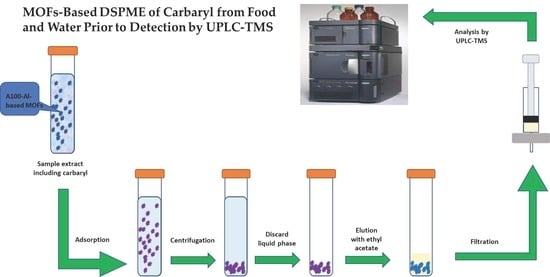
Metal Organic Framework-Based Dispersive Solid-Phase Microextraction of Carbaryl from Food and Water Prior to Detection by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Instruments
2.2. DSPME Preconcentration Process
3. Results and Discussion
3.1. Optimization of the Conditions for the Preconcentration of Carbaryl
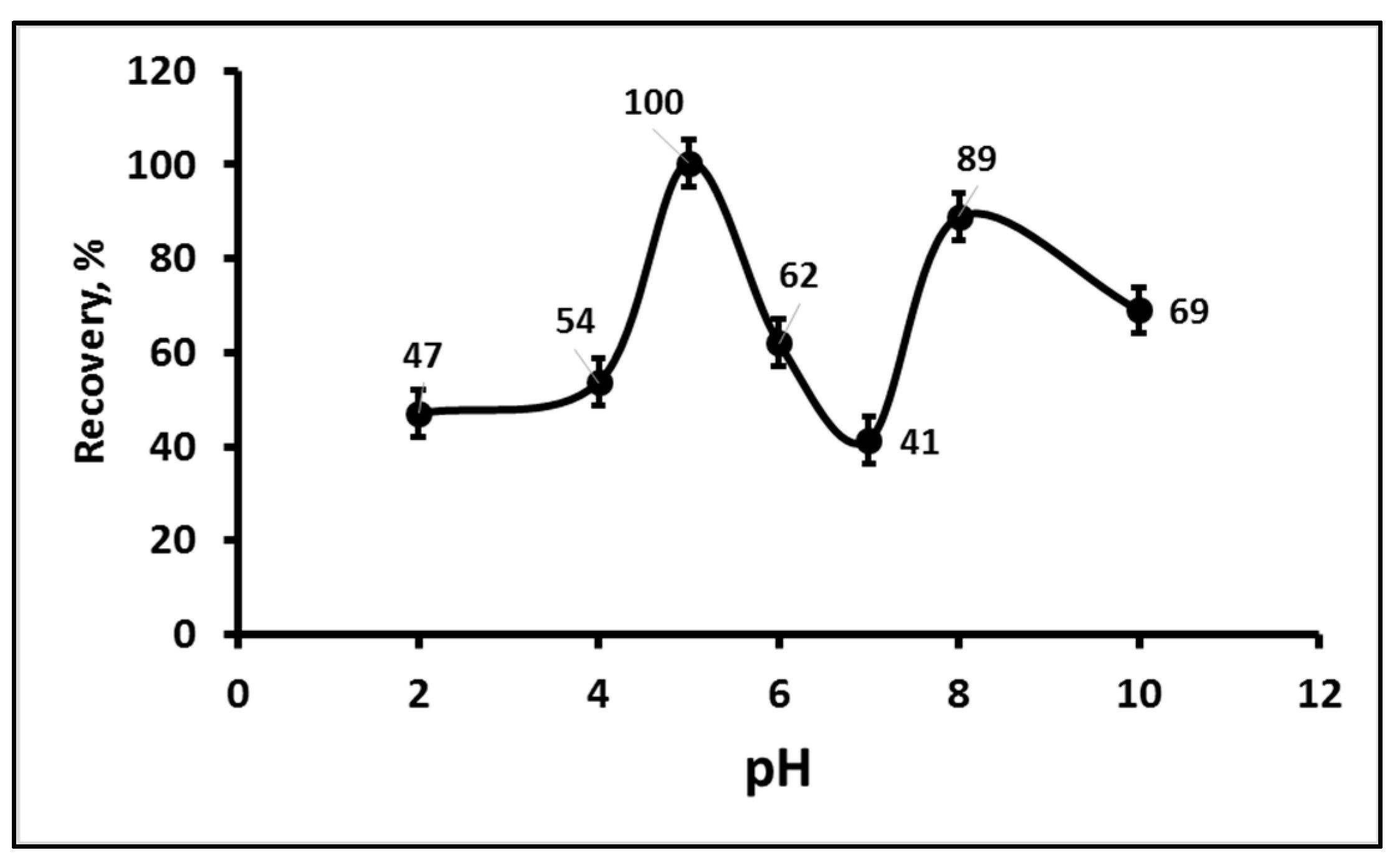
3.1.1. Effect of the Apparent pH on Carbaryl Recovery%
3.1.2. Effect of the Eluent Type on the Desorption of Carbaryl
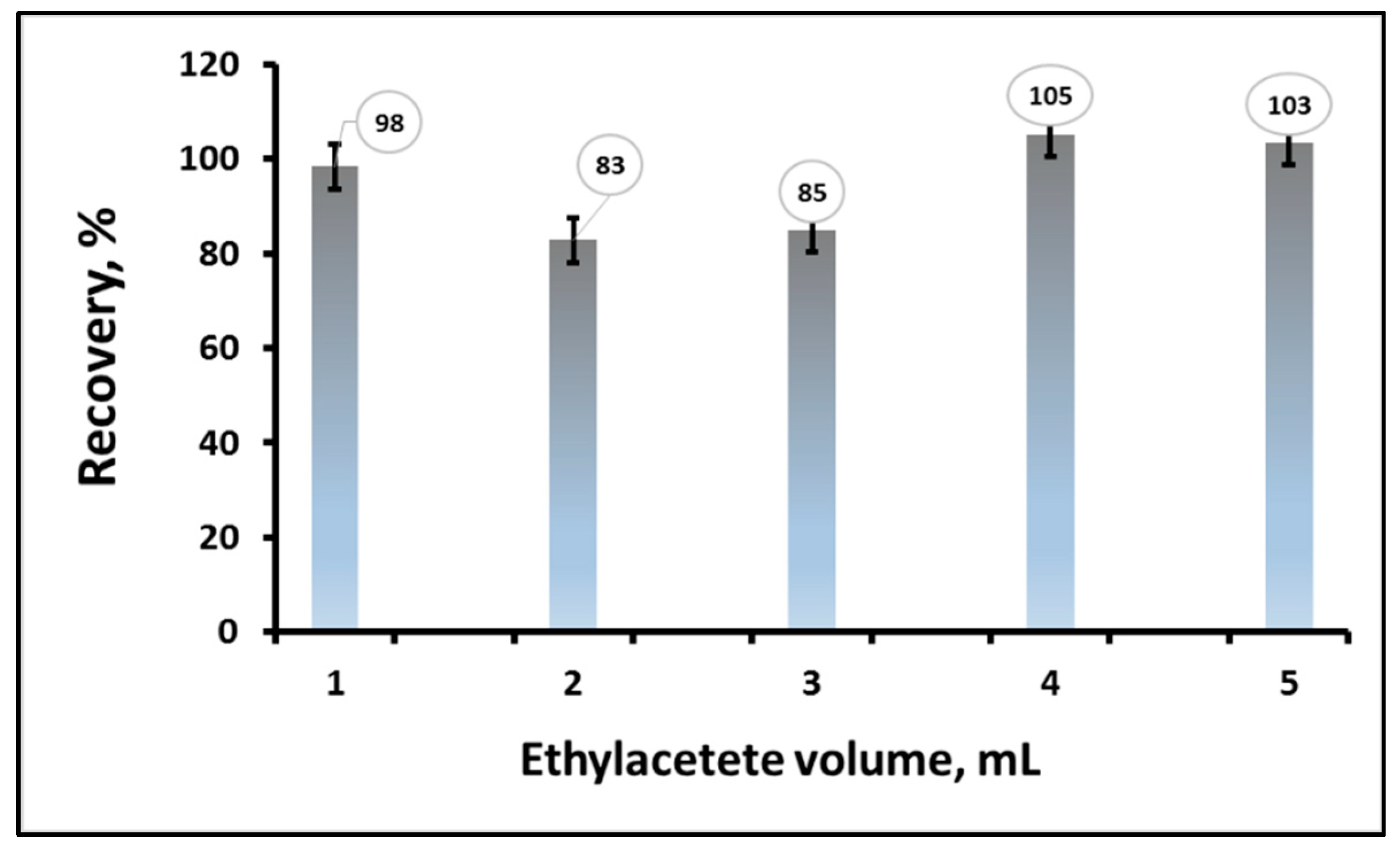
3.1.3. Effect of the Eluent Volume on Carbaryl Recovery%
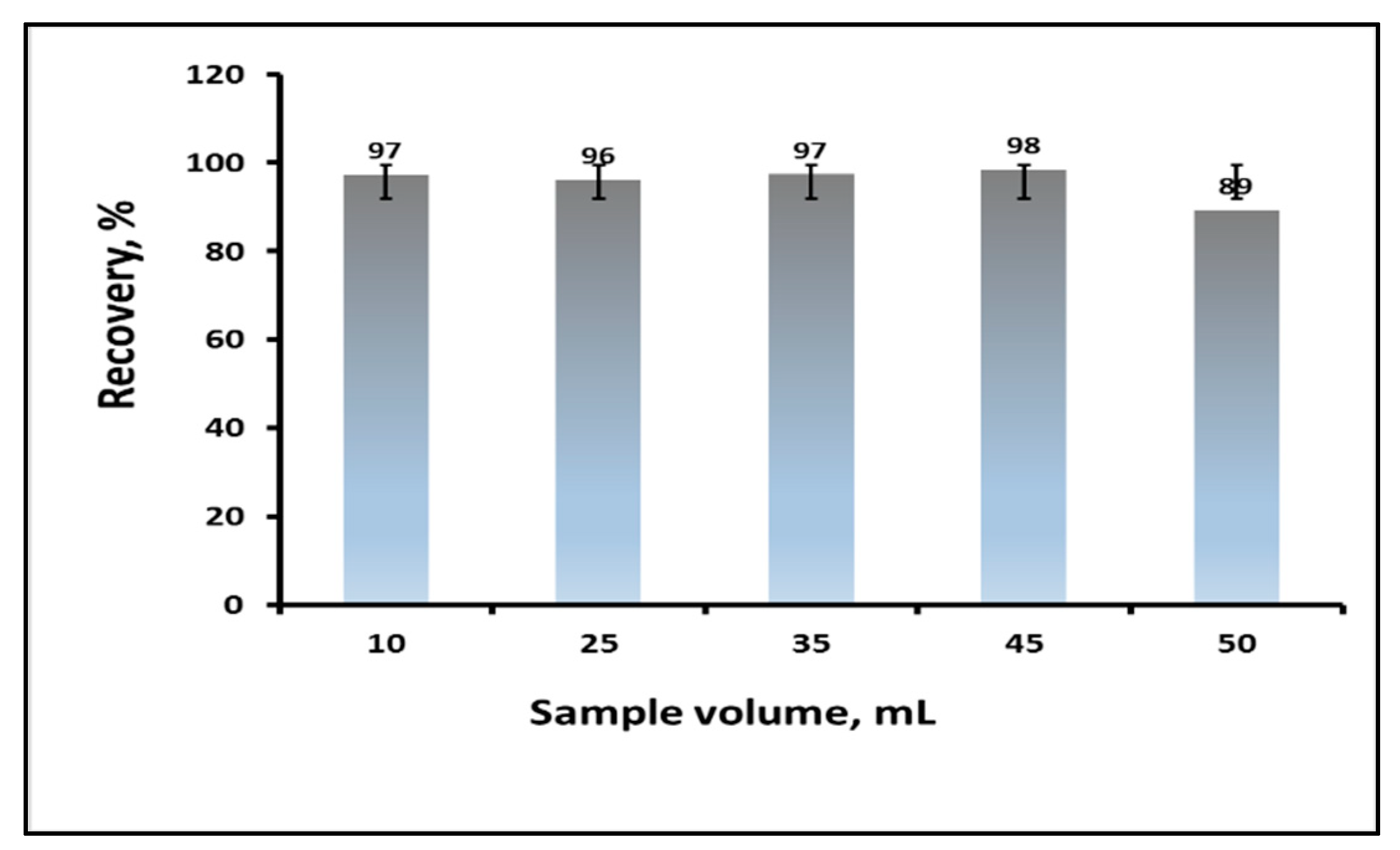
3.1.4. Effect of the Sample Volume on the Recovery% of Carbaryl
3.1.5. Effect of the Coexisting Ions on Carbaryl Recovery%
3.1.6. Analytical Features
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arustamov, Y.R.; Sukhanov, P.T.; Gubin, A.S.; Peregudov, Y.S.; Churilina, E.V.; Shatalov, G.V.; Koroleva, E.V. Sorption of carbaryl and naphthols by polymers based on N-vinylamides from aqueous solutions. Russ. J. Appl. Chem. 2013, 86, 1292–1297. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Pei, L.; Yue, S.; Ma, L.; Zhou, L.; Huang, Z.; He, Y.; Gao, J. Silver nanoparticles modified two-dimensional transition metal carbides as nanocarriers to fabricate acetycholinesterase-based electrochemical biosensor. Chem. Eng. J. 2018, 339, 547–556. [Google Scholar] [CrossRef]
- Chen, H.; He, X.; Rong, X.; Chen, W.; Cai, P.; Liang, W.; Li, S.; Huang, Q. Adsorption and biodegradation of carbaryl on montmorillonite, kaolinite and goethite. Appl. Clay Sci. 2009, 46, 102–108. [Google Scholar] [CrossRef]
- Bazrafshan, A.A.; Ghaedi, M.; Rafiee, Z.; Hajati, S.; Ostovan, A. Nano-sized molecularly imprinted polymer for selective ultrasound-assisted microextraction of pesticide carbaryl from water samples: Spectrophotometric determination. J. Colloid Interface Sci. 2017, 498, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Hatefi-Mehrjardi, A. Bienzyme self-assembled monolayer on gold electrode: An amperometric biosensor for carbaryl determination. Electrochim. Acta 2013, 114, 394–402. [Google Scholar] [CrossRef]
- Abdullah Alfaris, N.; Zaidan Altamimi, J.; Alothman, Z.A.; Wabaidur, S.M.; Ghafar, A.A.; Saleh Aldayel, T. Development of a sensitive liquid-liquid extraction and ultra-performance liquid chromatography-tandem mass spectrometry method for the analysis of carbaryl residues in fresh vegetables sold in Riyadh. J. King Saud Univ.-Sci. 2020, 32, 2414–2418. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, X.; Yuan, C.; Shi, R.; Wang, Y. Double responsive analysis of carbaryl pesticide based on carbon quantum dots and Au nanoparticles. Dye. Pigment. 2020, 181, 108529. [Google Scholar] [CrossRef]
- Ruengprapavut, S.; Sophonnithiprasert, T.; Pongpoungphet, N. The effectiveness of chemical solutions on the removal of carbaryl residues from cucumber and chili presoaked in carbaryl using the HPLC technique. Food Chem. 2020, 309, 125659. [Google Scholar] [CrossRef]
- Xiang, H.; Cai, Q.; Li, Y.; Zhang, Z.; Cao, L.; Li, K.; Yang, H. Review article sensors applied for the detection of pesticides and heavy metals in freshwaters. Hindawi J. Sens. 2020, 2020, 22. [Google Scholar] [CrossRef]
- Karnsa-Ard, S.; Santaladchaiyakit, Y.; Srijaranai, S.; Srijaranai, S. Modified QuEChERS and cloud-point extraction combined with visible spectrophotometric detection for carbaryl residue analysis in vegetables. Curr. Anal. Chem. 2012, 9, 150–156. [Google Scholar] [CrossRef]
- Mukdasai, S.; Thomas, C.; Srijaranai, S. Enhancement of sensitivity for the spectrophotometric determination of carbaryl using dispersive liquid microextraction combined with dispersive μ-solid phase extraction. Anal. Methods 2013, 5, 789–796. [Google Scholar] [CrossRef]
- Tabrizi, A.B.; Rashidi, M.R.; Ostadi, H. Nanoparticle-based solid-phase extraction procedure followed by spectrofluorimetry to determine carbaryl in different water samples. J. Braz. Chem. Soc. 2014, 25, 709–715. [Google Scholar] [CrossRef]
- Codex Alimentarius FAO-WHO. Pesticide Detail. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=8 (accessed on 1 December 2021).
- Guidelines for Canadian Drinking Water Quality: Guideline Technical Document–Carbaryl—Canada.Ca. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-carbaryl.html#fn7 (accessed on 1 December 2021).
- Wu, P.; Chen, Z.; Zhang, Y.; Wang, Y.; Zhu, F.; Cao, B.; Jin, L.; Hou, Y.; Wu, Y.; Li, N. Retraction notice to “carbaryl waste-water treatment by rhodopseudomonas sphaeroides” [Chemosphere 233 (October 2019) 597–602]. Chemosphere 2020, 257, 127394. [Google Scholar] [CrossRef]
- Derbalah, A.; Sunday, M.; Kato, R.; Takeda, K.; Sakugawa, H. Photoformation of reactive oxygen species and their potential to degrade highly toxic carbaryl and methomyl in river water. Chemosphere 2020, 244, 125464. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.S.; Givianrad, M.H.; Akhoundi, L.; Akhoundi, M. Preconcentration and determination of carbaryl and carbofuran in water samples using ionic liquids and in situ solvent formation microextraction. Anal. Methods 2013, 5, 2406–2412. [Google Scholar] [CrossRef]
- Fairbairn, J.W.; Relph, S.J. Sources of error in quantitative paper and thin layer chromatography II. Movement of solutes from initial to final spotfehlerquellen bei der quantitativen papier-und dünnschicht-chromatographie II. Bewegung der substanz vom start zum endflecksources d’erreurs en chromatographie quantitative sur papier et sur couche mince. II. Mouvement de solutes entre tache initiale et tache finale. Chromatographia 1969, 2, 204–207. [Google Scholar] [CrossRef]
- Asensio-Ramos, M.; Hernández-Borges, J.; González-Hernández, G.; Rodríguez-Delgado, M.Á. Hollow-fiber liquid-phase microextraction for the determination of pesticides and metabolites in soils and water samples using HPLC and fluorescence detection. Electrophoresis 2012, 33, 2184–2191. [Google Scholar] [CrossRef]
- Biswas, A.K.; Kondaiah, N.; Anjaneyulu, A.S.R.; Setty Rao, G.; Prakash Singh, R. A simple assay for analyzing residues of carbaryl insecticide in buffalo meat by liquid chromatography—Photodiode array detection. Anal. Methods 2010, 2, 393–396. [Google Scholar] [CrossRef]
- Bargańska, Ż.; Lambropoulou, D.; Namieśnik, J. Problems and challenges to determine pesticide residues in bumblebees. Crit. Rev. Anal. Chem. 2018, 48, 447–458. [Google Scholar] [CrossRef]
- Takino, M.; Yamaguchi, K.; Nakahara, T. Determination of carbamate pesticide residues in vegetables and fruits by liquid chromatography-atmospheric pressure photoionization-mass spectrometry and atmospheric pressure chemical ionization-mass spectrometry. J. Agric. Food Chem. 2004, 52, 727–735. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Yilmaz, E.; Habila, M.; Soylak, M. Solid Phase Extraction of Metal Ions in Environmental Samples on 1-(2-Pyridylazo)-2-Naphthol Impregnated Activated Carbon Cloth. Ecotoxicol. Environ. Saf. 2015, 112. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Dharmani, T.; Sharma, N. Extractive spectrophotometric method for the determination of carbaryl in environmental samples. Bull. Chem. Soc. Ethiop. 2015, 29, 173–186. [Google Scholar] [CrossRef]
- Shakouri, A.; Yazdanpanah, H.; Shojaee, M.H.; Kobarfard, F. Method development for simultaneous determination of 41 pesticides in rice using LC-MS/MS technique and its application for the analysis of 60 rice samples collected from tehran market. Iran. J. Pharm. Res. IJPR 2014, 13, 927. [Google Scholar] [PubMed]
- Barceló, D.; Petrovic, M. Challenges and achievements of LC-MS in environmental analysis: 25 years on. TrAC-Trends Anal. Chem. 2007, 26, 2–11. [Google Scholar] [CrossRef]
- Walorczyk, S.; Gnusowski, B. Development and validation of a multi-residue method for the determination of pesticides in honeybees using acetonitrile-based extraction and gas chromatography-tandem quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 6522–6531. [Google Scholar] [CrossRef]
- Walorczyk, S.; Kopeć, I.; Szpyrka, E. Pesticide residue determination by gas chromatography-tandem mass spectrometry as applied to food safety assessment on the example of some fruiting vegetables. Food Anal. Methods 2015, 9, 1155–1172. [Google Scholar] [CrossRef]
- Jing, X.; Wang, H.; Huang, X.; Chen, Z.; Zhu, J.; Wang, X. Digital image colorimetry detection of carbaryl in food samples based on liquid phase microextraction coupled with a microfluidic thread-based analytical device. Food Chem. 2021, 337, 127971. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Ogunneye, A.L.; Barbarinde, N.A.A.; Erenas, M.M.; Capitán-Vallvey, L.F. Bioactive microfluidic paper device for pesticide determination in waters. Talanta 2020, 218, 121108. [Google Scholar] [CrossRef]
- Habila, M.A.; Yilmaz, E.; AlOthman, Z.A.; Soylak, M. 1-Nitroso-2-Naphthol Impregnated Multiwalled Carbon Nanotubes (NNMWCNTs) for the Separation-Enrichment and Flame Atomic Absorption Spectrometric Detection of Copper and Lead in Hair, Water, and Food Samples. Desalin. Water Treat. 2017, 87, 285–291. [Google Scholar] [CrossRef]
- Bedassa, T.; Gure, A.; Megersa, N. The QuEChERS analytical method combined with low density solvent based dispersive liquid–liquid microextraction for quantitative extraction of multiclass pesticide residues in cereals. Bull. Chem. Soc. Ethiop. 2017, 31, 1–15. [Google Scholar] [CrossRef][Green Version]
- Farajzadeh, M.A.; Bamorowat, M.; Afshar Mogaddam, M.R. Development of a dispersive liquid-liquid microextraction method based on solidification of a floating ionic liquid for extraction of carbamate pesticides from fruit juice and vegetable samples. RSC Adv. 2016, 6, 112939–112948. [Google Scholar] [CrossRef]
- Khodadoust, S.; Hadjmohammadi, M. Determination of N-methylcarbamate insecticides in water samples using dispersive liquid-liquid microextraction and HPLC with the aid of experimental design and desirability function. Anal. Chim. Acta 2011, 699, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kongphonprom, K.; Burakham, R. Determination of carbamate insecticides in water, fruit, and vegetables by ultrasound-assisted dispersive liquid–liquid microextraction and high-performance liquid chromatography. Anal. Lett. 2016, 49, 753–767. [Google Scholar] [CrossRef]
- Ma, H.; Feng, W.; Tian, M.; Jia, Q. Determination of N-methylcarbamate pesticides in vegetables by poly(methacrylic acid-co-ethylene glycol dimethacrylate) monolith microextraction coupled with high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 929, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhou, X.; Ma, X.; Wang, C.; Wu, Q.; Wang, Z. Determination of carbamate pesticides in vegetables by octadecyl modified graphene reinforced hollow fiber liquid phase microextraction combined with high-performance liquid chromatography. Anal. Lett. 2015, 48, 1671–1685. [Google Scholar] [CrossRef]
- Soylak, M.; Elçi, L. Preconcentration and separation of trace metal ions from sea water samples by sorption on amberlite XAD-16 after complexation with sodium diethyl dithiocarbamate. Int. J. Environ. Anal. Chem. 2006, 66, 51–59. [Google Scholar] [CrossRef]
- Soylak, M.; Tuzen, M. Diaion SP-850 resin as a new solid phase extractor for preconcentration-separation of trace metal ions in environmental samples. J. Hazard. Mater. 2006, 137, 1496–1501. [Google Scholar] [CrossRef]
- Saracoglu, S.; Soylak, M.; Elci, L. Separation/preconcentration of trace heavy metals in urine, sediment and dialysis concentrates by coprecipitation with samarium hydroxide for atomic absorption spectrometry. Talanta 2003, 59, 287–293. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Li, Y.; Zang, X.; Wang, C.; Wang, Z. Application of dispersive liquid-liquid microextraction combined with high-performance liquid chromatography to the determination of carbamate pesticides in water samples. Anal. Bioanal. Chem. 2009, 393, 1755–1761. [Google Scholar] [CrossRef]
- Goulart, S.M.; Alves, R.D.; De Paula, W.X.; De Queiroz, J.H.; Neves, A.A.; De Queiroz, M.E.L.R. Determination of carbamates in beverages by liquid-liquid extraction with low temperature partitioning and liquid chromatography. J. Braz. Chem. Soc. 2012, 23, 1154–1165. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, E.; Zafar, F. Introductory chapter: Metal organic frameworks (MOFs). In Metal-Organic Frameworks; IntechOpen: London, UK, 2016. [Google Scholar]
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of metal-organic frameworks in food sample preparation. Molecules 2018, 23, 2896. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, H.; Yamini, Y.; Masoomi, M.Y.; Morsali, A. Nanostructured metal-organic frameworks, TMU-4, TMU-5, and TMU-6, as novel adsorbents for solid phase microextraction of polycyclic aromatic hydrocarbons. New J. Chem. 2017, 41, 12035–12043. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-organic frameworks in green analytical chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, J.H.; Zi, M.; Chen, X.X.; Yuan, L.M. Solid-phase extraction with metal–organic frameworks for the analysis of chiral compounds. Chirality 2016, 28, 778–783. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Termopoli, V. Metal–organic frameworks in solid-phase extraction procedures for environmental and food analyses. Chromatographia 2019, 82, 1191–1205. [Google Scholar] [CrossRef]
- Zha, J.; Yin, X.; Baltzegar, J.R.; Zhang, X. Coordinatively Unsaturated Metal Site-Promoted Selective Adsorption of Organic Molecules on Supported Metal–Organic Framework Nanosheets. Langmuir 2019, 35, 12908–12913. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Wang, W.; Wang, C.; Wang, Z. Covalent bonding of metal–organic framework-5/graphene oxide hybrid composite to stainless steel fiber for solid-phase microextraction of triazole fungicides from fruit and vegetable samples. J. Agric. Food Chem. 2016, 64, 2792–2801. [Google Scholar] [CrossRef]
- Rezaei Kahkha, M.R.; Oveisi, A.R.; Kaykhaii, M.; Rezaei Kahkha, B. Determination of carbamazepine in urine and water samples using amino-functionalized metal–organic framework as sorbent. Chem. Cent. J. 2018, 12, 77. [Google Scholar] [CrossRef]
- Sitnikov, D.G.; Monnin, C.S.; Vuckovic, D. Systematic assessment of seven solvent and solid-phase extraction methods for metabolomics analysis of human plasma by LC-MS. Sci. Rep. 2016, 6, 38885. [Google Scholar] [CrossRef]
- Caban, M.; Migowska, N.; Stepnowski, P.; Kwiatkowski, M.; Kumirska, J. Matrix effects and recovery calculations in analyses of pharmaceuticals based on the determination of β-blockers and β-agonists in environmental samples. J. Chromatogr. A 2012, 1258, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Chiadmi, F.; Schlatter, J. Determination and validation of a solid-phase extraction gas chromatography-mass spectrometry for the quantification of methadone and its principal metabolite in human plasma. Anal. Chem. Insights 2015, 10, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Deshmukh, N.I.; Shah, I.; Zachar, G.; Szekely, A.D.; Petroczi, A.; Naughton, D.P. LC-MS/MS-based assay for free and deconjugated testosterone and epitestosterone in rat urine and serum. J. Anal. Bioanal. Tech. 2014, s5, 006. [Google Scholar] [CrossRef]
- Zhou, G.; Tan, Z.R.; Zhang, W.; Ou-Yang, D.S.; Chen, Y.; Guo, D.; Liu, Y.Z.; Fan, L.; Deng, H.W. An improved LC-MS/MS method for quantitative determination of ilaprazole and its metabolites in human plasma and its application to a pharmacokinetic study. Acta Pharmacol. Sin. 2009, 30, 1330–1336. [Google Scholar] [CrossRef][Green Version]
- Habila, M.A.; ALOthman, Z.A.; Al-Tamrah, S.A.; Ghafar, A.A.; Soylak, M. Activated carbon from waste as an efficient adsorbent for malathion for detection and removal purposes. J. Ind. Eng. Chem. 2015, 32, 336–344. [Google Scholar] [CrossRef]
- Habila, M.; Yilmaz, E.; Alothman, Z.A.; Soylak, M. Flame atomic absorption spectrometric determination of Cd, Pb, and Cu in food samples after pre-concentration using 4-(2-thiazolylazo) resorcinol-modified activated carbon. J. Ind. Eng. Chem. 2014, 20, 3989–3993. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Yilmaz, E.; Alabdullkarem, E.A.; Soylak, M. A new amine based microextraction of lead (II) in real water samples using flame atomic absorption spectrometry. Microchem. J. 2019, 148, 214–219. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Habila, M.A.; Yilmaz, E.; Soylak, M.; Alfadul, S.M. Ultrasonic supramolecular microextration of nickel (II) as N,N′-dihydroxy-1,2-cyclohexanediimine chelates from water, tobacco and fertilizer samples before FAAS determination. J. Mol. Liq. 2016, 221, 773–777. [Google Scholar] [CrossRef]
- Lal, A.; Tan, G.; Chai, M. Multiresidue analysis of pesticides in fruits and vegetables using solid-phase extraction and gas chromatographic methods. Anal. Sci. 2008, 24, 231–236. [Google Scholar] [CrossRef]
- Ruiz-Medina, A.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Ortega-Barrales, P. Automated optosensor for the determination of carbaryl residues in vegetable edible oils and table olive extracts. J. Food Compos. Anal. 2012, 26, 66–71. [Google Scholar] [CrossRef]
- Paíga, P.; Domingues, V.F.; Wanderley, K.A.; Júnior, S.A.; Delerue-Matos, C.M. QuEChERS: A sample preparation for extraction of carbaryl from rat feces. Toxicol. Environ. Chem. 2015, 97, 687–699. [Google Scholar] [CrossRef]
- Dias, J.V.; Cutillas, V.; Lozano, A.; Pizzutti, I.R.; Fernández-Alba, A.R. Determination of pesticides in edible oils by liquid chromatography-tandem mass spectrometry employing new generation materials for dispersive solid phase extraction clean-up. J. Chromatogr. A 2016, 1462, 8–18. [Google Scholar] [CrossRef] [PubMed]

| Co-Existing Ion | Carbaryl Recovery% |
|---|---|
| Ca2+ | 97.0 ± 0.2 |
| Mg2+ | 99.0 ± 1.0 |
| Ni2+ | 98.0 ± 0.9 |
| Fe2+ | 96.0 ± 0.5 |
| Zn2+ | 96.0 ± 0.8 |
| Na2+ | 100.0 ± 0.2 |
| Cl2+ | 97.0 ± 0.5 |
| SO42− | 99 ± 0.09 |
| NO3− | 95.0 ± 1.2 |
| Samples | Carbaryl Concentration | Recovered Concentration after Spiking 0.300 mg.L−1 Carbaryl | Recovery% |
|---|---|---|---|
| Tap water | 0.00 mg.L−1 | 0.298 ± 0.050 mg.L−1 | 99 |
| Apple extract | 0.01 mg.kg−1 | 0.294 ± 0.050 mg.L−1 | 98 |
| Tomato extract | 0.02 mg.kg−1 | 0.279 ± 0.40 mg.L−1 | 93 |
| Red radish | 0.01 mg.kg−1 | 0.285 ± 0.040 mg.L−1 | 95 |
| Mango | 0.01 mg.kg−1 | 0.292 ± 0.010 mg.L−1 | 97 |
| Carrot | 0.01 mg.kg−1 | 0.274 ± 0.005 mg.L−1 | 91 |
| Carbaryl Solution (mg.L−1) | Intra-Day Analysis | Inter-Day Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Day | Third Day | Fifth Day | ||||||||||
| Detected Concentration (mg.L−1) | Accuracy% | Precision (RSD%) | Detected Concentration (mg.L−1) | Accuracy% | Precision (RSD%) | Detected Concentration (mg.L−1) | Accuracy% | Precision (RSD%) | Detected Concentration (mg.L−1) | Accuracy% | Precision (RSD%) | |
| 0.01 | 0.010 | 2.0 | 0.87 | 0.010 | 3.5 | 0.85 | 0.010 | 3.8 | 0.27 | 0.011 | 7.2 | 1.03 |
| 0.05 | 0.050 | 0.2 | 1.05 | 0.051 | 1.2 | 2.13 | 0.049 | 0.6 | 1.68 | 0.051 | 2.2 | 1.72 |
| 0.10 | 0.104 | 4.1 | 1.4 | 0.107 | 6.6 | 1.9 | 0.106 | 6.0 | 0.8 | 0.104 | 3.7 | 1.7 |
| Method Description | Limit of Detection | Limit of Quantification | RSD | References |
|---|---|---|---|---|
| SPE-GC | 0.05 mg.kg−1 | 0.15 mg.kg−1 | 4.7% | [62] |
| DLLME-HPLC | 0.4–1.0 μg.L−1 | 1.3 μg.L−1 | 5.1% | [41] |
| QuEChERS | 1.0 mg.kg−1 | - | 4% | [63] |
| LLE-LTP-HPLC-UV | 8.0 mg.L−1 | 25.0 mg.L−1 | - | [42] |
| QuEChERS-LC-FLD | 27.7 mg.kg−1 | 92.3 mg.kg−1 | 1.1% | [64] |
| UHPLC-QqQ-MS/MS | - | 10 mg.kg−1 | 4% | [65] |
| DSPME | 0.01 mg.L−1 | 0.03 mg.L−1 | 0.27–2.13% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habila, M.A.; Alhenaki, B.; El-Marghany, A.; Sheikh, M.; Ghfar, A.A.; ALOthman, Z.A.; Soylak, M. Metal Organic Framework-Based Dispersive Solid-Phase Microextraction of Carbaryl from Food and Water Prior to Detection by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Separations 2022, 9, 32. https://doi.org/10.3390/separations9020032
Habila MA, Alhenaki B, El-Marghany A, Sheikh M, Ghfar AA, ALOthman ZA, Soylak M. Metal Organic Framework-Based Dispersive Solid-Phase Microextraction of Carbaryl from Food and Water Prior to Detection by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Separations. 2022; 9(2):32. https://doi.org/10.3390/separations9020032
Chicago/Turabian StyleHabila, Mohamed A., Bushra Alhenaki, Adel El-Marghany, Mohamed Sheikh, Ayman A. Ghfar, Zeid A. ALOthman, and Mustafa Soylak. 2022. "Metal Organic Framework-Based Dispersive Solid-Phase Microextraction of Carbaryl from Food and Water Prior to Detection by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry" Separations 9, no. 2: 32. https://doi.org/10.3390/separations9020032
APA StyleHabila, M. A., Alhenaki, B., El-Marghany, A., Sheikh, M., Ghfar, A. A., ALOthman, Z. A., & Soylak, M. (2022). Metal Organic Framework-Based Dispersive Solid-Phase Microextraction of Carbaryl from Food and Water Prior to Detection by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Separations, 9(2), 32. https://doi.org/10.3390/separations9020032