Extensible LED-Induced Integrated Fluorescence Detection Module for Quantitative Analysis of Lucigenin Concentration
Abstract
:1. Introduction
2. Theoretical Method and Device
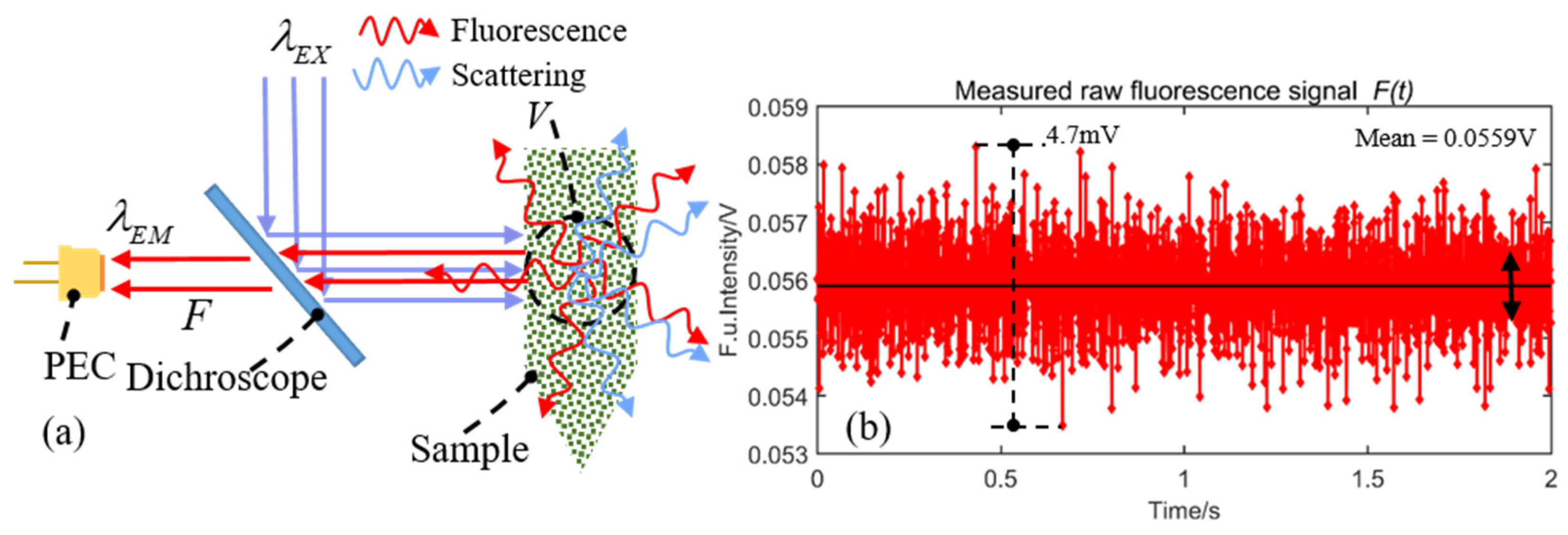
2.1. Measurement Analytic Theory
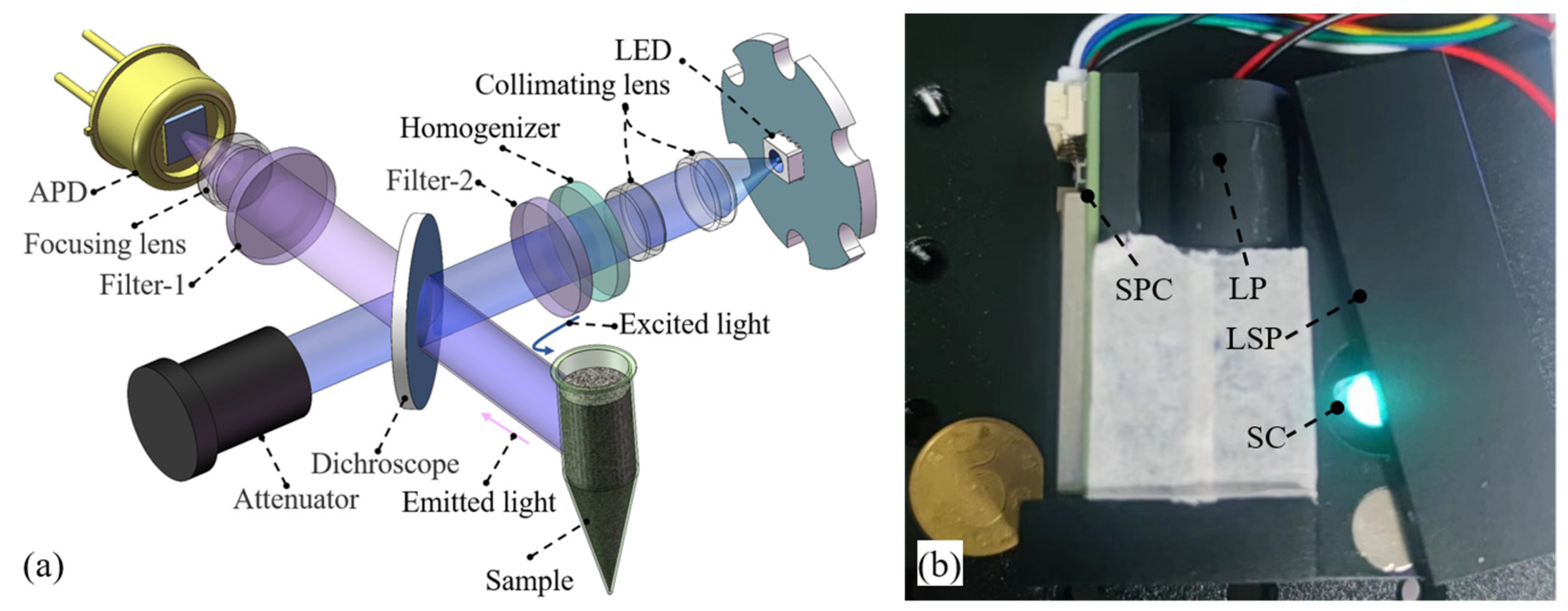
2.2. Integrated LED-Induced Fluorescence Module
- (i)
- Abundant selection of excitation wavelengths: the range of commercially available LED central wavelengths range from 255 to 4600 nm.
- (ii)
- Lower economic cost: LEDs often have a service life of up to 100,000 h at a cost of less than $1.
- (iii)
- Small size, higher power, and straightforward operation: an LED is only a few millimeters in size, yet it can easily provide watts of output power with a constant drive current.
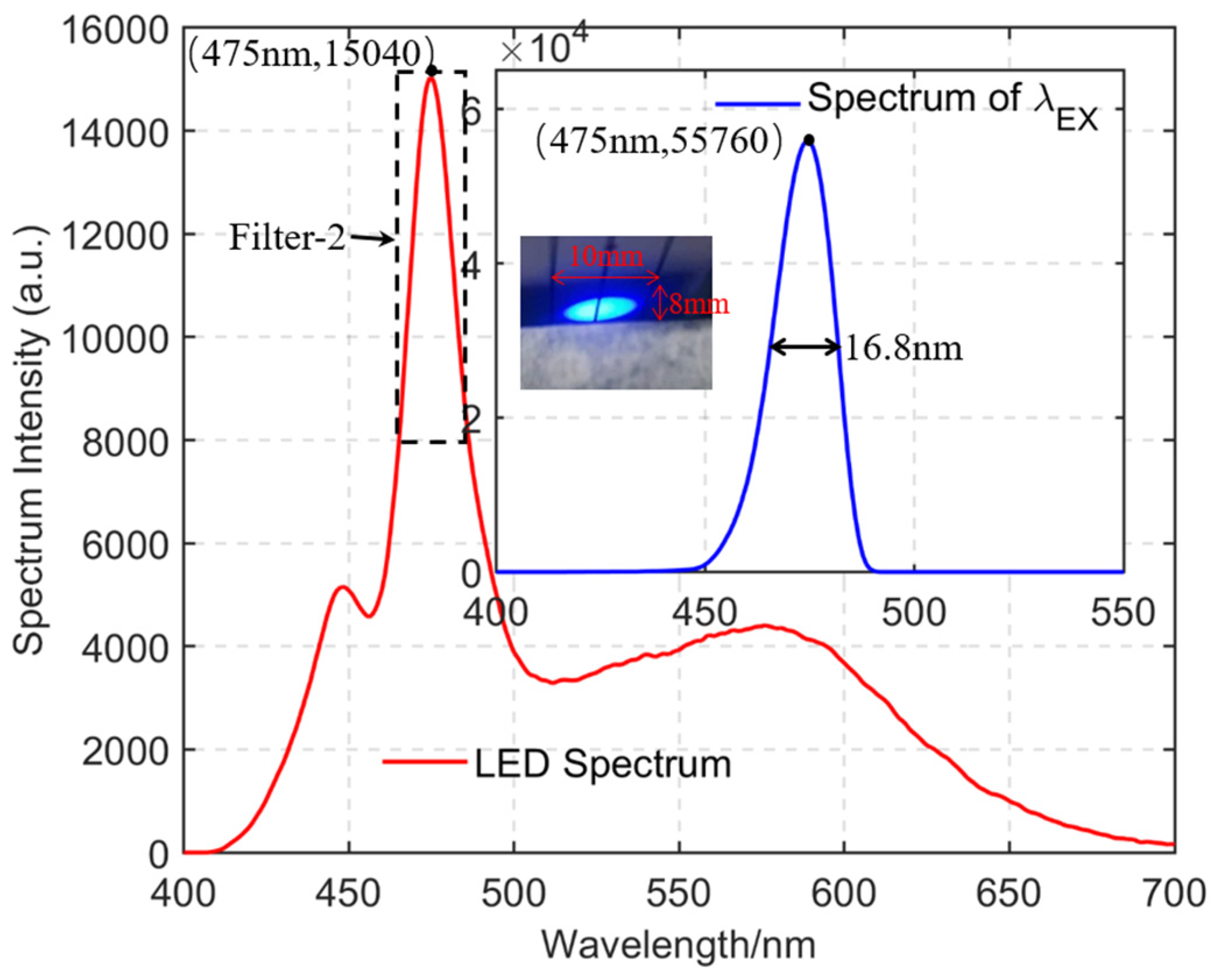
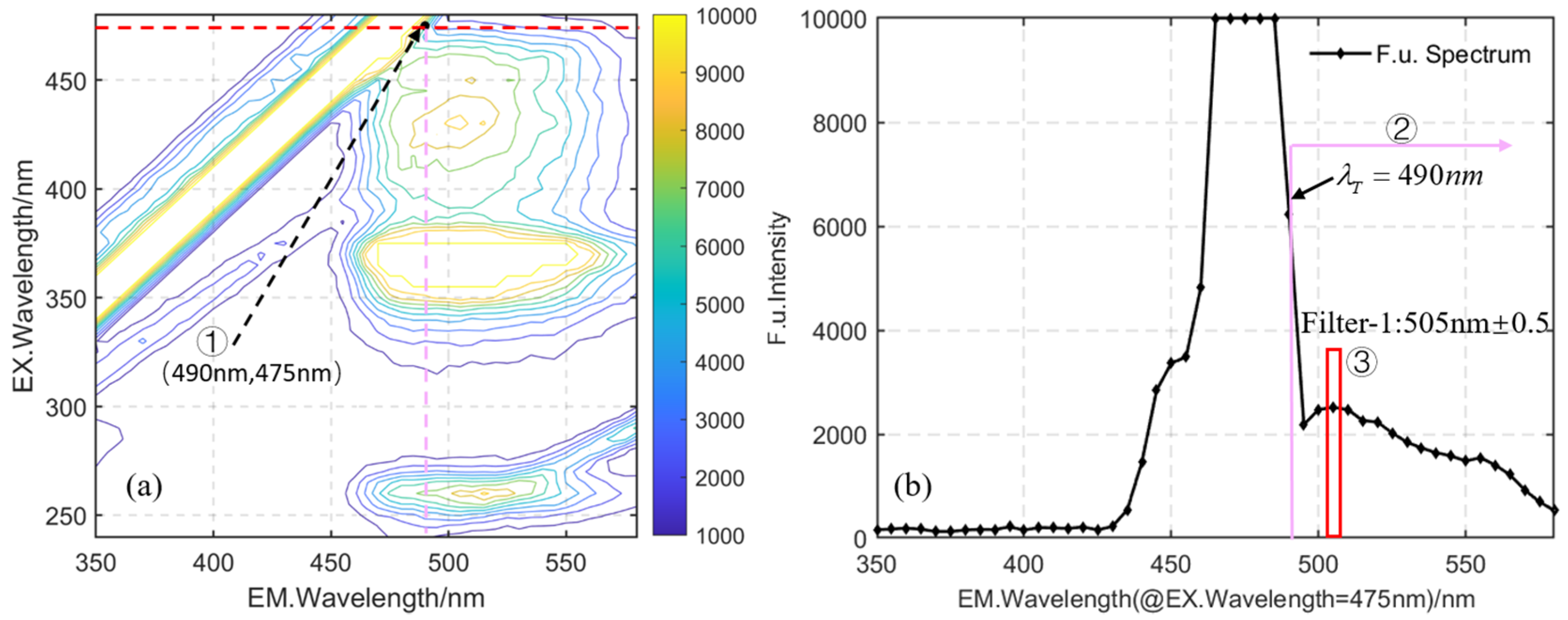
2.3. Target-Oriented Design Methods
3. Experimental Section
3.1. Materials
3.2. Integrated Fluorescence Module Testing
3.3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Liu, T.; Zhang, Z.; Li, H.; Li, W.; Huang, M. The crosstalk fluorescence spectroscopy analysis principle and an accurate fluorescence quantitative method for multi-composition fluorescence substances. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121472. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lei, Y.; Ma, Y.; Liu, M.; Zheng, J.; Dan, D.; Gao, P. A Comprehensive Review of Fluorescence Correlation Spectroscopy. Front. Phys. 2021, 9, 644450. [Google Scholar] [CrossRef]
- Inka, N.; Andreas, B.; Meike, S.; Olga, S.; Leon, C.; Manfred, W.; Manfred, S.; Volker, M.; Mark, H.; Matthias, B.; et al. Monitoring drug nanocarriers in human blood by near-infrared fluorescence correlation spectroscopy. Nat. Commun. 2018, 9, 5306. [Google Scholar]
- Yang, J.; Song, N.; Lv, X.; Jia, Q. UV-light-induced synthesis of PEI-CuNCs based on Cu2+-quenched fluorescence turn-on assay for sensitive detection of biothiols, acetylcholinesterase activity and inhibitor. Sens. Actuators B Chem. 2018, 259, 226–232. [Google Scholar] [CrossRef]
- Tu, J.; Dennis, S.; Saba, P.; Albert, C.L.; Brian, J.L.; Hannah, J.E.; Randall, T.P.; Kendall, N.H.; Raphael, M.F. Stable, Reactive, and Orthogonal Tetrazines: Dispersion Forces Promote the Cycloaddition with Isonitriles. Angew. Chem. Int. Ed. 2019, 58, 9043–9048. [Google Scholar] [CrossRef] [PubMed]
- Akira, K.; Masataka, K. State-of-the-Art Fluorescence Fluctuation-Based Spectroscopic Techniques for the Study of Protein Aggregation. Int. J. Mol. Sci. 2018, 19, 964. [Google Scholar]
- Cui, Y.; Kong, D.; Kong, L.; Wang, S. Excitation emission matrix fluorescence spectroscopy and parallel factor framework-clustering analysis for oil pollutants identification. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 253, 119586. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Thomas, P.; Raphael, P.; Gabriele, W. A Portable Sensor System for Measurement of Fluorescence Indices of Water Samples. IEEE Sens. J. 2020, 20, 9132–9139. [Google Scholar]
- Li, W.; Liu, T.; Fu, Y.; Huang, M. High sensitivity and wide range chlorophyll-a determination by simultaneous measurement of absorbance and fluorescence using a linear CCD. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 270, 120831. [Google Scholar] [CrossRef]
- Elliot, L.E. Fluorescence Correlation Spectroscopy: Past, Present, Future. Biophys. J. 2011, 101, 2855–2870. [Google Scholar]
- Cao, S.; Li, H.; Liu, Y.; Zhang, M.; Wang, M.; Zhou, Z.; Chen, J.; Zhang, S.; Xu, J.; Knutson, J.R. Femtosecond Fluorescence Spectra of NADH in Solution: Ultrafast Solvation Dynamics. J. Phys. Chem. B 2020, 124, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Darshan, C.M.; Jackson, R.; Vijay, K.J.; Chandavalli, R.R.; Krishna, K.M. A comprehensive review on LED-induced fluorescence in diagnostic pathology. Biosens. Bioelectron. 2022, 209, 114230. [Google Scholar]
- Heykel, A.; Frédérique, D.; Jérôme, W.; Patrick, F.; Neso, S.; Hervé, R. Optical-fiber-microsphere for remote fluorescence correlation spectroscopy. Opt. Express 2009, 17, 19085–19092. [Google Scholar]
- Chang, Y.C.; Ye, J.Y.; Thommey, T.; Chen, Y.; James, R.B., Jr.; Theodore, B.N. Two-photon fluorescence correlation spectroscopy through a dual-clad optical fiber. Opt. Express 2008, 16, 12640–12649. [Google Scholar] [CrossRef] [PubMed]
- Kossolapov, A.; Phillips, B.; Bucci, M. Can LED lights replace lasers for detailed investigations of boiling phenomena? Int. J. Multiph. Flow 2021, 135, 103522. [Google Scholar] [CrossRef]
- Li, W.; Jin, J.; Li, Q.; Wu, C.; Lu, H.; Zhou, Q.; Li, A. Developing LED UV fluorescence sensors for online monitoring DOM and predicting DBPs formation potential during water treatment. Water Res. 2016, 93, 1–9. [Google Scholar] [CrossRef]
- Duy, A.B.; Peter, C.H. Analytical devices based on light-emitting diodes-a review of the state-of-the-art. Anal. Chim. Acta 2014, 853, 46–58. [Google Scholar]
- Soumajit, M.; Emmanuel, B.; Eleonore, R.; Yves, M.; Halina, A. Imaging Viral Infection by Fluorescence Microscopy: Focus on HIV-1 Early Stage. Viruses 2021, 13, 213. [Google Scholar]
- Mirek, M.; Tomasz, P.; Purnendu, K.D. Light-Emitting Diodes for Analytical Chemistry. Rev. Anal. Chem. 2014, 7, 183–207. [Google Scholar]
- Hu, Y.; Zhao, D.; Qin, Y.; Wang, X. An order determination method in direct derivative absorption spectroscopy for correction of turbidity effects on COD measurements without baseline required. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 226, 117646. [Google Scholar] [CrossRef]
- Abubakar, A.; Chen, Y.; Yuan, F.; Ma, X.; Lou, B.; Xu, G. Dithiothreitol-Lucigenin Chemiluminescent System for Ultrasensitive Dithiothreitol and Superoxide Dismutase Detection. Anal. Chem. 2022, 94, 11023–11029. [Google Scholar]
- Ma, X.; Gao, W.; Mohamed, I.H.; Lan, Y.; Li, J.; Xu, G. Lucigenin fluorescent assay of tyrosinase activity and its inhibitor screening. Sens. Actuators B Chem. 2018, 280, 41–45. [Google Scholar] [CrossRef]
- Sergey, P.M.; Pavel, M.G.; Asiya, A.G.; Galina, V.K.; Galina, V.K.; Galina, M.K.; Vadim, V.P. Generation of the Reactive Oxygen Species on the surface of nanosized titanium(IV) oxides particles under UV-irradiation and their connection with photocatalytic properties. J. Photochem. Photobiol. A 2020, 393, 112424. [Google Scholar]
- Mohamed, I.H.; Wu, F.; Muhammad, N.Z.; Islam, M.M.; Abubakar, A.; Han, S.; Xu, G. Turn-on fluorescent glutathione detection based on lucigenin and MnO2 nanosheets. J. Mater. Chem. B 2019, 8, 3542–3549. [Google Scholar]
- Klaus-Peter, K.; Thomas, E.; Köhler, J.K. Microfluidically prepared sensor particles for determination of chloride by fluorescence quenching of matrix-embedded lucigenin. SN Appl. Sci. 2020, 2, 366. [Google Scholar]
- Chen, A.; Eberle, M.M.; Lunt, E.J.; Liu, S.; Leake, K.; Rudenko, M.I.; Hawkins, A.R.; Schmidt, H. Dual-color fluorescence cross-correlation spectroscopy on a planar optofluidic chip. Lab Chip 2011, 11, 1052–1056. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, C.; Yang, R.; Dong, G.; Yang, Y.; Liu, H.; Wu, N. Detection of pesticide in water using two-dimensional fluorescence correlation spectroscopy and N-way partial least squares. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117981. [Google Scholar] [CrossRef]
- Chen, W.; Xiong, Y.; Wang, W.; Wu, T.; Li, L.; Kang, Q.; Du, Y. Assembly of a UV-LED induced fluorescence system for rapid determination of amiloride in pharmaceutical tablet and human serum. Talanta 2019, 203, 77–82. [Google Scholar] [CrossRef]


| Device | Parameter | Values |
|---|---|---|
| LED | Peak wavelength: | 475 nm |
| Transmitted power: | 0.5 W | |
| Dichroscope | Transmission wavelength: | 490 nm |
| Filter-1 | Central wavelength: | 505 nm |
| Operation bandwidth: | 1 nm | |
| Filter-2 | Central wavelength: | 475 nm |
| Operation bandwidth: | 20 nm | |
| AD | Digitalizing bit | 24-bit |
| APD | Peak sensitivity | 565 nm |
| Spectral range | 420–675 nm | |
| MCU | Basic frequency | 32 MHz |
| Flash memory | 16 Kbyte | |
| RAM | 2 Kbyte |
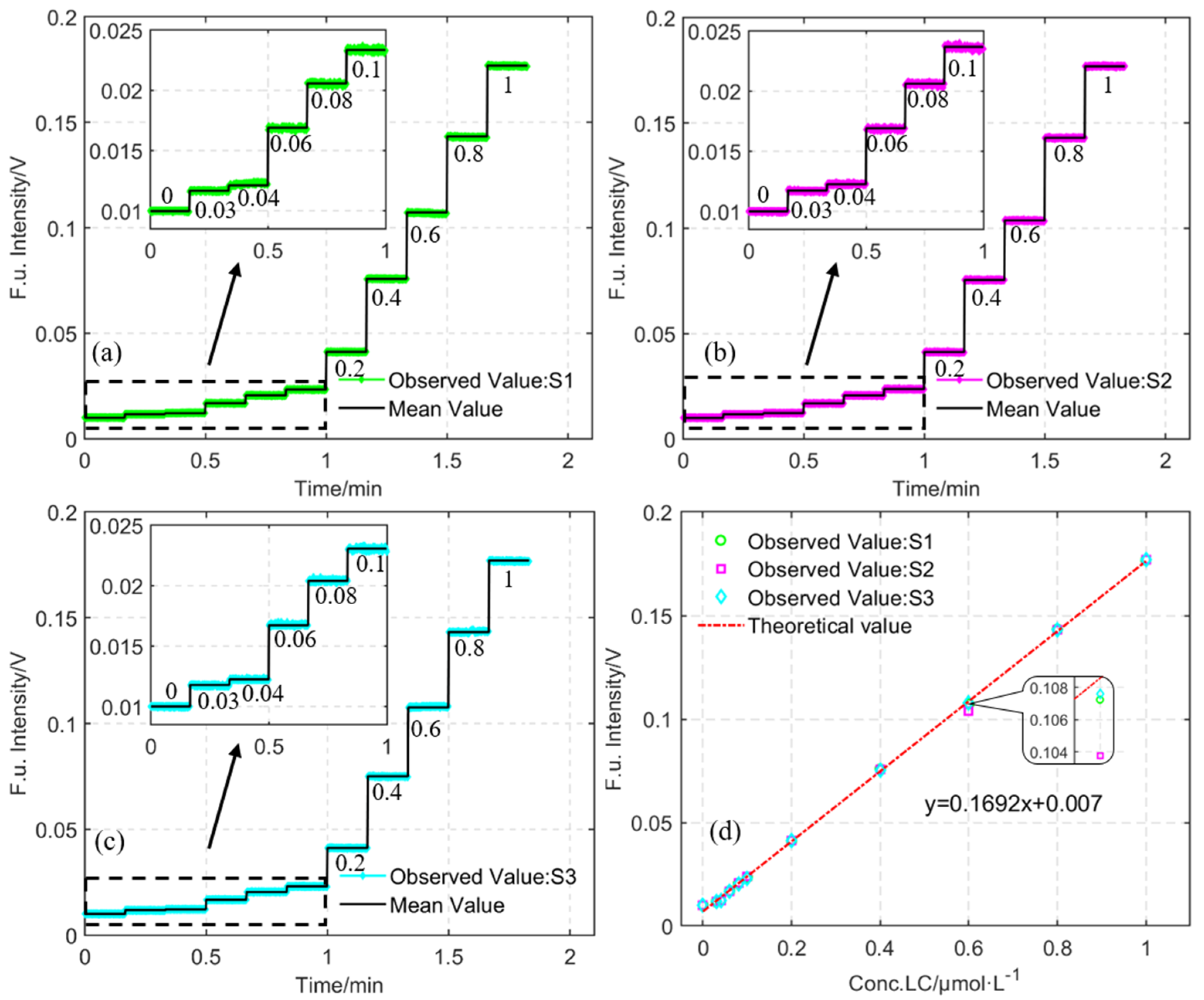
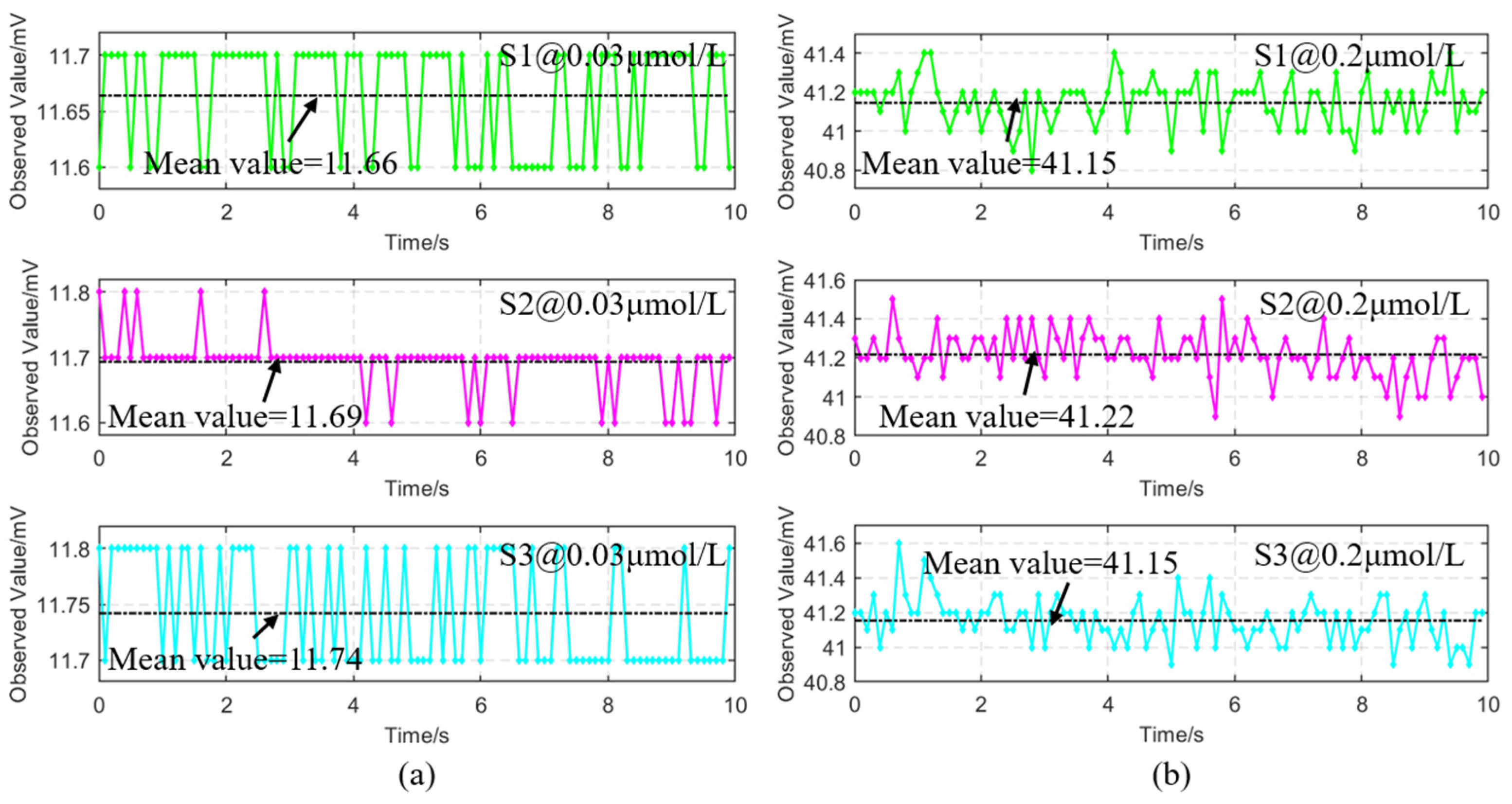
| Sample | R | RMSE | MAPE |
|---|---|---|---|
| S1 | 0.9998 | 1.2‰ | 5.1048% |
| S2 | 0.9995 | 1.8‰ | 5.0790% |
| S3 | 0.9998 | 1.2‰ | 5.0968% |
| Excitation | Sample | Performance indicators | Ref. | ||
|---|---|---|---|---|---|
| Sensitivity | Correlation | LOD | |||
| LED | Lucigenin | 0.1692 V/μM | >0.9995 | 0.03 μM | * |
| LED | Chlorophyll-a | / | 0.9917 | 0.0025 μg/L | [9] |
| LED | DTT-Lucigenin | 3.59 a.u./μg·mL−1 | 0.993 | 2.2 ng/mL | [21] |
| Xenon lamp | Tyrosinase | 1.04/μg·mL−1 | 0.9981 | 1.0 μg/mL | [22] |
| Laser | Glutathione | 2.61/μM | 0.995 | 180 nM | [24] |
| Xenon lamp | Carbaryl | / | 0.93 | 9.2 × 10−7g/L | [27] |
| LED | Amiloride | 23.3 a.u./ng·mL−1 | 0.9997 | 1.43 ng/mL | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Hao, X.; Zhang, M.; Jiang, L.; Gao, W.; Wu, C. Extensible LED-Induced Integrated Fluorescence Detection Module for Quantitative Analysis of Lucigenin Concentration. Photonics 2023, 10, 392. https://doi.org/10.3390/photonics10040392
Qi X, Hao X, Zhang M, Jiang L, Gao W, Wu C. Extensible LED-Induced Integrated Fluorescence Detection Module for Quantitative Analysis of Lucigenin Concentration. Photonics. 2023; 10(4):392. https://doi.org/10.3390/photonics10040392
Chicago/Turabian StyleQi, Xiaoguang, Xianglong Hao, Muzi Zhang, Lili Jiang, Wenyue Gao, and Chi Wu. 2023. "Extensible LED-Induced Integrated Fluorescence Detection Module for Quantitative Analysis of Lucigenin Concentration" Photonics 10, no. 4: 392. https://doi.org/10.3390/photonics10040392
APA StyleQi, X., Hao, X., Zhang, M., Jiang, L., Gao, W., & Wu, C. (2023). Extensible LED-Induced Integrated Fluorescence Detection Module for Quantitative Analysis of Lucigenin Concentration. Photonics, 10(4), 392. https://doi.org/10.3390/photonics10040392






