Abstract
Azanza garckeana (F. Hoffm). Exell and Hillc. is an important food and medicinal plant that has been used in tropical Africa. Therefore, this study aimed to investigate the nutritional value of jakjak fruit using different analytical techniques. The obtained results have demonstrated that jakjak fruit is very rich in total soluble sugar, constituting about 48% of the dry weight. Moreover, the chromatographic analysis revealed that jakjak fruit contained a high amount of glucose, fructose, maltose, and ascorbic acid. Further, GC-MS analysis detected four compounds related to secondary metabolites. Some of these detected constituents have medicinal value. For example, phenol, 2,4-bis (1,1-dimethylethyl) has been reported to have many functions such as antioxidant activity, anticancer, antifungal, and antibacterial properties. Furthermore, the antioxidant potential of different concentrations of deionized water and methanolic extracts was estimated using 2,2-diphenyl-1-picrylhydrazyl (DPPH). The results showed that the scavenging activity of the DPPH radical was found to be raised with increasing concentrations of fruit extracts. The concentration (50%) of both methanol and deionized water gave the best inhibition percentage (91.7 and 84.4%), respectively. In contrast, the methanolic extract has shown significant results compared to deionized water. This study concluded that jakjak fruit is very rich in total soluble sugar and phenolic compounds, which can be used as a source of polysaccharides and antioxidants for the human diet as well as raw materials for downstream industries.
1. Introduction
Azanza garckeana (F. Hoffm). Exell and Hillc., locally known as Goron Tula and Jakjak, considering different local names in tropical Africa, belong to the Malvaceae family. Azanza garckeana fruit is an important plant for food and herbal medicine in Africa. The tree is found in Sudan, Botswana, Kenya, Nigeria, Malawi, Mozambique, Namibia, South Africa, Tanzania, Zambia, and Zimbabwe [1,2,3,4,5]. Jakjak is an important food and medicinal plant in tropical Africa and a product sold in local markets [5,6]. In addition, jakjak is a popular multipurpose fruit, particularly used during seasonal food shortages, and is often the only available food source of high nutrients [7,8,9]. Moreover, A. garckeana has been used in traditional medicine as a remedy for chest pains, menstruation, and coughs [10,11,12]. Jakjak fruit is an important source of essential minerals, particularly Mg, P, Ca, and Na [13]. According to Branch, 1983 [2], the indigenous fruit-bearing tree species are rich in sugars, essential vitamins, and minerals. In context, a recent study showed that fruit could be used as a new source of pectin [14]. Multiple classes of phytochemical compounds such as ascorbic acid, amino acids, flavonoids, carotenoids, glucosides, phenols, lipids, and tannins have been isolated from A. garckeana [12]. In addition, very important sugars such as glucose, mannose, galactose, and fructose were detected in jakjak fruit [15]. Moreover, a recent study has reported that GC-MS analysis of jakjak fruit pulp revealed the presence of 22 bioactive compounds, which have been reported to have antioxidant, anticancer, antimicrobial, anti-inflammatory, and hepatoprotective properties [16]. Phenolic compounds derived from consuming fruits and vegetables have been associated with health benefits [17]. The beneficial impacts of phenolics have been assigned to their antioxidant activity [18]. In accordance, it has been determined that the antioxidant effect of plant products is mainly due to the radical scavenging activity of phenolic compounds such as flavonoids, polyphenols, tannins, and phenolic terpenes [19]. Over recent decades, an expanding body of evidence from epidemiological and laboratory studies has demonstrated that some edible plants as a whole, or their identified ingredients with antioxidant properties, have substantial protective effects on human carcinogenesis [20]. Phenolic compounds are an integral part of both the human and animal diet [21]. Although jakjak fruit has the potential to be utilized and used in the production and development of new food and beverage products, they still have not been fully exploited [22]. Van Wyk, 2011 [22] has stated that further research should be conducted because the plant is promising as some of its nutritional and pharmacological value may be used to explain and support the nutritional and medicinal uses. Therefore, the main objective of this study was to detect and estimate the nutritional value of A. garckeana fruit. Hence, phytochemical screening, separation, and estimation were performed using GC-MS, HPLC, and UV–visible spectroscopy. Phenolic compounds such as total phenolic content (TPC), total tannin content (TTC), total flavonoid content (TFC), total flavanol, and total soluble sugar were estimated using a UV–vis spectrophotometer. Moreover, individual compounds such as rutin, quercetin, gallic acid, and tannic acid were also determined. In addition, glucose, fructose, maltose, and ascorbic acid were separated with chromatography and quantified using high-performance liquid chromatography (HPLC) with authentic standards.
2. Materials and Methods
2.1. Chemical Reagents and Standards
Authentic standards include d-(−)-fructose, d-(+)-glucose, maltose, ascorbic acid, gallic acid, tannic acid, quercetin, and rutin. Moreover, solvents such as acetonitrile, glacial acetic acid, methanol, and deionized water (purchased from Sigma-Aldrich, St. Louis, MO, USA) were used as the mobile phase for separating and quantifying the targeted compounds in this study.
2.2. Fruit Collection and Extraction Process
Azanzagarckeana fruit (Figure 1) was obtained from the local market in South Darfur State, Sudan. Next, the fruit was washed and dried at room temperature. One gram of the powdered fruit was extracted in 100 mL of methanol and deionized water. The extraction process was achieved in an Innova 44 Incubator Shaker at 100 rpm, at 28 ± 2° for three days. Next, the aqueous and organic phases were separated by centrifugation at 5000 rpm for 15 min and filtered using a 0.45 µm nylon syringe for phytochemical screening and estimation using GC-MS, HPLC, and UV–visible spectrophotometry.

Figure 1.
Azanza garckeana fruit.
2.3. Phenolic Compounds Assay
2.3.1. Phenolic Contents Estimation
Total phenolic content (TPC) in aqueous extracts of A. garckeana fruit was carried out using the Ainsworth https://www.nature.com/articles/s41598-021-98607-3 (accessed on 6 June 2022)—ref-CR37 [23] method. Here, 0.1 mL of extracted fruit material was mixed with 0.1 mL of the Folin reagent. Next, the mixture was neutralized using 0.3 mL of sodium carbonate solution (20%, w/v). Further, the mixture was incubated in the dark at room temperature for 30 min. The wavelength of the resulting blue color was recorded at 765 nm. The phenolic content of the fruit was determined using the following linear equation (y = 0.0014x + 0.0114 with R2 = 0.999) of a standard curve prepared with gallic acid (25–400 µg/mL). The content of phenolic compounds was defined as mg/g gallic acid equivalent (GAE) of dry extract.
2.3.2. Estimation of the Total Flavonoid Content
Estimation of the total flavonoid content (TFC) in aqueous fruit extracts was conducted using methods described by Ordonez et al., 2006 [24]. A volume of 0.5 mL of extract was added to 0.5 mL of 2% AlCl3 water solution. After a 2 h incubation period at room temperature, the wavelength was measured at 420 nm. A calibration curve was constructed using quercetin (50–400 µg/mL). The total flavonoid content in the fruit was defined by quercetin (mg/g DW) using the following equation based on the standard curve (y = 0.0012x + 0.0595).
2.3.3. Estimation of Total Tannin Content
The total tannin content (TTC) in jakjak fruit was estimated using the Folin–Ciocalteu method described by Rodrigues et al., 2007 [25] with slight modifications. A total of 100 µL of fruit extract was added to a tube containing 1.5 mL of deionized water and 100 µL of the Folin–Ciocalteu phenol reagent. Then, 300 µL of 35% sodium carbonate solution was added to the mixture. Next, the mixture was shaken well and kept at room temperature for 20 min in the dark. Tannic acid was used as the reference for the standard curve (y = 0.0054x − 0.0095 with R2 = 0.992).
2.3.4. Estimation of Flavanols Content
The flavanols content was determined by the Kumaran and Karunakaran, 2007 [26] method. The rutin calibration curve was prepared by mixing 2 mL of various concentrations of ethanolic solutions of rutin with 2 mL (20 mg/mL) of aluminum trichloride and 6 mL (50 mg/mL) of sodium acetate. The absorbance was measured at 440 nm after 2.5 h. The same procedure was used for 2 mL of fruit extract (10 mg/mL) instead of the rutin solution. All determinations were carried out in duplicate. The content of flavanols was calculated using a standard curve obtained from various concentrations of rutin.
2.4. Sugar Content Determination Assay
Estimation of Total Soluble Sugar
The total sugar content in the fruit extracts was estimated using the method described by Dubois et al., 1956 [27]. Then, 1 mL of phenol 5% and 5 mL of sulfuric acid 96% were added sequentially to the fruit extract. The test tubes containing the reaction mixture were incubated for 20 min in a water bath set at 30 °C. In a hot acidic medium, glucose becomes dehydrated to hydroxymethylfurfural. The resulting yellow-brown color was measured at 490 nm. The total soluble sugar content was expressed as glucose (mg/g DW) using the following equation (y = 10.334x − 0.0906 with R2 = 0.993) based on the calibration curve prepared using the glucose standard solutions (0.025–0.3 µg/mL).
2.5. HPLC Equipment and Compounds Quantification
A liquid chromatographic system, Agilent, USA, controlled by G 4226A software and equipped with an SB-C18 column (1.8 μm, 4.6 × 150 mm) and mobile phases, was used for the separation, identification, and estimation of glucose, maltose, fructose, ascorbic acid, rutin, quercetin, and gallic acid content of fruit extracts as follows.
2.5.1. Quantification of Glucose
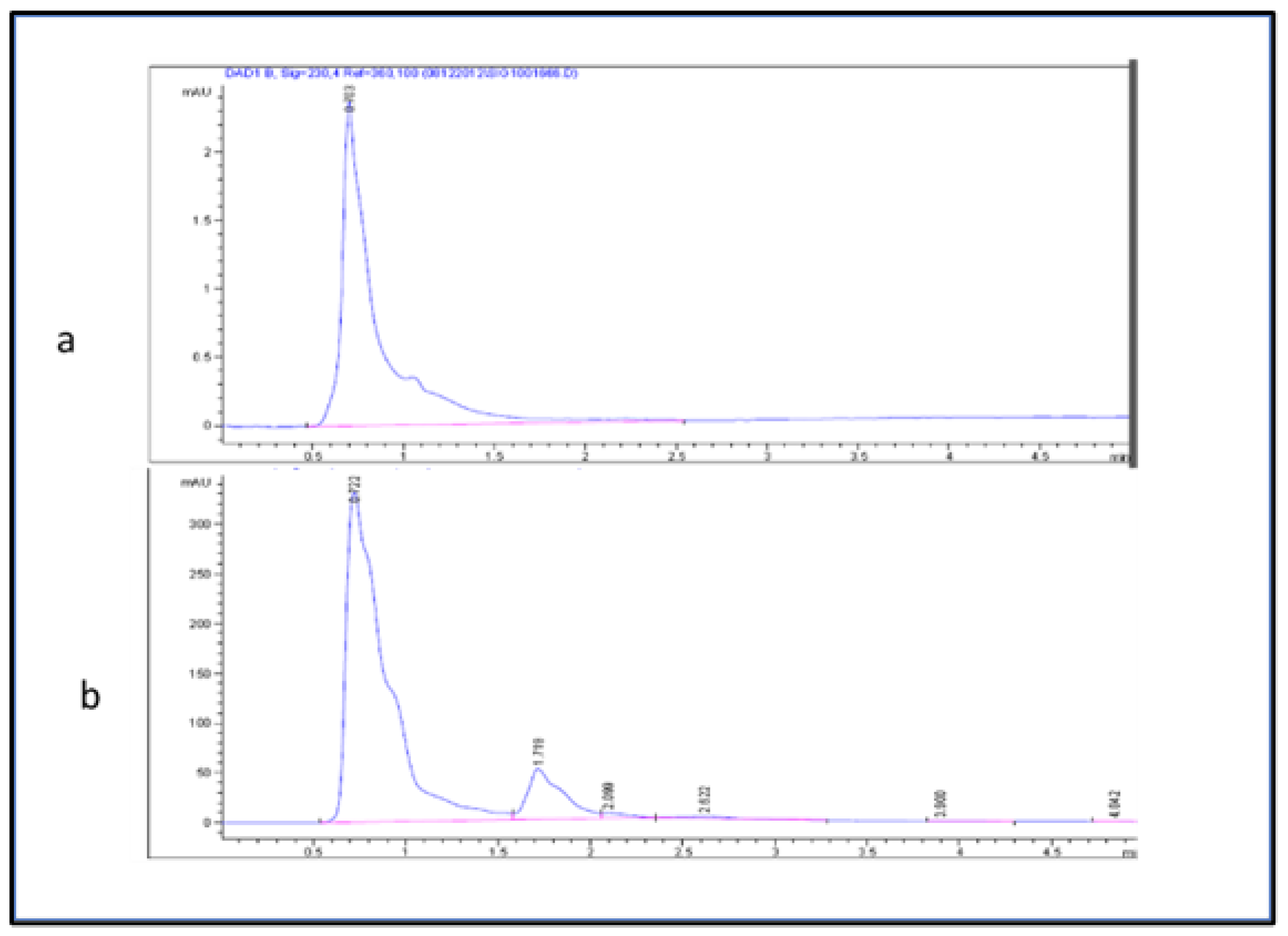
The glucose content in the fruit was separated and quantified using the method described by Montesano et al., 2016 [28] with minor modifications. The mobile phase consisted of deionized water and acetonitrile (B) (20:80) (v/v). Samples were eluted with the following conditions. The flow rate was 1.7 mL/min, and the injected volume was 20 µL with a 5 min run time. The column temperature was adjusted to be 27 °C. A chromatogram was acquired at a wavelength of 230 nm (Figure 2). The glucose sugar in the fruit extract was estimated using the following linear equation (y = 2.8295 − 10.595 with R2 = 0.9597) of a standard curve prepared with d-glucose (5–20 µL).
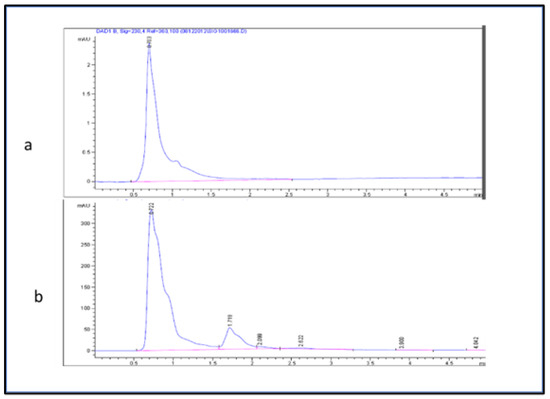
Figure 2.
HPLC chromatogram (a) d-glucose standard and (b) glucose in fruit extracts.
2.5.2. Quantification of Fructose
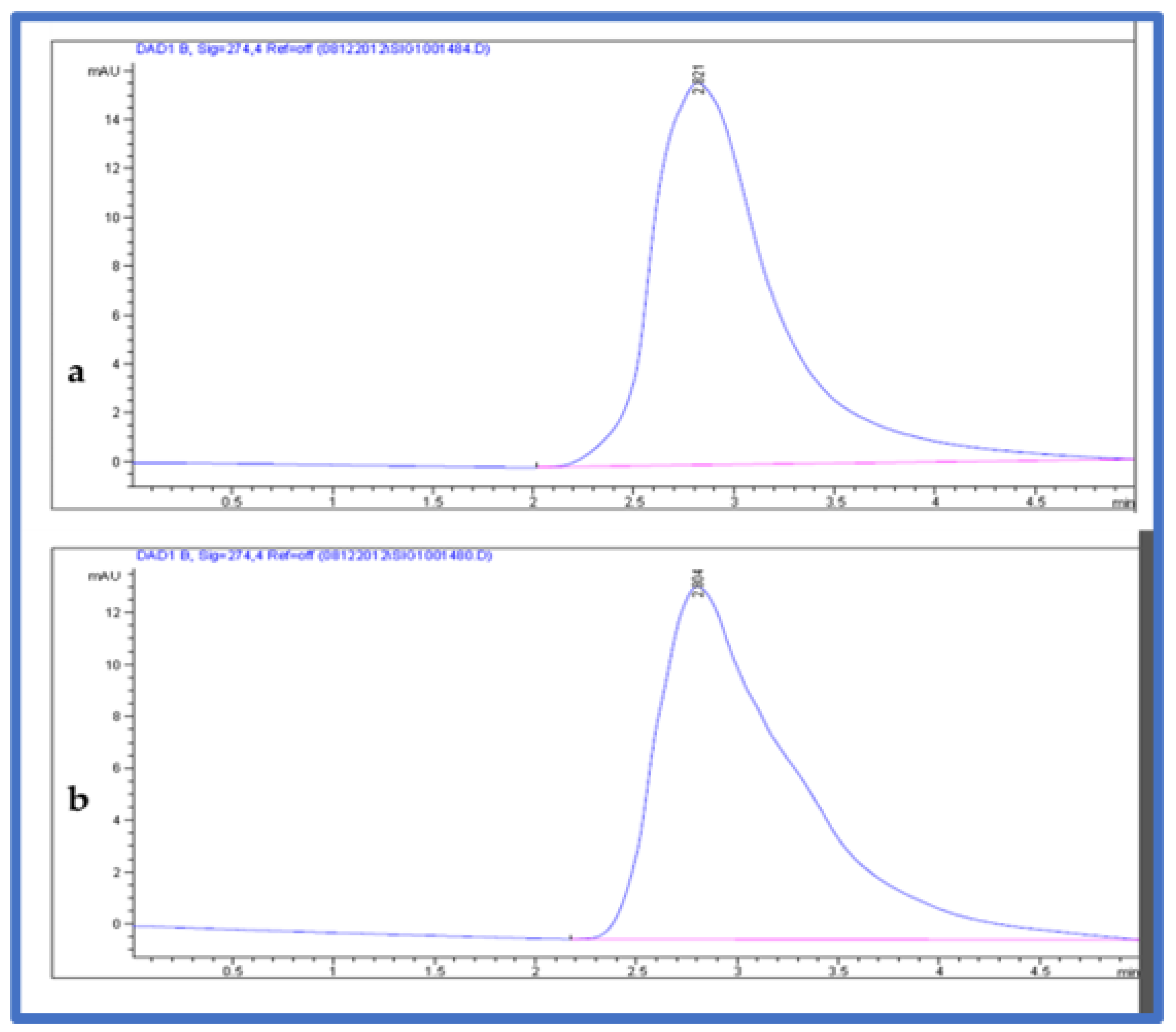
The mobile phase used for the separation and quantification of fructose sugar in the samples consisted of an acetonitrile solution and methanol (70:30) (v/v). Samples were eluted with the following conditions. The flow rate was 0.700 mL/min, with a 5 min run time. The injected volume was 10 µL. The column temperature was maintained at 25 °C. The chromatogram was acquired at a wavelength of 274 nm (Figure 3). The fructose in the plant material was estimated from the linear equation (y = 7.5x − 4 with R2 = 9995) of a standard curve prepared with d-fructose (5–15 µL).
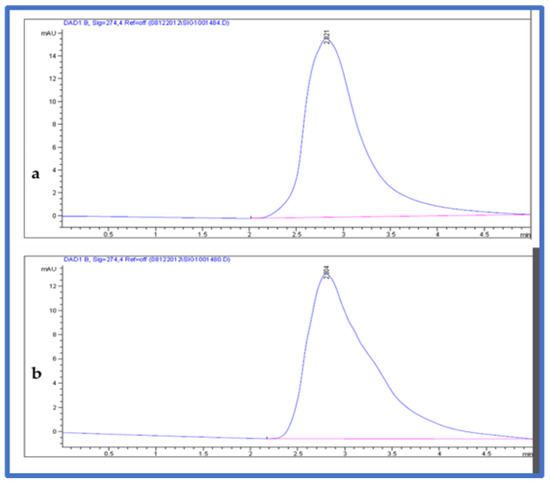
Figure 3.
HPLC chromatogram (a) fructose standard and (b) fructose from fruit extracts.
2.5.3. Quantification of Maltose
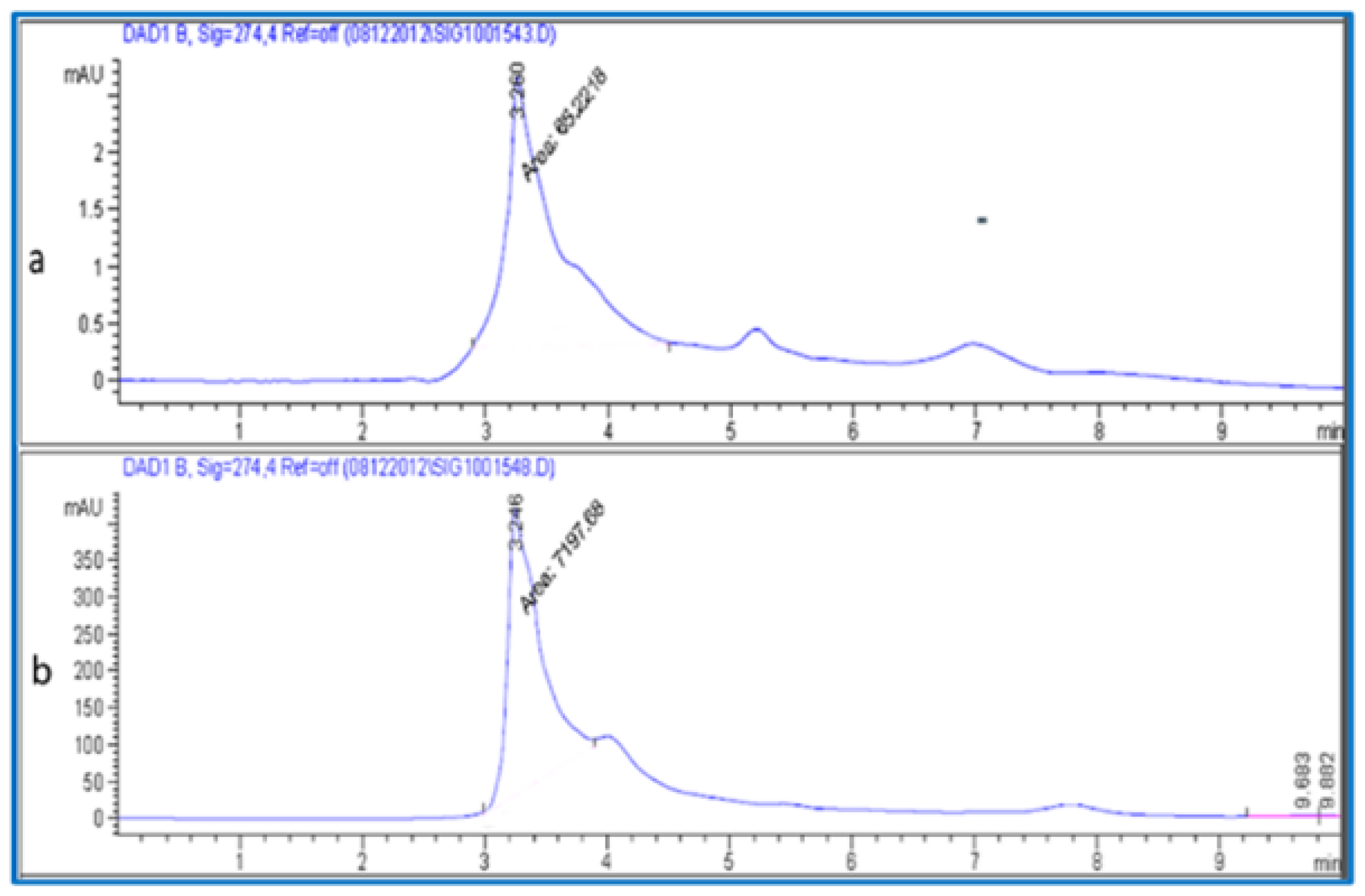
The maltose content in the fruit was separated and quantified using a mobile phase consisting of deionized water solvent (A) and methanol solvent (B) (50:50) (v/v). Samples were eluted with the following conditions. The flow rate was 0.400 mL/min with a 10 min run time. The injected volume was 20 µL. The column temperature was maintained at 25 °C. The chromatogram was acquired at a wavelength of 274 nm according to the absorption maxima analyzed (Figure 4). The maltose sugar in the plant material was estimated from the linear equation (y = 11.44x − 22.5 with R2 = 9898) of a standard curve prepared with d-maltose (5–20 µL).
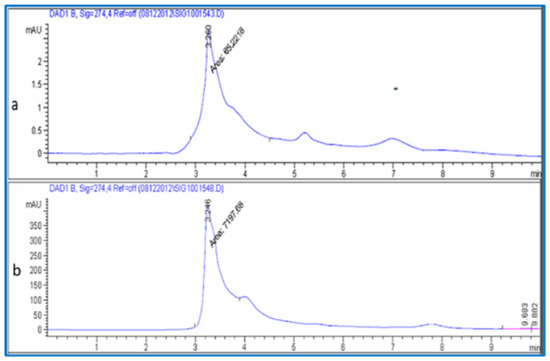
Figure 4.
HPLC chromatogram (a) Maltose standard and (b) maltose from fruit extracts.
2.5.4. Estimation of Ascorbic Acid
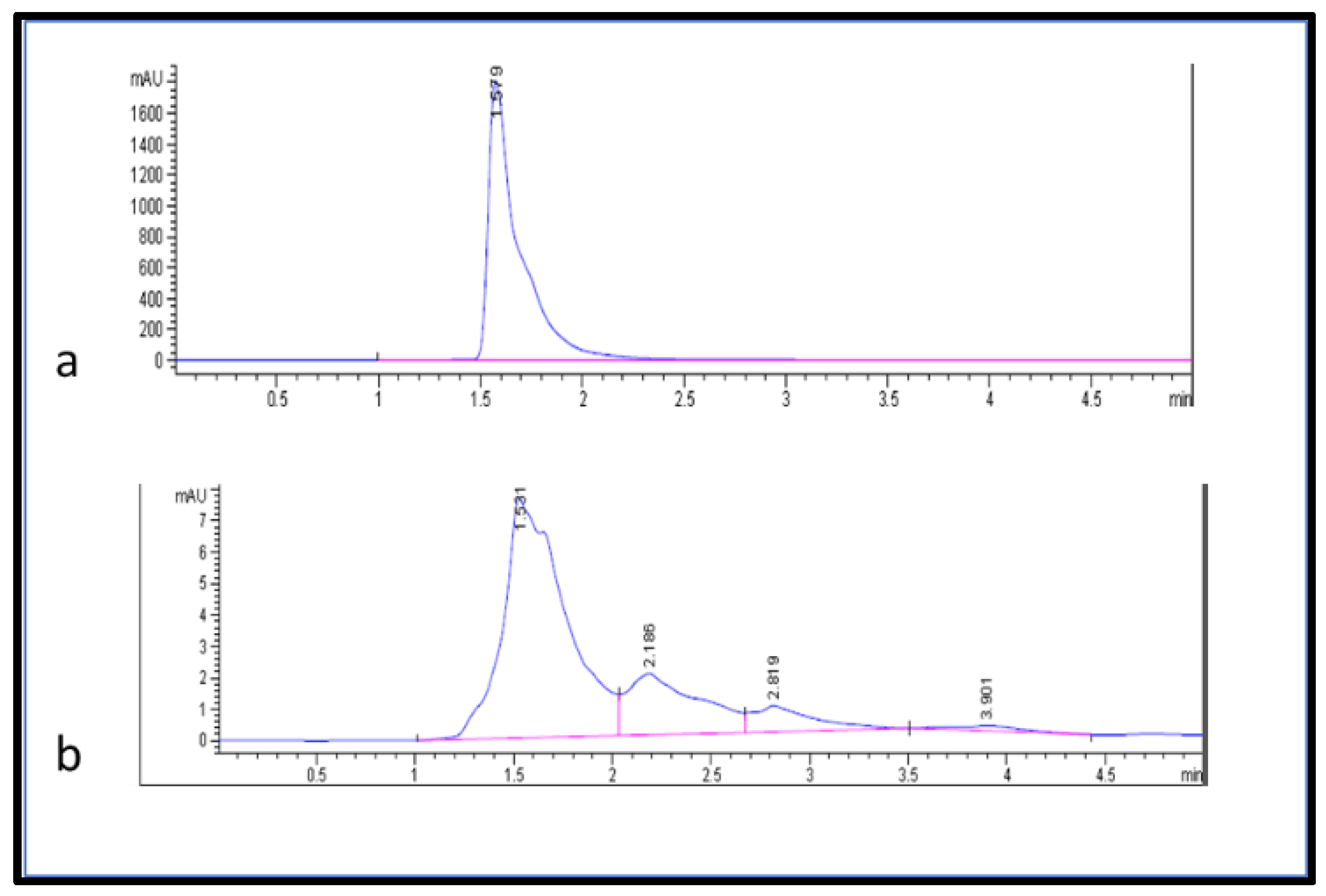
The separation and quantification of ascorbic acid in the samples were performed using high-performance liquid chromatography (HPLC) following Castro et al.’s [29] method with minor modifications. This method utilized UV detection at 254 nm, a C-18 column with a mobile phase consisting of a mixture of (15:85) (v/v) methanol and deionized water, an injection volume of 2.5 µL, a flow rate of 1.000 mL/min, and a 5 min run time (Figure 5). The ascorbic acid in the fruit extract was estimated from the linear equation (2.5437x − 0.3207 with R2 = 0.996) of a standard curve prepared using ascorbic acid (100–250 µg/mL).
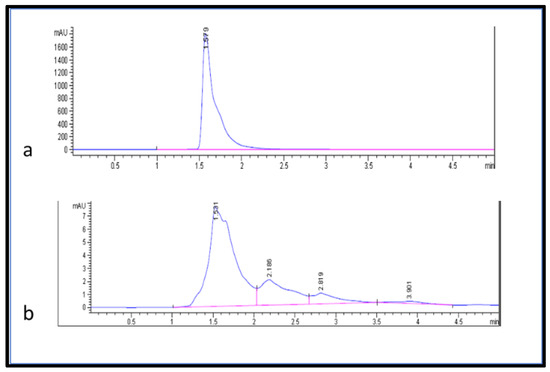
Figure 5.
HPLC chromatogram (a) ascorbic acid standard and (b) ascorbic acid from fruit extracts.
2.5.5. Quantification of Rutin
The mobile phase used for the separation and quantification of rutin in the samples consisted of acetonitrile solution (40) and methanol (60) (v/v). Samples were eluted with the following gradient. The flow rate was 0.500 mL/min, and the injection volume was 1 µL. The column temperature was maintained at 25 °C. The chromatogram was acquired at a wavelength of 274 nm. The rutin in the fruit extract was estimated from the linear equation (108.98x − 2.2449 with R2 = 0.9912) of a standard curve prepared with the rutin standard solutions (200–1000 µg/mL).
2.5.6. Quantification of Quercetin
The quercetin in the fruit samples was separated and quantified chromatographically using methanol (60%) and acetonitrile (40%) (v/v). The sample injected volume was 1 µL with a 0.800 mL/min flow rate, 5 min run time, and the temperature was maintained at around 25 °C. The chromatogram was recorded at wavelengths of 274 nm. The quercetin was identified by its retention time compared to the quercetin standard under similar conditions. The quercetin in the fruit extract was quantified from the linear equation (y = 1925.7x − 30,714 with R2 = 0.9987) prepared from the quercetin standard solutions (200–1000 µg/mL).
2.5.7. Quantification of Gallic Acid
The gallic acid in each fruit sample was identified and quantified chromatographically using methanol and 0.6% aqueous acetic acid (80:20) (v/v). The standard and samples were acquisitioned with the following conditions. The injected volume was 1 µL with a 1.000 mL/min flow rate. The column temperature was adjusted to around 25 °C. The chromatogram was measured at 274 nm. The gallic acid in the fruit extracts was identified by its retention compared to the gallic acid standard with the same conditions using the method described by Nour et al., 2013 [30]. The gallic acid was estimated from the linear equation (y = 266.3x − 141.63 with R2 = 0.9989) of a standard curve prepared with gallic acid.
2.6. GC-MS Analysis of Fruit Extracts
The methanol extract of jakjak fruit was selected for GC-MS analysis based on UV–visible spectrophotometer results. A GC-MS 7890A; Agilent Technologies-USA, with a 5975 mass-selective detector and a 7693 automated liquid sampler, fitted with a DB-5MS GC column (30 m length, 0.25 mm inner diameter, and 0.25 μm film thickness), was used. The extract was filtered using a 2 µm membrane filter. Then, a 1.0 µL aliquot of the methanol extract was injected into the system. Initially, the oven temperature was programmed at 80 °C, the final column temperature was adjusted to 300 °C, and the total run time was 40 min. Helium gas was used as the carrier with a flow rate of 1 mL/min. The electron ionization energy was 70 eV. The identification of the bioactive constituents in the methanolic fruit extracts was performed using commercial libraries and comparison of mass spectra, matches percentage (>80%), and retention times of reference compounds.
2.7. DPPH Radical Scavenging Assay
The antioxidant potential of the deionized water and the methanolic extracts of A. garckeana fruit was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) according to the Liyana-Pathirana and Shahidi, 2005 method (Liyana-Pathirana and Shahidi, 2005). In this experiment, different concentrations of extracts (12.5, 25, 50, and 100%) were mixed with 1 mL of 0.135 mM of DPPH. Next, mixtures were vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the samples and control (1 mL methanol + 1 mL DPPH) solutions were measured at 517 nm. The percentage of DPPH scavenging activity of the extracts and standard was calculated using the following formula: DPPH scavenging activity (%) = [(Abs control − Abs sample)/Abs control] × 100, where Abs control is the absorbance of DPPH + methanol and Abs sample is the absorbance of DPPH radical + sample.
2.8. Statistical Analysis
The experiment was conducted in triplicate, and results were reported by the table means value ± (SD) using the one-way ANOVA–Duncan test for estimating statistical significance differences at (p < 0.05).
3. Results and Discussion
3.1. Sugar Content Estimation
Medicinal plants are considered a rich resource of bioactive compounds with potential for drug discovery and development. In the present study, the total sugar content in the fruit extract of jakjak was estimated, as detailed in the methodology section. The obtained results from methanol and deionized water extracts showed that jakjak fruit is very rich in total soluble sugar content, about (48%) and (40%) out of the dried weight (Table 1), respectively. In agreement with our findings, a previous study reported that jakjak fruit contains high percentages of total carbohydrates (87.56%) and total sugars (61.39%) [31]. Moreover, the glucose, fructose, and maltose content were chromatographically separated and quantified using HPLC and authentic standards (Figure 2, Figure 3 and Figure 4), which revealed that the fruit of A. garckeana contained about (978.21, 428.57 µg/g DW), (202.27, 65.60 µg/g DW), and (209.12, ND µg/g DW), respectively (Table 1). In the literature, glucose, mannose, galactose, and fructose sugars were detected in jakjak fruit extracts [15]. These sugars detected in the fruit extracts of jakjak are good sources of energy [15], particularly in rural areas. It has been reported that the polysaccharides obtained from plant foods are major constituents of the human diet, with limited contributions of related constituents from fungal and algal sources [32]. In context, carbohydrates are the most abundant bioactive compounds in all kinds of natural plants, and are the building units of natural products, complex carbohydrates, and molecules. According to Kilic et al., 2007 [33], maltose is one of the most abundant sugars in the human diet. This present work and previous reports have confirmed that jakjak fruit is a good source of sugars.

Table 1.
Total sugar content (%), glucose, fructose, and maltose in the fruit of A. garckeana in methanol and deionized water extracts (µg/g DW).
3.2. Phenolic Compounds of Jakjak Fruit
Here, in this study, the phenolic compounds (phenol, tannin, flavonoid, and flavanol contents) in deionized water and methanol extracts of jakjak fruit were estimated using a UV–visible spectrophotometer to evaluate their medicinal and nutritional value. The recorded results demonstrated that the jakjak fruit contained valuable nutritional value. For example, the fruit contained a high amount of phenolic, tannin, flavonoid, and flavanol content, as shown in (Table 2). In contrast, Lawal et al. [34] reported that the fruit pulp of jakjak contained about 260.80 ± 2.23 mg/100 g of total phenol and 10.28 ± 1.29 mg/100 g of total flavonoid content. The results of this study were lower than our findings; this variation might be due to the plant environment, fruit collection, and handling process. Moreover, molecules such as quercetin, rutin, gallic acid, tannic acid, and ascorbic acid were separated with chromatography before being identified and estimated using HPLC with authentic standards (Table 3). It has been stated that rutin and quercetin are two of the flavonoid components in the human diet [35]. In addition, quercetin is one of the natural antioxidants found in various food products [36]. Gallic acid has antioxidant and antifungal activity, as reported by Gunckel et al. and Shukla et al., 1999 [37,38]. We found that the fruit of jakjak contained about (12.18 µg/g DW) of ascorbic acid. Suliman et al., 2012 reported that jakjak fruit juice contained appreciable amounts of ascorbic acid (21.1344 mg/100 g) [7]. Mojerewane and Tshwenyane, 2004 stated that the ascorbic acid content of jakjak fruit is about 20.5 mg/100 [13], while Jacob et al., 2016 reported that the ascorbic acid content of jakjak fruit was found to be 28.5–30.5 mg/50 g [39]. The findings of these studies are higher than our results. Ascorbic acid is known to be one of the most important vitamins in the human diet [40]. In general, the methanol solvent was best for the extraction of bioactive compounds from jakjak fruit compared to using deionized water. The deionized water achieved the highest yield of ascorbic acid (12.18 µg/g DW) compared to the methanolic extract (6.1 µg/g DW) (Table 3). The optimal solvent for extraction of phytochemical compounds from plant material might depend on the constituents that are to be isolated, and the part of the plant used. Solvents have shown a significant effect in the extraction of phenolic compounds [41]. Our results have shown that jakjak fruit is a good source of antioxidant compounds which are suitable to be used as dietary supplements.

Table 2.
TPC, TTC, TTFC, and total flavanol content of A. garckeana fruit (mg/g DW).

Table 3.
Quercetin, rutin, gallic acid, tannic acid, and ascorbic acid in methanol and water of A. garckeana fruit (µg/g DW).
3.3. Antioxidant Activity Assay
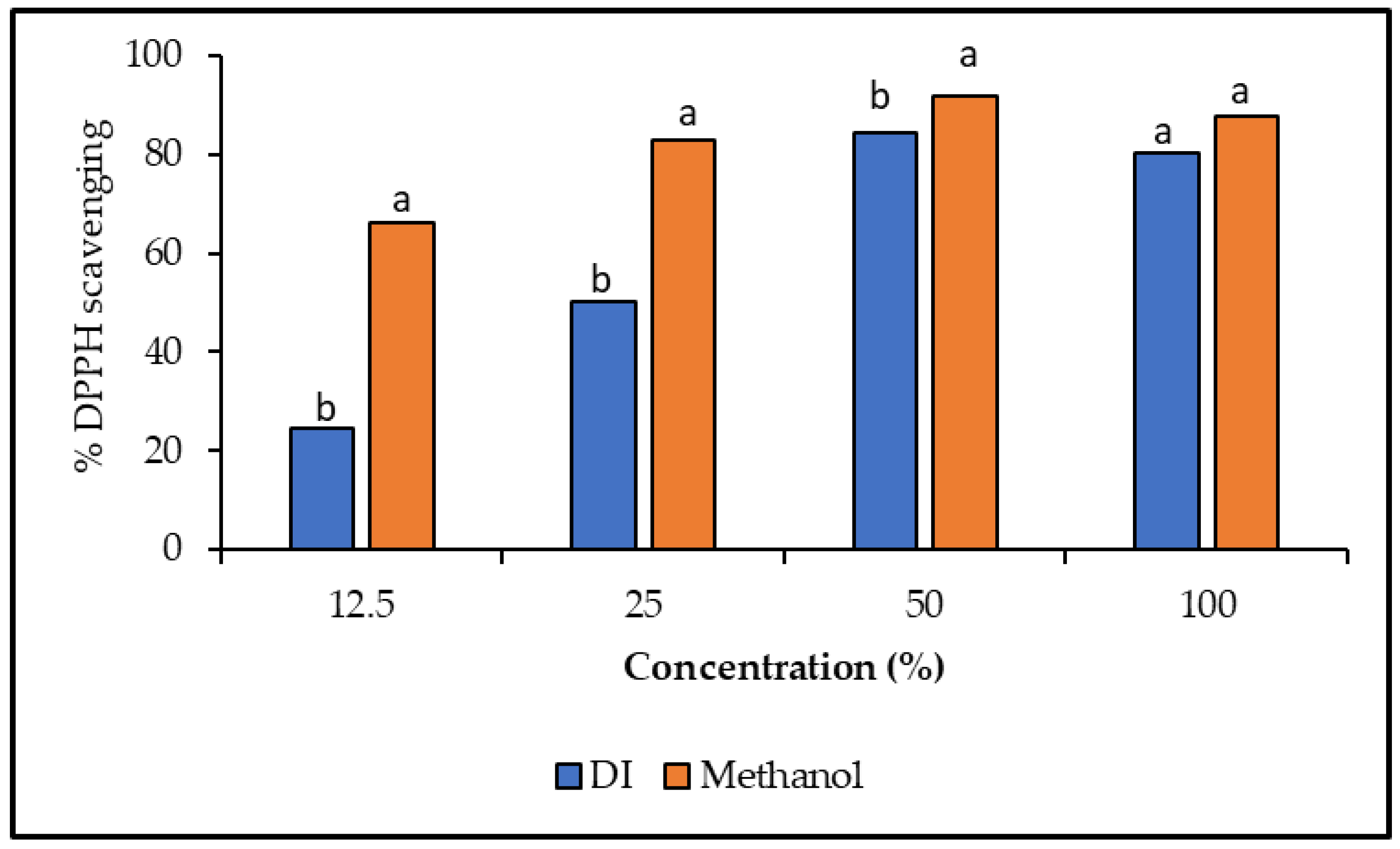
Antioxidants in food and medicinal plants fight against free radicals, which protect us from a wide range of diseases [42]. Here, in this present research, the antioxidant potential of different concentrations (12.5, 25, 50, and 100%) of deionized water and methanolic extracts of A. garckeana fruit was estimated using 2,2-diphenyl-1-picrylhydrazyl (DPPH). The recorded results have shown that DPPH radical scavenging activity was raised with the concentration of the plant extracts. The concentration (50%) of both methanol and deionized water gave the best inhibition percentages (91.7 and 84.4%), respectively (Figure 6). However, the methanolic extract has shown significant results compared to the deionized extract (Figure 6). These findings are consistent with the results of the phenolic compounds. Methanol extracts of jakjak fruit might possess a higher ability to donate protons to the free radicals than the deionized water extract. These results confirm the major role in the antioxidant activity of jakjak fruit. Maroyi, 2017 stated that the methanol and ethyl-acetate extract of jajak fruit exhibited noteworthy DPPH scavenging activities, thus providing scientific evidence and validation for their uses in traditional medicine in treatments of various human diseases [6].
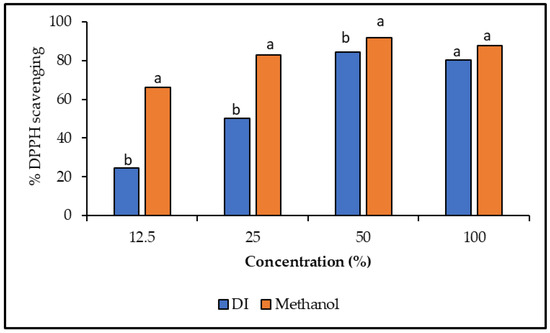
Figure 6.
DPPH radical scavenging activity (%). DPPH scavenging activity of different concentrations of DI and methanolic extracts from Azanza garckeana fruit. Results are presented as means ± SD (n = 3). a,b Means in the same column with different letters are significantly different at (p < 0.05).
3.4. Gas Chromatography–Mass Spectrometry Analysis of Fruit Extracts of Azanza garckeana
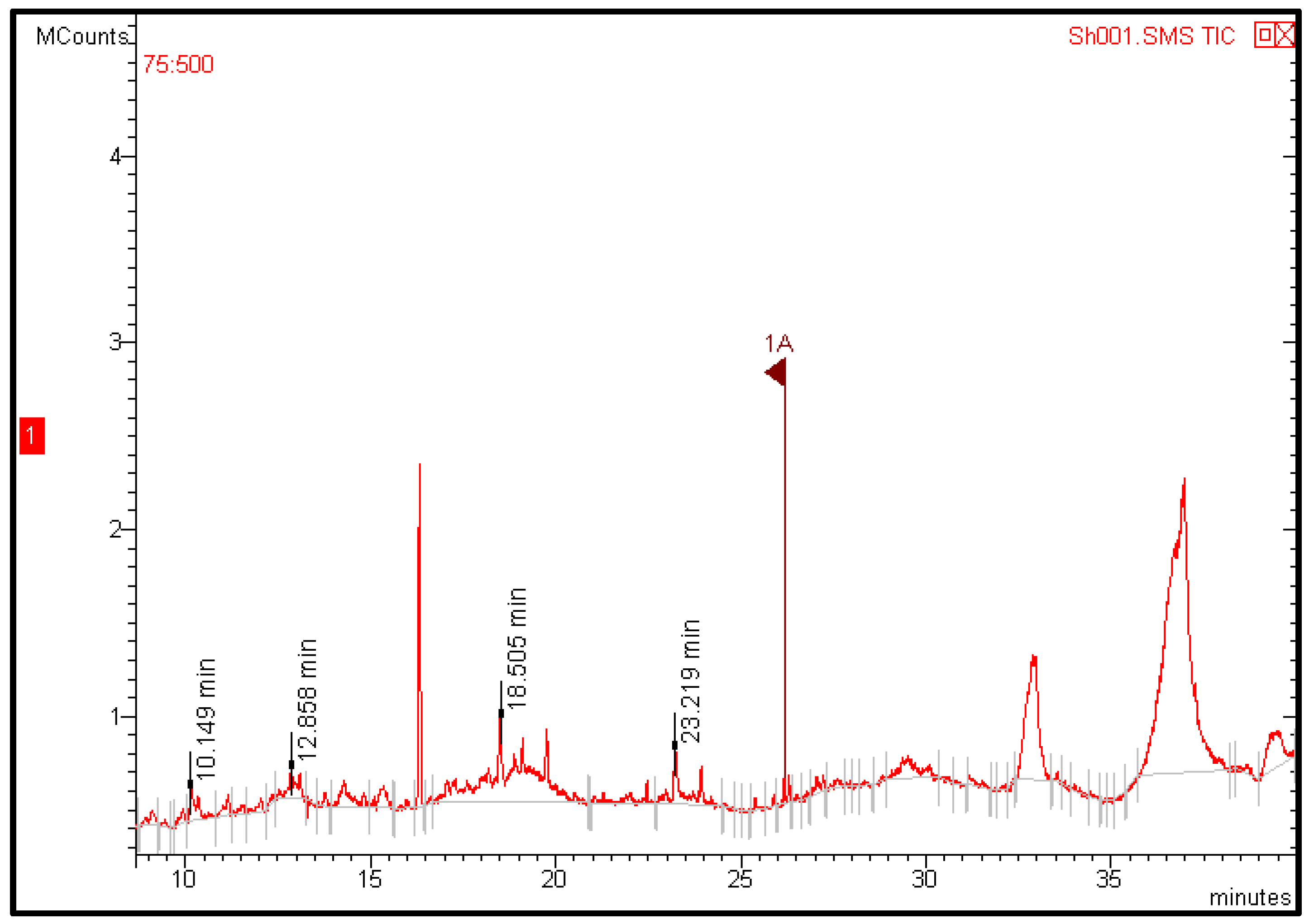
GC-MS analysis of fruit extracts showed four peaks indicating the presence of four bioactive constituents related to secondary metabolites (Table 4 and Figure 7). Some of the detected constituents contributed to the nutritional and medicinal value of the fruit. For example, phenol, 2,4-bis (1,1-dimethylethyl) has been reported to have medicinal and nutritional value. It has antioxidant activity [43,44], anticancer [45], antifungal [46], and antibacterial properties [47]. A recent study reported that GC-MS analysis of jakjak fruit pulp revealed the presence of 22 bioactive compounds related to secondary metabolites, which have been reported to have antioxidant, anticancer, antimicrobial, anti-inflammatory, and hepatoprotective properties [16]. The variation between these findings and our results may refer to the plant environment, the extraction process, as well as the GC-MS reference libraries used for the identification of bioactive compounds. These findings may support and justify the utilization of jakjak fruit in traditional medicine.

Table 4.
Phytochemical compounds of A. garckeana fruit detected by GC-MS analysis.
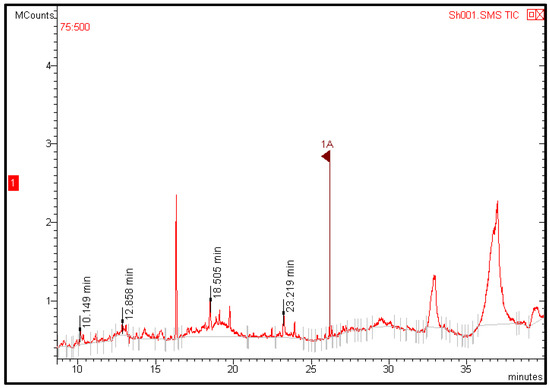
Figure 7.
Chromatogram of Azanza garckeana fruit methanolic extract.
4. Conclusions
Azanza garckeana is an important food and medicinal plant that has been used in tropical Africa. Polysaccharides and phenolic compounds obtained from plant foods are major components of the human diet, which have been associated with health benefits due to their antioxidant activity. This research concluded that jakjak fruit is very rich in secondary metabolites, particularly sugars, which can be used as a source of polysaccharides and antioxidants for the human diet as well as a source of raw materials for downstream industries. Further, more studies should be conducted to investigate the medicinal and nutritional value of Azanza garckeana fruit.
Author Contributions
A.M.S. was responsible for the conceptualization as well as the research that was proposed and planned; A.M.S., M.T. and H.O.S. contributed to the methodology; A.M.S. wrote the original manuscript; F.A.-Q. acted as the supervisor; A.H. supervised the lab. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by (RSP-2021/73) at King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their appreciation to Researchers supporting project number (RSP-2021/73) at King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Taylor, F.; Kwerepe, B. Towards domestication of some indigenous fruit trees in Botswana. In Proceedings of the Conference on the Improvement of Indigenous Fruit Trees of the Miombo Woodlands of Southern Africa, Mangochi, Malawi, 23–27 January 1994. [Google Scholar]
- Agriculture Organization of the United Nations. Food and Fruit-Bearing Forest Species; Food and Agriculture Organization of the United Nations: Rome, Italy, 1983; Volume 44. [Google Scholar]
- Sulieman, A.-H. Azanza garckeana L.: Distribution, Composition, Nutritive Value, and Utilization. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Berlin/Heidelberg, Germany, 2019; pp. 379–393. [Google Scholar]
- Ochokwu, I.; Dasuki, A.; Oshoke, J. Azanza garckeana (Goron Tula) as an edible indigenous fruit in North Eastern Part of Nigeria. J. Biol. Agric. Healthc. 2015, 5, 26–31. [Google Scholar]
- Dikko, Y.; Khan, M.; Tor-Anyiin, T.; Anyam, J.; Linus, U. In vitro antimicrobial activity of fruit pulp extracts of Azanza garckeana (f. hoffm.) exell & hillc. and isolation of one of its active principles, betulinic acid. Methodology 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Maroyi, A. Azanza garckeana fruit tree: Phytochemistry, pharmacology, nutritional and primary healthcare applications as herbal medicine: A Review. Res. J. Med. Plants 2017, 11, 115–123. [Google Scholar] [CrossRef]
- Suliman, A.M.E.; Difa, I.Y.; Salih, Z.A. The Nutritive Value of Jakjak (Azanza garckeana L.) Fruit and its Utilization in Juice Production. Asian J. Biol. Sci. 2012, 5, 209–215. [Google Scholar]
- Lee, K.; Roth, R.A.; LaPres, J.J. Hypoxia, drug therapy and toxicity. Pharmacol. Ther. 2007, 113, 229–246. [Google Scholar] [CrossRef]
- Glew, R.S.; Vanderjagt, D.J.; Chuang, L.-T.; Huang, Y.-S.; Millson, M.; Glew, R.H. Nutrient content of four edible wild plants from West Africa. Plant Foods Hum. Nutr. 2005, 60, 187–193. [Google Scholar] [CrossRef]
- Gelfland, M.; Mavi, S.; Drummond, R.; Ndemera, B. The Traditional Medical Practitioner in Zimbabwe: His Principles of Practice and Pharmacopoeia; Mambo Press: Gweru, Zimbabwe, 1985. [Google Scholar]
- Mshelia, E.; Watirahyel, E.; Maigari, A.; Yohanna, C.; Ismail, F. Cytotoxicity and antioxidant activity of stem bark extracts of Azanza garckeana (kola of Tula). Eur. J. Pure Appl. Chem. 2016, 3, 16–24. [Google Scholar]
- World Health Organization. This year’s malaria report at glance. In World Malaria Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Mojeremane, W.; Tshwenyane, S. Azanza garckeana: A Valuable Edible Indigenous Fruit Tree of Botswana. Pak. J. Nutr. 2004, 3, 264–267. [Google Scholar] [CrossRef]
- Chawafambira, A. Extraction and Characterization of Pectin from Snot Apple (Azanza garckeana) Fruits with Potential Use in Zimbabwe. Int. J. Fruit Sci. 2021, 21, 791–803. [Google Scholar] [CrossRef]
- Karu, E.; Maitale, J.; Maigari, F. Extraction and Identification of Reducing Sugars in Azanza garckeana Fruit. BIMA J. Sci. Technol. (2536–6041) 2019, 3, 87–95. [Google Scholar]
- Momodu, I.; Okungbowa, E.; Agoreyo, B.; Maliki, M. Gas Chromatography–Mass Spectrometry Identification of Bioactive Compounds in Methanol and Aqueous Seed Extracts of Azanza garckeana Fruits. Niger. J. Biotechnol. 2022, 38, 25–38. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Rahman, M.A.A.; Moon, S.-S. Antioxidant polyphenol glycosides from the plant Draba nemorosa. Bull. Korean Chem. Soc. 2007, 28, 827–831. [Google Scholar] [CrossRef]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of cancer. CA A Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Van Wyk, B.-E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Ordonez, A.; Gomez, J.; Vattuone, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Rodrigues, C.I.; Marta, L.; Maia, R.; Miranda, M.; Ribeirinho, M.; Máguas, C. Application of solid-phase extraction to brewed coffee caffeine and organic acid determination by UV/HPLC. J. Food Compos. Anal. 2007, 20, 440–448. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers Pt Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Montesano, D.; Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.; Blasi, F. A simple HPLC-ELSD method for sugar analysis in goji berry. J. Chem. 2016, 2016, 6271808. [Google Scholar] [CrossRef]
- Castro, R.; Azeredo, L.; Azeredo, M.; De Sampaio, C. HPLC assay for the determination of ascorbic acid in honey samples. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1015–1020. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Abass, A.A.; Ahmed, G.A. Assessment of the Suitability of Using Jakjak Fruits (Azanza garcheana) for Production of Lokum Candy; Sudan University of Science and Technology: Khartoum, Sudan, 2017. [Google Scholar]
- Lovegrove, A.; Edwards, C.; De Noni, I.; Patel, H.; El, S.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Kilic, A.O.; Honeyman, A.L.; Tao, L. Overlapping substrate specificity for sucrose and maltose of two binding protein-dependent sugar uptake systems in Streptococcus mutans. FEMS Microbiol. Lett. 2007, 266, 218–223. [Google Scholar] [CrossRef][Green Version]
- Lawal, B.; Sani, S.; Onikanni, A.S.; Ibrahim, Y.O.; Agboola, A.R.; Lukman, H.Y.; Olawale, F.; Jigam, A.A.; Batiha, G.E.-S.; Babalola, S.B. Preclinical anti-inflammatory and antioxidant effects of Azanza garckeana in STZ-induced glycemic-impaired rats, and pharmacoinformatics of it major phytoconstituents. Biomed. Pharmacother. 2022, 152, 113196. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 457–479. [Google Scholar]
- Baghel, S.S.; Shrivastava, N.; Baghel, R.S.; Agrawal, P.; Rajput, S. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Gunckel, S.; Santander, P.; Cordano, G.; Ferreira, J.; Munoz, S.; Nunez-Vergara, L.; Squella, J. Antioxidant activity of gallates: An electrochemical study in aqueous media. Chem. Biol. Interact. 1998, 114, 45–59. [Google Scholar] [CrossRef]
- Shukla, Y.; Srivastava, A.; Kumar, S.; Kumar, S. Phytotoxic and antimicrobial constituents of Argyreia speciosa and Oenothera biennis. J. Ethnopharmacol. 1999, 67, 241–245. [Google Scholar] [CrossRef]
- Jacob, C.; Shehu, Z.; Danbature, W.; Karu, E. Proximate analysis of the fruit Azanza garckeana (“Goron Tula”). Bayero J. Pure Appl. Sci. 2016, 9, 221–224. [Google Scholar] [CrossRef]
- Foyer, C.H. Ascorbic acid. In Antioxidants in Higher Plants; CRC Press: Boca Raton, FL, USA, 2017; pp. 31–58. [Google Scholar]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization Method for Phenolic Compounds Extraction from Medicinal Plant (Juniperus procera) and Phytochemicals Screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J. Antioxidant and antiproliferative properties of a tocotrienol-rich fraction from grape seeds. Food Chem. 2009, 114, 1386–1390. [Google Scholar] [CrossRef]
- Kadoma, Y.; Ito, S.; Atsumi, T.; Fujisawa, S. Mechanisms of cytotoxicity of 2- or 2,6-di-tert-butylphenols and 2-methoxyphenols in terms of inhibition rate constant and a theoretical parameter. Chemosphere 2009, 74, 626–632. [Google Scholar] [CrossRef]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1724. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Z.; Du, L.; Xie, Y.; Zhang, Q.; Ye, X. Allelopathy of root exudates from different resistant eggplants to Verticillium dahliae and the identification of allelochemicals. Afr. J. Biotechnol. 2011, 10, 8284–8290. [Google Scholar]
- Abdullah, A.-S.H.; Mirghani, M.E.S.; Jamal, P. Antibacterial Activity of Malaysian mango kernel. Afr. J. Biotechnol. 2011, 10, 18739–18748. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).