Abstract
A rapid and simplified sample preparation method was developed for the simultaneous determination of 26 beta-agonists in swine muscle using a multi-functional filter (MFF) based on quick, easy, cheap, effective, rugged, and safe methods (QuEChERS). MFF integrated the cleanup and filter procedures, thereby significantly improving the efficiency of sample preparation compared with traditional solid-phase extraction. The sample was processed via enzymatic hydrolysis, purified with the optimized MFF containing 150 mg magnesium sulfate, 50 mg PSA, and 50 mg C18, and then analyzed using ultra-high performance liquid chromatography-tandem mass spectrometry. All procedures can be completed in 6.5 h. Good linearity (R2 > 0.99) was detected in all analytes. The recoveries ranged from 71.2% to 118.6%, with relative standard deviations (RSDs) of less than 18.37% in all spiked concentrations. The limits of detection (LOD) and the limits of quantitation (LOQ) were 0.01–0.10 and 0.10–0.50 μg/kg, respectively. The decision limit (CCα) and detection capacity (CCβ) values fluctuated in the range of 3.44–25.71 and 6.38–51.21 μg/kg, respectively. This method is a good alternative for detecting beta-agonist residues in swine muscle and can be successfully applied to the national risk monitoring of agro-product quality and safety in China.
1. Introduction
Beta-agonists are a group of phenylethanolamine compounds used as bronchodilatory and tocolytic agents for therapeutic purposes. However, over the past few decades, clenbuterol and other beta-agonists have been illegally added to animal feeds to improve growth rate and reduce the carcass fat of farm animals. This addition leads to the generation of residues in animal meat and giblets, such as liver [1], thereby resulting in adverse effects for consumers, such as muscle twitching, heart disease, central nervous diseases, or even death [2,3,4].
Regulations were formulated for the strict administration of beta-agonists in many countries, including China and European Union (EU) countries. For example, beta-agonists have been banned for promoting animal growth since March 1997 in China. According to Announcement No. 235 of the Ministry of Agriculture, beta-agonists such as clenbuterol, ractopamine, salbutamol, and cimaterol should not be detected in animal foods [5]. Additionally, clenbuterol has been banned as a growth promoter in EU countries since 1988. Ractopamine and other beta-agonists are also banned according to the Council Directive 1996/22/EC [6]. However, the use of some beta-agonists such as rctopamine as feed additives, is legal in some countries, such as the USA.
Among the various meat matrices that may contain beta-agonist residue, swine muscle is the most widely-eaten meat, accounting for over 36% of the world’s meat intake. Therefore, monitoring the beta-agonist residues in muscle is essential to support the enforcement of these regulations, which is also significant to international trade. The illegal use of beta-agonists still exists in some countries and districts. For example, in Portugal, the consumption of lamb and bovine meat containing clenbuterol residues caused toxicity to 50 people, who showed symptoms such as gross tremors in the extremities, tachycardia, nausea, headaches, and dizziness [7]. Furthermore, the Ministry of Agriculture and Rural Affairs of PR China has monitored the beta-agonist residues in swine muscle samples collected from all over the country for more than 10 consecutive years to ensure food safety. The qualified rates for beta-agonist residues in China remained lower than the satisfactory level, which is higher than 98%. In addition, many research institutions and universities in China analyzed beta-agonist residues in swine muscle samples collected from local supermarkets, traditional markets, and slaughterhouses [8,9,10,11] and confirmed that clenbuterol, salbutamol, and ractopamine were detected in a few meat samples. Therefore, the strict monitoring and control of beta-agonist residues are still required to meet the regulations in different countries.
Various analytical methods were developed for detecting beta-agonist residue in different food matrices. These methods included the enzyme-linked immunosorbent assay (ELISA) [12], lateral flow test strips [13], high-performance liquid chromatography coupled with UV or electrospray ionization mass spectrometry detectors (HPLC-UV, HPLC-ESI-MS) [14,15], gas chromatography-mass spectrometry (GC-MS) [16,17,18], and liquid chromatography-tandem mass spectrometry (LC-MS/MS) [19,20,21]. ELISA is simple, quick, and cheap and is mostly suitable for rapid screening purposes [22]. HPLC-UV has lower sensitivity, whereas GC-MS needs the derivative process and is only suitable for easy gasification solvents. In contrast, LC-MS/MS has many advantages for detecting and confirming multi beta-agonist residues in different food matrices [19,20,23]. Ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS) is one of the most effective methods because of the high resolution and rapid separation of UHPLC. Moreover, it has a shorter chromatographic run time with high accuracy compared with other methods.
Additionally, sample preparation has a significant influence on the beta-agonist determination. For LC-MS/MS, obtaining a cleaner extracted solution can avoid false-positive results effectively. Two principal sample preparation methodologies are used for detecting beta-agonists in animal-derived foods. Solid-phase extraction (SPE) with different classes of sorbents has become the principal method in recent decades. Mixed-mode SPE cartridges called mixed-mode cation exchangers (MCX) [2,24] are commonly used. The traditional hydrophilic–lipophilic balance [25] and a new type of SPE column called PRiME (process, robustness, improvements, matrix effects, ease of use) [26] are also used for cleanup. Moreover, the QuEChERS methodology introduced by Anastassiades et al. in 2003 [27] is a green chemical extraction and cleanup method for the detection of veterinary residue. Some QuEChERS methods use new materials synthesized in-house as cleanup sorbents to detect beta-agonists [21]. Others use traditional cleanup sorbents, including C18 sorbent (C18) and primary secondary amine (PSA). However, in some cases, these methods require the manual weighing of the sorbent, which may enlarge the sample preparation time. Furthermore, they were developed for the detection of a limited kind of beta-agonists in swine muscle [24,28,29].
Recently, a kind of multi-functional filter (MFF) developed by our laboratory was used for the cleanup of 58 pesticides and relevant metabolites in eggs [30]. By comparing the effect of three different mixtures of sorbents, MFF containing 150 mg magnesium sulfate, 50 mg PSA, and 50 mg C18 was selected as the optimal cleanup method. It saved pretreatment time and reduced matrix interference significantly. However, the amount and combination of sorbents still need to be optimized to make them more suitable for the detection of beta-agonists in swine muscle.
Above all, this study aims to develop a rapid method with MFFs to efficiently monitor beta-agonist residues in swine muscle. The method includes a time-saving and effective enzymatic hydrolysis procedure, a fast and straightforward one-step solid–liquid extraction with 0.1% formic acid in acetonitrile, and a d-SPE cleanup procedure based on MFF. All procedures were completed within 6.5 h, thereby shortening the sample preparation time by half compared with other methods [2,31]. The method was fully validated according to EU Commission Decision 2002/657/EC [32].
2. Materials and Methods
2.1. Materials and Chemical Reagents
All reagents and solvents were HPLC grade unless otherwise specified. Acetonitrile (MeCN), n-hexane, and MS-grade methanol were obtained from Merck (Darmstadt, Germany). Acetic acid (>99%) and ammonium formate (>99%) were purchased by Sigma-Aldrich (St. Louis, MO, USA). β-Glucuronidase/arylsulfatase (>100,000 units/mL) was procured from Helix Pomatia (Merck, Germany). Ultra-pure water was obtained using a Milli-Q purification apparatus (Millipore Direct-Q UV, Bedford, MA, USA). QuSEL QuEChERS AOAC extraction kits containing 6.0 g of anhydrous magnesium sulfate (MgSO4) and 1.5 g of sodium acetate were acquired from Alta Scientific Co., Ltd. (Tianjin, China). Cleanup was performed using a QuSEL MFF we developed and produced in collaboration with Alta Scientific Co., Ltd. (Tianjin, China). The MFF contained MgSO4, PSA, and/or C18, along with a 0.22 μm filter. The specific types and sorbents used were as follows: (1) MFF 3201 (150 mg MgSO4 and 50 mg PSA); (2) MFF 3202 (150 mg MgSO4, 50 mg PSA, and 50 mg C18); (3) MFF 3101 (150 mg MgSO4 and 25 mg PSA); (4) MFF 3102 (150 mg MgSO4, 25 mg PSA, and 25 mg C18).
The analytical standard solutions of 32 compounds included 26 beta-agonists (cimaterol, terbutaline, zilpaterol, salbutamol, cimbuterol, fenoterol, ritodrine, clencyclohexerol, clenbuterolhydroxymethyl, isoxsuprine, ractopamine, clenproperol, clorprenaline, formoterol, clenbuterol, metoprolol tartrate, bromchlorbuterol, bromobuterol, tulobuterol, mabuterol, bambuterol, clenpenterol, labetalol, clenhexerol, salmeterol, and penbutolol) and six internal standards (ISs) (clenbuterol-d9, ractopamine-d6, salbutamol-d3, fenoterol-d6, cimaterol-d7, and terbutaline-d9). These beta-agonists were also provided by Alta Scientific Co., Ltd. (Tianjin, China).
2.2. Standard Preparation
The standard stock solutions of 26 beta-agonists (1000 mg/L) and their isotopic ISs (1000 mg/L) were individually prepared with methanol and stored in the dark at −20 °C. The mixed working standard solutions (I) were obtained by diluting stock solutions with methanol at a 1.0 mg/L concentration. The working solution (II) of the ISs at a concentration of 1.0 mg/L was also prepared using the same method and stored in the dark at 4 °C.
2.3. Sample Preparation
The homogenized samples (2.00 ± 0.01 g) spiked 10 μL of ISs working solution (II) were enzymatically hydrolyzed with 8 mL of 0.2 M ammonium acetate buffer (pH 5.2) and 50 μL of β-glucuronidase/arylsulfatase at 55 °C in a shaking water bath for 6 h. Afterward, a grain of ceramic homogenizers, 8 mL of 0.1% acetic acid in MeCN, and extraction kits were successively added to each sample. Then, the samples were homogenized via a vortex for 30 s and centrifuged for 10 min at 10,000 rpm, and 4 °C. 2 mL of supernatant was mixed with 2 mL of n-hexane saturated with MeCN by vortex for 30 s and centrifuged for 2 min at 10,000 rpm and 4 °C. A quantity of 1 mL of MeCN extracts were drained and passed through MFF 3202 (allowing the liquid to drip out). Finally, they were dried with nitrogen under 40 °C. The residue was redissolved using 0.25 mL of 50% methanol in water containing 0.1% formic acid and subjected to UHPLC-MS/MS analysis.
2.4. UHPLC-MS/MS Analysis
The UHPLC separations were carried out on a C18 Zorbax EclipsePlus RRHD (3.0 × 150 mm, 1.8 μm) column from Agilent Technologies. The mobile phase consisted of 0.1% volume ratio of formic acid and 5 mM ammonium acetate in water (eluent A) and 0.1% volume ratio of formic acid in acetonitrile (eluent B), and was supplied at a flow rate of 0.4 mL/min. The following gradient program was selected in order to accomplish the separations: 0.0 min, 5% (v/v) B; 0.5 min, 5% (v/v) B; 1.5 min, 15% (v/v) B; 5.0 min, 100% (v/v) B; 8.0 min, 100% (v/v) B; 8.1 min, 5% (v/v) B; and 10 min, 5% (v/v) B. Column temperature was set to 40 °C, and an injection volume of 2 μL was selected.
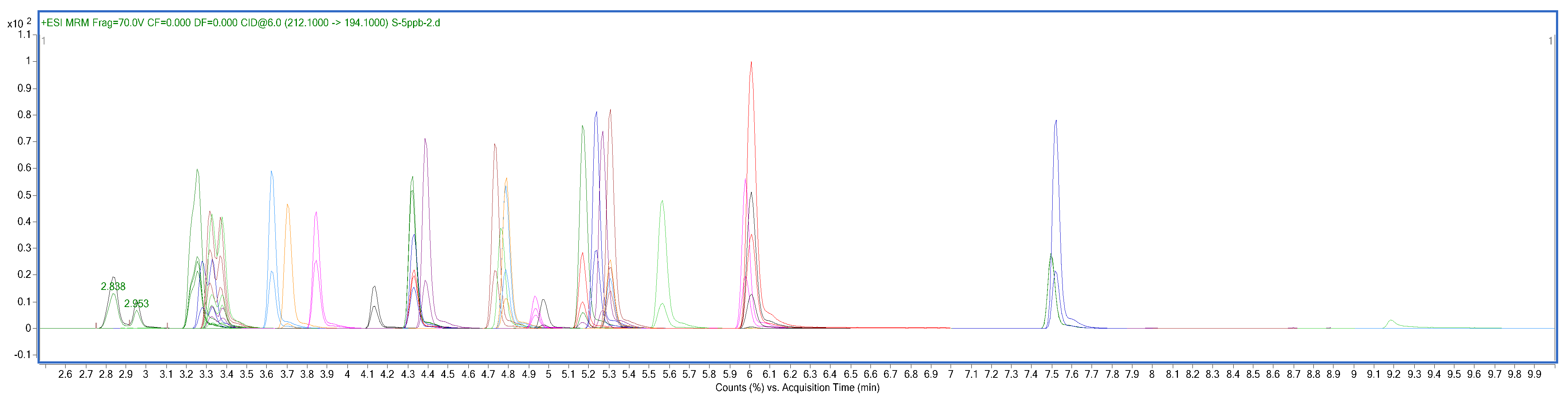
Furthermore, analyses were carried out under electrospray ion source positive conditions (ESI+). Ionization source parameters were established as follows: gas temperature, 250 °C; capillary voltage, 3.5 kV; nebulizing gas (nitrogen) and drying gas (nitrogen) pressure, 35 psi; and sheath gas temperature, 325 °C. Dynamic multiple reaction monitoring (dMRM) was selected as the acquisition mode. The protonated molecular ion ([M + H]+) was considered the precursor ion for all beta-agonists. The m/z of targeted analytes and their related MRM parameters are summarized in Table 1. A typical dMRM chromatographic of a mixture of 26 beta-agonists and ISs is shown in Figure 1, and relevant extracted ion chromatograms (EIC) of targeted analytes and ISs are illustrated in Figure S1.

Table 1.
Retention time and m/z of targeted analytes and their related MRM parameter.
Figure 1.
Total ion chromatogram (TIC) of the fortified swine muscle-based sample. Concentration levels: 5 μg/L of 26 beta-agonists and ISs.
2.5. Method Validation
The method was validated following the European Commission Decision 2002/657/EC, which has specific procedures for quantitative confirmatory methods. The following parameters were conducted: specificity, selectivity, linearity, precision, trueness, LOD, LOQ, CCα, and CCβ.
First of all, one precursor ion and two transition products were selected to reach four identification points required by Regulation (EU) 2002/657/EC for the quantification and confirmation of forbidden substances in food products derived from animals. The retention times between analytes and standard solution should be identified within the margin of ±0.5%.
Selectivity was confirmed based on the ratio between the transition products at the correct retention times corresponding to beta-agonists. Blank pork-based samples spiked at five different levels were injected into UHPLC-MS/MS. The mean calculated ion ratios for the solvent and matrix are shown in Table S1. All of the ratios were within the tolerance fixed by the EU criteria. Moreover, 20 blank samples derived from different origins (purchased from local markets) were analyzed using dMRM. The results are shown in Figure S2 and indicate that there was no obvious interference in the chromatograms of the blank samples.
A solvent calibration curve with seven or eight concentration levels (0.01, 0.05, 0.1, 0.2, 1, 5, 10, and 20 μg/L for terbutaline, clencyclohexerol, clorprenaline, and clenbuterol; 0.1, 0.2, 1, 2, 5, 10, 20, and 50 μg/L for fenoterol, ritodrine, clenbuterolhydroxymethyl, clenproperol, bromchlorbuterol, bambuterol, clenpenterol, labetalol, clenhexerol, salmeterol, penbutolol; 0.2, 0.5, 1, 5, 10, 20, and 50 μg/L for cimaterol, salbutamol, zilpaterol, cimbuterol, isoxsuprine, ractopamine, formoterol, metoprolol tartrate, bromobuterol, tulobuterol, mabuterol) with 5 μg/L IS was chosen to evaluate the linearity of the method. Each was treated and analyzed using UHPLC-MS/MS in triplicate. When the coefficient of determination (R2) is greater than 0.99, the linearity of the calibration curves can be considered to meet the guidelines of regulatory agencies.
Trueness was evaluated by both the certified reference material (CRM) purchased from the National Institute of Metrolog China (https://www.ncrm.org.cn/Web/Material/Components?autoID=13614&pageIndex=1 accessed on 12 November 2020) and recovery experiments at low, medium, and high concentration levels. Additionally, it is considered to be accepted when trueness or recoveries are in the range of 70%~120%. Six replicates of each sample were tested following the test method.
Intra-day and inter-day precision were compared in terms of investigating repeatability and reproducibility. They were evaluated using blank sample fortified analytes at three to seven levels and should meet the criteria of RSD ≤ 20%. Six swine muscle samples per concentration were analyzed using UHPLC-MS/MS over three separate days.
LOD was calculated using the IUPAC approach, in which the signals/noises of three were calculated based on the peak area of quantifiers, while LOQ was calculated as spiked concentrations with signals/noises of 10 under the acceptable accuracy and precision.
Furthermore, we calculated CCα and CCβ in combination with Regulation 2002/657/EC [32] and the guidance paper [33]. The blank samples were spiked at and above the lowest possible level. Since beta-agonists are group A substances with no maximum residue limits (MRLs) [34], LOQ was used as the first spiking level. CCα and CCβ were determined using the following equation according to Jedziniak et al. [35]:
where SDLOQ was the standard deviation at the LOQ level.
CCα = LOQ + 1.64 × SDLOQ and CCβ = CCα + 1.64 × SDLOQ
The matrix effects (MEs) were evaluated by comparing the signals of the pure solution standard (Ssolvent) and the matrix-matched standards (Smatrix) at the same concentrations using the following equation:
ME% = (Smatrix/Ssolvent) × 100%.
3. Results and Discussion
3.1. Optimization of the Enzymatic Hydrolysis Process
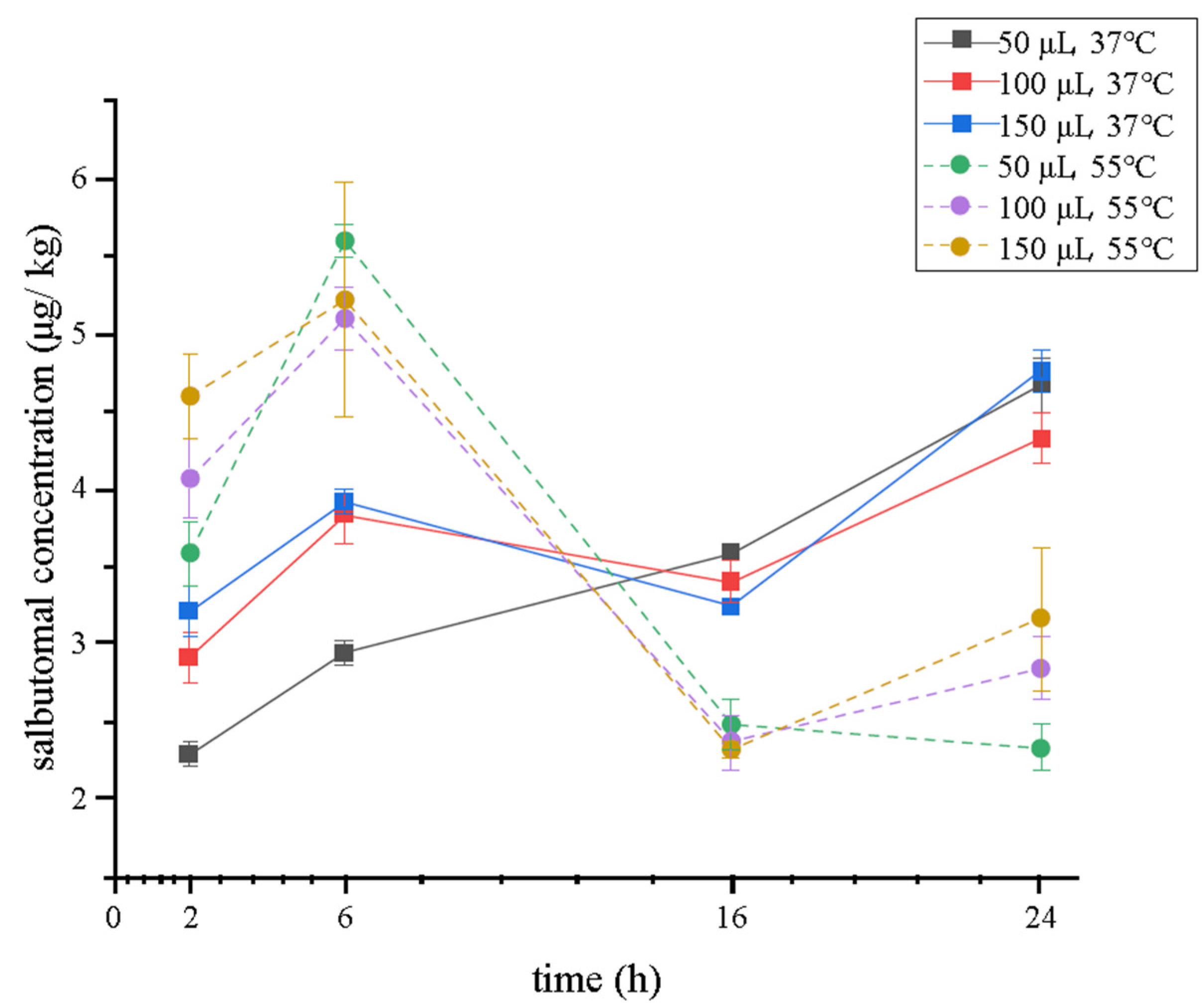
Enzymatic hydrolysis procedures are essential to release the conjunction between beta-agonists and glucose acid or sulfate [36] before the sample preparation. They are always operated at 37 °C for 16 h, which can be quite time-consuming and ineffective. Moreover, in previous studies, nearly all these procedures were optimized using blank matrix spiked standard solution other than using positive samples. It can be tough to keep beta-agonists in the same state within real samples. Therefore, in this study, we used a positive sample containing 5.26 μg/kg of salbutamol, which was detected using a traditional validated method [37] as a matrix to obtain an optimal enzymatic hydrolysis result. Furthermore, the concentration of enzymes, temperature, and time can affect this process. So, 50, 100, and 150 μL of β-glucuronidase/arylsulfatase were chosen to obtain the maximum catalytic activity under 37 °C and 55 °C, respectively. The concentration of salbutamol was monitored for 2, 6, 16, and 24 h. As shown in Figure 2, the hydrolysis temperature had a visible effect on salbutamol, and its concentration was higher at 55 °C from 2 to approximately 12 h and peaked at 5.60 μg/mL at 6 h. This result was in accordance with that of another report, which showed that the β-glucuronidase had higher efficiency when the samples were incubated at 55 °C than at 37 °C [21]. Between 12 and 24 h, the concentration showed the opposite trend. However, the samples (37 °C) did not exceed the highest concentration at 55 °C. The reason may be that some enzyme activity was inhibited at high temperatures as the time lasted for more than 12 h. Therefore, 55 °C was chosen for this experiment. Time and enzyme concentration can also affect the catalytic activity. When enzymatic hydrolysis lasted for 6 h, 50 μL of enzyme obtained the optimum, a practical volume to deconjugate salbutamol-glucuronides in the tested samples (salbutamol increased by 9.83% and 10.68% in comparison with 100 and 150 μL, respectively). Therefore, the enzyme hydrolysis condition was finally chosen to be 50 μL, 55 °C, and 6 h. This alternative method is better than the traditional one [37] because the cost of β-glucuronidase/arylsulfatase is reduced by half, and the time of the digestion process is shortened from 16 h to 6 h.
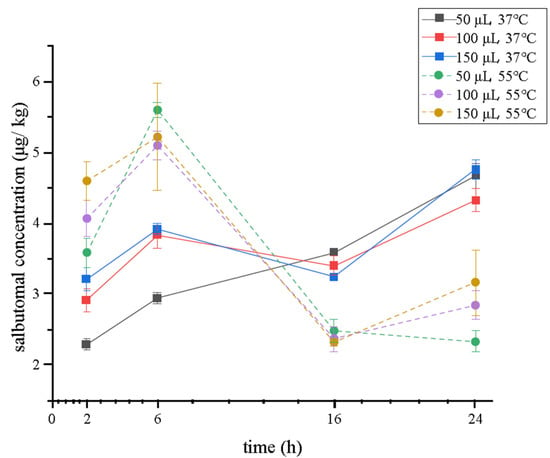
Figure 2.
Salbutamol concentration when a different volume of β-glucuronidase/arylsulfatase was used under 37 °C or 55 °C (n = 3).
3.2. Optimization of Cleanup Method
Cleanup is a crucial step in multi-residue drug analyses because of the high rate of fat and protein. A practical and straightforward purified method is needed to remove the interfering substance in the swine muscle. We investigated four different mixtures of sorbents based on MFFs and compared their cleanup effects.
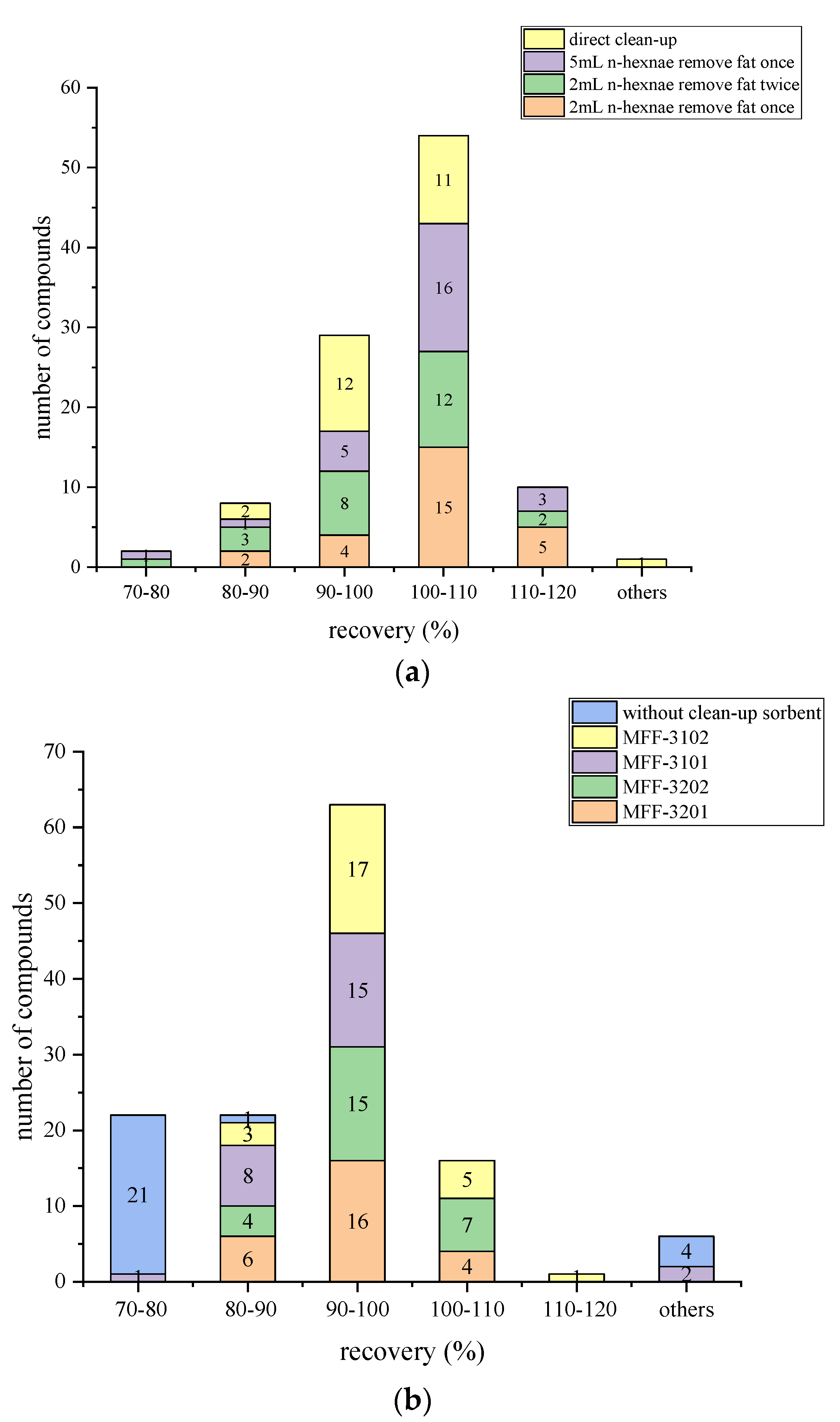
The addition of n-hexane is an effective way to remove fat and improve the recovery of compounds. Thus, before 1 mL of the extracted solution was directly passed through MFFs, 2 mL supernatant mixed with different volumes of n-hexane solution were tested. The results in Figure 3a showed that when n-hexane was used, all recoveries were within the range of 71.2–120.0%. Meanwhile, more satisfied and stable recoveries (81.0–117.4%) than others were found when using 2 mL of n-hexane to remove fat once. For clencyclohexerol, using n-hexane to remove fat caused a significant drop in recovery. The recovery decreased to 71.3% when 2 mL of n-hexane was used to remove fat twice compared with using 2 mL of n-hexane to remove fat once (84.0%), and 5 mL of n-hexane was used to remove fat once (77.5%). Thus, 2 mL of n-hexane was chosen to remove the fat before cleaning up with MFFs.
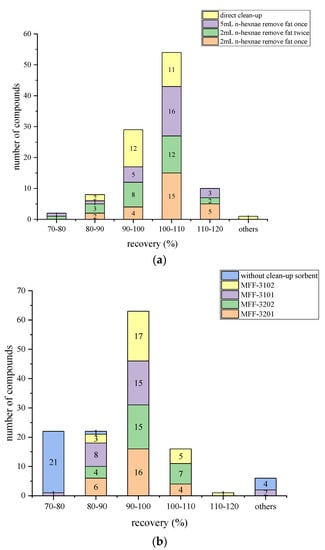
Figure 3.
Comparison of the number of compounds with various recoveries using different fat removal methods (a) and purification materials (b).
As shown in Figure 3b, the use of sorbents can increase the recoveries of compounds dramatically. When 150 mg MgSO4 combined with 25 mg PSA (MFF 3101) was used, the number of compounds with recoveries ranging from 70% to 120% rose to 24. As the amount of PSA increased to 50 mg (MFF 3201), all recoveries varied in the range of 70–120%. Moreover, when C18 was added, the results were more stable and nearer to 100%. When MgSO4 combined with 50 mg PSA and 50 mg C18 (MFF 3202) was used, the recoveries of 26 beta-agonists ranged from 86.6% to 104.3%. Furthermore, the time of using MFFs (<1 min) can shorten the cleanup time by half compared to the traditional QuEChERS method (manual combination of cleanup sorbents or commercial cleanup tube). Therefore, purification with MFF 3202 (150 mg MgSO4, 50 mg PSA, and 50 mg C18) was ultimately selected as the optimal cleanup method. C18 is a reverse-phase sorbent that can remove non-polar or moderately polar compounds such as grease because of van der Waals and dispersion interactions. PSA can retain polar compounds and anions and exhibit a negative charge at pH 8 or lower as it acts as both a normal phase sorbent and weak anionic exchanger. PSA can also remove carbohydrate impurities, pigments, and organic acids effectively [38]. MgSO4 can further reduce the water in samples.
3.3. Selection of Redissolved Solvent
Redissolved solvents can affect the peak shape and response of target analytes in UHPLC-MS/MS methods. Thus, a series of redissolved solvents mixed with water was tested for optimization. The results indicated that the recoveries ranged from 87.0% to 209.3% when 100% of MeCN was used. Meanwhile, as the proportion of water increased, the number of compounds with recoveries in the range of 70–120% increased gradually. When MeCN/water (50/50, v/v) was used, all recoveries ranged from 83.4% to 119.4%. As presented in Figure S3, higher responses were observed for salbutamol and terbutaline when 0.1% volume of formic acid was added. Thus, MeCN/water (50/50, v/v) containing 0.1% volume of formic acid (FA) was used as a redissolved solvent for higher sensitivity to detect beta-agonists.
3.4. Matrix Effect
Matrix effect (ME) was used to estimate the effects caused by co-eluted components in mass spectrum methods. According to the method used by Ferrer-Amate H et al. [39], ME values in the deviation of 10–20% could be considered “soft”, and ME ranging in the region of 0–10% can be considered “negligible”. In this study, instead of a specific concentration level, matrix-matched and standard solution calibration curves were used for calculation. The results are summarized in Table 2 and indicate the ME of this method showed soft signal suppression for three compounds (terbutaline, zilpaterol, and formoterol) with ME values in the deviation of 11.3–19%. Additionally, the ME of the other 23 beta-agonists can be neglected because all slope ratios (93.4–99.3%) were within the −10% of 100%. This result may be attributed to the use of ISs and effective cleanup sorbents, both of which reduce the effect of ME.

Table 2.
Statistical and performance characteristics of the proposed UHPLC–MS/MS method for beta-agonists analysis in pork samples according to the applied sample treatment.
3.5. Method Validation
Seven-point solvent linearity was obtained to evaluate the linearity of the method. As shown in Table 2, in all cases, linearity with a coefficient of determination (R2) exceeding 0.9978 was achieved. This result indicated that all targeted compounds had a good linear range.
Recovery was evaluated by spiking blank muscle samples with the appropriate amount of sipking solution (26 beta-agonists) on a series of concentration levels (0.1, 0.2, 1, 2, 5, 10, and 20 μg/kg) on three separate days before extraction. Since beta-agonists were group A substances, as described above, no MRLs were built for them. Therefore, considering the comprehensive factors, the individual screening and confirming requirements, the concentration detected in real samples, and the expected sensitivity limitation of the method, three or seven specific levels covering low to high concentrations were chosen. As shown in Table 3, recoveries ranged from 71.2% to 118.6%, with relative standard deviations (RSDs) < 20% for both intra- and inter-day precisions. This finding indicated that the precision and repeatability of this method are satisfactory.

Table 3.
Recovery, intra-day, inter-day precisions for beta-agonists.
Additionally, CRM containing clenbuterol (3.59 ± 0.34 μg/kg) was used to validate the developed method. By analyzing six replicates of CRM, we calculated the trueness values using the equitation described in the EU Commission Decision 2002/657/EC. All values were within the range of 102.3% to 108.5%, with RSDs in the range of 20%, which indicated that this method could detect clenbuterol effectively.
The LOD and LOQ values are shown in Table 2, ranging from 0.01 μg/kg to 0.10 μg/kg and 0.10 μg/kg to 0.50 μg/kg, respectively. In total, 60% of compounds showed lower LOD, and 30% of compounds had lower LOQ than those reported by others [2,21,31]. The detailed performance of our method for beta-agonist determination over existing methods is shown in Table 4. Particularly, the LOQ value for fenoterol dropped dramatically from 2.50 μg/kg to 0.20 μg/kg, indicating this method has good sensitivity for monitoring beta-agonists.

Table 4.
Performance of MFF-based method for beta-agonists determination over existing methods.
In the alternative method we used for calculating CCα and CCβ, the limit at and above CCα indicated an error probability of α (5%), thereby indicating that the sample was non-compliant. CCβ represented the lowest concentration at which the method was able to detect truly contaminated samples with a statistical certainty of 1-β (95%) [33]. No MRLs were set for the beta-agonists as they are non-permitted analytes. This alternative method was confirmed to be more time-saving and easier to operate. The results are summarized in Table 2. The CCα and CCβ values all varied within the ranges of 3.44–25.71 and 6.38–51.21 μg/kg, respectively, indicating that the developed method had 95% statistical certainty for successfully detecting beta-agonists in swine muscle.
3.6. Application to Actual Samples
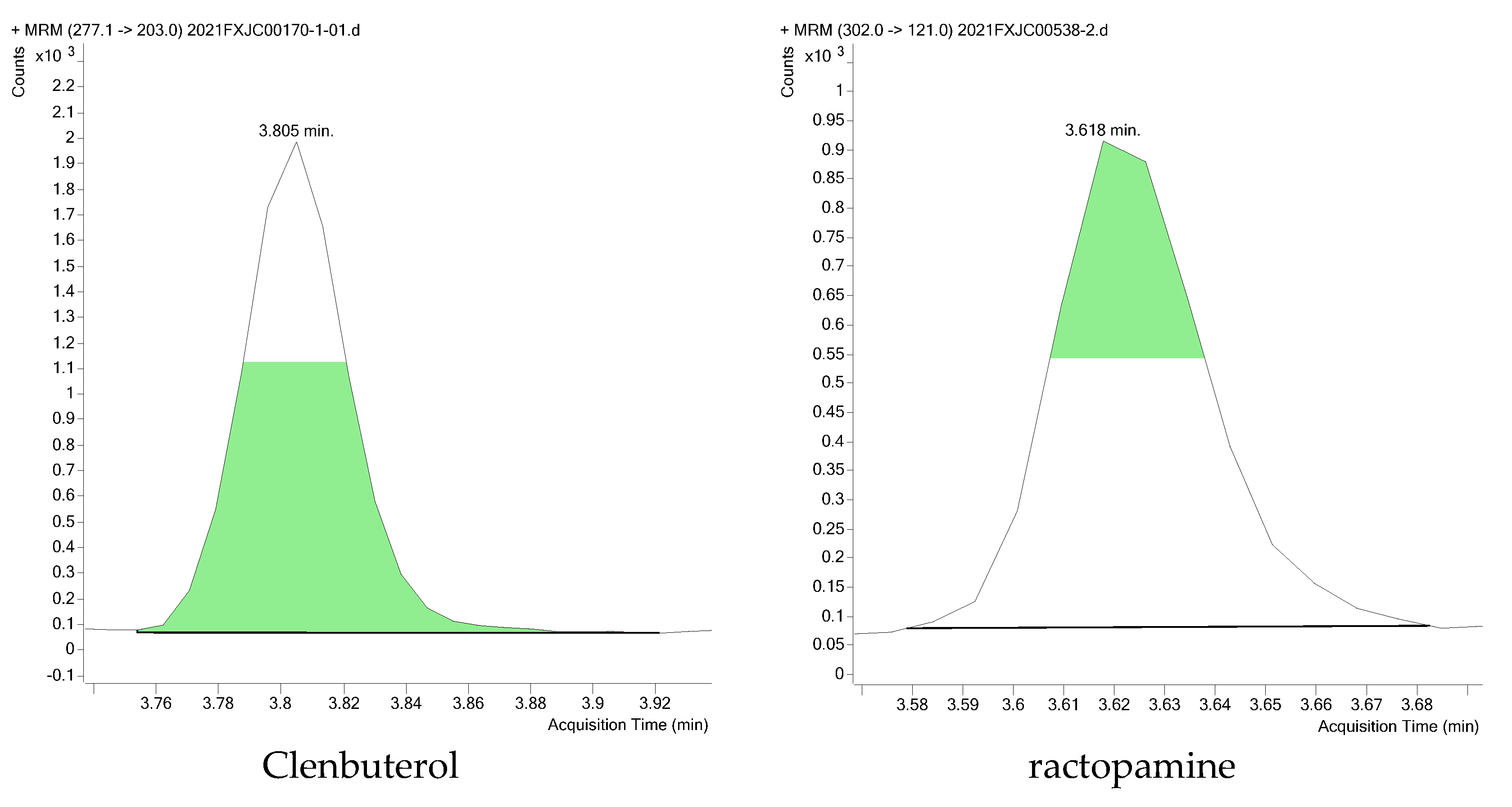
A total of 200 swine muscle samples were collected from 10 different markets in China and tested according to the national risk monitoring plan for agro-product quality and safety to demonstrate the applicability of the method. The recoveries of the quality control sample spiked at 1.0 μg/kg were in the range of 81.6–117.2%, with RSD < 19.35%. Fortunately, only two beta-agonists were detected in two samples, including clenbuterol and ractopamine, at concentrations of 0.94 μg/kg and 0.17 μg/kg, respectively. They all exceeded the LOQs set by our method, and their related Extracted ion chromatogram (EIC) is shown in Figure 4. The application to actual samples proved that the method has good practicality.
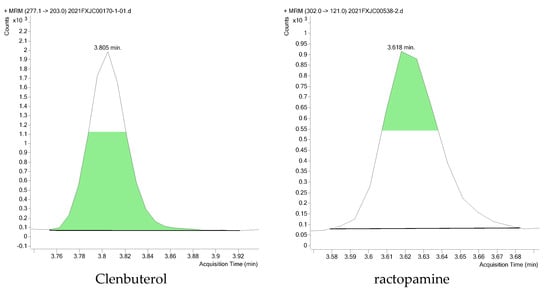
Figure 4.
Extracted ion chromatogram (EIC) of clenbuterol and ractopamine in two actual samples.
4. Conclusions
A fast and simplified sample preparation method using MFF based on QuEChERS was developed to analyze 26 beta-agonists in swine muscle via UHPLC-MS/MS. The enzymatic hydrolysis procedure was optimized using β-glucuronidase/arylsulfatase enzymolysis at 55 °C for 6 h. Extraction was performed using 0.1% acetic acid in MeCN solution, and anhydrous MgSO4 and sodium acetate were used for salting out. After repartitioning with n-hexane, the extracted solution was directly cleaned up with MFF 3202, which obviously shortened the sample preparation time compared with the traditional QuEChERS and SPE method. The analytical method was validated using CRM and based on Regulation 2002/65/EC. The results showed that the developed method had good sensitivity, precision, and accuracy for 26 beta-agonists and can be applied for effective monitoring of beta-agonist residue in swine muscle.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9050121/s1, Figure S1: Extracted ion chromatogram (EIC) of 26 beta-agonists and ISs. Concentration levels: 5 μg/L of 26 beta-agonists and ISs; Figure S2: Chromatograms of 20 blank samples derived from different origins; Figure S3: Chromatograms of Salbutamol and Terbutaline extract from swine samples by using different redissoved solvent; Table S1: Ion ratios of two transition products in solvent standard and fortified matrix for 26 beta-agonists.
Author Contributions
Methodology, L.Z.; validation, Q.J. and G.L.; formal analysis, L.Z. and G.L.; data curation, Q.J.; writing—original draft preparation, L.Z.; writing—review and editing, Q.J. and J.Q.; visualization, L.Z.; supervision, Y.Q. and J.Q.; funding acquisition, Y.Q. and J.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Scientific and Technical Innovation Project of the Chinese Academy of Agricultural Sciences] grant number [CAAS-ASTIP-IQSTAP] and [Coordinated Research Project from the International Atomic Energy Agency] grant number [D52041].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data shown in this study are contained within the article.
Acknowledgments
The authors are thankful to the Chinese Academy of Agricultural Sciences, and the reviewers for their comments and suggestions to the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martinez-Navarro, J.F. Food poisoning related to consumption of illicit beta-agonist in liver. Lancet 1990, 336, 1311. [Google Scholar] [CrossRef]
- Guo, P.; Wan, J.; Zhan, C.; Zhu, C.; Jiang, W.; Ke, Y.; Ding, S.; Wang, D. A simplified sample pretreatment for the rapid determination of 22 beta-agonist residues in swine muscle and liver tissues by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1096, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Miao, H.; Zhao, Y.; Chen, H.; Wu, Y. Simultaneous Detection of Residues of 25 beta(2)-Agonists and 23 beta-Blockers in Animal Foods by High-Performance Liquid Chromatography Coupled with Linear Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 1898–1905. [Google Scholar]
- Shao, B.; Jia, X.; Zhang, J.; Meng, J.; Wu, Y.; Duan, H.; Tu, X. Multi-residual analysis of 16 β-agonists in pig liver, kidney and muscle by ultra performance liquid chromatography tandem mass spectrometry. Food Chem. 2009, 114, 1115–1121. [Google Scholar] [CrossRef]
- The Ministry of Agriculture, PR China. Maximum Residue Limits of Veterinary Drugs in Animal Food; Announcement No. 235; The Ministry of Agriculture: Beijing, China, 2002.
- Directive, E. Council Directive 96/22EC of 29 April 1996 Concerning the Prohibition on the Use in Stockfarming of Certain Substances Having a Hormonal Other Thyrostatic Action and of Beta-Agonist, and Repealing Directives 81/602. Off. J. Eur. Union 1996, 125, 3–9. Available online: https://eur-lex.europa.eu/eli/dir/1996/22/oj (accessed on 21 April 2022).
- Barbosa, J.; Cruz, C.; Martins, J.; Silva, J.M.; Neves, C.; Alves, C.; Ramos, F.; da Silveira, M.I.N. Food poisoning by clenbuterol in Portugal. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2005, 22, 563–566. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, D.; Wu, K.; Yang, H.; Du, H.; Zhao, K.; Li, J.; Deng, A. Development of a sensitive monoclonal antibody-based ELISA for the determination of a beta-adrenergic agonist brombuterol in swine meat, liver and feed samples. Anal. Methods 2016, 8, 6941–6948. [Google Scholar] [CrossRef]
- Liu, C.; Wu, X.-H.; Xu, W.-D.; Chai, Y.-F. LC-MS/MS determination of 15 beta-agonists in animal borne food. Chin. J. Pharm. Anal. 2008, 28, 2085–2089. [Google Scholar]
- Li, H.; Zhang, J.; Song, H.; Gao, W. HPLC—TMS determination of 6 kinds of beta—agonist residues in muscle tissue. Chin. J. Pharm. Anal. 2011, 31, 2273–2277. [Google Scholar]
- Xu, C.; Gao, H.; Pan, N.; Jiang, M.; Huang, Y.; Zhu, K.; Gong, P.; Lv, S. Clenbuterol, salbutamol, and ractopamine in fresh meat products in Jilin province, China. Int. J. Food Prop. 2019, 22, 1183–1194. [Google Scholar] [CrossRef]
- Wang, X.; Liufu, T.; Beloglazova, N.V.; Luo, P.; Qu, J.; Jiang, W. Development of a Competitive Indirect Enzyme-Linked Immunosorbent Assay for Screening Phenylethanolamine A Residues in Swine muscle Samples. Food Anal. Methods 2016, 9, 3099–3106. [Google Scholar] [CrossRef]
- Ngom, B.; Guo, Y.; Wang, X.; Bi, D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: A review. Anal. Bioanal. Chem. 2010, 397, 1113–1135. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Saleem, K.; Gaitonde, V.D.; Aboul-Enein, H.Y.; Hussain, I. Chiral Separations of Some beta-Adrenergic Agonists and Antagonists on AmyCoat Column by HPLC. Chirality 2010, 22, 24–28. [Google Scholar] [CrossRef]
- Garcia, P.; Paris, A.C.; Gil, J.; Popot, M.A.; Bonnaire, Y. Analysis of beta-agonists by HPLC/ESI-MSn in horse doping control. Biomed. Chromatogr. 2011, 25, 147–154. [Google Scholar] [CrossRef]
- VanVyncht, G.; Preece, S.; Gaspar, P.; MaghuinRogister, G.; DePauw, E. Gas and liquid chromatography coupled to tandem mass spectrometry for the multiresidue analysis of beta-agonists in biological matrices. J. Chromatogr. A 1996, 750, 43–49. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P.; Kwiatkowski, M.; Migowska, N.; Kumirska, J. Determination of beta-blockers and beta-agonists using gas chromatography and gas chromatography-mass spectrometry—A comparative study of the derivatization step. J. Chromatogr. A 2011, 1218, 8110–8122. [Google Scholar] [CrossRef]
- Karamolegou, F.; Dasenaki, M.; Belessi, V.; Georgakilas, V.; Thomaidis, N. Multi-Residue Determination of 7 beta-Agonists in Liver and Meat Using Gas Chromatography-Mass Spectrometry. Food Anal. Methods 2018, 11, 2925–2942. [Google Scholar] [CrossRef]
- Wang, L.; Pu, R.-C.; Wang, X.-X.; Luo, C.-Y.; Zhang, L.-C.; Zhang, X.-S. Multiresidue Determination of beta(2)-Agonists Including Phenylethanolamine A in Animal-Derived Food by Ultra-High Performance Liquid Chromatography/Tandem Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 925–931. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.-P.; Lee, Y.-L.; Hung, C.-Y.; Huang, W.-J.; Lin, S.-C. Determination of multiresidue analysis of beta-agonists in muscle and viscera using liquid chromatograph/tandem mass spectrometry with Quick, Easy, Cheap, Effective, Rugged, and Safe methodologies. J. Food Drug Anal. 2017, 25, 275–284. [Google Scholar] [CrossRef]
- Xiong, L.; Gao, Y.-Q.; Li, W.-H.; Yang, X.-L.; Shimo, S.P. Simple and sensitive monitoring of beta(2)-agonist residues in meat by liquid chromatography-tandem mass spectrometry using a QuEChERS with preconcentration as the sample treatment. Meat Sci. 2015, 105, 96–107. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Jiang, W.; Zhang, S.; Shen, J.; Wen, K.; Wang, Z. Development and Application of a Gel-Based Immunoassay for the Rapid Screening of Salbutamol and Ractopamine Residues in Swine muscle. J. Agric. Food Chem. 2015, 63, 10556–10561. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Gao, Y.-Q.; Li, W.-H.; Guo, T.-F.; Yang, X.-L. A method for multiple identification of four beta(2)-Agonists in goat muscle and beef muscle meats using LC-MS/MS based on deproteinization by adjusting pH and SPE for sample cleanup. Food Sci. Biotechnol. 2015, 24, 1629–1635. [Google Scholar] [CrossRef]
- Valese, A.C.; Oliveira, G.A.P.; Kleemann, C.R.; Molognoni, L.; Daguer, H. A QuEChERS/LC-MS method for the analysis of ractopamine in swine muscle. J. Food Compos. Anal. 2016, 47, 38–44. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.-L.; Yang, T.; Zhang, Y.; Huang-Fu, W.-G. Simultaneous determination of clenbuterol, salbutamol and ractopamine in milk by reversed-phase liquid chromatography tandem mass spectrometry with isotope dilution. J. Chromatogr. A 2010, 1217, 7873–7877. [Google Scholar] [CrossRef]
- Castilla-Fernandez, D.; Moreno-Gonzalez, D.; Beneito-Cambra, M.; Molina-Diaz, A. Critical assessment of two sample treatment methods for multiresidue determination of veterinary drugs in milk by UHPLC-MS/MS. Anal. Bioanal. Chem. 2019, 411, 1433–1442. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. Aoac Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Mastrianni, K.R.; Metavarayuth, K.; Brewer, W.E.; Wang, Q. Analysis of 10 beta-agonists in swine muscle meat using automated dispersive pipette extraction and LC-MS/MS. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1084, 64–68. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Su, Y.-Q.; Ding, X.-M.; Xia, W.-S.; Liu, H.-M.; Zhang, Y.-B. Single-Step Multiresidue Determination of beta-Lactam Antibiotics and beta-Agonists in Porcine Muscle by Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 2185–2193. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Jia, Q.; Zhang, L.; Zhang, W.; Mu, P.; Jia, Y.; Qian, Y.; Qiu, J. Simultaneous determination of 58 pesticides and relevant metabolites in eggs with a multi-functional filter by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1593, 81–90. [Google Scholar] [CrossRef]
- Gressler, V.; Feddern, V.; Peixoto, J.d.O.; Ledur, M.C.; Costa, O.A.D.; Mello Monteiro de Lima, G.J. Application of Enzyme Digestion and Deconjugation Followed by Quick, Easy, Cheap, Effective, Rugged, Safe Extraction and Liquid Chromatography-Tandem Mass Spectrometry Methodology To Determine Ractopamine Residue in Swine muscle. J. Food Prot. 2018, 81, 1258–1263. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 2002/657/EC of 12 August 2002. Off. J. Eur. Communities 2002, 50, 8–36. [Google Scholar]
- European Union Reference Laboratories. CRLs View on State of the Art Analytical Methods for National Residue Control Plans. CRL Guidance Paper. December 2007, pp. 1–8. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/07_Untersuchungen/EURL_Empfehlungen_Konzentrationsauswahl_Methodenvalierungen_EN.html?nn=11011448 (accessed on 21 April 2022).
- de Brabander, H.F.; Noppe, H.; Verheyden, K.; Bussche, J.V.; Wille, K.; Okerman, L.; Vanhaecke, L.; Reybroeck, W.; Ooghe, S.; Croubels, S. Residue analysis: Future trends from a historical perspective. J. Chromatogr. A 2009, 1216, 7964–7976. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Morante-Zarcero, S.; Perez-Quintanilla, D.; Sierra, I. Application of a hybrid ordered mesoporous silica as sorbent for solid-phase multi-residue extraction of veterinary drugs in meat by ultra-high-performance liquid chromatography coupled to ion-trap tandem mass spectrometry. J. Chromatogr. A 2016, 1459, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Mauro, D.; Ciardullo, S.; Civitareale, C.; Fiori, M.; Pastorelli, A.A.; Stacchini, P.; Palleschi, G. Development and validation of a multi-residue method for determination of 18 beta-agonists in bovine urine by UPLC-MS/MS. Microchem. J. 2014, 115, 70–77. [Google Scholar] [CrossRef]
- The Ministry of Agriculture and Rural Affairs, PR China. Determination of Beta-Agonists Residues in Animal Derived Food by Liquid Chromatography-Tandem Mass Spectrometry; The Ministry of Agriculture: Beijing, China, 2008.
- Gabriela, I.; Ibarra, I.S.; Prisciliano, H.; Miranda, J.M.; Alberto, C. Dispersive solid phase extraction for the analysis of veterinary drugs applied to food samples: A review. Int. J. Anal. Chem. 2017, 2017, 1–16. [Google Scholar]
- Amate, C.F.; Unterluggauer, H.; Fischer, R.J.; ARFernández-Alba Masselter, S. Development and validation of a lc-ms/ms method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal. Bioanal. Chem. 2010, 397, 93–107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).