Abstract
The aim of this study was to investigate the correlation between solid materials and micropollutants, aiming to enhance the removal of the latter during the application of the ozonation process. For that purpose, two solid materials (SiO2 and Al2O3) presenting catalytic activity were used for the removal of eight micropollutants from natural potable water, containing them either separately or in a mixture, by ozonation. The studied micropollutants, presenting different physicochemical properties, are atrazine, ibuprofen, p-CBA (ozone-resistant compounds), benzotriazole, caffeine (with moderate ozone reactivity), carbamazepine, fluoxetine, paracetamol (easily oxidized by ozone). The residual concentrations of carbamazepine, paracetamol, and fluoxetine were found to be lower than 5.9 μg/L, 1.2 μg/L, and 15.5 μg/L, respectively, after 1 min of oxidation time in all studied systems. In contrast, benzotriazole and caffeine removal was enhanced by the addition of catalysts; in both cases the best catalyst was SiO2. Regarding the ozone-resistant compounds, both examined materials enhanced the removal of ibuprofen and p-CBA; however, the best was found to be SiO2 and Al2O3, respectively. In contrast, Al2O3 cannot be considered as an effective catalyst for the removal of atrazine, which presents chemical affinity only with SiO2 and for this reason it can be removed to a higher extent by its presence. Similar results were observed in the study of the mixture, although in this system, the residual concentration of all micropollutants was found to be under the detection limit after the application of catalytic ozonation.
1. Introduction
Micropollutants are chemical compounds that co-exist in small concentrations in aquatic ecosystems; they have become an important issue of concern [1]. They exist in almost all consumer products and are used to facilitate everyday life. Therefore, the control of their sources in the aquatic environment is rather difficult [2]. Their continuous entry into the ecosystem has negative effects, because several micropollutants are compounds of high toxicity that can affect the nervous system or provoke endocrine disorders, cancer, etc. [3,4]. However, until now, there have been no specific provisions enacted against them. The Urban Wastewater Directive (Council Directive 91/271/EEC, concerning urban wastewater treatment) sets requirements for wider challenges related to water pollution [5]. However, this directive is currently under consideration for revision, so in the next few years, it is possible that this legislation gap will be addressed [6]. Up to now, a Strategic Approach to Pharmaceuticals in the Environment [7] has been in place, but this strategy focuses on the whole lifecycle of the pharmaceuticals and not on the treatment of them.
Ozonation is considered as an effective oxidation process that can degrade most organic compounds existing at low concentrations, known also as micropollutants, either by their reaction with ozone molecules (direct mechanism) or by the (more effective) hydroxyl radicals (indirect mechanism). The hydroxyl radicals that are produced by the decomposition of ozone are more powerful oxidants, and therefore, they are sought to predominate in respective oxidation reactions [8]. However, the application of single ozonation requires a high amount of energy to produce ozone. The removal of pollutants and the economic efficiency of the ozonation process can be further improved by the addition of an appropriate catalyst [9]. Catalytic ozonation is an Advanced Oxidation Process (AOP) that uses solid (heterogeneous)- or liquid (homogeneous)-appropriate catalytic materials to enhance the production of hydroxyl radicals [10,11].
Regarding behavior towards the ozonation processes, the target micropollutants can be divided into three main groups based on the respective reaction rate constant with ozone, i.e., (1) ozone-resistant compounds (kO3 < 10 M−1s−1), which almost do not participate in direct reactions with ozone; (2) compounds with moderate ozone reactivity (10 M−1s−1 < kO3 < 104 M−1s−1), which participate in both direct and indirect reactions; and (3) highly ozone-reactive compounds (kO3 > 104 M−1s−1), which are mainly oxidized by the presence of ozone [12,13].
Al2O3 and SiO2 are two commonly used inexpensive metal oxides that have been added as catalysts in similar research works [8,14,15,16]. In one of our previously published studies, it was proven that Al2O3 and SiO2 materials can be characterized as effective catalysts for the removal of refractory organic compound p-CBA from deionized water by the application of the ozonation process [17]. These metal oxides present different surface charges at the common pH values of natural waters (i.e., pH close to 7.5). Al2O3 has a Point-of-Zero Charge (PZC) value of 7; therefore, it is slightly negatively charged, while the PZC value of SiO2 is 2.6, and hence, it is strongly negatively charged. The wettability of silica (SiO2) is much higher than alumina (Al2O3). The PZC values and the wettability are two properties that can greatly influence the contact of ozone with the surface of the catalyst, enhancing its subsequent decomposition towards hydroxyl radicals [17]. As a result, it is considered that the contact of ozone with the surface of alumina is favored due to the higher PZC value (being almost neutrally charged), whereas in the case of SiO2, the contact is favored by the higher wettability of the solid.
The aim of this study was to evaluate the catalytic activity of these two metal oxides (Al2O3 and SiO2) in natural potable water matrix (and not in simulated conditions) for the improvement of the ozonation process. The investigation was performed with a variety of micropollutants with different physicochemical properties and reaction rate constants with ozone and hydroxyl radicals. For this aim, eight rather commonly detected micropollutants, considered as being representative of the aforementioned three main categories and presenting different reaction rate constants with ozone and hydroxyl radicals and different charges (pKa values), were used as probe compounds: atrazine, benzotriazole, carbamazepine, caffeine, fluoxetine, ibuprofen, p-CBA, and paracetamol, either separately or in mixture. Among them, p-CBA is an organic molecule that has been used in similar studies for the indirect estimation of •OH production in ozonation systems. It reacts slowly with molecular ozone (<0.15 M−1s−1), while at the same time presenting a much higher reactivity constant with the hydroxyl radicals (5.2 × 109 M−1s−1). Therefore, an enhancement of p-CBA removal corresponds to an increase in the production of hydroxyl radicals.
2. Materials and Methods
2.1. Materials
The organic compounds atrazine, benzotriazole, carbamazepine, caffeine, fluoxetine, ibuprofen, paracetamol, and p-chlorobenzoic acid (p-CBA), used as typical probe compounds for the study of catalytic ozonation experiments, were obtained from Sigma-Aldrich (St. Louis, MO, USA). Their physicochemical characteristics and the respective reaction rate constants with ozone and hydroxyl radicals are presented in Table 1. A stock solution of 50 mg/L was prepared by dissolving each micropollutant into deionized water.

Table 1.
The main physicochemical properties and the reaction rate constants with ozone and hydroxyl radicals of examined micropollutants.
The used matrix was dechlorinated natural potable water and its major characteristics are shown in Table 2. Ozone was produced from pure oxygen (99.9%) by using the corona discharge method in the laboratory ozonator Ozonia Triogen (Model TOGC2A) from Ozonia Triogen Ltd. (Glasgow, UK). Al2O3 and SiO2 materials (examined as catalysts) were commercially available. Both have been shown to enhance the production of hydroxyl radicals, and they present different PZC values and wettability. More details about their specific physicochemical characteristics can be found in Psaltou et al. [17].

Table 2.
The major characteristics of the water matrix (dechlorinated natural potable water) used for the experiments.
The examined solid materials were used without further purification; however, they were subjected to appropriate preparation by the application of preliminary ozonation before the experiments. Pre-ozonation is an important step in the relevant studies because it helps to simulate the real conditions of the respective full-scale applications (see Psaltou et al. [17]). Acetonitrile, phosphoric acid, methanol, and water were used for the determination of micropollutants by the application of HPLC analytical technique; they were purchased from Chem-Lab (Zedelgem, Belgium) and Sigma-Aldrich (St. Louis, MO, USA). Indigo reagent and stock solution were prepared using potassium indigo trisulfonate (TCI, Tokyo, Japan), based on the respective method described in the Standard Methods’ Handbook [24].
2.2. Ozonation Procedure
The catalytic ozonation experiments were conducted in 250 mL dark glass vessels in batch mode, at ambient temperature and applying initial ozone concentrations 2 mg/L. The pH of natural water was 7.8 without the need for further adjustment. Al2O3 and SiO2 solid materials were used as catalysts in fine-powdered form (size: <0.63 μm) and introduced into the reaction vessel just before the addition of the appropriate amount of dissolved (in deionized water) ozone. The initial micropollutants’ concentrations were either 500 μg/L or 50 μg/L, depending on the needs of each experiment, to be able to study the kinetics of ozonation processes without deviating from the concentrations commonly detected in real aquatic systems The solution was continuously stirred magnetically at 250 rpm to ensure a homogenous mixture and simultaneously to avoid the escaping of high ozone concentrations in the gaseous phase. The duration of each oxidation reaction depended on the respective probe compound under examination. Appropriate samples taken at different time intervals, according to the examined ozonation system and the oxidation reaction, were quenched by the addition of small amounts of indigo stock solution. The samples were filtered through 0.45 μm membranes; then, the residual concentrations of ozone and of micropollutants were determined accordingly. Single ozonation and adsorption experiments were also conducted by the same manner, but without the addition of catalysts or ozone, respectively. The amount of adsorbed micropollutant (μg/g, adsorption capacity) was calculated by Equation (1):
where qt is the adsorption capacity (μg/g), C0 is the initial concentration of micropollutant (μg/L), Ct is the residual concentration of micropollutant (μg/L), V is the volume of the solution (L), and W is the dry weight of the respective catalyst (g).
The presented data in the Figures are the average values obtained during independent experiments, conducted in triplicate, and the error bars represent the standard deviation between them. The red dashed line in the micropollutant removal figures represents the detection limit of the HPLC analytical technique for each micropollutant.
2.3. Analytical Techniques
The aqueous dissolved ozone concentration was determined by the application of the common indigo method [24]. The color change of indigo was measured at 600 nm with a UV-Vis spectrophotometer (Hach-Lange, model DR3900, Manchester, UK). The pH was measured using a Crison (model MM41) pH meter. The micropollutants’ residual concentrations were measured by HPLC (THERMO apparatus, Thermo, Waltham, MA, USA). A 4.6 × 250 mm reversed phase column (AGILENT, model Eclipse Plus C18) was used and the mobile phase was either a mixture of acetonitrile and phosphoric acid or methanol and water. The HPLC measurement parameters for each micropollutant and the respective linear calibration curves are presented in Table S1 and Figure S1, respectively (Supplementary Materials File). The NO3− and PO43− were measured spectrophotometrically by the appropriate Hach Lange LCK procedures with the use of a Lange model DR3900 spectrophotometer (Hach, Loveland, CO, USA). The Dissolved Organic Carbon (DOC) and the conductivity were measured by TOC-Vcsh Total Organic Carbon Analyzer (Shimadzu, Kyoto, Japan) and the CON 6+meter (Oatkon, VA, USA), respectively.
3. Results
3.1. Adsorption
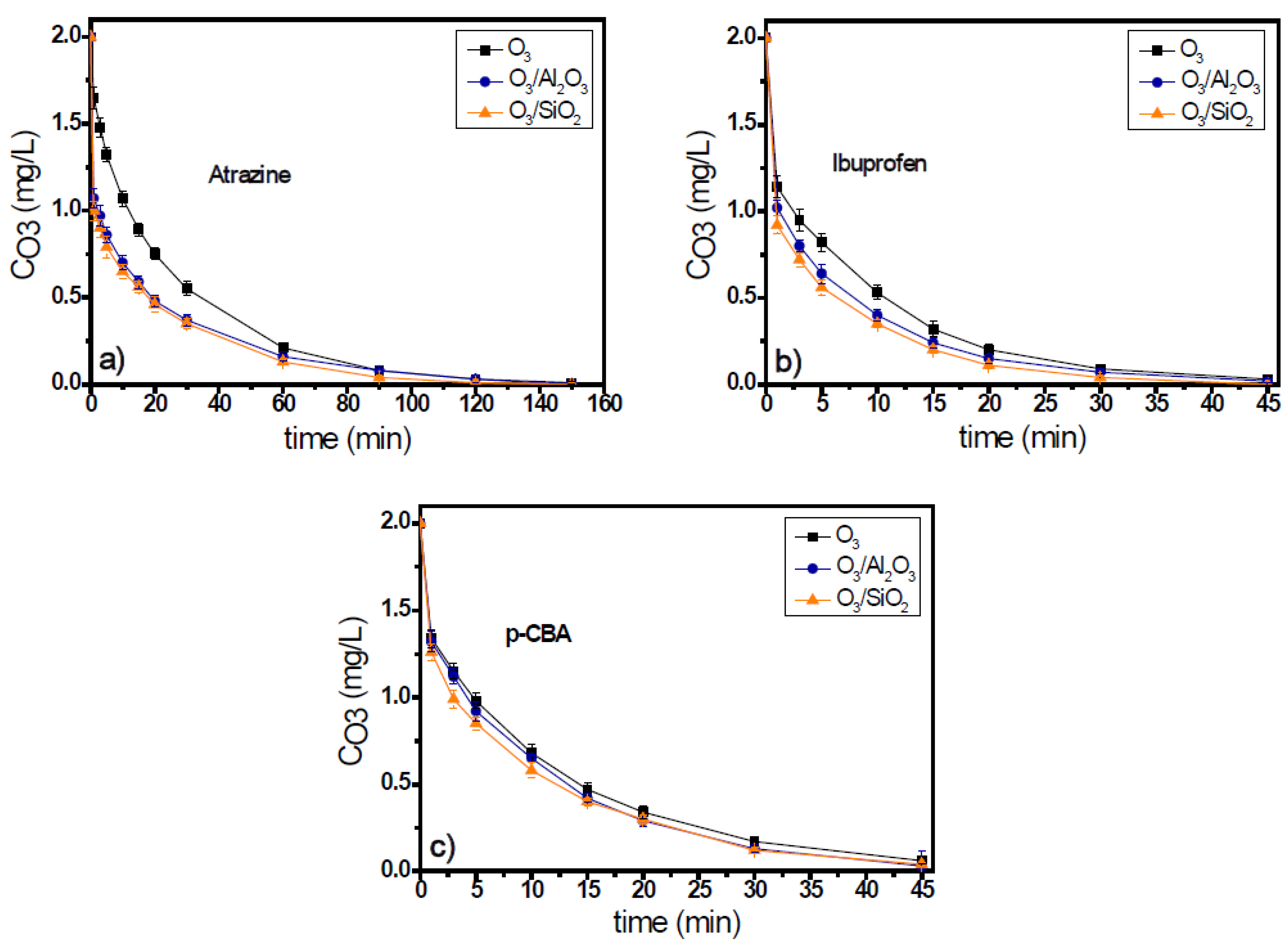
The adsorption capacity of SiO2 and Al2O3, regarding the eight examined micropollutants, and the duration of adsorption process are presented in Table 3. The duration of adsorption process was set equal to the respective oxidation time that applied for each micropollutant. Τhe decomposition time of ozone in the natural potable water, leading to the production of radicals, is different for each examined micropollutant and depends on them; e.g., it was 150 min in the presence of atrazine for the initial ozone concentration of 2 mg/L (see Figure 1), while in the case of ibuprofen, the respective oxidation process lasted 45 min (see Figure 1).

Table 3.
Adsorption capacity of Al2O3 and SiO2 materials for the examined micropollutants, presenting different physicochemical properties. Experimental conditions: Cmicr. 500 μg/L, Ccat. 0.5 g/L, pH 7.8, T 23 ± 2 °C.
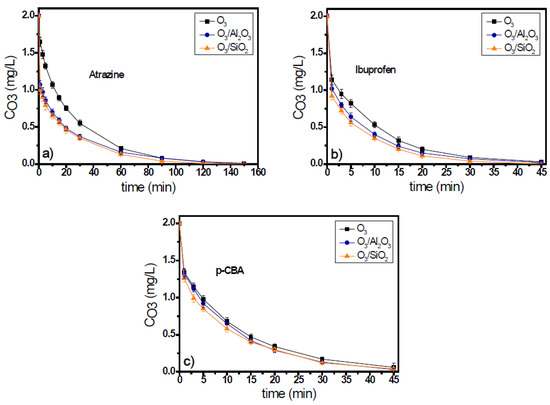
Figure 1.
Ozone decomposition during catalytic ozonation of (a) atrazine, (b) ibuprofen, and (c) p-CBA with the use of Al2O3 and SiO2 as catalysts compared to single ozonation in natural potable water. Experimental conditions: CMP 500 μg/L, Ccat. 0.5 g/L, CO3 2 mg/L, pH 7.8, T 23 ± 2 °C.
Paracetamol presented a higher adsorption for both examined solid materials during the first 20 min of the adsorption process. The efficiency of examined solids was similar to those reported in relevant studies regarding this organic compound [25,26]. The removal efficiency of paracetamol due to the adsorption process reached 76.3% and 79.4% with the use of Al2O3 or SiO2, respectively. As a result, both examined materials can be considered as rather effective adsorptive materials for the case of paracetamol, and the removal efficiency during the catalytic ozonation can be largely attributed due to the adsorption process. At pH 7.8, paracetamol is mainly in its neutral form; hence, neither repulsion nor adsorption is favored [27]. However, the amide group present in paracetamol is an activated (charged) group in the aromatic ring, and hence, its adsorption is influenced to a large extent by the electrostatic interactions with the adsorbent [28]. Therefore, the pH value plays a crucial role to the adsorption process of this molecule. According to Bernal et al. [29], the adsorption of paracetamol is favored at pH values near to neutrality.
p-CBA is a pollutant that is generally difficult to be adsorbed onto solids’ surfaces, as previously reported [30], and as Table 3 shows, the solid materials present the lowest adsorption capacity for this molecule compared to the other examined micropollutants. Other strongly charged pollutants at the applied experimental pH value were fluoxetine and ibuprofen (Table 1). Fluoxetine with pKa equal to 9.8 was positively charged, whereas ibuprofen with pKa 4.9 was negatively charged. For this reason, fluoxetine can be adsorbed to a higher extent than ibuprofen. Since the other examined micropollutants were not ionized at the experimental pH value [31,32], they presented neutral charge during the adsorption process. Among them, paracetamol as aforementioned presented higher adsorption rates for both examined solids. However, it should be noted that the adsorption process did not reach the equilibrium stage for any micropollutant, so the respective efficiencies cannot be evaluated properly. The study of the adsorption process was mainly conducted to investigate the possibility of solid materials to act as adsorbents and not as catalysts, as shown for the case of paracetamol.
3.2. Oxidation of Probe Compounds (Examined Separately)
3.2.1. Ozone-Resistant Micropollutants
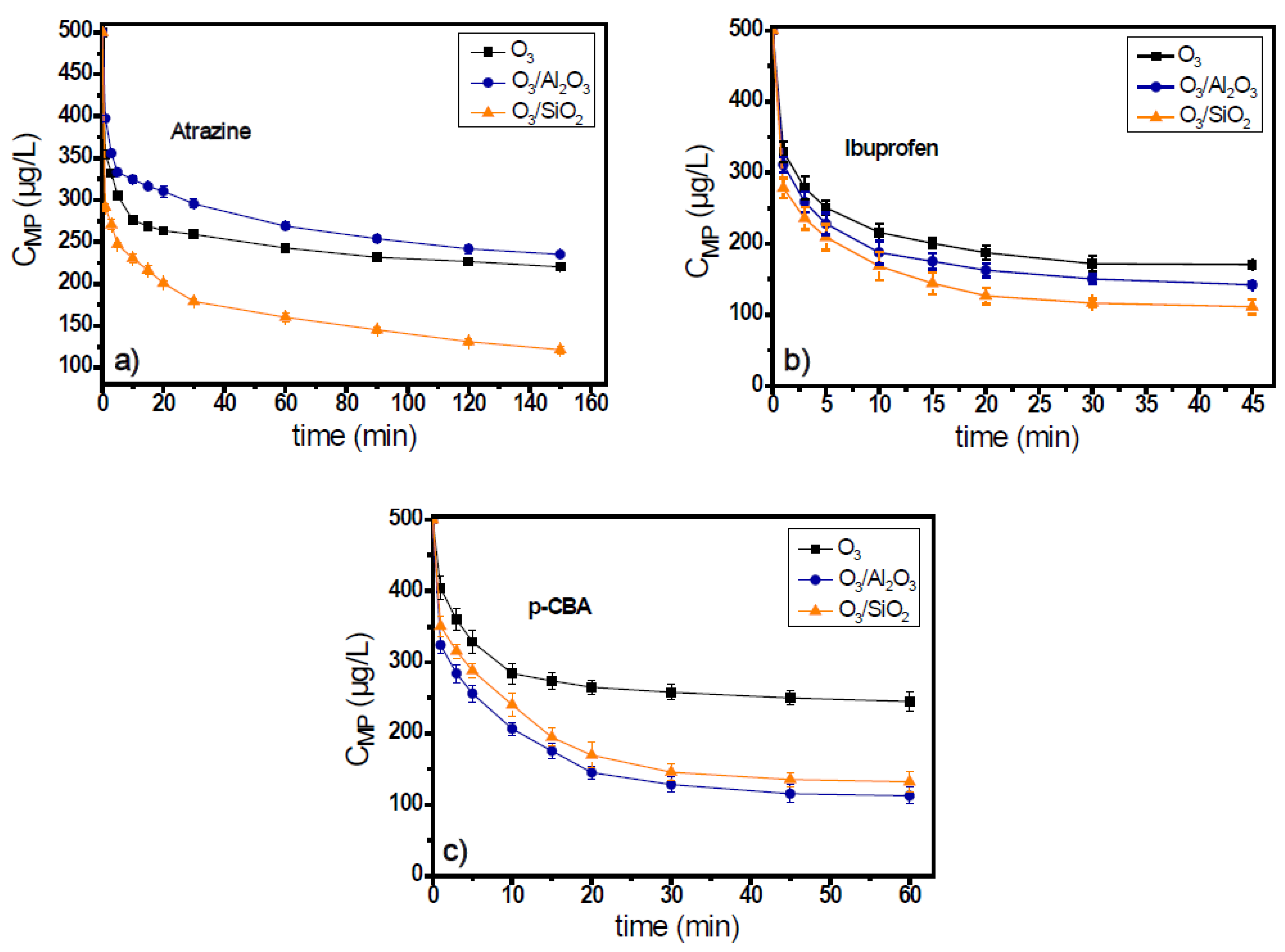
Atrazine, ibuprofen, fluoxetine, and p-CBA are generally classified as ozone-resistant organic compounds due to their very low ozone response (kinetic) rates. However, in the case of fluoxetine, due to its variable charge, according to the pH value of the used matrix, its kinetic constant at pH 7.8 was rather high (kO3 = 1.6 × 104 M−1s−1) [33], and for that reason, in this study, it will be classified with the compounds of high ozone reactivity. Figure 1a and Figure 2a show the decomposition of ozone and the degradation of atrazine during the application of catalytic ozonation with the use of Al2O3 and SiO2 materials, applied as catalysts, and the obtained results compared to that of single ozonation (i.e., without their presence).
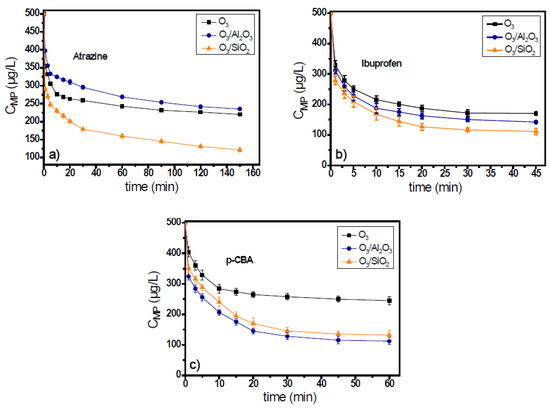
Figure 2.
Degradation of ozone-resistant micropollutants: (a) atrazine, (b) ibuprofen, and (c) p-CBA during the application of catalytic ozonation with the use of Al2O3 or SiO2 as potential catalysts, compared to the single ozonation in natural potable water matrix. Experimental conditions: CMPs 500 μg/L, Ccatalyst 0.5 g/L, CO3 2 mg/L, pH 7.8, Temperature 23 ± 2 °C.
Ozone consumption in the presence of atrazine in the ozonation systems lasted for 150 min for initial ozone concentration 2 mg/L (Figure 1a), independent of the solid material used as catalyst. Both solids increased the ozone decomposition, and after the first 30 min of oxidation process, when the major part of decomposition occurred (Figure 1a), the ozone concentration was 0.55 mg/L, 0.3 mg/L, and 0.35 mg/L (i.e., 72.5%, 85%, and 82.5% consumption) in the O3, O3/Al2O3, and O3/SiO2 systems, respectively. Although ozone decomposition was almost independent from the solid material added to the ozonation system, the efficiencies of examined materials presented differences regarding the removal of atrazine. Between the two examined materials, only SiO2 can be considered as catalyst for the case of atrazine as it increases the micropollutant’s removal when compared to the application of single ozonation. In contrast, the presence of alumina seems to inhibit the removal of atrazine (Figure 2a). The concentration of atrazine at the end of the oxidation reaction was 220 μg/L, 253 μg/L, and 121 μg/L (56%, 49.4%, and 75.8% removal), respectively.
In the case when ibuprofen was used as the probe compound, the ozone consumption was faster than in the case of atrazine. The oxidation process completed within 45 min (Figure S1b of Supplementary Materials). Both solids can enhance the decomposition of ozone, and the consumption of oxidant was quite independent of the solid material used. Similar results were observed regarding the removal of ibuprofen. Both solid materials were found to increase its removal efficiency compared to single ozonation, but SiO2 was also the best catalyst for this case. The concentration of ibuprofen at the end of the oxidation reaction was 170 μg/L, 142 μg/L, and 112 μg/L (65.9%, 71.6%, and 77.5% removal) for the O3, O3/Al2O3, and O3/SiO2 ozonation systems, respectively. However, in all these systems, the removal of ibuprofen was lower than 80% (Figure 2b).
The 3rd examined micropollutant, presenting the lowest ozone response rate constant (kO3 < 0.15 M−1s−1), was p-CBA. Figure 1c and Figure 2c show the ozone decomposition and the p-CBA removal, respectively, during the application of heterogeneous catalytic ozonation with the use of Al2O3 and SiO2 as (potential) catalysts. Similarly, for the two aforementioned cases, the consumption of ozone increased with the addition of examined solid materials, although it was independent of their nature. Regarding the removal of micropollutants, the degradation of p-CBA exhibited opposite behavior to atrazine. As Figure 2c shows, the best catalyst in the case of p-CBA removal (although only slightly) was the Al2O3 material and not the SiO2, although both can increase the degradation of ozone compared to single ozonation. The increase of p-CBA removal in the presence of alumina proves the higher hydroxyl radical’s production due to its presence in both examined matrixes (i.e., in the natural and in the deionized water), which is in agreement with a previous relevant publication [17].
3.2.2. Micropollutants with Moderate Reactivity against Ozone
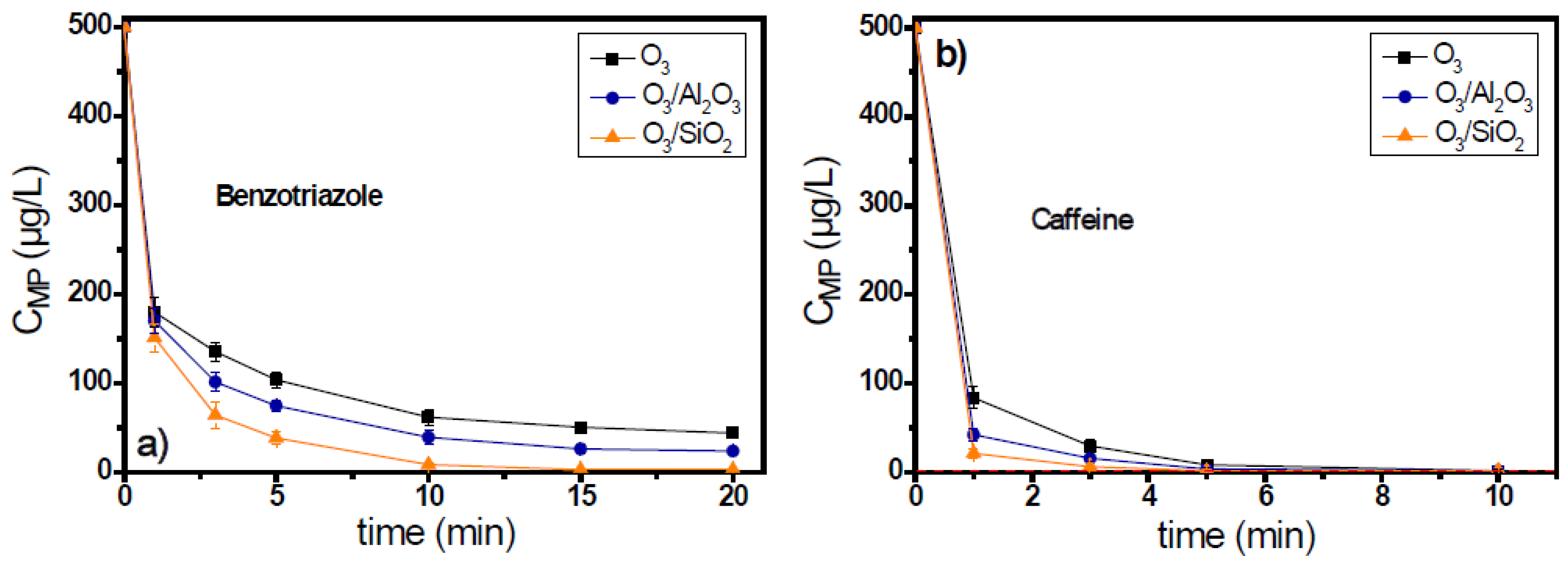
Benzotriazole and caffeine are two micropollutants which present moderate reactivity against ozone; their reaction rate constants with ozone are 20 M−1s−1 and 650 M−1s−1, respectively. Figure S2a shows the consumption of ozone during the application of catalytic ozonation for the case of benzotriazole, depending on the specific solid used as catalyst. In the presence of alumina, the concentration of ozone after 5 min of oxidation reaction was 0.3 mg/L (i.e., 85% consumption), while in the O3/SiO2 system, it was 0.1 mg/L (i.e., 95% consumption) under the same conditions, i.e., similarly to the results of micropollutant removal. The best catalyst for the removal of benzotriazole was SiO2 (Figure 3), with which the pollutants’ residual concentration reached 8 μg/L (i.e., 98.3% removal) after 10 min reaction/oxidation time, whereas after the same time, the removal with the use of alumina as catalyst was 92.1% (i.e., 39 μg/L residual concentration).
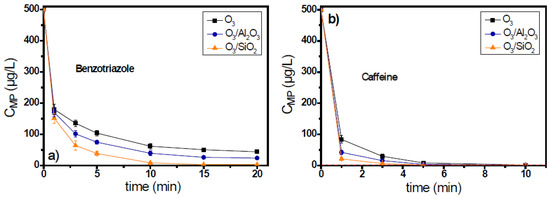
Figure 3.
Degradation of micropollutants presenting moderate ozone reactivity, (a) benzotriazole, and (b) caffeine, during the application of catalytic ozonation with the use of Al2O3 and SiO2 as potential catalysts, compared to the single ozonation in natural potable water matrix. Experimental conditions: CMPs 500 μg/L, Ccatalyst 0.5 g/L, CO3 2 mg/L, pH 7.8, Temperature 23 ± 2 °C.
The addition of caffeine in the ozonation system resulted in the extension of respective ozone decomposition time up to 60 min (Figure S2b of Supplementary Materials). Both examined solid materials were found to increase the decomposition of ozone, but in contrast with the previous case of benzotriazole, the rate of ozone decomposition was similar for both materials. The results regarding the removal of caffeine were similar (Figure 3b). Thus, both solids can be characterized as catalysts for the case of caffeine because they presented similar results throughout the oxidation reaction. Caffeine, with a reaction rate constant towards ozone equal to 650 M−1s−1, can be more easily degraded than benzotriazole by the interaction with molecular ozone; even the single ozonation process can cause its almost complete removal (<1.6 μg/L) within 5 min of the oxidation reaction.
3.2.3. Micropollutants Presenting High Reactivity against Ozone
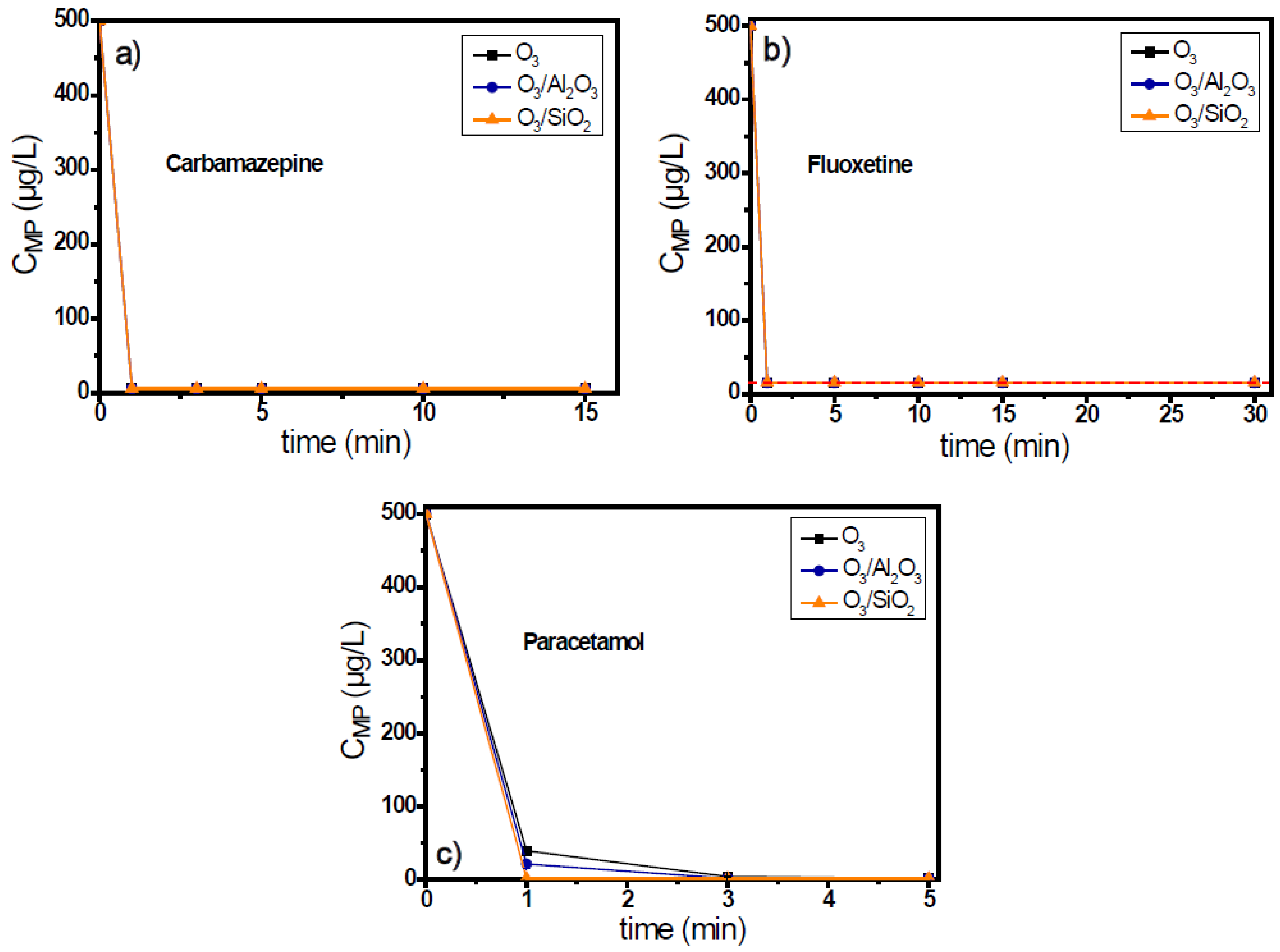
The remaining three examined micropollutants were carbamazepine, fluoxetine, and paracetamol. These compounds present high reaction rate constants with ozone and are easily degraded by its presence. In all examined cases, the addition of both solid materials was found to increase the decomposition of ozone compared to the single ozonation process, whereas higher decomposition rates were presented by the case of SiO2 (Figure S3). However, when fluoxetine was used as the probe compound, the decomposition of ozone was found to be similar for both examined materials (Figure S3b).
The residual concentration of micropollutants was lower than the respective detection limit of analytical method, independently of the used ozonation system, even after the first min of oxidation reaction (Figure 4). The only difference between these three cases was the different detection limit by the application of HPLC analytical method. Paracetamol presents the lowest detection limit among these organic compounds (equal to 1.2 μg/L), while the concentration of carbamazepine can be detected up to 5.9 μg/L lowest concentration. In contrast, fluoxetine presented the highest detection limit, equal to 15.5 μg/L, and therefore, the concentration of fluoxetine degradation cannot be accurately identified with this technique when below this concentration.
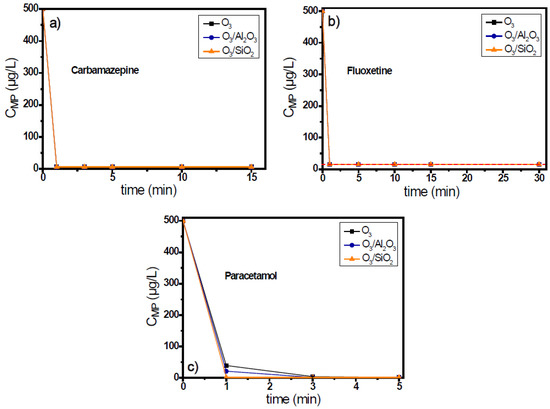
Figure 4.
Degradation of micropollutants presenting high ozone reactivity, (a) carbamazepine, (b) fluoxetine, and (c) paracetamol, during the application of catalytic ozonation with the use of Al2O3 and SiO2 as potential catalysts compared to single ozonation in natural potable water matrix. Experimental conditions: CMPs 500 μg/L, Ccatalyst 0.5 g/L, CO3 2 mg/L, pH 7.8, Temperature 23 ± 2 °C.
3.3. Oxidation of Micropollutant Mixture
This section examines the performance of catalysts in a mixed pollution system, containing all the aforementioned eight micropollutants, considering an initial concentration of 50 μg/L for each of them. The concentration of each micropollutant was actually 1/10 of the initial concentration used in the previous experiments, and the total concentration of all of them approached the initial concentration of the previous experiments.
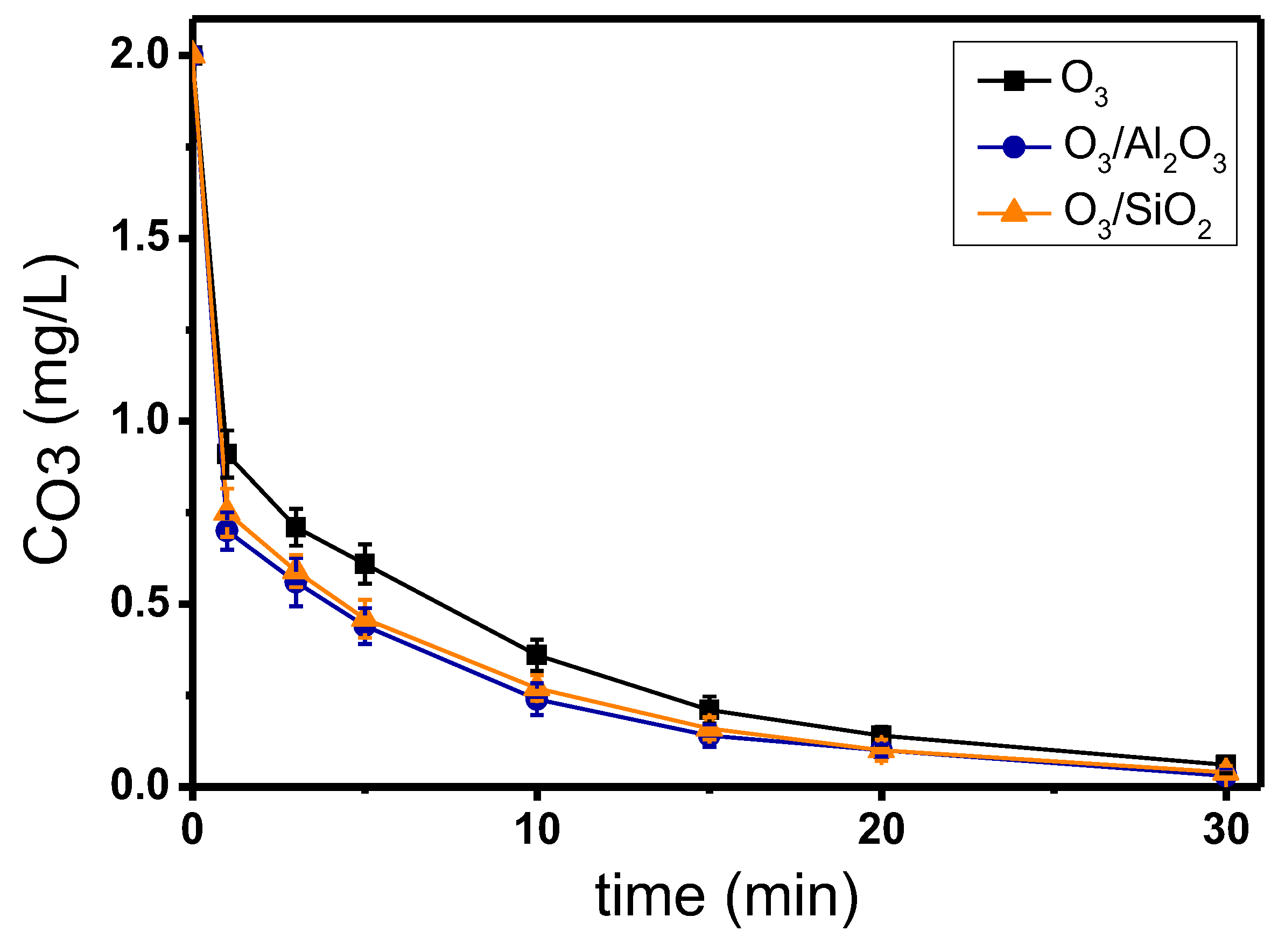
Figure 5 shows the decomposition of ozone during the application of single and catalytic ozonation experiments, regarding the micropollutant mixture, by using Al2O3 or SiO2 as potential catalysts. Although each micropollutant was found to lead the oxidation reaction towards different completion times, the combined influence of all the examined organic compounds together as a mixture had an overall ozone decomposition time to 30 min. Furthermore, as in the previous experiments, the addition of catalysts increased the decomposition of ozone when compared to single ozonation, but without significant differences between the two examined solids. The 1st-order kinetic constants of ozone decomposition in this set of experiments were 0.094 min−1, 0.107 min−1, and 0.104 min−1 for the processes of O3, O3/Al2O3, and O3/SiO2, respectively, which are quite close to the average sum of 1st-order kinetic constants of ozone decomposition in the separate experiments using one probe compound each time (Table S2).
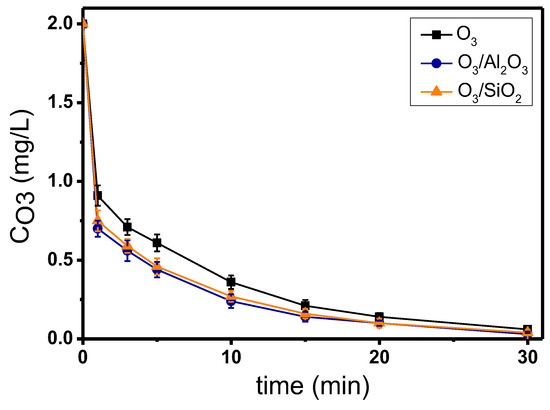
Figure 5.
Ozone decomposition during catalytic ozonation of the mixture of the 8 examined micropollutants with the use of Al2O3 and SiO2 as catalysts compared to single ozonation in natural potable water. Experimental conditions: CMP. 50 μg/L, Ccat. 0.5 g/L, CO3 2 mg/L, pH 7.8, T 23 ± 2 °C.
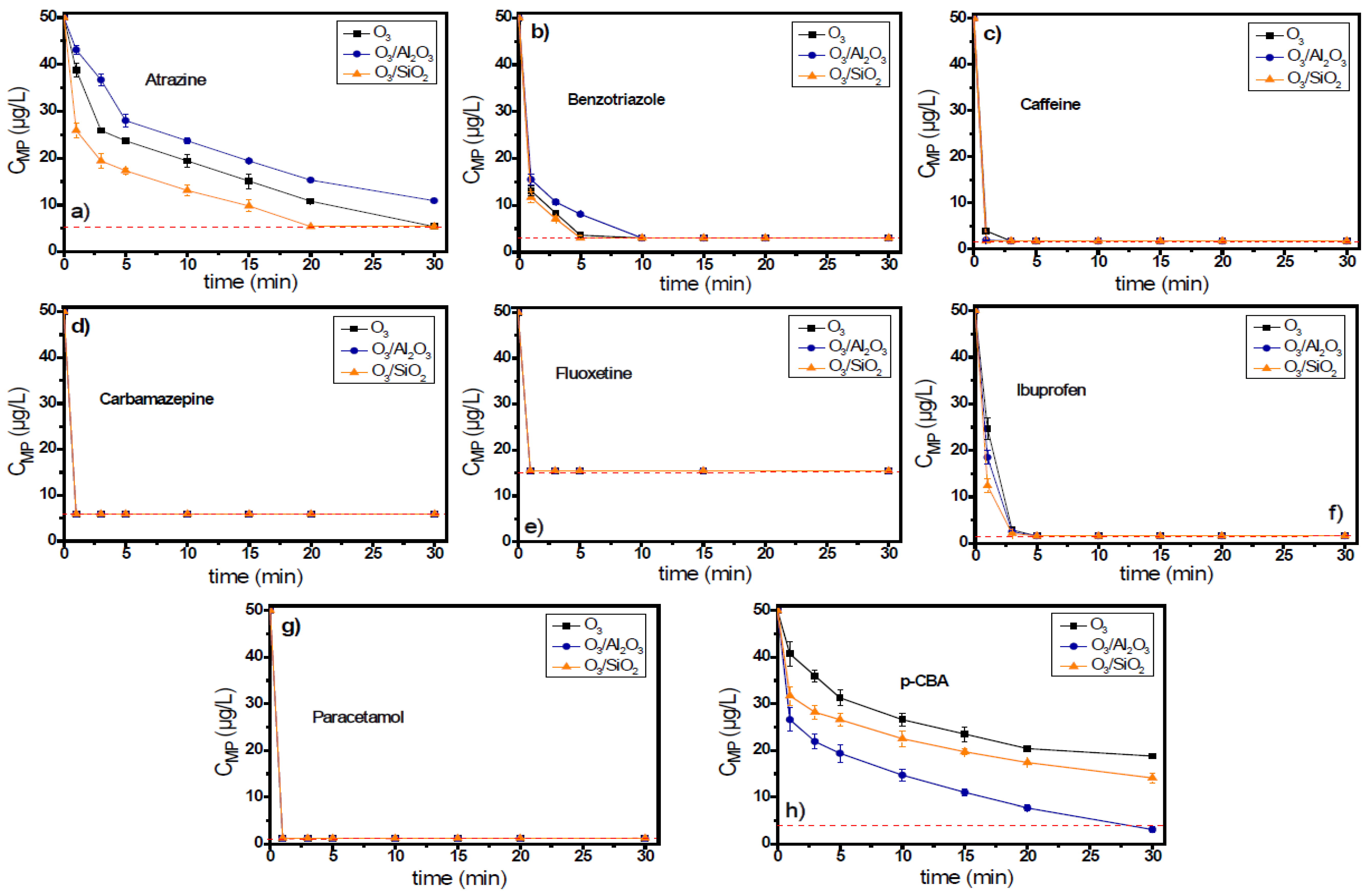
Figure 6 shows the removal of each micropollutant separately in the mixed system during the application of single or catalytic ozonation experiments. The red dashed line represents the detection limit of the respective analytical technique for each micropollutant. In most cases, the reduction of initial concentration of micropollutants increased their removal rates, and as a result, the residual concentrations of all examined micropollutants in the mixture were found to be below the respective analytical detection limits.
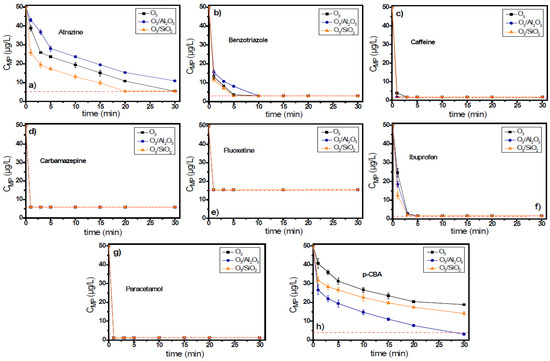
Figure 6.
The degradation of (a) atrazine, (b) benzotriazole, (c) caffeine, (d) carbamazepine, (e) fluoxetine, (f) ibuprofen, (g) paracetamol, and (h) p-CBA in a mixed solution during the application of catalytic ozonation with the use of Al2O3 or SiO2 as potential catalysts compared to the application of single ozonation in natural potable water matrix. Experimental conditions: CMPs 50 μg/L, Ccatalyst 0.5 g/L, CO3 2 mg/L, pH 7.8, Temperature 23 ± 2 °C.
Atrazine presented the same behavior as in the previous experiments. It was removed by 78.2% in this case (i.e., 10.9 μg/L residual concentration) after 30 min of oxidation reaction, although a similar residual concentration (10.8 μg/L) was observed also for the application of single ozonation process after 20 min of oxidation/contact time. With the application of single ozonation, the concentration of atrazine after the completion of the oxidation reaction (30 min) was under the respective analytical detection limit (5.4 μg/L). In contrast, the addition of SiO2 can remove this pollutant by at least 89.2% (i.e., leading to <5.4 μg/L residual concentration) after 20 min of reaction time. Similar results, but to a lower extent, were also observed for the cases of benzotriazole. Benzotriazole can be also be oxidized directly by ozone, and as a result, it presents higher removal rates and smaller differences between the different examined ozonation processes. In this case, the addition of alumina in the ozonation process was also found to reduce the removal rate of this compound compared to single ozonation. After the initial 3 min of the oxidation process, the residual concentration of benzotriazole by the application of O3, O3/Al2O3, and O3/SiO2 systems was 8.3 μg/L, 10.7 μg/L, and 7.1 μg/L (83.3%, 78.6%, and 85.7% removal), respectively.
The compound removed with the highest rate in the examined mixture of micropollutants by the application of a O3/Al2O3 system was p-CBA, similarly to the experiments performed separately. p-CBA presented a reaction rate constant with hydroxyl radicals higher than that of atrazine (Table 1), and hence, it can react faster with them in the bulk solution, inhibiting the removal of co-existing atrazine to some extent. After the completion of oxidation reaction, the p-CBA residual concentrations for the O3, O3/Al2O3, and O3/SiO2 systems were 18.8 μg/L, ≤3.1 μg/L, and 14.1 μg/L, respectively.
The difference between the two performed sets of experiments (separately and mixture) was best observed for the case of benzotriazole. In the mixture, the removal rates of this organic compound in the presence of alumina were found to be lower than that of the single ozonation case, probably due to competition with the other co-existing organic compounds. All other examined micropollutants were removed effectively, even from the 1st minute of reaction, independent of the ozonation process examined. Even ibuprofen, which is considered to be an ozone-resistant compound, was able to be removed within the initial 3 min of the oxidation reaction by applying all (three) different oxidation processes.
Reducing the concentration of micropollutants in the mixture to 1/10 of the initial concentrations of the separate pollutants’ experiments (Section 3.1) resulted in an increase in removal for certain examined organic compounds. This is because the micropollutants presenting a higher reactivity against ozone can be removed quickly, even during the 1st min of oxidation reaction, due to their reaction with both oxidation agents (ozone and hydroxyl radicals). Therefore, the remaining oxidizing agents can react significantly with the other five compounds after the removal of the first three micropollutants, i.e., with those belonging to either the group of moderately active compounds, or with the group of organic compounds resistant to ozone. Furthermore, the addition of different micropollutants in the ozonation system changed the overall ozone decomposition rate, and consequently, that of the production of hydroxyl radicals, hence enhancing the removal of ozone-resistant compounds. However, a further reduction in the initial concentration of micropollutants does not necessarily imply a further increase in removal efficiencies, as the even smaller initial concentrations can make efficient contact between the oxidizing species and the pollutants more difficult.
4. Discussion
Catalytic ozonation is a water/wastewater treatment method for the removal of micropollutants which has shown promising results [34]. The key to this process is to find the right catalyst for the removal of these low-concentration organic compounds [35] that can enhance the production of hydroxyl radicals and the contact between the catalyst and the compound. In the present study, a variety of micropollutants with different properties and reaction rate constants with ozone and hydroxyl radicals were used as probe compounds in catalytic ozonation processes for the evaluation of SiO2 and Al2O3 as potential catalysts under real conditions.
In terms of micropollutant removal, it was observed that when the ozonation process is applied as a treatment method, the ozone resistant compounds are what determine the effectiveness of it. Atrazine is a pollutant which reacts rather slowly with ozone, whereas it presents a higher ozone reactivity constant with the hydroxyl radicals. In a previously published study [17], where deionized water was used as the matrix, it was proven that SiO2 and Al2O3 can increase the production of hydroxyl radicals compared to the single ozonation process. As shown in Table 3, the adsorption capacity of SiO2 regarding atrazine is higher than that of Al2O3. To a certain extent, this can help to explain the improvement of catalytic ozonation efficiency but cannot sufficiently justify the large difference of micropollutants’ removal efficiencies as observed between these two materials.
In order for a material to be an efficient catalyst in the heterogeneous catalytic ozonation process, in some cases, such as those of ozone-resistant organic compounds, it must not only be able to increase the production of hydroxyl radicals, but also to bring the pollutant into a close encounter with these radicals to be efficiently oxidized. Therefore, the contact between the pollutant and the catalyst surface is necessary. The improvement regarding the removal of atrazine only by the presence of SiO2 (Figure 2) suggests that the organic molecule can be bonded (through hydrogen bonds) with the Si surface, but not with the Al, as other relevant research has also previously reported [36,37,38].
Atrazine at pH 7.8 is neutrally charged [39], whereas the surface of silica is mostly occupied by silanol groups. This suggests that atrazine is mainly physisorbed to the silanol groups and/or surface water. In environments where the silica surface is fully hydrated, the driving force of adsorption for water-almost-insoluble compounds, such as atrazine, is the exclusion of these compounds from the aqueous phase, due to the hydrophobicity effect [37]. Clausen et al. [38] observed that when atrazine was not charged, it could be adsorbed on the kaolin surface, but not on the surface of alumina. The non-adsorption on alumina suggests that in the case of kaolin, which contains both silica and alumina, the adsorption occurs due to the surface groups of silica. This absence of interaction between alumina and atrazine was also observed by Czaplicka et al. [36], who simultaneously confirmed through respective FT-IR measurements that the 1,3,5–azidine ring of the atrazine molecule interacts with the SiO2 molecules, possibly through the formation of hydrogen bonds between atrazine and silica, such as the one shown in Figure S4.
Another micropollutant in this study that contains nitrogen in its ring as does atrazine is benzotriazole. The results revealed that for this compound, SiO2 showed the highest efficiency, while Al2O3 presents similar results to single ozonation.
In contrast to atrazine and benzotriazole, the p-CBA molecule was oxidized mainly in the bulk solution, and it can be removed to a higher extent by the process using the catalyst, leading to greater production of radicals. The p-CBA was adsorbed to lesser extent, and therefore, the adsorption process in this case cannot be considered to contribute significantly to its removal; therefore, the best catalyst is alumina. Alumina at pH 7.8 was almost neutrally charged, enhancing the contact of ozone with its surface [17] and the consequent increase in the production of hydroxyl radicals.
The 3rd ozone-resistant micropollutant, ibuprofen, similarly to p-CBA, is a nitrogen-free compound in structure, and although it is as difficult to be degraded by ozone molecules (kO3= 9.6 M−1s−1) as atrazine, both solid materials were found to increase its removal, proving once again that the two metal oxides can enhance the production of hydroxyl radicals. The solid materials presented a higher adsorption capacity for ibuprofen than for p-CBA, contributing to the overall removal of the organic compound.
These observations highlight the significant role of chemical affinity between the catalyst and the pollutant in the catalytic ozonation process. This oxidation technology is promising for reduction of the occurrence of the micropollutants in water bodies, but to be efficient, the appropriate catalysts must be chosen depending on the type of micropollutants that are present. Therefore, a diligent micropollutant detection study is considered necessary before the application of catalytic ozonation.
5. Conclusions
The removal of different micropollutants by the application of catalytic ozonation depends on their physicochemical properties and their reactivity with ozone. Those with high ozone reactivity (i.e., carbamazepine, paracetamol, fluoxetine) were removed effectively even after the 1st min of the oxidation reaction by single ozonation. On the other hand, the removal of benzotriazole and caffeine (i.e., typical micropollutants presenting moderate ozone reactivity) was enhanced by the addition of the examined catalysts; in this case, the best catalyst for both compounds was SiO2. Regarding the ozone-resistant micropollutants (i.e., atrazine, ibuprofen, p-CBA) both examined solid materials presented sufficient catalytic activity for ibuprofen and p-CBA; however, the best material was SiO2 and Al2O3, respectively. In contrast, Al2O3 cannot be considered as an effective catalyst for the removal of atrazine. The oxidation of p-CBA is based on the oxidation reactions in the bulk solution, and it is favored by the addition of alumina in the ozonation system, leading to higher production of •OH. In contrast, atrazine, which can create a bond with the surface of silica (chemical affinity), is largely removed by its presence. However, the high catalytic activity of SiO2 also suggests the important role of wettability in the ozonation process, a significant parameter which will be under consideration in future relevant research. When treating a mixture of micropollutants, the results were found to be similar to the separate ones (containing only one compound), although higher removal rates were observed, due to the smaller initial concentrations. Even the ozone-resistant micropollutants were removed effectively because of the simultaneous presence of several organic compounds increasing their removal by changing the overall ozone decomposition rate.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/separations9070173/s1. Figure S1: Linear calibration curve for the determination of (a) atrazine, (b) benzotriazole, (c) caffeine, (d) carbamazepine, (e) fluoxetine, (f) ibuprofen, (g) paracetamol, and (h) p-CBA in the concentration range of 0–600 μg/L; Figure S2: Ozone decomposition during catalytic ozonation of (a) benzotriazole and (b) caffeine with the use of Al2O3 and SiO2 as catalysts compared to single ozonation in natural potable water; Figure S3: Ozone decomposition during catalytic ozonation of (a) carbamazepine, (b) fluoxetine, and (c) paracetamol with the use of Al2O3 and SiO2 as catalysts compared to single ozonation in natural potable water; Figure S4: Hydrogen bonds between atrazine and silica surface; Table S1: HPLC measurement parameters for the determination of the concentration of the examined 8 micropollutants; Table S2: 1st order reaction rate constants of ozone decomposition during catalytic ozonation with the use of SiO2 and Al2O3 as catalysts for ozone concentration of 2 mg/L in experiments with one probe compound.
Author Contributions
Conceptualization, S.P., M.M., and A.Z.; methodology, S.P. and M.M.; validation, S.P., M.M., and A.Z.; formal analysis, S.P. and E.K.; investigation, S.P. and A.T.; resources, M.M. and A.Z.; data curation, S.P. and E.K.; writing—original draft preparation, S.P.; writing—review and editing, M.M. and A.Z.; visualization, S.P.; supervision, M.M. and A.Z.; project administration, A.Z.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the name RESEARCH-CREATE-INNOVATE (project code: T1EDK-02397).
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetileke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Zoh, K. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Hess-Wilson, J.K.; Knudsen, K.E. Endocrine disrupting compounds and prostate cancer. Cancer Lett. 2006, 241, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S.; Thomaidis, N.S.; Arvaniti, O.S.; Asimakopoulos, A.G.; Samaras, V.G.; Ajibola, A.; Mamais, D.; Lekkas, T.D. Contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in sewage treatment plant. Sci. Total Environ. 2013, 463–464, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Underman, E.; Rasmusson, K.; Kokorite, I.; Leppänen, M.T.; Larsen, M.M.; Pazdro, K.; Siedlewicz, G. Micropollutants in urban wastewater: Large-scale emission estimates and analysis of measured concentrations in the Baltic Sea catchment. Mar. Pollut. Bull. 2022, 178, 113559. [Google Scholar] [CrossRef] [PubMed]
- European Union Strategic Approach to Pharmaceuticals in the Environment as Required by Article 8c of Directive 2008/105/EC as Amended by Directive 2013/39/EU. Available online: https://ec.europa.eu/environment/water/water-dangersub/pharmaceuticals.htm (accessed on 8 June 2002).
- Urban Waste Water Treatment Directive—Review. Available online: https://ec.europa.eu/environment/water/water-urbanwaste/evaluation/index_en.htm (accessed on 8 June 2022).
- Nawrocki, J. Catalytic ozonation in water: Controversies and questions. Discussion paper. Appl. Catal. B Environ. 2013, 142–143, 465–471. [Google Scholar] [CrossRef]
- Ghupe, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents—Application of mesoporous materials: A review. J. Environ. Manag. 2018, 211, 83–102. [Google Scholar] [CrossRef]
- Roshani, B.; McMaster, I.; Rezaei, E.; Soltan, J. Catalytic ozonation of benzotriazole over alumina supported transition metal oxide catalysts in water. Sep. Purif. Technol. 2014, 135, 158–164. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, R.; Zhao, X.; Qu, J. Effect of manganese ion on the mineralization of 2.4 dichlorophenol by ozone. Chemosphere 2008, 72, 1006–1012. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Wang, B.; Deng, S.; Huang, J.; Yu, G.; Wang, Y. Prediction of micropollutant abatement during homogeneous catalytic ozonation by a chemical kinetic model. Water Res. 2018, 142, 383–395. [Google Scholar] [CrossRef]
- Hübner, U.; Zucker, I.; Jekel, M. Options and limitations of hydrogen peroxide addition to enhance radical formation during ozonation of secondary effluents. J. Water Reuse Desalin. 2015, 12, 127–134. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, J.; Hua, X.; Liang, D.; Dong, D.; Guo, Z.; Zheng, N.; Zhang, L. Catalytic ozonation for the degradation of polyvinyl alcohol in aqueous solution using catalyst based on copper and manganese. J. Clean. Prod. 2020, 272, 122856. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Zhang, L. Enhanced mineralization of pharmaceuticals by surface oxidation over mesoporous γ-Ti-Al2O3 suspension with ozone. Appl. Catal. B Environ. 2017, 202, 118–126. [Google Scholar] [CrossRef]
- Xiong, P.; Fan, S.; Song, J.; Dai, Q. Mechanism of catalytic ozonation for elimination of methyldopa with Fe3O4@SiO2@CeO catalyst. Water Environ. Res. 2021, 93, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Psaltou, S.; Kaprara, E.; Triantafyllidis, K.; Mitrakas, M.; Zouboulis, A. Heterogeneous catalytic ozonation: The significant contribution of PZC value and wettability of the catalysts. J. Environ. Chem. Eng. 2021, 9, 106173. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Gonzalo, M.S.; García-Calvo, E. Catalytic ozonation of naproxen and carbamazepine on titanium dioxide. Appl. Catal. B 2008, 84, 48–57. [Google Scholar] [CrossRef]
- Mandal, S. Reaction rate constants of hydroxyl radicals with micropollutants and their significance in advanced oxidation processes. J. Adv. Oxid. Technol. 2018, 21, 178–195. [Google Scholar] [CrossRef]
- Du, M.S.; Chen, K.P.; Lin, Y.P. Degradation of ibuprofen and acetlsulfamethoxazole by multi-walled carbon nanotube catalytic ozonation: Surface properties, kinetics, and modeling. Environ. Sci. Water Res. 2019, 5, 1758–1768. [Google Scholar] [CrossRef]
- Zoumpouli, A.G.; Souza, F.S.; Petrie, B.; Féris, L.A.; Kasprzyk-Hordern, B.; Wenk, J. Simultaneous ozonation of 90 organic micropollutants including illicit drugs and their metabolites in different water matrices. Environ. Sci. Water Res. Technol. 2020, 6, 2465–2478. [Google Scholar] [CrossRef]
- Lan, B.; Huang, R.; Li, L. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chem. Eng. J. 2013, 219, 346–354. [Google Scholar] [CrossRef]
- El Najjar, N.H.; Touffet, A.; Deborde, M.; Journel, R.; Vel Leitner, N.K. Kinetics of paracetamol oxidation by ozone and hydroxyl radicals, formation of transformation products and toxicity. Sep. Purif. Technol. 2014, 136, 137–143. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Trussel, R.R.; Greenberg, A. Standard Methods: For Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Elamin, M.R.; Abdulkhair, B.; Algethami, F.K.; Khezami, L. Linear and nonlinear investigations for the adsorption of paracetamol and metformin from water on acid-treated clay. Sci. Rep. 2021, 11, 13606. [Google Scholar] [CrossRef] [PubMed]
- Sumalinog, D.A.; Capareda, S.C.; de Luna, M.D. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 2010, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Iovino, P.; Canzano, S.; Capasso, S.; Erto, A.; Musmarra, D. A modeling analysis for the assessment of ibuprofen adsorption mechanism onto activated carbon. Chem. Eng. J. 2015, 277, 360–367. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Couto, O.M.; Carvalho, K.Q.; Arroyo, P.A.; Barros, M.A.S.D. Effect of solution pH on the removal of paracetamol by activated carbon of dende coconut mesocarp. Chem. Biochem. Eng. Q. 2015, 29, 47–53. [Google Scholar] [CrossRef]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of solution pH on the adsorption of paracetamol on chemically modified activated carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Pines, D.S.; Reckhow, D.A. Solid phase catalytic ozonation process for the destruction of a model pollutant. Ozone Sci. Eng. 2003, 25, 25–39. [Google Scholar] [CrossRef]
- Tan, B.; Guo, L.; Yin, D.; Ma, T.; Zhang, S.; Wang, C. Environmentally sustainable corrosion inhibitors used for electronics industry (Chap.). In Environmentally Sustainable Corrosion Inhibitors—Fundamental and Industrial Applications; Chaudhery, H., Chandrabhan, V., Jeenat, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 359–381. ISBN 9780323854054. [Google Scholar] [CrossRef]
- Mennickent, S.; Vega, M.; Godoy, C.G. Development and validation of a method using instrumental planar chromatography for quantitative analysis of carbamazepine in saliva. J. Chil. Chem. Soc. 2003, 48, 71–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, G.; Chen, S.; Zhang, S.; Wang, B.; Huang, J.; Deng, S.; Wang, Y. Ozonation of antidepressant fluoxetine and its metabolite product norfluoxetine: Kinetics, intermediates and toxicity. Chem. Eng. J. 2017, 316, 951–963. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Fuentes, I.; Aguilar, C.M.; Valenzuela, M.A.; Poznyak, T.; Chairez, I. Catalytic Ozonation as a Promising Technology for Application in Water Treatment: Advantages and Constrains. In Ozone in Nature and Practice; Derco, J., Koman, M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar] [CrossRef] [Green Version]
- Czaplicka, M.; Barchanska, H.; Jaworek, K.; Kaczmarczyk, B. The interaction between atrazine and the mineral horizon of soil: A spectroscopic study. J. Soils Sediments 2018, 18, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Casillas-Ituarte, N.N.; Allen, H.C. Water, chloroform, acetonitrile, and atrazine adsorption to the amorphous silica surface studied by vibrational sum frequency generation spectroscopy. Chem. Phys. Lett. 2009, 483, 84–89. [Google Scholar] [CrossRef]
- Clausen, L.; Fabricius, I.; Madsen, L. Adsorption of pesticides onto quartz, calcite, kaolinite, and alpha-alumina. J. Environ. Qual. 2001, 30, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.; Silva, B.; Tavares, T. Cleaner approach for atrazine removal using recycling biowaste/waste permeable barriers. Recycling 2021, 6, 41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).