder-Level Assisted Thermal Coupling and Thermal-Enhanced Luminescence in NaYF4: Nd3+. Opt. Express 2014, 22, 30333–30345. [Google Scholar] [CrossRef] [PubMed]
- Runowski, M.; Martín, I.R.; Sigaev, V.N.; Savinkov, V.I.; Shakhgildyan, G.Y.; Lis, S. Luminescent-Plasmonic Core–Shell Microspheres, Doped with Nd3+ and Modified with Gold Nanoparticles, Exhibiting Whispering Gallery Modes and SERS Activity. J. Rare Earths 2019, 37, 1152–1156. [Google Scholar] [CrossRef]
- Marciniak, L.; Prorok, K.; Bednarkiewicz, A.; Kowalczyk, A.; Hreniak, D.; Strek, W. Water Dispersible LiNdP4O12 Nanocrystals: New Multifunctional NIR-NIR Luminescent Materials for Bio-Applications. J. Lumin. 2016, 176, 144–148. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Golyeva, E.V.; Kalinichev, A.A.; Kurochkin, M.A.; Lähderanta, E.; Mikhailov, M.D. Nd3+ Single Doped YVO4 Nanoparticles for Sub-Tissue Heating and Thermal Sensing in the Second Biological Window. Sens. Actuators B Chem. 2016, 243, 338–345. [Google Scholar] [CrossRef]
- Tejeda, E.M.; Arámburo, M.; Vargas, E.; Contreras, O.E.; Hirata1, G.A. Novel Bifunctional Nd:YAG/Fe3O4 Nanocomposite as Nanothermometer/Nanoheater for Potential Biomedical Applications. J. Phys. D Appl. Phys. Lett. 2018, 51, 1361–6463. [Google Scholar] [CrossRef]
- Gomes, M.A.; Carvalho, I.S.; Domingos, L.F.A.; Brandão-Silva, A.C.; Avila, J.F.M.; Rodrigues, J.J.; Alencar, M.A.R.C.; Valerio, M.E.G.; Macedo, Z.S. Temperature-Sensitive Luminescence of Y2O3:Nd3+ Nanocrystals Produced by an Eco-Friendly Route. Opt. Mater. 2019, 89, 536–542. [Google Scholar] [CrossRef]
- Nunes, L.A.O.; Souza, A.S.; Carlos, L.D.; Malta, O.L. Neodymium Doped Fluoroindogallate Glasses as Highly-Sensitive Luminescent Non-Contact Thermometers. Opt. Mater. 2017, 63, 42–45. [Google Scholar] [CrossRef]
- Kalinichev, A.A.; Kurochkin, M.A.; Golyeva, E.V.; Kurochkin, A.V.; Lähderanta, E.; Mikhailov, M.D.; Kolesnikov, I.E. Near-Infrared Emitting YVO4: Nd3+ Nanoparticles for High Sensitive Fluorescence Thermometry. J. Lumin. 2018, 195, 61–66. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Lozano-Gorrín, A.D.; Martín, I.R.; Rodríguez-Mendoza, U.R.; Lavín, V. Comparison of the Sensitivity as Optical Temperature Sensor of Nano-Perovskite Doped with Nd3+ Ions in the First and Second Biological Windows. Sens. Actuators B Chem. 2018, 255, 970–976. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Kalinichev, A.A.; Kurochkin, M.A.; Mamonova, D.V.; Kolesnikov, E.Y.; Kurochkin, A.V.; Lähderanta, E.; Mikhailov, M.D. Y2O3:Nd3+ Nanocrystals as Ratiometric Luminescence Thermal Sensors Operating in the Optical Windows of Biological Tissues. J. Lumin. 2018, 204, 506–512. [Google Scholar] [CrossRef]
- Orlova, A.V.; Kozhevnikova, V.Y.; Goloveshkin, A.S.; Lepnev, L.S.; Utochnikova, V.V. NIR Luminescence Thermometers Based on Yb–Nd Coordination Compounds for the 83–393 K Temperature Range. Dalton Trans. 2022, 51, 5419–5425. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, P.M.; Starsich, F.H.L.; Keitel, R.C.; Pratsinis, S.E. Nd3+-Doped BiVO4 Luminescent Nanothermometers of High Sensitivity. Chem. Commun. 2019, 55, 7147–7150. [Google Scholar] [CrossRef]
- Saruwatari, M.; Kimura, T. LED Pumped Lithium Neodymium Tetraphosphate Lasers. IEEE J. Quantum Electron. 1976, 12, 584–591. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Palmer, A.W. Infrared Fluorescence “Decay-Time” Temperature Sensor. Rev. Sci. Instrum. 1985, 56, 1784–1787. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Palmer, A.W.; Wilson, C.A. A Miniaturised Microcomputer-Based Neodymium “decay-Time” Temperature Sensor. J. Phys. E 1987, 20, 1201–1205. [Google Scholar] [CrossRef]
- Gomes, M.A.; Brandão-Silva, A.C.; Avila, J.F.M.; Alencar, M.A.R.C.; Rodrigues, J.J.; Macedo, Z.S. Particle Size Effect on Structural and Optical Properties of Y2O3:Nd3+ Nanoparticles Prepared by Coconut Water-Assisted Sol-Gel Route. J. Lumin. 2018, 200, 43–49. [Google Scholar] [CrossRef]
- de Andrade Gomes, M.; Valerio, M.E.G.; Macedo, Z.S. Particle Size Control of Y2O3:Eu3+ Prepared via a Coconut Water-Assisted Sol-Gel Method. J. Nanomater. 2011, 2011, 469685. [Google Scholar] [CrossRef]
- Dantas, N.O.; Silva, V.A.; Neto, O.O.D.; Nascimento, M.L.F. Control of Crystallization Kinetics and Study of the Thermal, Structural and Morphological Properties of an Li2O-B2O3-Al2O3 Vitreous System. Braz. J. Phys. 2012, 42, 347–354. [Google Scholar] [CrossRef]
- Laia, A.S.; Maciel, G.S.; Rodrigues, J.J.; Dos Santos, M.A.C.; Machado, R.; Dantas, N.O.; Silva, A.C.A.; Rodrigues, R.B.; Alencar, M.A.R.C. Lithium-Boron-Aluminum Glasses and Glass-Ceramics Doped with Eu3+: A Potential Optical Thermometer for Operation over a Wide Range of Temperatures with Uniform Sensitivity. J. Alloys Compd. 2022, 907, 164402. [Google Scholar] [CrossRef]
- Allison, S.W. A Brief History of Phosphor Thermometry. Meas. Sci. Technol. 2019, 30, ab1d02. [Google Scholar] [CrossRef]
- Skrzypczak, U.; Pfau, C.; Seifert, G.; Schweizer, S. Comprehensive Rate Equation Analysis of Upconversion Luminescence Enhancement Due to BaCl2 Nanocrystals in Neodymium-Doped Fluorozirconate-Based Glass Ceramics. J. Phys. Chem. C 2014, 118, 13087–13098. [Google Scholar] [CrossRef]
- Brandão-Silva, A.C.; Gomes, M.A.; Novais, S.M.V.; Macedo, Z.S.; Avila, J.F.M.; Rodrigues, J.J.; Alencar, M.A.R.C. Size Influence on Temperature Sensing of Erbium-Doped Yttrium Oxide Nanocrystals Exploiting Thermally Coupled and Uncoupled Levels’ Pairs. J. Alloys Compd. 2018, 731, 478–488. [Google Scholar] [CrossRef]
- Kusama, H.; Sovers, O.J.; Yoshioka, T. Line Shift Method for Phosphor Temperature Measurements. Jpn. J. Appl. Phys. 1976, 15, 2349–2358. [Google Scholar] [CrossRef]
- Laia, A.S.; Hora, D.A.; Rezende, M.V.d.S.; Xing, Y.; Rodrigues, J.J., Jr.; Maciel, G.S.; Alencar, M.A.R.C. Nd3+-Doped LiBaPO4 Phosphors for Optical Temperature Sensing within the First Biological Window: A New Strategy to Increase the Sensitivity. Chem. Eng. J. 2020, 399, 125742. [Google Scholar] [CrossRef]
- Laia, A.S.; Felix, N., J.; Brandão-Silva, A.C.; Rodrigues, J.J.; dos Santos, M.A.C.; Dantas, N.O.; Silva, A.C.A.; Alencar, M.A.R.C. Obtaining the Spectroscopic Quality Factor through the Luminescent Thermometry in 50Li2O·45B2O3·5Al2O3 Glasses Doped with Nd3+ and Fluorides. Appl. Opt. 2023, 62, C21. [Google Scholar] [CrossRef]


| Sample | B | Uncertainty | Uncertainty | |
|---|---|---|---|---|
| Y2O3:Nd3+ (Laser 532 nm) | 3.11 | 0.02 | 1524 | 7 |
| Y2O3:Nd3+ (LED 730 nm) | 3.26 | 0.02 | 1641 | 8 |
| LiBaPO4:Nd3+ (Laser 532 nm) | 2.18 | 0.04 | 1688 | 14 |
| LiBaPO4:Nd3+ (LED 730 nm) | 1.90 | 0.01 | 1678 | 3 |
| LBA:Nd3+ (Laser 532 nm) | 1.93 | 0.02 | 1601 | 8 |
| LBA:Nd3+ (LED 730 nm) | 1.99 | 0.04 | 1636 | 16 |
| Material | Excitation (nm) | Involved Transitions | Temperature Range (K) | Sr (%.K−1) at 294 K | Ref. |
|---|---|---|---|---|---|
| NaYF4 | 830 | Starks 4F3/2–4I9/2 | 273–423 | 0.10 | [19] |
| LaF3 | 808 | Starks 4F3/2–4I9/2 | 283–333 | 0.10 | [12] |
| YAG | 808 | Starks 4F3/2–4I9/2 | 283–343 | 0.16 | [18] |
| YVO4 | 808 | Starks 4F3/2–4I11/2 | 298–328 | 0.25 | [41] |
| Y2O3 | 800 | Starks 4F3/2–4I9/2 | 303–333 | 0.34 | [43] |
| NaYF4 | 574.8 | 4F7/2–4I9/2/4F3/2–4I9/2 | 323–673 | 3.24 | [38] |
| Gd2O3 | 580 | 4F5/2–4I9/2/4F3/2–4I9/2 | 288–323 | 1.68 | [17] |
| YVO4 | 532 | 4F5/2–4I9/2/4F3/2–4I9/2 | 123–873 | 1.6 | [45] |
| SrF2 | 532 and 750 | 4F5/2–4I9/2/4F3/2–4I9/2 | 298–573 | 1.69 and 1.32 | [36] |
| BiVO4 | 750 | 4F5/2–4I9/2/4F3/2–4I9/2 | 310–573 | 1.7 | [49] |
| Y2O3 | 532 and 730 | 4F5/2–4I9/2/4F3/2–4I9/2 | 294–423 | 1.75 and 1.92 | This work |
| LBA | 532 and 730 | 4F5/2–4I9/2/4F3/2–4I9/2 | 294–423 | 1.87 and 1.92 | This work |
| LiBaPO4 | 532 and 730 | 4F5/2–4I9/2/4F3/2–4I9/2 | 294–423 | 2.01 and 1.94 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laia, A.S.; Hora, D.A.; Rezende, M.V.d.S.; Gomes, M.A.; Brandão-Silva, A.C.; Santos, M.A.C.d.; Dantas, N.O.; Silva, A.C.A.; Rodrigues, J.J., Jr.; Valerio, M.E.G.; et al. Investigating the Thermometric Performance of Inorganic Materials Doped with Nd3+ under Infrared LED Excitation: An Alternative for Deep Tissue Luminescent Thermometry. Photonics 2023, 10, 485. https://doi.org/10.3390/photonics10050485
Laia AS, Hora DA, Rezende MVdS, Gomes MA, Brandão-Silva AC, Santos MACd, Dantas NO, Silva ACA, Rodrigues JJ Jr., Valerio MEG, et al. Investigating the Thermometric Performance of Inorganic Materials Doped with Nd3+ under Infrared LED Excitation: An Alternative for Deep Tissue Luminescent Thermometry. Photonics. 2023; 10(5):485. https://doi.org/10.3390/photonics10050485
Chicago/Turabian StyleLaia, André S., Daniela A. Hora, Marcos V. dos S. Rezende, Maria A. Gomes, Antônio C. Brandão-Silva, Marcos A. C. dos Santos, Noelio O. Dantas, Anielle C. A. Silva, José J. Rodrigues, Jr., Mário E. G. Valerio, and et al. 2023. "Investigating the Thermometric Performance of Inorganic Materials Doped with Nd3+ under Infrared LED Excitation: An Alternative for Deep Tissue Luminescent Thermometry" Photonics 10, no. 5: 485. https://doi.org/10.3390/photonics10050485
APA StyleLaia, A. S., Hora, D. A., Rezende, M. V. d. S., Gomes, M. A., Brandão-Silva, A. C., Santos, M. A. C. d., Dantas, N. O., Silva, A. C. A., Rodrigues, J. J., Jr., Valerio, M. E. G., Macedo, Z. S., & Alencar, M. A. R. C. (2023). Investigating the Thermometric Performance of Inorganic Materials Doped with Nd3+ under Infrared LED Excitation: An Alternative for Deep Tissue Luminescent Thermometry. Photonics, 10(5), 485. https://doi.org/10.3390/photonics10050485







