Speciation of Selenium in Selenium-Enriched Foods by High-Performance Liquid Chromatography-Inductively Coupled Plasma-Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Instruments
2.2. Chromatographic Separation
2.3. Instrument Conditions
2.4. Extraction of Selenium for Chromatographic Speciation
3. Results and Discussion
3.1. The Effect of Methanol in the Mobile Phase
3.2. Influence of Ionic Strength in Mobile Phase
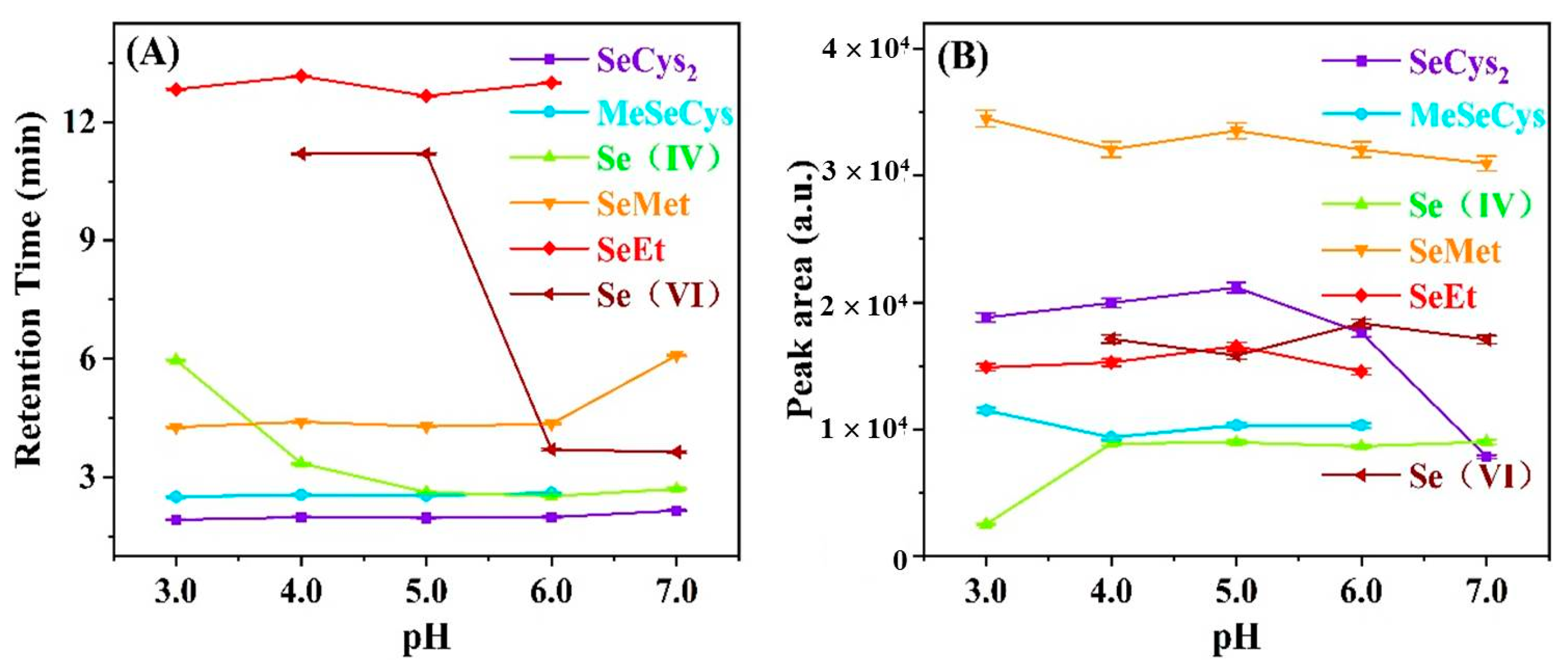
3.3. Effect of PH
3.4. Analytical Performance and Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xu, F.; Wang, S.; Xu, K.; Hou, X.; Wu, P. Gold Nanoparticle-Based Colorimetric Assay for Selenium Detection via Hydride Generation. Anal. Chem. 2017, 89, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Plummer, J.D.; Postnikoff, S.D.; Tyler, J.K.; Johnson, J.E. Selenium supplementation inhibits IGF-1 signaling and confers methionine restriction-like healthspan benefits to mice. eLife 2021, 10, e62483. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-X.; Meharg, A.; Li, G.; Chen, Z.; Yang, L.; Chen, S.-C.; Zhu, Y.-G. Distribution of soil selenium in China is potentially controlled by deposition and volatilization? Sci. Rep. 2016, 6, 20953. [Google Scholar] [CrossRef]
- Ye, M.; Li, J.; Yu, R.; Cong, X.; Huang, D.; Li, Y.; Chen, S.; Zhu, S. Selenium Speciation in Selenium-Enriched Plant Foods. Food Anal. Methods 2022, 15, 1377–1389. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, Y.-D.; Deng, J.; Wang, C.-L. Marketing Healthy Diets: The Impact of Health Consciousness on Chinese Consumers’ Food Choices. Sustainability 2022, 14, 2059. [Google Scholar] [CrossRef]
- Xu, F.; Hu, J.; Zhang, J.; Hou, X.; Jiang, X. Nanomaterials in speciation analysis of mercury, arsenic, selenium, and chromium by analytical atomic/molecular spectrometry. Appl. Spectrosc. Rev. 2018, 53, 333–348. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Liquid chromatographic analysis of selenium species in plant materials. TrAC Trends Anal. Chem. 2019, 111, 128–138. [Google Scholar] [CrossRef]
- Moreno-Martin, G.; Sanz-Landaluze, J.; León-González, M.E.; Madrid, Y. In vivo quantification of volatile organoselenium compounds released by bacteria exposed to selenium with HS-SPME-GC-MS. Effect of selenite and selenium nanoparticles. Talanta 2021, 224, 121907. [Google Scholar] [CrossRef] [PubMed]
- Grønbæk-Thorsen, F.; Hansen, R.H.; Østergaard, J.; Gammelgaard, B.; Møller, L.H. Analysis of selenium nanoparticles in human plasma by capillary electrophoresis hyphenated to inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 2247–2255. [Google Scholar] [CrossRef]
- Li, M.; Xia, H.; Luo, J.; Yang, X.; Li, H.; Liu, X.; Xu, F. Homogeneous catalysis for photochemical vapor generation for speciation of inorganic selenium by high performance liquid chromatography-atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2021, 36, 2210–2215. [Google Scholar] [CrossRef]
- Proch, J.; Niedzielski, P. Multi–mode Sample Introduction System (MSIS) as an interface in the hyphenated system 2 HPLC–MSIS–ICP–OES in simultaneous determination of metals and metalloids species. Anal. Chim. Acta 2021, 1147, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, Z.; Xu, F.; Geng, D.; Tang, X. MIL-125-NH2 catalyzed photochemical vapor generation coupled with HPLC-ICPMS for speciation analysis of selenium. Microchem. J. 2022, 174, 107053. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, X.; Zhao, Q.; Han, Y.; Zhan, T.; Li, Y.; Tang, C.; Zhang, J. Development and application of a HPLC-ICP-MS method to determine selenium speciation in muscle of pigs treated with different selenium supplements. Food Chem. 2020, 302, 125371. [Google Scholar] [CrossRef]
- Krata, A.A.; Wojciechowski, M.; Karasinski, J.; Bulska, E. Comparative study of high performance liquid chromatography species-specific and species-unspecific isotope dilution inductively coupled plasma mass spectrometry. A case study of selenomethionine and the origin of its oxidized form. Microchem. J. 2018, 143, 416–422. [Google Scholar] [CrossRef]
- Bolea-Fernandez, E.; Balcaen, L.; Resano, M.; Vanhaecke, F. Interference-free determination of ultra-trace concentrations of arsenic and selenium using methyl fluoride as a reaction gas in ICP–MS/MS. Anal. Bioanal. Chem. 2015, 407, 919–929. [Google Scholar] [CrossRef]
- Bamonti, L.; Theiner, S.; Rohr-Udilova, N.; Keppler, B.K.; Koellensperger, G. Accurate high throughput quantification of selenium in biological samples—The potential of combining isotope dilution ICP-tandem mass spectrometry with flow injection. J. Anal. At. Spectrom. 2016, 31, 2227–2232. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Li, X.; Gao, Y.; Wei, C.; Li, H.; Jiao, H. The compound-independent calibration of five selenium species in rice using ion-pairing reversed phase chromatography coupled to inductively coupled plasma tandem mass spectrometry. J. Chromatogr. A 2022, 1674, 463134. [Google Scholar] [CrossRef]
- Bolea-Fernandez, E.; Rua-Ibarz, A.; Resano, M.; Vanhaecke, F. To shift, or not to shift: Adequate selection of an internal standard in mass-shift approaches using tandem ICP-mass spectrometry (ICP-MS/MS). J. Anal. At. Spectrom. 2021, 36, 1135–1149. [Google Scholar] [CrossRef]
- Warburton, E.; Goenaga-Infante, H. Methane mixed plasma—improved sensitivity of inductively coupled plasma mass spectrometry detection for selenium speciation analysis of wheat-based food. J. Anal. At. Spectrom. 2007, 22, 370–376. [Google Scholar] [CrossRef]
- Grindlay, G.; Mora, J.; de Loos-Vollebregt, M.; Vanhaecke, F. A systematic study on the influence of carbon on the behavior of hard-to-ionize elements in inductively coupled plasma–mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2013, 86, 42–49. [Google Scholar] [CrossRef]
- Stadlober, M.; Sager, M.; Irgolic, K.J. Effects of selenate supplemented fertilisation on the selenium level of cereals—Identification and quantification of selenium compounds by HPLC–ICP–MS. Food Chem. 2001, 73, 357–366. [Google Scholar] [CrossRef]



| ICP-MS/MS | |
|---|---|
| Forward power | 1550 W |
| Carrier gas (Ar) flow rate | 1.0 L min−1 |
| Reaction gas (O2) flow rate | 0.3 mL min−1 |
| Isotopes monitored | 80Se (Q1), 80Se16O (Q3) |
| HPLC | |
| Column | Hamilton PRP-X100 (10 μm, 4.1 × 250 mm) |
| Mobile phase | 25 mM Sodium citrate-2% Methanol (pH = 4.0) |
| Flow rate | 1.0 mL min−1 |
| Column temperature | 30 °C |
| Injection volume | 25 μL |
| Component | Linear Range (ng mL−1) | R2 | LOD (ng mL−1) | LOQ (ng mL−1) |
|---|---|---|---|---|
| SeCys2 | 0.07–100 | 0.995 | 0.02 | 0.07 |
| MeSeCys | 0.07–100 | 0.998 | 0.03 | 0.11 |
| Se(IV) | 0.07–100 | 0.997 | 0.02 | 0.07 |
| SeMet | 0.35–500 | 0.999 | 0.05 | 0.17 |
| SeEt | 0.35–500 | 0.995 | 0.15 | 0.50 |
| Se(VI) | 0.14–200 | 0.998 | 0.04 | 0.14 |
| Sample | SeCys2 | MeSeCys | Se (IV) | SeMet | SeEt | Se (VI) |
|---|---|---|---|---|---|---|
| ChongYan iodine-enriched selenium salt | - | - | 17.9 | - | 11.9 | - |
| XinJiang Tianshan snow crystal salt | - | - | - | - | 17.2 | 71.96 |
| LuJing selenium-enriched sea salt | 4.09 | - | 8.57 | - | - | - |
| ZhongYan iodized table salt | 5.26 | 6.33 | 2.63 | - | - | - |
| JiYan selenium-enriched edible salt | 3.77 | - | 2.87 | - | - | - |
| Sample | SeCys2 | MeSeCys | Se (IV) | SeMet | SeEt | Se (VI) | Total Se | Extraction Rate (%) |
|---|---|---|---|---|---|---|---|---|
| ZiYang selenium-enriched green tea | 0.77 | 1.13 | 3.83 | 6.62 | - | 1.51 | 52.7 | 26.3 |
| EnShi selenium tea (fried green) | 0.66 | 0.67 | 1.95 | 4.18 | - | 0.40 | 32.6 | 24.1 |
| EnShi selenium tea (premium green tea) | 0.41 | 0.82 | - | 1.56 | - | 0.30 | 11.2 | 27.5 |
| EnShi alpine selenium tea | 0.45 | 1.27 | 1.62 | 3.56 | - | 1.45 | 34.4 | 24.3 |
| LiangPing alpine green tea | 0.34 | 0.65 | 0.47 | 1.53 | - | 1.00 | 22.9 | 17.4 |
| BoChuan EnShi selenium tea | 0.36 | - | - | 1.10 | - | 1.67 | 12.1 | 25.9 |
| XiKeXiKe EnShi selenium tea | 0.26 | 1.10 | 0.68 | 2.88 | - | 0.53 | 26.7 | 20.4 |
| JiYe selenium-enriched green tea | 0.38 | 0.77 | 0.99 | 1.67 | - | - | 14.8 | 26.1 |
| ZiYang green tea | 2.13 | 3.53 | 1.96 | 5.90 | - | 6.39 | 87.7 | 22.7 |
| WuHan selenium-enriched tea | 0.67 | - | 1.14 | 4.32 | - | 0.27 | 45.0 | 14.2 |
| Hubei EnShi selenium tea | 0.56 | 0.22 | 0.50 | 1.71 | - | - | 23.5 | 13.1 |
| LvWanjia selenium chrysanthemum | 4.56 | 0.14 | 7.90 | 2.05 | - | 10.52 | 108 | 23.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Chen, G.; Deng, X.; Cai, H.; Fu, X.; Xu, F.; Xiao, X.; Huo, Y.; Luo, J. Speciation of Selenium in Selenium-Enriched Foods by High-Performance Liquid Chromatography-Inductively Coupled Plasma-Tandem Mass Spectrometry. Separations 2022, 9, 242. https://doi.org/10.3390/separations9090242
Luo Y, Chen G, Deng X, Cai H, Fu X, Xu F, Xiao X, Huo Y, Luo J. Speciation of Selenium in Selenium-Enriched Foods by High-Performance Liquid Chromatography-Inductively Coupled Plasma-Tandem Mass Spectrometry. Separations. 2022; 9(9):242. https://doi.org/10.3390/separations9090242
Chicago/Turabian StyleLuo, Yue, Gang Chen, Xiuqing Deng, Hanqing Cai, Xueheng Fu, Fujian Xu, Xiaonian Xiao, Yumeng Huo, and Jin Luo. 2022. "Speciation of Selenium in Selenium-Enriched Foods by High-Performance Liquid Chromatography-Inductively Coupled Plasma-Tandem Mass Spectrometry" Separations 9, no. 9: 242. https://doi.org/10.3390/separations9090242
APA StyleLuo, Y., Chen, G., Deng, X., Cai, H., Fu, X., Xu, F., Xiao, X., Huo, Y., & Luo, J. (2022). Speciation of Selenium in Selenium-Enriched Foods by High-Performance Liquid Chromatography-Inductively Coupled Plasma-Tandem Mass Spectrometry. Separations, 9(9), 242. https://doi.org/10.3390/separations9090242






