Release of Selected Non-Intentionally Added Substances (NIAS) from PET Food Contact Materials: A New Online SPE-UHPLC-MS/MS Multiresidue Method
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
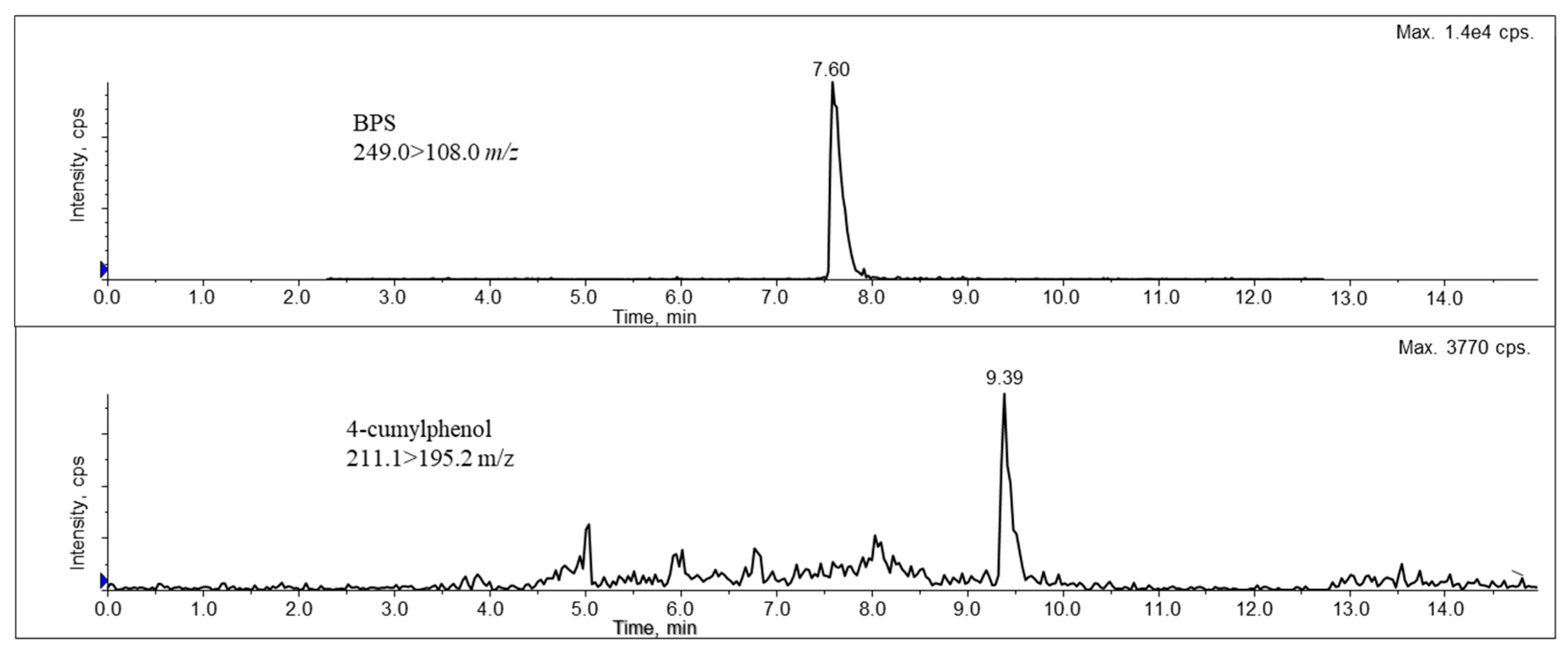
2.2. Online SPE-UHPLC-MS/MS Method
2.3. PET Samples Treatment
2.4. Overall and Specific Migration Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lahimer, M.C.; Ayed, N.; Horriche, J.; Belgaied, S. Characterization of plastic packaging additives: Food contact, stability and toxicity. Arab. J. Chem. 2017, 10, S1938. [Google Scholar] [CrossRef] [Green Version]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; García, R.S.; Cooper, I.; Franz, R.; Losada, P.P. Compilation of analytical methods and guidelines for the determination of selected model migrants from plastic packaging. Trends Food Sci. Technol. 2006, 17, 535. [Google Scholar] [CrossRef]
- Sablani, S.S. Food preservation and processing using membranes. In Handbook of Food Preservation; Rahmna, M.S., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Harper, C.A. Plastic additives. In Modern Plastics Handbook; McGraw-Hill Companies, Inc: New York, NY, USA, 2000. [Google Scholar]
- Boussoum, M.O.; Atek, D.; Belhaneche-Bensemra, N. Interactions between poly(vinyl chloride) stabilised with epoxidised sunflower oil and food simulants. Polym. Degrad. Stab. 2006, 91, 579. [Google Scholar] [CrossRef]
- Kudłak, B.; Szczepańska, N.; Owczarek, K.; Mazerska, Z.; Namieśnik, J. Revision of biological methods for determination of EDC presence and their endocrine potential. Crit. Rev. Anal. Chem. 2015, 45, 191. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Identification of non volatile migrant compounds and NIAS in polypropylene films used as food packaging characterized by UPLC-MS/QTOF. Talanta 2018, 188, 750. [Google Scholar] [CrossRef]
- Piergiovanni, L.; Limbo, S.; Montesi, E. Food Packing. In Materiali, Tecnologie e Qualità Degli Alimenti, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Commission Regulation (EU) No. 10/2011. In Commission Regulation (EU) No. 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food; Commission Regulation (EU): Brussels, Belgium, 2011; pp. 1–89.
- Bach, C.; Dauchy, X.; Chagnon, M.C.; Etienne, S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: A source of controversy reviewed. Water Res. 2012, 46, 571. [Google Scholar] [CrossRef] [Green Version]
- Bach, C.; Dauchy, X.; Severin, I.; Munoz, J.F.; Etienne, S.; Chagnon, M.C. Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: Chemical analysis and potential toxicity. Food Chem. 2013, 139, 672. [Google Scholar] [CrossRef]
- Tisler, S.; Christensen, J.H. Non-target screening for the identification of migrating compounds from reusable plastic bottles into drinking water. J. Hazard. Mater. 2022, 429, 128331. [Google Scholar] [CrossRef]
- Yusà, V.; López, A.; Dualde, P.; Pardo, O.; Fochi, I.; Miralles, P.; Coscollá, C. Identification of 24 Unknown Substances (NIAS/IAS) from food contact polycarbonate by LC-Orbitrap Tribrid HRMS-DDMS3: Safety sssessment. Int. J. Anal. Chem. 2021, 2021, 6654611. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerin, C.; Dreolin, N.; Goshawk, J. The detection and elucidation of oligomers migrating from biodegradable multilayer teacups using liquid chromatography coupled to ion mobility time-of-flight mass spectrometry and gas chromatography-mass spectrometry. Food Chem. 2022, 374, 131777. [Google Scholar] [CrossRef] [PubMed]
- Pack, E.C.; Lee, K.Y.; Jung, J.S.; Jang, D.Y.; Kim, H.S.; Koo, Y.J.; Lee, H.G.; Kim, Y.S.; Lim, K.M.; Lee, S.H.; et al. Determination of the migration of plastic additives and non-intentionally added substances into food simulants and the assessment of health risks from convenience food packaging. Food Packag. Shelf Life 2021, 30, 100736. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Nuñez, A.; Johnston, J. Screening of chemicals migrating from plastic food contact materials for oven and microwave applications by liquid and gas chromatography—Orbitrap mass spectrometry. J. Chromat. A 2021, 1651, 462261. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Yusà, V.; Sanchís, Y.; Coscollà, C. Determination of 60 Migrant substances in plastic food contact materials by vortex-assisted Liquid-Liquid Extraction and GC-Q-Orbitrap HRMS. Molecules 2021, 26, 7640. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chen, J.R.; Zheng, C.; Yang, Z.B.; Xu, X.X.; Wong, M.H. Bioaccumulation and health risk assessment of phthalate esters in cultured low trophic level fish feded with food waste-based diets. Chemosphere 2021, 276, 130189. [Google Scholar] [CrossRef] [PubMed]
- Bridson, J.H.; Gaugler, E.C.; Smith, D.A.; Northcott, G.L.; Gaw, S. Leaching and extraction of additives from plastic pollution to inform environmental risk: A multidisciplinary review of analytical approaches. J. Hazard. Mater. 2021, 414, 125571. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- García Ibarra, V.; Sendón, R.; Bustos, J.; Paseiro Losada, P.; de Quirós, A.R.B. Estimates of dietary exposure of Spanish population to packaging contaminants from cereal based foods contained in plastic materials. Food Chem. Toxicol. 2019, 128, 180–192. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services F and DA. Bioanalytical Method Validation Guidance for Industry; US Dep Heal Hum Serv Food Drug Adm: Washington, DC, USA, 2018; pp. 1–41.
- Marín-Morocho, K.; Domenek, S.; Salazar, R. Identification of potential migrants in polyethylene terephthalate samples of Ecuadorian market. Polymers 2021, 13, 3769. [Google Scholar] [CrossRef]
- Cao, X.L.; Corriveau, J. Migration of bisphenol A from polycarbonate baby and water bottles into water under severe conditions. J. Agric. Food Chem. 2008, 56, 6378–6381. [Google Scholar] [CrossRef]
- Česen, M.; Lambropoulou, D.; Laimou-Geraniou, M.; Kosjek, T.; Blaznik, U.; Heath, D.; Heath, E. Determination of Bisphenols and Related Compounds in Honey and Their Migration from Selected Food Contact Materials. J. Agric. Food Chem. 2016, 64, 8866–8875. [Google Scholar] [CrossRef] [PubMed]
- Dreolin, N.; Aznar, M.; Moret, S.; Nerin, C. Development and validation of a LC-MS/MS method for the analysis of bisphenol a in polyethylene terephthalate. Food Chem. 2019, 274, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Vázquez Loureiro, P.; Sendón, R.; Paseiro Losada, P.; de Quirós, A.R.B. Application of chromatographic analysis for detecting components from polymeric can coatings and further determination in beverage samples. J. Chromatogr. A 2021, 1638, 461886. [Google Scholar] [CrossRef]
- Siddique, S.; Zhang, G.; Coleman, K.; Kubwabo, C. Investigation of the migration of bisphenols from baby bottles and sippy cups. Curr. Res. Nutr. Food Sci. 2021, 4, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Staniszewska, M.; Bodziach, K.; Calka, J.; Gonkowski, S. Concentrations of bisphenol a (BPA) in fresh pork loin meat under standard stock-farming conditions and after oral exposure—A preliminary study. Chemosphere 2022, 295, 133816. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Inácio, T.; Almada, M.; Ferreira, R.; Fernandes, J.O. Gas chromatography-mass spectrometry analysis of nine bisphenols in canned meat products and human risk estimation. Int. Food Res. J. 2020, 135, 109293. [Google Scholar] [CrossRef]
- Fattore, M.; Russo, G.; Barbato, F.; Grumetto, L.; Albrizio, S. Monitoring of bisphenols in canned tuna from Italian markets. Food Chem. Toxicol. 2015, 83, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Mohiuddin, I.; Kaur, K.; Lee, J.; Brown, R.J.; Malik, A.K.; Kim, K.H. Dual-template magnetic molecularly imprinted polymer-based sorbent for simultaneous and selective detection of phenolic endocrine disrupting compounds in foodstuffs. Environ. Pollut. 2021, 275, 116613. [Google Scholar] [CrossRef]
- Su, Q.; Vera, P.; Salafranca, J.; Nerín, C. Decontamination efficiencies of post-consumer high-density polyethylene milk bottles and prioritization of high concern volatile migrants. Resour. Conserv. Recycl. 2021, 171, 105640. [Google Scholar] [CrossRef]
- Su, Q.; Vera, P.; Nerín, C.; Lin, Q. Huai-Ning Zhong. Safety concerns of recycling postconsumer polyolefins for food contact uses: Regarding (semi-)volatile migrants untargetedly screened. Resour. Conserv. Recycl. 2021, 167, 105365. [Google Scholar] [CrossRef]
- Ramskov Tetzlaff, C.N.; Svingen, T.; Vinggaard, A.M.; Rosenmai, A.K.; Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 2020, 35, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yeung, K.; Chan, K.M. Toxic effects of octocrylene on zebrafish larvae and liver cell line (ZFL). Aquat. Toxicol. 2021, 236, 105843. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Junker, T.; Coors, A. Review of the environmental fate and effects of two UV filter substances used in cosmetic products. Sci. Total Environ. 2022, 808, 151931. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R. Towards standard methods for the classification of aquatic toxicity for biologically active household chemicals (BAHC) present in plastics, pharmaceuticals, and cosmetic products. Environ. Monit. Assess. 2021, 193, 685. [Google Scholar] [CrossRef]
- Carve, M.; Nugegoda, D.; Allinson, G.; Shimeta, J. A systematic review and ecological risk assessment for organic ultraviolet filters in aquatic environments. Environ. Pollut. 2021, 268, 115894. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; DiNardo, J.C.; Stien, D.; Rodrigues, A.; Lebaron, P. Benzophenone accumulates over time from the degradation of octocrylene in commercial sunscreen products. Chem. Res. Toxicol. 2021, 34, 1046–1054. [Google Scholar] [CrossRef]
- Harvey, J.A. Chemical and physical aging of plastics. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew Inc.: New York, NY, USA, 2005; pp. 153–163. [Google Scholar] [CrossRef]
- Nawrocki, J.; Dabrowska, A.; Borcz, A. Investigation of carbonyl compounds in bottled waters from Poland. Water Res. 2002, 36, 4893. [Google Scholar] [CrossRef]
- Dabrowska, A.; Borcz, A.; Nawrocki, J. Aldehyde contamination of mineral water stored in PET bottles. Food Addit. Contam. 2004, 20, 1170. [Google Scholar] [CrossRef]
- Mutsuga, M.; Kawamura, Y.; Sugita-Konishi, Y.; Hara-Kudo, Y.; Takatori, K.; Tanamoto, K. Migration of formaldehyde and acetaldehyde into mineral water in polyethylene terephthalate (PET) bottles. Food Addit. Contam. 2006, 23, 212. [Google Scholar] [CrossRef]
- Shotyk, W.; Krachler, M. Contamination of bottled waters with antimony leaching from polyethylene terephthalate (pet) increases upon storage. Environ. Sci. Technol. 2007, 41, 1560. [Google Scholar] [CrossRef]
- Keresztes, S.; Tatár, E.; Mihucz, V.; Virág, I.; Majdik, C. Leaching of antimony from polyethylene terephthalate (PET) bottles into mineral water. Sci. Total Environ. 2009, 407, 4731. [Google Scholar] [CrossRef] [PubMed]
| Compounds | [M + H]+ (m/z) | Product Ions (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|
| 2,4-TDI | 175.4 | 132.2 | 170 | 7 | 21 | 7 |
| 147.0 | 14 | 8 | ||||
| 104.2 | 25 | 6 | ||||
| 2,6-TDI | 175.4 | 132.2 | 170 | 7 | 21 | 7 |
| 147.0 | 14 | 8 | ||||
| 104.2 | 25 | 6 | ||||
| HDI | 169.1 | 126.2 | 122 | 12 | 8 | 6 |
| 98.2 | 13 | 7 | ||||
| Octocrylene | 362.2 | 232.2 | 94 | 14 | 27 | 12 |
| 250.0 | 13 | 13 | ||||
| Homosalate | 263.2 | 139.2 | 114 | 6 | 10 | 6 |
| 120.9 | 35 | 12 | ||||
| BEHT | 319.2 | 113.1 | 125 | 12 | 11 | 13 |
| 166.7 | 15 | 12 | ||||
| 279.1 | 10 | 9 |
| Compounds | [M-H]− (m/z) | Product Ions (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|
| BPA | 227.3 | 133.0 | −80 | −7 | −33 | −14 |
| 211.1 | −21 | −20 | ||||
| BPS | 249.0 | 108.0 | −75 | −3 | −35 | −13 |
| 156.0 | −27 | −12 | ||||
| 92.0 | −38 | −13 | ||||
| 4-cumylphenol | 211.1 | 195.2 | −104 | −8 | −22 | −17 |
| 133.1 | −32 | −11 | ||||
| 2,4-dicumylphenol | 329.2 | 313.2 | −94 | −9 | −46 | −16 |
| 250.9 | −37 | −14 | ||||
| 237.0 | −44 | −15 | ||||
| BHT | 219.0 | 203.0 | −133 | −11 | −35 | −11 |
| 163.0 | −35 | −13 | ||||
| 2-ethylhexyl salicylate | 249.2 | 93.0 | −120 | −13 | −28 | −10 |
| 137.0 | −24 | −14 | ||||
| 127.1 | −35 | −7 |
| Identification Name | Sample Characteristics |
|---|---|
| P1 | 1.5 L clear PET bottle (32.5 g preform) |
| P2 | 0.5 L clear PET bottle (19 g preform) |
| P3 | 0.5 L dark blue PET bottle (12 g preform) |
| P4 | 0.5 L light blue PET bottle (10.4 g preform) |
| P5 | 1.5 L light blue PET bottle (23 g preform) |
| P6 | 2 L light blue PET bottle (27.5 g preform) |
| P7 | 1.5 L dark blue PET bottle (32 g preform) |
| P8 | 0.5 L cleat PET bottle (7.2 g preform) |
| Compounds | SEL% | DIFF% | BIASLLOQ% | RSDLLOQ% | LOD (µg/L) 1 | LOQ (µg/L) 1 | LLOQ (µg/L) 1 |
|---|---|---|---|---|---|---|---|
| 4-cumylphenol | 2.10 | 5.00 | 20.2 | 13.4 | 0.38 | 1.27 | 10 |
| BHT | 19.5 | 4.62 | 15.8 | 13.0 | 13.6 | 42.2 | 100 |
| 2-ethylhexyl salicylate | 4.90 | 10.5 | 12.9 | 25.6 | 2.70 | 9.01 | 50 |
| 2,4-dicumylphenol | 1.13 | 6.51 | 8.15 | 0.18 | 0.18 | 0.61 | 10 |
| BPA | 0.01 | 5.65 | 14.6 | 0.11 | 0.12 | 0.40 | 10 |
| BPS | 0.27 | 9.50 | 10.5 | 0.1 | 0.002 | 0.01 | 0.25 |
| 2,4-TDI | 6.40 | 2.72 | 1.81 | 6.05 | 1.29 | 4.31 | 10 |
| 2,6-TDI | 0.16 | 3.36 | 3.73 | 14.9 | 0.27 | 0.91 | 10 |
| HDI | 0.11 | 11.8 | 12.8 | 8.69 | 0.82 | 2.74 | 10 |
| Homosalate | 0.83 | 18.9 | 10.4 | 26.4 | 0.21 | 0.70 | 10 |
| Octocrylene | 16.7 | 26.1 | 12.2 | 14.7 | 5.83 | 19.4 | 25 |
| Sample | Migration in Simulant A (mg/dm2) | Migration in Simulant B (mg/dm2) |
|---|---|---|
| Blank | <<0.1 | <<0.1 |
| P1 | <0.1 | <0.1 |
| P2 | <0.1 | 0.468 |
| P3 | <0.1 | <0.1 |
| P4 | <0.1 | 0.107 |
| P5 | 0.153 | 0.132 |
| P6 | <0.1 | 0.117 |
| P7 | <0.1 | <0.1 |
| P9 | <0.1 | 0.176 |
| Compound | P1 Sim. A/B | P2 Sim. A/B | P3 Sim. A/B | P4 Sim. A/B | P5 Sim. A/B | P6 Sim. A/B | P7 Sim. A/B | P8 Sim. A/B |
|---|---|---|---|---|---|---|---|---|
| 4-cumylphenol (ng/L) | nd/nd | nd/4.78 | 7.21/7.00 | 4.77/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| 2,4-dicumylphenol (µg/L) | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| 2-ethylhexyl salicylate (µg/L) | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| BPA (µg/L) | nd/nd | nd/0.03 | 0.01/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| BPS (µg/L) | nd/nd | 0.04/nd | 0.50/0.05 | nd/nd | 0.59/nd | nd/nd | 0.02/nd | nd/nd |
| BHT (µg/L) | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| Octocrylene (µg/L) | nd/nd | 0.04/nd | nd/nd | nd/nd | nd/nd | nd/nd | 0.01/nd | nd/nd |
| Homosalate (µg/L) | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| HDI (µg/L) | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| 2,4-TDI (µg/L) | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| 2,6-TDI(µg/L) | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aigotti, R.; Giannone, N.; Asteggiano, A.; Mecarelli, E.; Dal Bello, F.; Medana, C. Release of Selected Non-Intentionally Added Substances (NIAS) from PET Food Contact Materials: A New Online SPE-UHPLC-MS/MS Multiresidue Method. Separations 2022, 9, 188. https://doi.org/10.3390/separations9080188
Aigotti R, Giannone N, Asteggiano A, Mecarelli E, Dal Bello F, Medana C. Release of Selected Non-Intentionally Added Substances (NIAS) from PET Food Contact Materials: A New Online SPE-UHPLC-MS/MS Multiresidue Method. Separations. 2022; 9(8):188. https://doi.org/10.3390/separations9080188
Chicago/Turabian StyleAigotti, Riccardo, Nicola Giannone, Alberto Asteggiano, Enrica Mecarelli, Federica Dal Bello, and Claudio Medana. 2022. "Release of Selected Non-Intentionally Added Substances (NIAS) from PET Food Contact Materials: A New Online SPE-UHPLC-MS/MS Multiresidue Method" Separations 9, no. 8: 188. https://doi.org/10.3390/separations9080188
APA StyleAigotti, R., Giannone, N., Asteggiano, A., Mecarelli, E., Dal Bello, F., & Medana, C. (2022). Release of Selected Non-Intentionally Added Substances (NIAS) from PET Food Contact Materials: A New Online SPE-UHPLC-MS/MS Multiresidue Method. Separations, 9(8), 188. https://doi.org/10.3390/separations9080188








