Real-Time Chlorophyll-a Pigment Monitoring of Chlamydomonas reinhardtii in a Controlled Environment Using Pulsed LED Fluorescence LiDAR System
Abstract
1. Introduction
2. Experimental Preparation and Detection of C. reinhardtii
2.1. C. reinhardtii Sample Preparation and Cultivation Parameters
2.2. LED Fluorescence LiDAR System
2.3. Pulsed LED Fluorescence LiDAR Equation
2.4. Absorbance and EEM Fluorescence Measurements
2.5. Other Parameters Such as Water Quality and Weather Measurements
3. Results
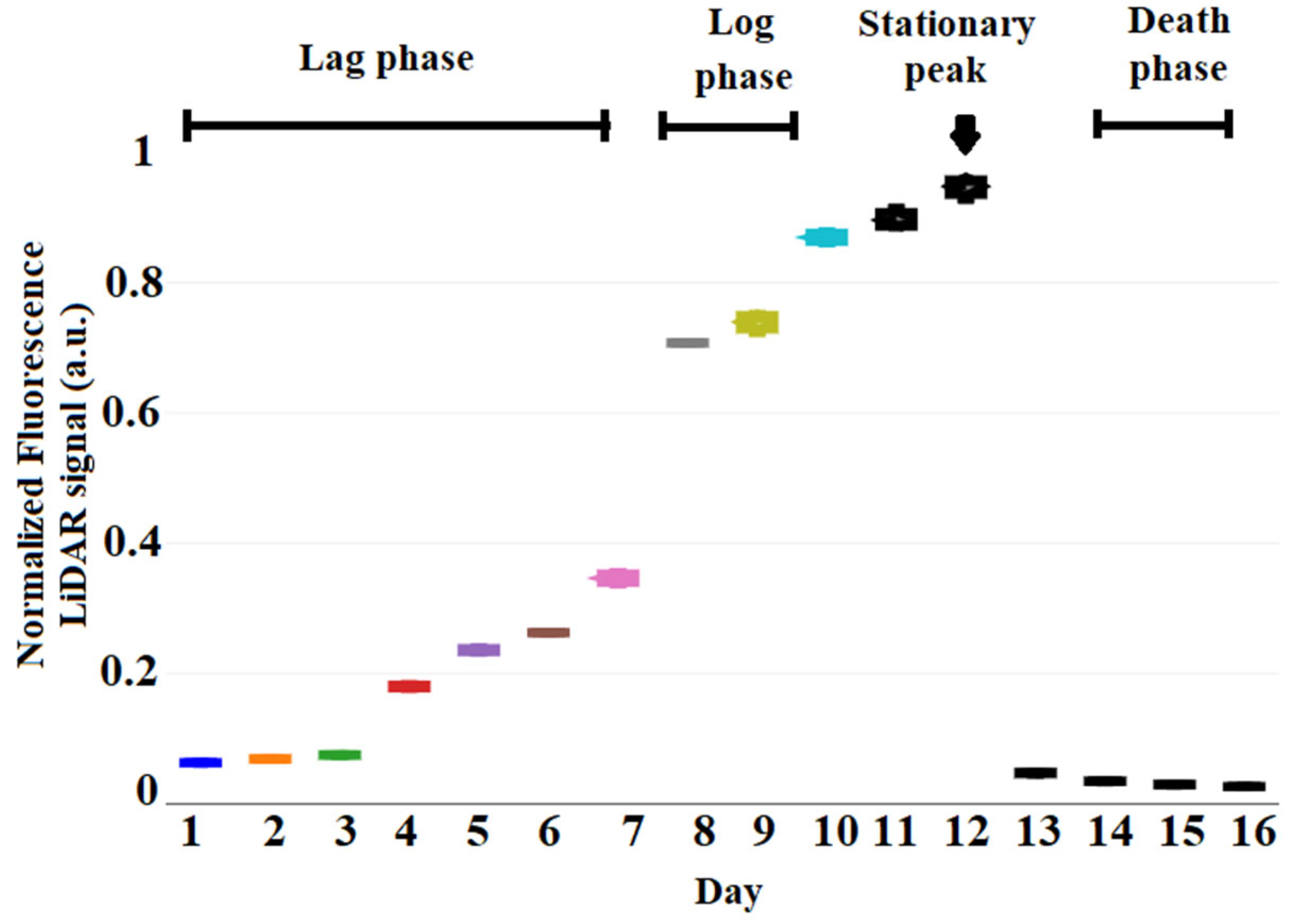
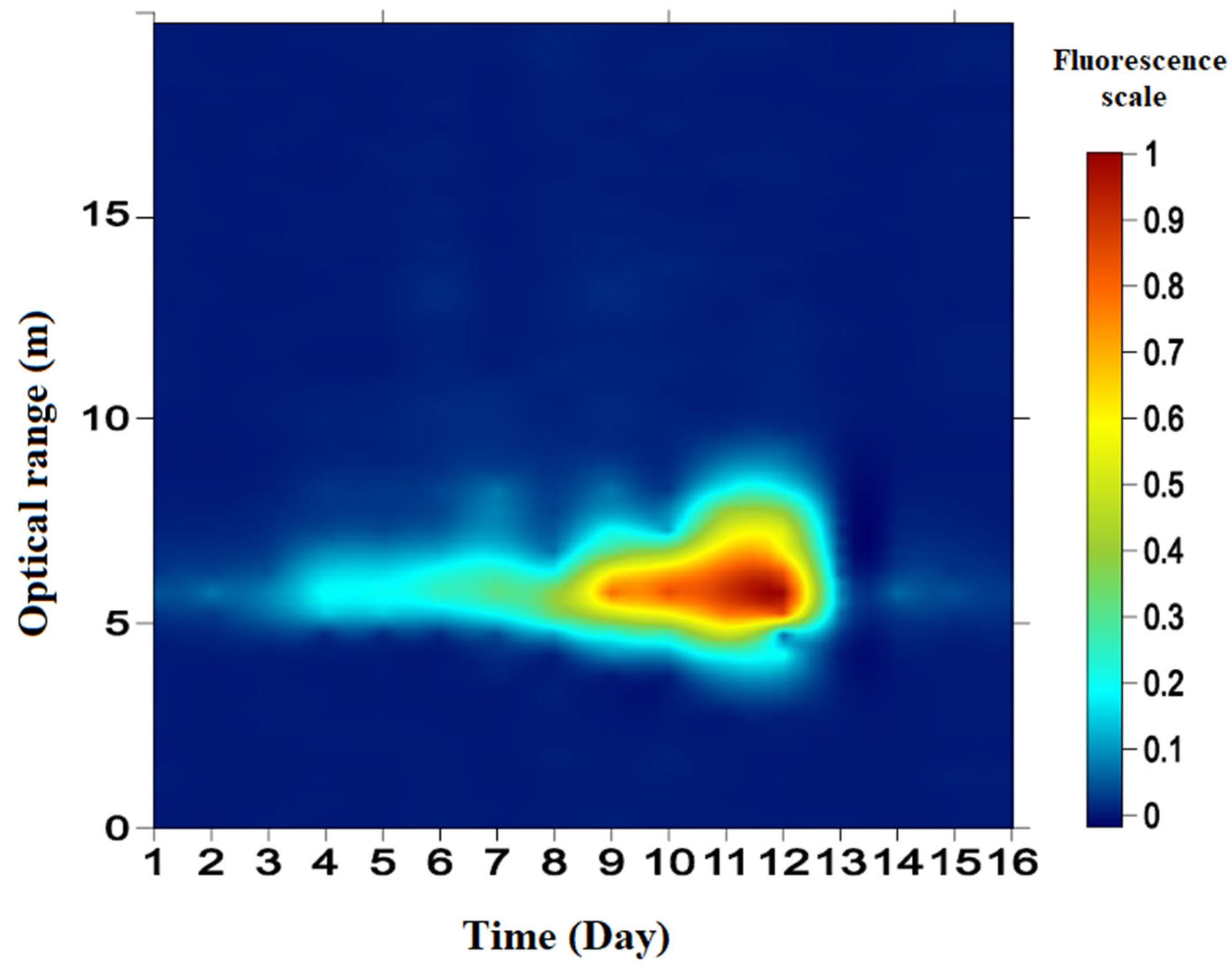
3.1. Pulsed LED Fluorescence LiDAR Signal of C. reinhardtii
3.2. Optical Density and EEM Fluorescence Measurements
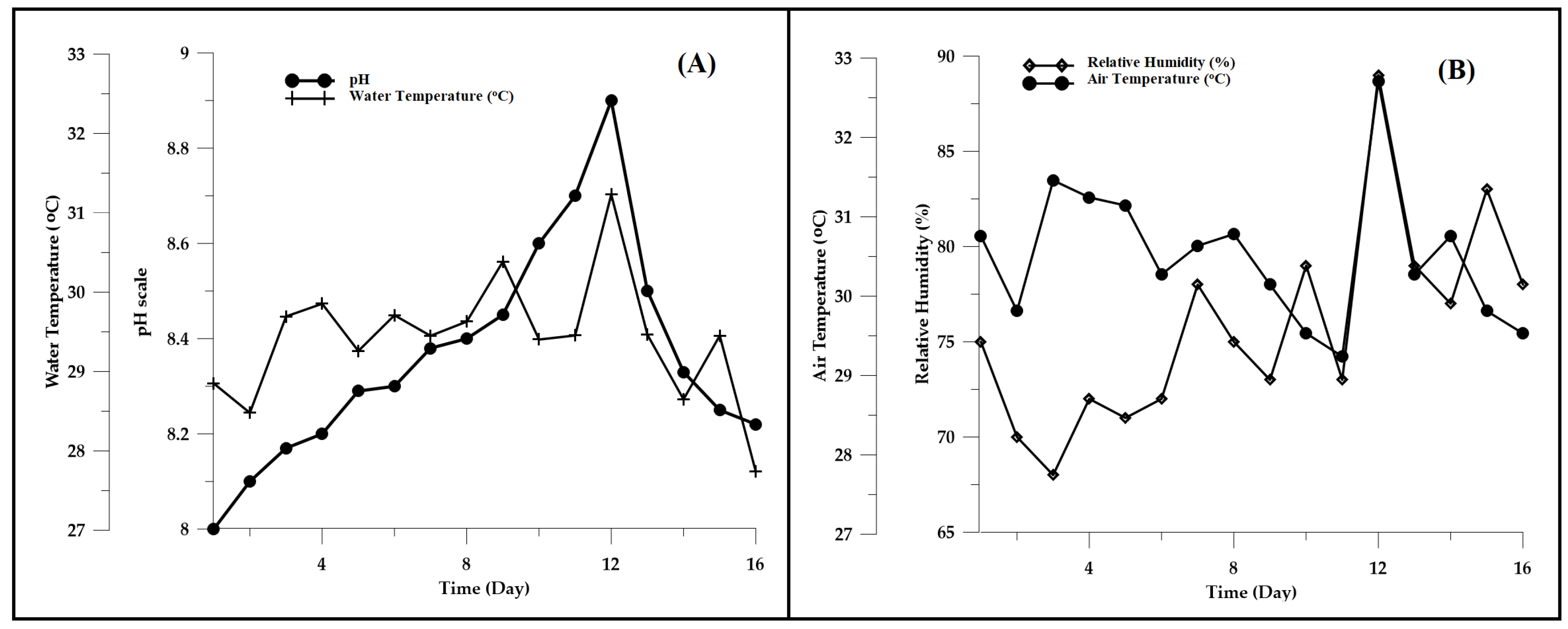
3.3. Water Quality and Weather Observations
3.4. Correlation between Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the microalgae Chlamydomonas on gastrointestinal health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as health ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Caporgno, M.P.; Mathys, A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Sarmast, E.; Habibian Dehkordi, S.; Engardeh, J.; Mahmoodnia, L.; Khaledifar, A.; Jafari, T. Effect of Chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1892–1901. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymudo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Springer Nature. Chlamydomonas reinhardtii. 2019. Available online: https://www.nature.com/subjects/chlamydomonas-reinhardtii (accessed on 26 July 2022).
- Scaife, M.A.; Nguyen, G.T.; Rico, J.; Lambert, D.; Helliwell, K.E.; Smith, A.G. Establishing Chlamydomnas reinhardtii as an industrial biotechnology host. Plant J. 2015, 82, 532–546. [Google Scholar] [CrossRef]
- Harris, E.H. Chlamydomonas as a model organism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 97–112. [Google Scholar] [CrossRef]
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibeling, B.; Jeppesen, E.; Lurling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the plankton ecology group (PEG) model: Mechanism driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Coleman, D.C.; Callaham, M.A., Jr.; Crossley, D.A., Jr. Fundamental of Soil Ecology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Buysschaert, B.; Vermijs, L.; Naka, A.; Boon, N.; De Gusseme, B. Online flow cytometric monitoring of microbial water quality in full-scale water treatment plant. Clean Water 2018, 1, 16. [Google Scholar] [CrossRef]
- Helmi, K.; David, F.; Di Matrino, P.; Jaffrezic, M.-P.; Ingrand, V. Assessment of flow cytometry for microbial water quality monitoring in cooling tower water and oxidizing treatment facility. J. Microbiol. Methods 2018, 152, 201–209. [Google Scholar] [CrossRef]
- Cadondon, J.G.; Napal, J.P.D.; Abe, K.; De Lara, R.; Vallar, E.A.; Orbecido, A.H.; Belo, L.P.; Galvez, M.C.D. Characterization of water quality and fluorescence measurements of dissolved organic matter in Cabuyao river and its tributaries using excitation-emission matrix spectroscopy. J. Phys. Conf. Ser. 2020, 1593, 012033. [Google Scholar] [CrossRef]
- Cadondon, J.G.; Vallar, E.A.; Belo, L.P.; Orbecido, A.H.; Galvez, M.C.D. UV-vis absorbance and fluorescence characterization of Pasig River surface water samples towards the development of an LED fluorescence lidar system. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 968–980. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Coble, P.; Lead, J.; Baker, A.; Reynolds, D.; Spencer, R. Aquatic Organic Matter Fluorescence; Cambridge Environmental Chemistry Series; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Campbell, J.B.; Wynne, R.H. Introduction to Remote Sensing, 5th ed.; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Rantajarvi, E.; Olsonen, R.; Hallfors Leppanen, J.-M.; Raateja, M. Effect of sampling frequency on detection of natural variability in phytoplankton: Unattended high-frequency measurements on board ferries in the Baltic Sea. ICES J. Mar. Sci. 1998, 55, 697–704. [Google Scholar] [CrossRef]
- Boss, E.; Haentjens, N.; Ackleson, S.G.; Balch, B.; Chase, A.; Dall’Olmo, G.; Freeman, S.; Liu, Y.; Loftin, J.; Neary, W.; et al. Inherent optical property measurements and protocols: Best practices for the collection and processing of ship-based underway flow-through optical data. In Ocean Optics & Biogeochemistry Protocols for Satellite Ocean Colour Sensor Validation; IOCCG Protocol Series; Neeley, A.R., Mannino, A., Eds.; IOCCG: Darmouth, NS, Canada, 2019; Volume 4.0. [Google Scholar]
- Li, Y.; Yu, K.; Zhang, W.; Li, D.; Zhao, Z.; Jiang, X.; Jin, D.; Shang, B.; Wang, H. Progress of airborne lidar of ocean chlorophyll observations including algorithms and instruments. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012046. [Google Scholar] [CrossRef]
- Yoder, J.A.; Aiken, J.; Swift, R.N.; Hoge, F.E.; Stegmann, P.M. Spatial variability in near surface chlorophyll-a fluorescence measured by the Airborne Oceanographic Lidar (AOL). Deep. Se Res. Part II Tp. Stud. Oceanogr. 1993, 40, 37–53. [Google Scholar] [CrossRef]
- Papenfus, M.; Schaeffer, B.; Pollard, A.I.; Loftin, K. Exploring the potential value of satellite remote sensing to monitor chlorophyll-a for US lakes and reservoirs. Environ. Monit. Assess. 2020, 192, 808. [Google Scholar] [CrossRef]
- Topp, S.N.; Pavelsky, T.M.; Jensen, D.; Simard, M.; Ros, M.R.V. Research trends in the use of remote sensing for inland water quality science: Moving towards multidisciplinary. Water 2019, 12, 169. [Google Scholar] [CrossRef]
- Raimondi, V.; Cecchi, G.; Lognoli, D.; Palombi, L.; Gronlund, R.; Johansson, A.; Svanberg, S.; Barup, K.; Hallstrom, J. The fluorescence lidar technique for the remote sensing of photoautotrophic biodeteriogens in the outdoor cultural heritage: A decade of in situ experiments. Inter. Biodeterior. Biodegrad. 2009, 63, 823–835. [Google Scholar] [CrossRef]
- Saito, Y.; Takano, K.; Kobayashi, F.; Kobayashi, K.; Prk, H.-D. Development of a UV laser-induced fluorescence for monitoring blue-green algae in Lake Suwa. Appl. Opt. 2014, 53, 7030–7036. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Pelevin, V.V.; Goncharenko, I.; Kovacs, A.W.; Zlinsky, A.; Presing, M.; Horvath, H.; Nicolas-Pera, V.; Balzier, H.; Toth, V.R. Ultraviolet fluorescence LiDAR (UFL) as a measurement tool for water quality parameters in Turbid Lake conditions. Remote Sens. 2013, 5, 4405–4422. [Google Scholar] [CrossRef]
- Cadondon, J.G.; Ong, P.M.B.; Vallar, E.A.; Shiina, T.; Galvez, M.C.D. Chlorophyll-a pigment measurement of spirulina in algal growth monitoring using portable pulsed LED fluorescence lidar system. Sensors 2022, 22, 2940. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ichihara, K.; Morishita, K.; Uchiyama, K.; Kobayashi, F.; Tomida, T. Remote detection of the fluorescence spectrum of natural pollens floating in the atmosphere using a laser-induced-fluorescence spectrum (LIPS) Lidar. Remote Sens. 2018, 10, 1533. [Google Scholar] [CrossRef]
- Shiina, T. LED mini lidar for atmospheric application. Sensors 2019, 19, 569. [Google Scholar] [CrossRef]
- Koyama, M.; Shiina, T. Light source module for LED mini-lidar. Rev. Laser Eng. 2011, 39, 617–621. [Google Scholar] [CrossRef]
- Ong, P.M.B.; Shiina, T.; Manago, N.; Kuze, H.; Senshu, H.; Otobe, N.; Hashimoto, G.; Kawabata, Y. A compact LED Lidar system fitted for a mars rover—Design and ground experiment. EPJ Web Conf. 2018, 176, 02013. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Measures, R. Laser Remote Sensing; Wiley Publishing: Hoboken, NJ, USA, 1984. [Google Scholar]
- Janssen, P.J.D.; Laambreva, M.D.; Plumere, N.; Bartolucci, C.; Antonacci, A.; Buonasera, K.; Frese, R.N.; Scognamiglio, V.; Rea, G. Photosynthesis at the forefront of a sustainable life. Front. Chem. 2014, 2, 36. [Google Scholar] [CrossRef]
- Donohue, J.M. Water conditioning, industrial. In Encyclopedia of Physical Science and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 671–697. [Google Scholar] [CrossRef]
- Miller, S.D.; Stephens, G.L. Multiple scattering effects in the lidar pulse stretching problem. J. Geophys. Res. 1999, 104, 22205–22219. [Google Scholar] [CrossRef]
- Kalra, I.; Wang, X.; Cvetkovska, M.; Jeong, J.; McHargue, W.; Zhang, R.; Huner, N.; Yuan, J.S.; Morgan-Kiss, R. Chlamydomonas sp. UWO 241 exhibits high cyclic electron flow and rewired metabolism under high salinity. Plant Physiol. 2020, 183, 588–601. [Google Scholar] [CrossRef]
- Young, E.B.; Reed, L.B.J.A. Growth Parameters and responses of green algae across a gradient of phototrophic, mixotrophic and heterotrophic conditions. Peer J 2022, 10, e13776. [Google Scholar] [CrossRef]
- Omar, W.M. Perspectives on the use of algae as biological indicators for monitoring and protecting aquatic environments, with special reference to Malaysian freshwater ecosystems. Trop. Life Sci. Res. 2010, 21, 51–67. [Google Scholar]
- Stevenson, J. Ecological assessments with algae: A review and synthesis. J. Phycol. 2014, 50, 437–461. [Google Scholar] [CrossRef]
- Krishnan, V.; Uemura, Y.; Thanh, N.T.; Khalid, N.A.; Osman, N.; Mansor, N. Three types of marine microalgae and Nannocholoropsis oculate cultivation for potential source of biomass production. J. Phys. Conf. Ser. 2015, 622, 012034. [Google Scholar] [CrossRef]
- Lurling, M.; Beekman, W. Palmelloids formation in Chlamydomonas reinhardtii: Defense against rotifer predators? Ann. Limnol. Int. J. Limnol. 2006, 42, 65–72. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopacova, E.; Ali, S.S. Estimating biomass and vitality of microalgae for monitoring cultures: A roadmap for reliable measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef]



| Compound | Final Concentration (g/L Distilled H2O) |
|---|---|
| NaNO3 | 1.5 |
| K2HPO4 | 0.04 |
| MgSO4.7H2O | 0.075 |
| CaCl2.2H2O | 0.036 |
| Citric acid | 0.006 |
| FeSO4.7H2O | 0.006 |
| EDTA | 0.001 |
| Na2CO3A5 micronutrient solution | 0.021 mL |
| Transmitter | |
| LED Name/Brand | Nichia, NCSU034C |
| Wavelength | 385 nm |
| Peak power | 830 mW |
| Resolution | 1.2 m |
| Bandwidth | 10.92 ns |
| Repetition | 500 kHz |
| Beam diameter | 50 mm𝛷 |
| Beam divergence | 5 mrad |
| Receiver | |
| Telescope | Schmidt-Cassegrain |
| Beam diameter | 100 mm𝛷 |
| Beam divergence | 3 mrad |
| Bandpass filters: At 680 nm: -Thorlabs (FB680-10) | 680 ± 5 nm |
| Detection device | Photomultiplier tube, Hamamatsu (R6350P) |
| Photon Counting Board | |
| Photon Counting Device/Brand | Spartan 6 (FPGA device) Trimatiz Co. Ltd. (Chiba, Japan), Photon tracker |
| System lock | 550 MHz |
| BIN Width | 5 ns (0.75 m) |
| BIN length | 50 |
| Acquisition count | 167777214 (max) |
| Trigger Input Threshold level | 300 mV |
| N = 16 | Mean/SD | Range-Resolved Fluorescence LiDAR Peak Value | OD680 | EEM Chlorophyll-a Pigment (680 nm) | pH | Water Temp (°C) | Rel. Humidity (%) | Air Temp (°C) |
|---|---|---|---|---|---|---|---|---|
| Range-resolved fluorescence LiDAR peak value | 0.38/0.31 | 1 | 0.00049 a,b,c | 0.0008 a,b,c | 0.00006 a,b,c | 0.0089 a,b,c | 0.2930 | 0.0211 |
| OD680 | 0.35/0.32 | 0.77 * | 1 | 0.00001 a,b,c | 0.000019 a,b,c | 0.035 b,c | 0.01 b,c | 0.48 |
| EEM Chlorophyll-a pigment (680 nm) | 0.17/0.15 | 0.75 * | 0.93 * | 1 | 0.00001 a,b,c | 0.01 a,b,c | 0.03 b,c | 0.27 |
| pH | 8.36/0.23 | 0.83 * | 0.86 * | 0.93 * | 1 | 0.012 b,c | 0.021 b,c | 0.61 |
| Water temp (°C) | 29.42/0.79 | 0.63 * | 0.53 * | 0.62 * | 0.61 * | 1 | 0.259 | 0.019 b,c |
| Rel Humidity (%) | 75.75/5.30 | 0.28 * | 0.62 * | 0.54 * | 0.57 * | 0.30 * | 1 | 0.435 |
| Air temp (°C) | 30.51/0.88 | 0.07 * | 0.19 * | 0.27 * | 0.14 * | 0.58 * | 0.21 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadondon, J.G.; Vallar, E.A.; Shiina, T.; Galvez, M.C.D. Real-Time Chlorophyll-a Pigment Monitoring of Chlamydomonas reinhardtii in a Controlled Environment Using Pulsed LED Fluorescence LiDAR System. Photonics 2023, 10, 144. https://doi.org/10.3390/photonics10020144
Cadondon JG, Vallar EA, Shiina T, Galvez MCD. Real-Time Chlorophyll-a Pigment Monitoring of Chlamydomonas reinhardtii in a Controlled Environment Using Pulsed LED Fluorescence LiDAR System. Photonics. 2023; 10(2):144. https://doi.org/10.3390/photonics10020144
Chicago/Turabian StyleCadondon, Jumar G., Edgar A. Vallar, Tatsuo Shiina, and Maria Cecilia D. Galvez. 2023. "Real-Time Chlorophyll-a Pigment Monitoring of Chlamydomonas reinhardtii in a Controlled Environment Using Pulsed LED Fluorescence LiDAR System" Photonics 10, no. 2: 144. https://doi.org/10.3390/photonics10020144
APA StyleCadondon, J. G., Vallar, E. A., Shiina, T., & Galvez, M. C. D. (2023). Real-Time Chlorophyll-a Pigment Monitoring of Chlamydomonas reinhardtii in a Controlled Environment Using Pulsed LED Fluorescence LiDAR System. Photonics, 10(2), 144. https://doi.org/10.3390/photonics10020144







